Characterization of PDGF-Induced Subcellular Calcium Regulation through Calcium Channels in Airway Smooth Muscle Cells by FRET Biosensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Chemical Reagents
2.3. Construction of Calcium FRET Plasmids
2.4. FRET Plasmid Transfection
2.5. Intracellular FRET Imaging of Calcium Biosensors
2.6. Data Processing
3. Results
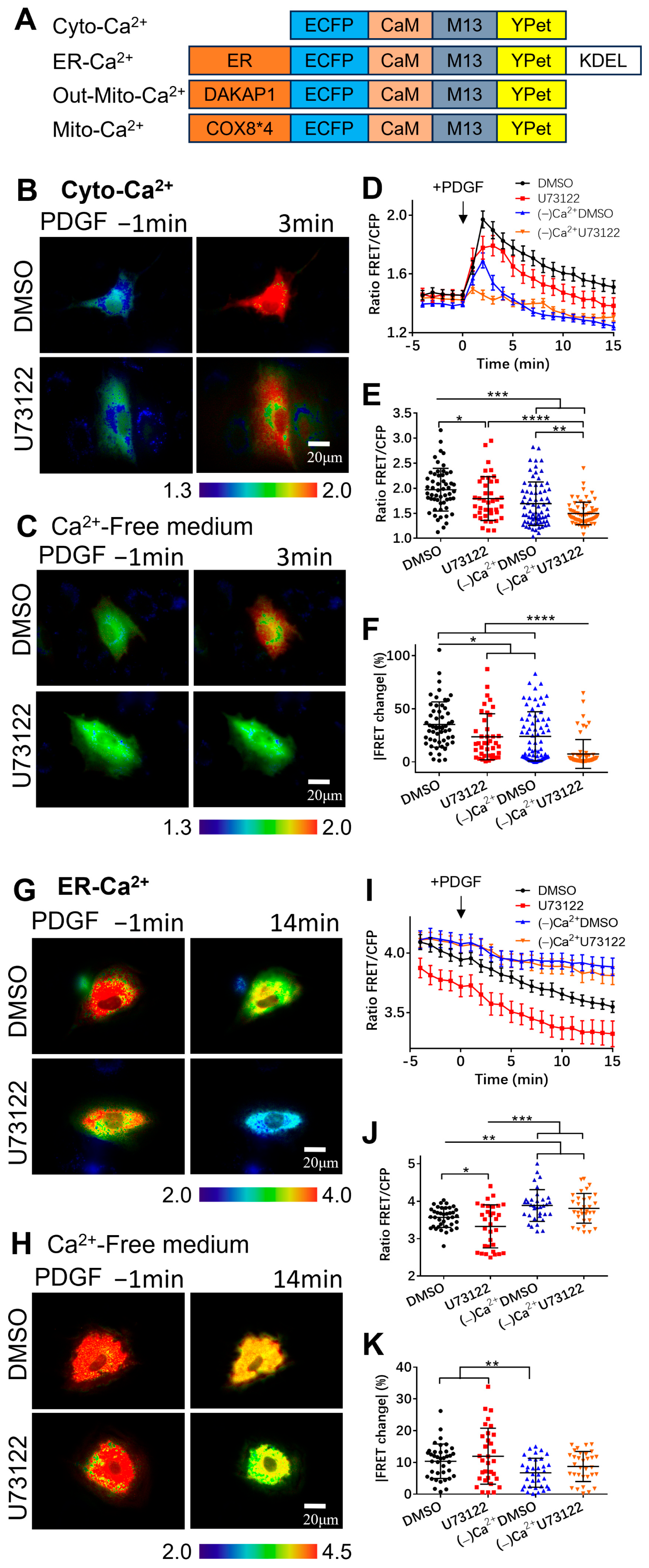
3.1. Extracellular Calcium Entry Affects Both Calcium Upregulation in Cytoplasm and Calcium Release from ER Storage
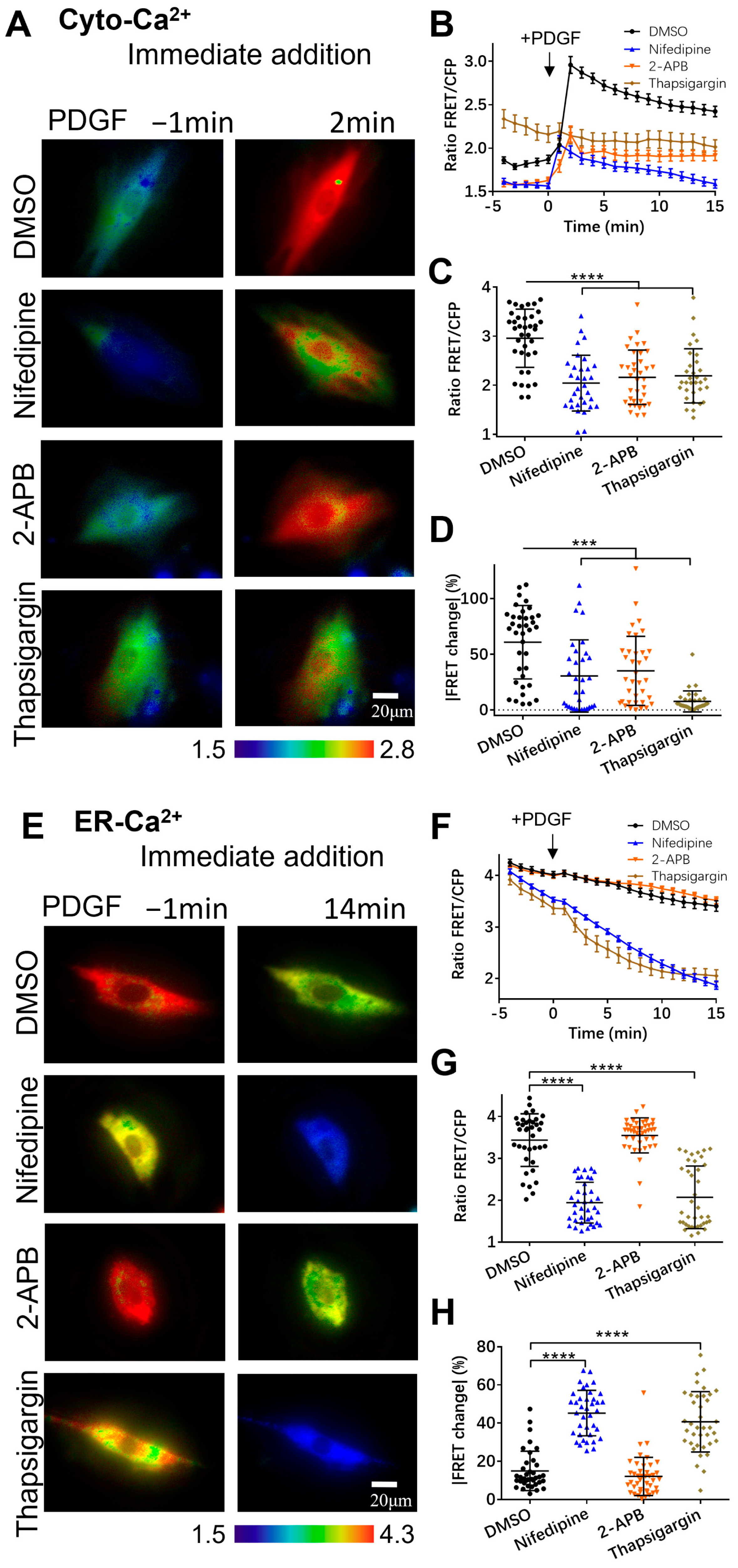
3.2. PDGF-Induced Increase in Cytoplasmic Calcium Is Regulated by ER Calcium Release and Extracellular Calcium Flow through Their Calcium Channels
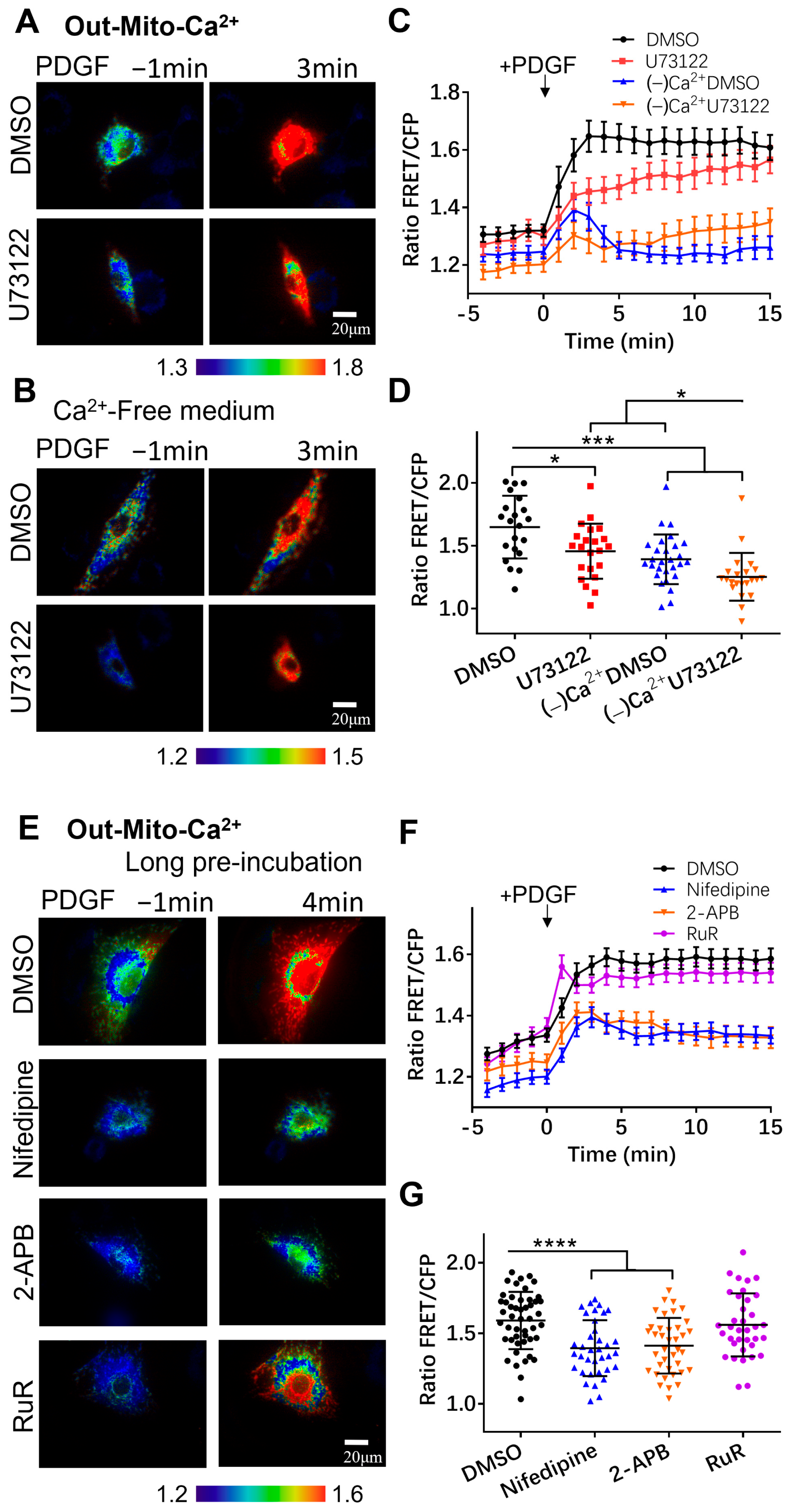
3.3. Regulation of Mitochondrial Calcium by Calcium Channels
3.4. Characterization of Calcium Exchange in Cellular Compartments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bidaux, G.; Flourakis, M.; Thebault, S.; Zholos, A.; Beck, B.; Gkika, D.; Roudbaraki, M.; Bonnal, J.L.; Mauroy, B.; Shuba, Y.; et al. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J. Clin. Investig. 2007, 117, 1647–1657. [Google Scholar] [CrossRef]
- Egan, C.G.; Wainwright, C.L.; Wadsworth, R.M.; Nixon, G.F. PDGF-induced signaling in proliferating and differentiated vascular smooth muscle: Effects of altered intracellular Ca2+ regulation. Cardiovasc. Res. 2005, 67, 308–316. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef]
- Pingel, J.; Vandenrijt, J.; Kampmann, M.L.; Andersen, J.D. Altered gene expression levels of genes related to muscle function in adults with cerebral palsy. Tissue Cell 2022, 76, 101744. [Google Scholar] [CrossRef]
- Du, X.; Zhi, J.; Yang, D.; Wang, Q.; Luo, X.; Deng, X. Research progress in the mechanism of calcium ion on contraction and relaxation of airway smooth muscle cells. J. Recept. Signal Transduct. Res. 2021, 41, 117–122. [Google Scholar] [CrossRef]
- Wang, I.Y.; Bai, Y.; Sanderson, M.J.; Sneyd, J. A mathematical analysis of agonist- and KCl-induced Ca2+ oscillations in mouse airway smooth muscle cells. Biophys. J. 2010, 98, 1170–1181. [Google Scholar] [CrossRef]
- Pepe, C.; Foley, S.; Shannon, J.; Lemiere, C.; Olivenstein, R.; Ernst, P.; Ludwig, M.S.; Martin, J.G.; Hamid, Q. Differences in airway remodeling between subjects with severe and moderate asthma. J. Allergy Clin. Immunol. 2005, 116, 544–549. [Google Scholar] [CrossRef]
- Kardas, G.; Daszyńska-Kardas, A.; Marynowski, M.; Brząkalska, O.; Kuna, P.; Panek, M. Role of Platelet-Derived Growth Factor (PDGF) in Asthma as an Immunoregulatory Factor Mediating Airway Remodeling and Possible Pharmacological Target. Front. Pharmacol. 2020, 11, 47. [Google Scholar] [CrossRef]
- Spinelli, A.M.; González-Cobos, J.C.; Zhang, X.; Motiani, R.K.; Rowan, S.; Zhang, W.; Garrett, J.; Vincent, P.A.; Matrougui, K.; Singer, H.A.; et al. Airway smooth muscle STIM1 and Orai1 are upregulated in asthmatic mice and mediate PDGF-activated SOCE, CRAC currents, proliferation, and migration. Pflug. Arch. Eur. J. Physiol. 2012, 464, 481–492. [Google Scholar] [CrossRef]
- Wang, W.A.; Agellon, L.B.; Michalak, M. Organellar Calcium Handling in the Cellular Reticular Network. Cold Spring Harb. Perspect. Biol. 2019, 11, a038265. [Google Scholar] [CrossRef]
- Case, R.M.; Eisner, D.; Gurney, A.; Jones, O.; Muallem, S.; Verkhratsky, A. Evolution of calcium homeostasis: From birth of the first cell to an omnipresent signalling system. Cell Calcium 2007, 42, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Drago, I.; Pizzo, P.; Pozzan, T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007, 14, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Wieckowski, M.R.; Chinopoulos, C.; Kepp, O.; Kroemer, G.; Galluzzi, L.; Pinton, P. Molecular mechanisms of cell death: Central implication of ATP synthase in mitochondrial permeability transition. Oncogene 2015, 34, 1475–1486. [Google Scholar] [CrossRef]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed]
- Mykytyn, K.; Askwith, C. G-Protein-Coupled Receptor Signaling in Cilia. Cold Spring Harb. Perspect. Biol. 2017, 9, a028183. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta 2009, 1793, 933–940. [Google Scholar] [CrossRef]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Schmitz, E.A.; Takahashi, H.; Karakas, E. Structural basis for activation and gating of IP3 receptors. Nat. Commun. 2022, 13, 1408. [Google Scholar] [CrossRef]
- Ozawa, T. Ryanodine-sensitive Ca2+ release mechanism in non-excitable cells (Review). Int. J. Mol. Med. 2001, 7, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Mekahli, D.; Bultynck, G.; Parys, J.B.; De Smedt, H.; Missiaen, L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a004317. [Google Scholar] [CrossRef] [PubMed]
- Kamer, K.J.; Mootha, V.K. The molecular era of the mitochondrial calcium uniporter. Nat. Rev. Mol. Cell Biol. 2015, 16, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Patergnani, S.; Pinton, P. The endoplasmic reticulum-mitochondria connection: One touch, multiple functions. Biochim. Biophys. Acta 2014, 1837, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Madec, A.M.; Perrier, J.; Panthu, B.; Dingreville, F. Role of mitochondria-associated endoplasmic reticulum membrane (MAMs) interactions and calcium exchange in the development of type 2 diabetes. Int. Rev. Cell Mol. Biol. 2021, 363, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shyy, J.Y.; Chien, S. Fluorescence proteins, live-cell imaging, and mechanobiology: Seeing is believing. Annu. Rev. Biomed. Eng. 2008, 10, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Wan, R.; Qin, Q.; Peng, Q.; Wang, P.; Wu, J.; Allen, M.; Shi, Y.; Laub, S.; Deng, L.; et al. Sensitive FRET Biosensor Reveals Fyn Kinase Regulation by Submembrane Localization. ACS Sens. 2019, 4, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots prepared for fluorescence and chemiluminescence sensing. Sci China Chem. 2019, 62, 968–981. [Google Scholar] [CrossRef]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Tay, L.H.; Griesbeck, O.; Yue, D.T. Live-cell transforms between Ca2+ transients and FRET responses for a troponin-C-based Ca2+ sensor. Biophys. J. 2007, 93, 4031–4040. [Google Scholar] [CrossRef]
- Miyawaki, A.; Llopis, J.; Heim, R.; McCaffery, J.M.; Adams, J.A.; Ikura, M.; Tsien, R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997, 388, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Rice, K.G. Peptide-guided gene delivery. AAPS J. 2007, 9, E18–E29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Araki, S.; Jiahui, W.H.; Teramoto, T.; Chang, Y.F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An Expanded Palette of Genetically Encoded Ca2+ Indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef]
- Ouyang, M.X.; Sun, J.; Chien, S.; Wang, Y.X. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc. Natl. Acad. Sci. USA 2008, 105, 14353–14358. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Joo, C.; Seong, J.; Vafabakhsh, R.; Botvinick, E.L.; Berns, M.W.; Palmer, A.E.; Wang, N.; Ha, T.; Jakobsson, E.; et al. Distinct mechanisms regulating mechanical force-induced Ca2⁺ signals at the plasma membrane and the ER in human MSCs. eLife 2015, 4, e04876. [Google Scholar] [CrossRef]
- Mehta, S.; Aye-Han, N.N.; Ganesan, A.; Oldach, L.; Gorshkov, K.; Zhang, J. Calmodulin-controlled spatial decoding of oscillatory Ca2+ signals by calcineurin. eLife 2014, 3, e03765. [Google Scholar] [CrossRef]
- Ashrafi, G.; de Juan-Sanz, J.; Farrell, R.J.; Ryan, T.A. Molecular Tuning of the Axonal Mitochondrial Ca2+ Uniporter Ensures Metabolic Flexibility of Neurotransmission. Neuron 2020, 105, 678–687.e5. [Google Scholar] [CrossRef]
- Qin, Q.; Laub, S.; Shi, Y.W.; Ouyang, M.X.; Peng, Q.; Zhang, J.; Wang, Y.X.; Lu, S.Y. Fluocell for Ratiometric and High-Throughput Live-Cell Image Visualization and Quantitation. Front. Phys. 2019, 7, 154. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Fan, M.R.; Zhang, J.R.; Tsai, C.W.; Orlando, B.J.; Rodriguez, M.; Xu, Y.; Liao, M.F.; Tsai, M.F.; Feng, L. Structure and mechanism of the mitochondrial Ca uniporter holocomplex. Nature 2020, 582, 129–133. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnóczky, G. Intracellular Ca Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell. 2017, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.; Blum, R. Shaped by leaky ER: Homeostatic Ca fluxes. Front. Physiol. 2022, 13, 972104. [Google Scholar] [CrossRef] [PubMed]
- Van Petegem, F. Ryanodine Receptors: Structure and Function. J. Biol. Chem. 2012, 287, 31624–31632. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting calcium signaling in cancer therapy. Acta Pharm. Sin. B 2017, 7, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Mahn, K.; Ojo, O.O.; Chadwick, G.; Aaronson, P.I.; Ward, J.P.; Lee, T.H. Ca2+ homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax 2010, 65, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Emter, C.A.; Bowles, D.K. Store-operated Ca2+ entry is not essential for PDGF-BB induced phenotype modulation in rat aortic smooth muscle. Cell Calcium 2010, 48, 10–18. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, H.; Jiang, D.; Yang, K.; Wang, S.T.; Zhang, Y.X.; Wang, Y.; Liu, H.; Guo, C.; Tang, T.S. ER Ca2+ overload activates the IRE1α signaling and promotes cell survival. Cell Biosci. 2023, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ni, K.; Guo, J.; Li, Y.Q.; Zhou, Y.; Sheng, H.; Bu, B.; Luo, M.Z.; Ouyang, M.X.; Deng, L.H. FRET Visualization of Cyclic Stretch-Activated ERK via Calcium Channels Mechanosensation While Not Integrin β1 in Airway Smooth Muscle Cells. Front. Cell Dev. Biol. 2022, 10, 847852. [Google Scholar] [CrossRef]
- Kamishima, T.; Quayle, J.M. Ca-induced Ca release in cardiac and smooth muscle cells. Biochem. Soc. Trans. 2003, 31, 943–946. [Google Scholar] [CrossRef]
- Iida-Tanaka, N.; Namekata, I.; Kaneko, M.; Tamura, M.; Kawanishi, T.; Nakamura, R.; Shigenobu, K.; Tanaka, H. Involvement of intracellular Ca2+ in the regulatory volume decrease after hyposmotic swelling in MDCK cells. J. Pharmacol. Sci. 2007, 104, 397–401. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, M.; Zhou, B.; Li, C.; Deng, L. Characterization of PDGF-Induced Subcellular Calcium Regulation through Calcium Channels in Airway Smooth Muscle Cells by FRET Biosensors. Biosensors 2024, 14, 179. https://doi.org/10.3390/bios14040179
Ouyang M, Zhou B, Li C, Deng L. Characterization of PDGF-Induced Subcellular Calcium Regulation through Calcium Channels in Airway Smooth Muscle Cells by FRET Biosensors. Biosensors. 2024; 14(4):179. https://doi.org/10.3390/bios14040179
Chicago/Turabian StyleOuyang, Mingxing, Binqian Zhou, Chunmei Li, and Linhong Deng. 2024. "Characterization of PDGF-Induced Subcellular Calcium Regulation through Calcium Channels in Airway Smooth Muscle Cells by FRET Biosensors" Biosensors 14, no. 4: 179. https://doi.org/10.3390/bios14040179
APA StyleOuyang, M., Zhou, B., Li, C., & Deng, L. (2024). Characterization of PDGF-Induced Subcellular Calcium Regulation through Calcium Channels in Airway Smooth Muscle Cells by FRET Biosensors. Biosensors, 14(4), 179. https://doi.org/10.3390/bios14040179




