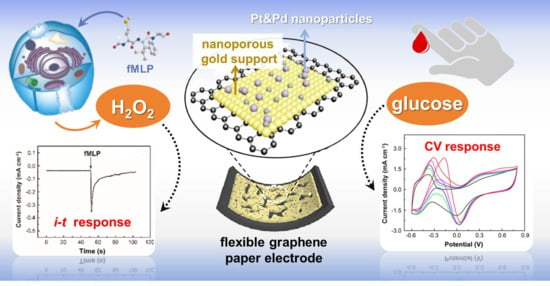
Flexible Graphene Paper Modified Using Pt&Pd Alloy Nanoparticles Decorated Nanoporous Gold Support for the Electrochemical Sensing of Small Molecular Biomarkers
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Freestanding GP
2.2. Preparation of Freestanding NPG/GP
2.3. Preparation of Pt&Pd-NPs–NPG/GP
2.4. Cell Culture
2.5. Treatment of Human Body Fluid Samples
3. Results and Discussion
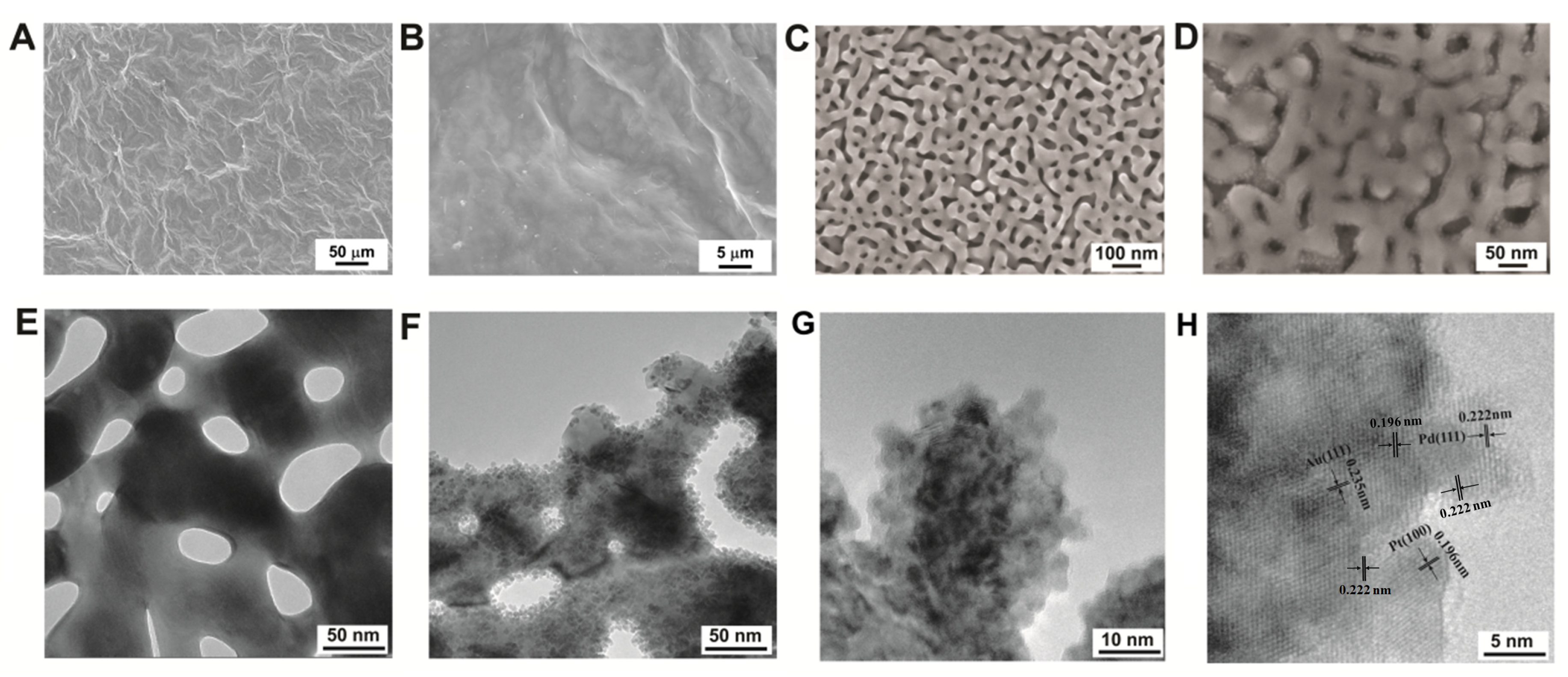
3.1. Physical Characterization of the Nanohybrid Paper Electrode
3.2. Electrochemical Characterization of the Nanohybrid Paper Electrode
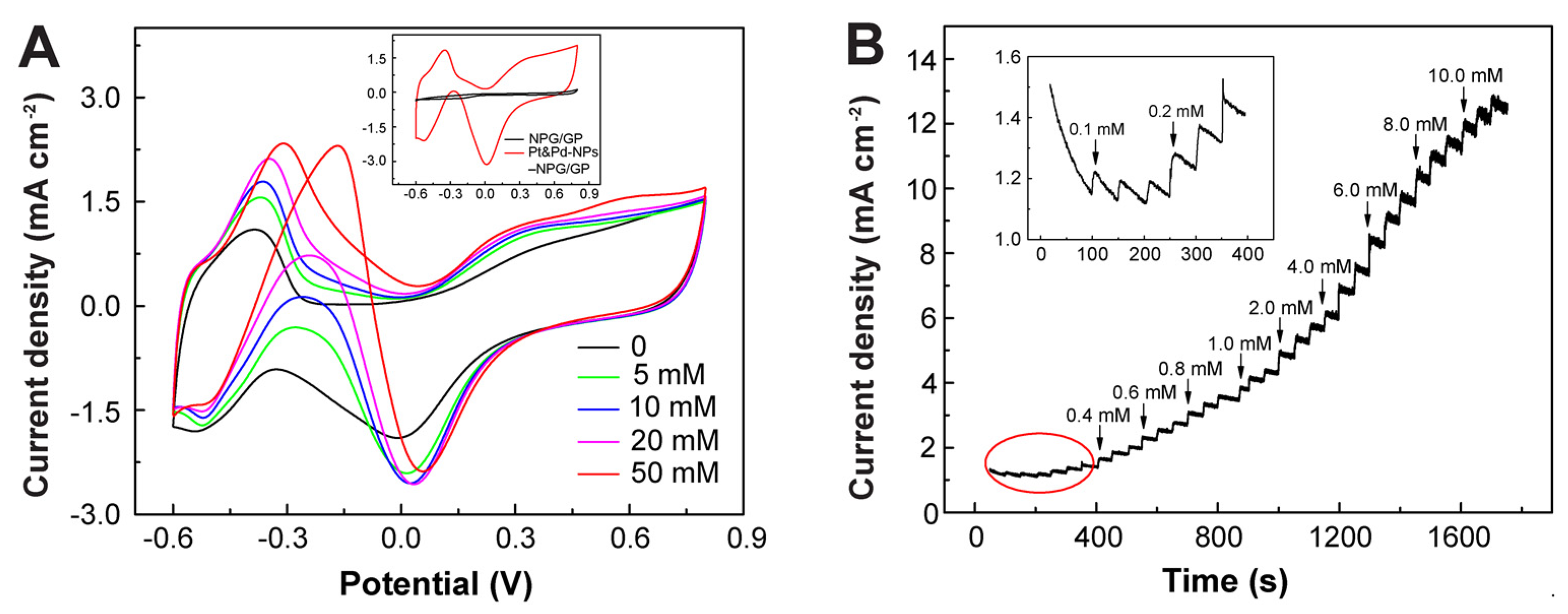
3.3. Electrochemical Sensing Performances of Pt&Pd-NPs–NPG/GP towards H2O2
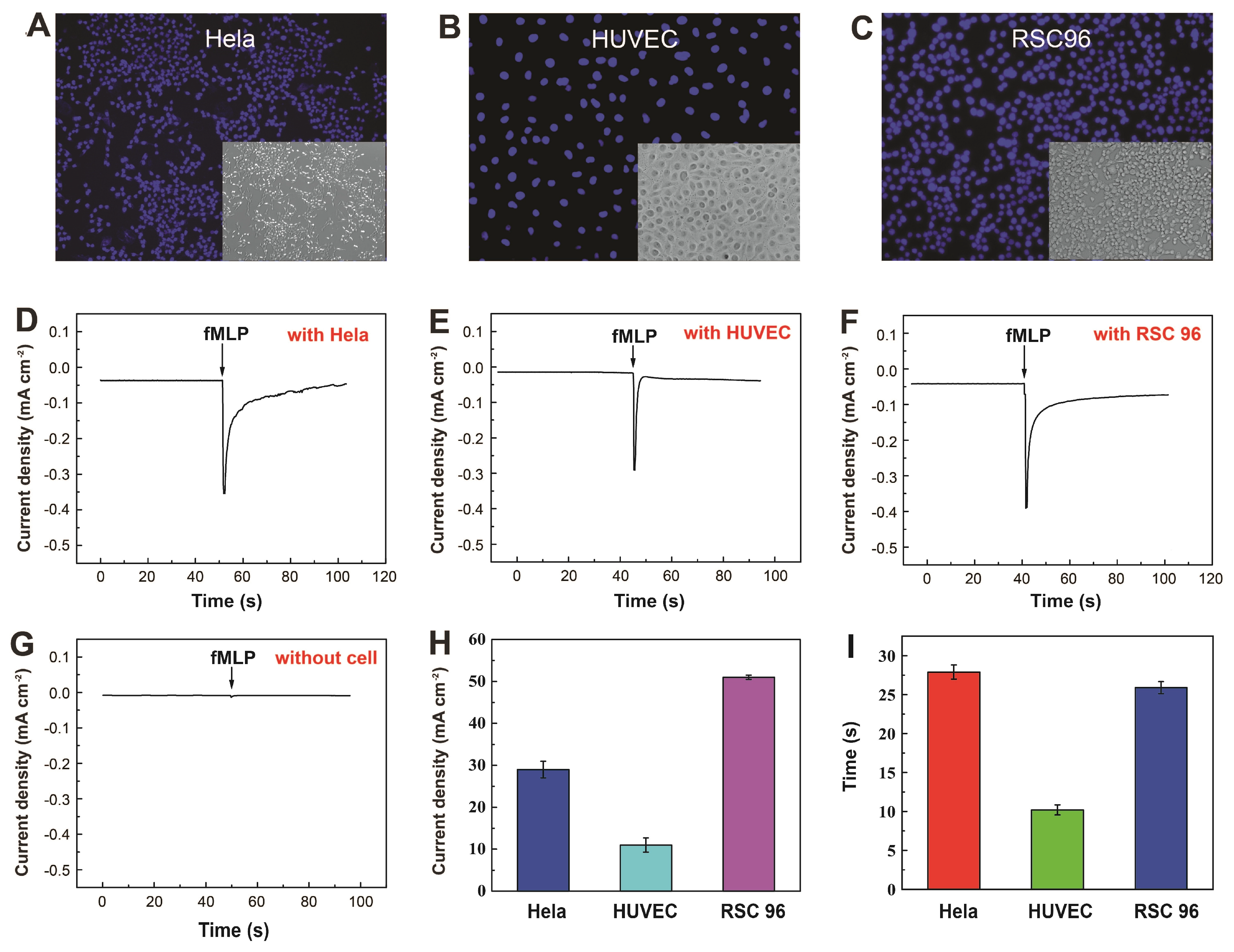
3.4. Real-Time Electrochemical Detection of H2O2 Secretion by Live Cells
3.5. Electrochemical Sensing Performances of Pt&Pd-NPs–NPG/GP towards Glucose
3.6. Electrochemical Detection of Glu in Different Human Body Fluid Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lennicke, C.; Cocheme, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Y.; Su, L.; Zhao, D.; Zhao, L.; Zhang, X. Microfluidic chip-based wearable colorimetric sensor for simple and facile detection of sweat glucose. Anal. Chem. 2019, 91, 14803–14807. [Google Scholar] [CrossRef]
- Lipani, L.; Dupont, B.G.R.; Doungmene, F.; Marken, F.; Tyrrell, R.M.; Guy, R.H.; Ilie, A. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 2018, 13, 504–511. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Biomed. Eng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Li, D.; Xiong, Q.; Liu, W.; Liang, L.; Duan, H. Nanozymatic magnetic nanomixers for enzyme immobilization and multiplexed detection of metabolic disease biomarkers. Biosens. Bioelectron. 2023, 219, 114795. [Google Scholar] [CrossRef]
- Saha, T.; Del Caño, R.; Mahato, K.; De la Paz, E.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable electrochemical glucose sensors in diabetes management: A comprehensive review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Zhu, M.; Kuang, Z.; Li, X.; Xu, F.; Miao, S.; Zhang, Z.; Lou, X.; Li, H.; et al. Electrochemical biosensors for whole blood analysis: Recent progress, challenges, and future perspectives. Chem. Rev. 2023, 123, 7953–8039. [Google Scholar] [CrossRef]
- Liu, Y.L.; Huang, W.H. Stretchable electrochemical sensors for cell and tissue detection. Angew. Chem. Int. Ed. 2020, 60, 2757–2767. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Chen, W. Recent advances in graphene-based nanomaterials for fabricating electrochemical hydrogen peroxide sensors. Biosens. Bioelectron. 2017, 89, 249–268. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lasalde-Ramírez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional nanomaterials and nanostructures enhancing electrochemical biosensors and lab-on-chip perperformances: Recent progress, applications, and future perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Amali, R.K.A.; Lim, H.N.; Ibrahim, I.; Huang, N.M.; Zainal, Z.; Ahmad, S.A.A. Significance of nanomaterials in electrochemical sensors for nitrate detection: A review. Trends Environ. Anal. Chem. 2021, 31, e00135. [Google Scholar] [CrossRef]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.-H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef]
- Dong, T.; Matos Pires, N.M.; Yang, Z.; Jiang, Z. Advances in electrochemical biosensors based on nanomaterials for protein biomarker detection in saliva. Adv. Sci. 2022, 10, 2205429. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. TrAC Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, Z.; Liu, Q.; Jia, Y.; Li, Y.; Liu, K.; Lin, Y.; Liu, M.; Li, G.; Su, C.Y. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 2021, 12, 1369. [Google Scholar] [CrossRef]
- Yi, J.; Xianyu, Y. Gold nanomaterials-implemented wearable sensors for healthcare applications. Adv. Funct. Mater. 2022, 32, 2113012. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B. Recent advances in porous Pt-based nanostructures: Synthesis and electrochemical applications. Chem. Soc. Rev. 2014, 43, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, H.; Wang, C.; Yang, M.; Zhou, A.; Duan, H. Ultrasonic-electrodeposition of PtPd alloy nanoparticles on ionic liquid-functionalized graphene paper: Towards a flexible and versatile nanohybrid electrode. Nanoscale 2016, 8, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hiekel, K.; Hübner, R.; Sun, H.J.; Ferancova, A.; Sillanpää, M. Pt and Au bimetallic and monometallic nanostructured amperometric sensors for direct detection of hydrogen peroxide: Influences of bimetallic effect and silica support. Sens. Actuators B Chem. 2018, 255, 1325–1334. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Y.; She, J.; Xu, Y.; Zhang, Y.; Zhao, A.; Manoj, D.; Xi, J.; Sun, Y.; Ren, J.; et al. 3D nitrogen-doped carbon nanofoam arrays embedded with PdCu alloy nanoparticles: Assembling on flexible microelectrode for electrochemical detection in cancer cells. Anal. Chim. Acta 2021, 1158, 338420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, W.; Si, C.; Zhang, J.; Yan, X.; Jin, C.; Ding, Y.; Zhang, Z. Self-supporting nanoporous gold-palladium overlayer bifunctional catalysts toward oxygen reduction and evolution reactions. Nano Res. 2016, 9, 3781–3794. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.; Kasuga, T.; Nogi, M.; Koga, H. All-nanochitin-derived, super-compressible, elastic, and robust carbon honeycombs and their pressure-sensing properties over an ultrawide temperature range. ACS Appl. Mater. Interfaces 2023, 15, 41732–41742. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, L.; Kasuga, T.; Nogi, M.; Koga, H. Frequency-tunable and absorption/transmission-switchable microwave absorber based on a chitin-nanofiber-derived elastic carbon aerogel. Chem. Eng. J. 2023, 469, 144010. [Google Scholar] [CrossRef]
- Luc, W.; Jiao, F. Nanoporous metals as electrocatalysts: State-of-the-art, opportunities, and challenges. ACS Catal. 2017, 7, 5856–5861. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Ren, L.Q.; Huang, Y.P.; Li, X.D.; Wu, S.H.; Sun, J.J. 3D nanoporous gold-supported Pt nanoparticles as highly accelerating catalytic Au-Pt micromotors. Adv. Mater. Interfaces 2018, 5, 1701689. [Google Scholar] [CrossRef]
- Chen, Q. Bicontinuous nanoporous metals with self-organized functionalities. Chem. Mater. 2022, 34, 10237–10248. [Google Scholar] [CrossRef]
- Flores-Hernandez, D.R.; Santamaria-Garcia, V.J.; Melchor-Martinez, E.M.; Sosa-Hernandez, J.E.S.; Parra-Saldivar, R.; Bonilla-Rios, J. Paper and other fibrous materials—A complete platform for biosensing applications. Biosensors 2021, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Xu, Y.; Zhao, A.; He, W.; Xiao, F. Flexible electrochemical sensors integrated with nanomaterials for in situ determination of small molecules in biological samples: A review. Anal. Chim. Acta 2022, 1207, 339461. [Google Scholar] [CrossRef] [PubMed]
- Kongkaew, S.; Meng, L.; Limbut, W.; Liu, G.; Kanatharana, P.; Thavarungkul, P.; Mak, W.C. Craft-and-stick xurographic manufacturing of integrated microfluidic electrochemical sensing platform. Biosensors 2023, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Poletti, F.; Scidà, A.; Zanfrognini, B.; Kovtun, A.; Parkula, V.; Favaretto, L.; Melucci, M.; Palermo, V.; Treossi, E.; Zanardi, C. Graphene-paper-based electrodes on plastic and textile supports as new platforms for amperometric biosensing. Adv. Funct. Mater. 2021, 32, 2107941. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, S.; Zhang, Z.; Liu, H.; Xiao, J.; Wan, L.; Luo, J.; Wang, S.; Liu, Y. Scalable synthesis of freestanding sandwich-structured graphene/polyaniline/ graphene nanocomposite paper for flexible all-solid-state supercapacitor. Sci. Rep. 2015, 9, 9359. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.; Simon, F.; Formanek, P.; Müller, M.; Gupta, S.; Stamm, M.; Uhlmann, P. Catalytically active nanocomposites based on palladium and platinum nanoparticles in poly(2-vinylpyridine) brushes. Macromol. Chem. Phys. 2013, 214, 2301–2311. [Google Scholar] [CrossRef]
- Park, I.S.; Lee, K.S.; Jung, D.S.; Park, H.Y.; Sung, Y.E. Electrocatalytic activity of carbon-supported Pt–Au nanoparticles for methanol electro-oxidation. Electrochim. Acta 2007, 52, 5599–5605. [Google Scholar] [CrossRef]
- Friedrich, K.A.; Henglein, F.; Stimming, U.; Unkauf, W. Investigation of Pt particles on gold substrates by IR spectroscopy particle structure and catalytic activity. Colloid. Surface A Physicochem. Eng. Asp. 1998, 134, 193–206. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Baeck, S.H.; Cuenya, B.R.; McFarland, E.W. Catalytic activity of supported Au nanoparticles deposited from block copolymer micelles. J. Am. Chem. Soc. 2003, 125, 7148–7149. [Google Scholar] [CrossRef] [PubMed]
- Oesch, U.; Janata, J. Electrochemical study of gold electrodes with anodic oxide films-I. Formation and reduction behavior of anodic oxides on gold. Electrochim. Acta 1983, 28, 1237–1246. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Vogelsang, L. H2O2 sensing in immunity. Nat. Plants 2022, 8, 1140–1141. [Google Scholar] [CrossRef]
- Lin, L.-S.; Huang, T.; Song, J.; Ou, X.-Y.; Wang, Z.; Deng, H.; Tian, R.; Liu, Y.; Wang, J.-F.; Liu, Y.; et al. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J. Am. Chem. Soc. 2019, 141, 9937–9945. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ye, J.; Tang, Y.; Yuan, H.; Wen, D. Superhydrophilicity regulation of carbon nanotubes boosting electrochemical biosensing for real-time monitoring of H2O2 released from living cells. Anal. Chem. 2023, 95, 17851–17859. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xu, Y.; Wang, Z.; Liao, K.; Zhang, Y.; Sun, Y. Dual nanozyme based on ultrathin 2D conductive MOF nanosheets intergraded with gold nanoparticles for electrochemical biosensing of H2O2 in cancer cells. Talanta 2022, 249, 123612. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dong, J.; Chi, K.; Wang, L.; Xiao, F.; Wang, S.; Zhao, Y.; Liu, Y. Electrically conductive metal–organic framework thin film-based on-chip micro-biosensor: A platform to unravel surface morphology-dependent biosensing. Adv. Funct. Mater. 2021, 31, 2102855. [Google Scholar] [CrossRef]
- Daemi, S.; Ghasemi, S.; Akbar Ashkarran, A. Electrospun CuO-ZnO nanohybrid: Tuning the nanostructure for improved amperometric detection of hydrogen peroxide as a non-enzymatic sensor. J. Colloid Interface Sci. 2019, 550, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dong, X.; He, H.; Zhang, Y.; Chi, K.; Xu, Y.; Asif, M.; Yang, X.; He, W.; Liao, K.; et al. 2D carbon network arranged into high-order 3D nanotube arrays on a flexible microelectrode: Integration into electrochemical microbiosensor devices for cancer detection. NPG Asia Mater. 2023, 15, 6. [Google Scholar] [CrossRef]
- Li, J.; Shen, C.; Luo, J.; Pan, T.; Deng, J.; Cao, Z. Metal-organic framework-derived brain platygyra coral-like porous carbon architectures for real-time monitoring of hydrogen peroxide in biological matrices. Chem. Eng. J. 2023, 471, 144805. [Google Scholar] [CrossRef]
- Han, N.; Hua, S.Y.; Zhang, L.Y.; Yi, S.S.; Zhang, Z.T.; Wang, Y.; Zhou, Y.; Chen, D.L.; Gao, Y.F. CuCo-Cu@CoCH stamen-like nanoarray prepared by co-reduction for electrochemical detection of hydrogen peroxide. Appl. Surf. Sci. 2022, 576, 151879. [Google Scholar] [CrossRef]
- Patella, B.; Buscetta, M.; Di Vincenzo, S.; Ferraro, M.; Aiello, G.; Sunseri, C.; Pace, E.; Inguanta, R.; Cipollina, C. Electrochemical sensor based on rGO/Au nanoparticles for monitoring H2O2 released by human macrophages. Sens. Actuators B Chem. 2021, 327, 128901. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Chai, X.; Wang, T.; Cao, T.; Li, Y.; Zhang, L.; Fan, F.; Fu, Y.; Qi, W. An electrochemical sensor for H2O2 based on Au nanoparticles embedded in UiO-66 metal–organic framework films. ACS Appl. Nano Mater. 2021, 4, 6103–6110. [Google Scholar]
- He, G.; Gao, F.; Li, W.; Li, P.; Zhang, X.; Yin, H.; Yang, B.; Liu, Y.; Zhang, S. Electrochemical sensing of H2O2 released from living cells based on AuPd alloy-modified PDA nanotubes, Anal. Methods 2019, 11, 1651–1656. [Google Scholar]
- Xiao, F.; Song, J.; Gao, H.; Zan, X.; Xu, R.; Duan, H. Coating graphene paper with 2D-assembly of electrocatalytic nanoparticles: A modular approach toward high-performance flexible electrodes. ACS Nano 2012, 6, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.; Hernández-Rodríguez, J.F.; Della Pelle, F.; Del Carlo, M.; Compagnone, D.; Escarpa, A. Oxidative stress on-chip: Prussian blue-based electrode array for in situ detection of H2O2 from cell populations. Biosens. Bioelectron. 2020, 170, 112669. [Google Scholar] [CrossRef] [PubMed]
- DeJournett, J.; Nekludov, M.; DeJournett, L.; Wallin, M. Performance of a closed-loop glucose control system, comprising a continuous glucose monitoring system and an AI-based controller in swine during severe hypo- and hyperglycemic provocations. J. Clin. Monit. Comput. 2021, 35, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ju, Y.; Chen, J.; Liu, D.; Liu, H. Nonenzymatic wearable sensor for electrochemical analysis of perspiration glucose. ACS Sens. 2018, 3, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Habrioux, A.; Sibert, E.; Servat, K.; Vogel, W.; Kokoh, K.B.; Alonso, V.N. Activity of platinum–gold alloys for glucose electrooxidation in biofuel cells. J. Phys. Chem. B. 2007, 111, 10329–10333. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Feng, J.; Li, H.; Xiao, Z.; Qiu, Y.; Yu, X.; Xiang, J. Novel synthesis of carbon nanofiber aerogels from coconut matrix for the electrochemical detection of glucose. Diam. Relat. Mater. 2021, 111, 108180. [Google Scholar] [CrossRef]
- Long, M.; Tan, L.; Liu, H.; He, Z.; Tang, A. Novel helical TiO2 nanotube arrays modified by Cu2O for enzyme-free glucose oxidation. Biosens. Bioelectron. 2014, 59, 243–250. [Google Scholar] [CrossRef]
- Wang, L.; Fu, J.; Song, Y. A facile strategy to prepare Cu2O/Cu electrode as a sensitive enzyme-free glucose sensor. Int. J. Electrochem. Sci. 2012, 7, 12587–12600. [Google Scholar] [CrossRef]
- Belkhalfa, H.; Teodorescu, F.; Quéniat, G.; Co nier, Y.; Dokhan, N.; Sam, S.; Abderrahmani, A.; Boukherroub, R.; Szunerits, S. Insulin impregnated reduced graphene oxide/Ni(OH)2 thin films for electrochemical insulin release and glucose sensing. Sens. Actuat. B-Chem. 2016, 237, 693–701. [Google Scholar] [CrossRef]
- Kim, Y.J.; Chinnadayyala, S.R.; Le, H.T.N.; Cho, S. Sensitive electrochemical non-Enzymatic detection of glucose based on wireless data transmission. Sensors 2022, 22, 2787. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.J.; Wang, A.N.; Liao, Q.L.; Chuang, K.-Y. Non-enzymatic glucose sensor composed of carbon-coated nano-zinc oxide. Nanomaterials 2017, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.S.; Liu, Z.T.; Lai, C.C.; Lo, C.T.; Lee, C.L. Diameter effect of electrospun carbon fiber support for the catalysis of Pt nanoparticles in glucose oxidation. Chem. Eng. J. 2016, 283, 304–312. [Google Scholar] [CrossRef]
- Chen, K.J.; Lee, C.F.; Rick, J.; Wang, S.-H.; Liu, C.C.; Hwang, B.J. Fabrication and application of amperometric glucose biosensor based on a novel PtPd bimetallic nanoparticle decorated multi-walled carbon nanotube catalyst. Biosens. Bioelectron. 2012, 33, 75–81. [Google Scholar] [CrossRef]
- Xiao, F.; Li, Y.; Gao, H.; Ge, S.; Duan, H. Growth of coral-like PtAu-MnO2 binary nanocomposites on free-standing graphene paper for flexible nonenzymatic glucose sensors. Biosens. Bioelectron. 2013, 41, 417–423. [Google Scholar] [CrossRef]



| Clinical Measurement Method (mM) | The Proposed Method | |||
|---|---|---|---|---|
| Spiked (mM) | Found (mM) | Recovery (%) | ||
| Urine I | 0 | 0 | 0 | / |
| 0.2 | 0.18 | 90 | ||
| 0.5 | 0.54 | 108 | ||
| Urine II | 0 | 0 | 0 | / |
| 1 | 1.09 | 109 | ||
| 2 | 1.95 | 97.5 | ||
| Urine III | 0 | 0 | 0 | / |
| 5 | 5.17 | 103.4 | ||
| 10 | 9.56 | 95.6 | ||
| Fingertip blood I | 4.62 | 0 | 4.53 | 98.1 |
| 1 | 5.67 | 100.9 | ||
| 5 | 10.35 | 107.6 | ||
| Fingertip blood II | 8.35 | 0 | 8.95 | 107.2 |
| 1 | 9.64 | 103.1 | ||
| 5 | 14.52 | 108.8 | ||
| Fingertip blood II | 10.56 | 0 | 9.86 | 93.37 |
| 1 | 11.97 | 103.5 | ||
| 5 | 16.5 | 106 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, E.; Gu, Z.; Li, H.; Liu, X.; Li, Y.; Xiao, F. Flexible Graphene Paper Modified Using Pt&Pd Alloy Nanoparticles Decorated Nanoporous Gold Support for the Electrochemical Sensing of Small Molecular Biomarkers. Biosensors 2024, 14, 172. https://doi.org/10.3390/bios14040172
Sun E, Gu Z, Li H, Liu X, Li Y, Xiao F. Flexible Graphene Paper Modified Using Pt&Pd Alloy Nanoparticles Decorated Nanoporous Gold Support for the Electrochemical Sensing of Small Molecular Biomarkers. Biosensors. 2024; 14(4):172. https://doi.org/10.3390/bios14040172
Chicago/Turabian StyleSun, Encheng, Zhenqi Gu, Haoran Li, Xiao Liu, Yuan Li, and Fei Xiao. 2024. "Flexible Graphene Paper Modified Using Pt&Pd Alloy Nanoparticles Decorated Nanoporous Gold Support for the Electrochemical Sensing of Small Molecular Biomarkers" Biosensors 14, no. 4: 172. https://doi.org/10.3390/bios14040172
APA StyleSun, E., Gu, Z., Li, H., Liu, X., Li, Y., & Xiao, F. (2024). Flexible Graphene Paper Modified Using Pt&Pd Alloy Nanoparticles Decorated Nanoporous Gold Support for the Electrochemical Sensing of Small Molecular Biomarkers. Biosensors, 14(4), 172. https://doi.org/10.3390/bios14040172