Research Progress on the Application of Covalent Organic Framework Nanozymes in Analytical Chemistry
Abstract
1. Introduction
2. Synthesis of COF Nanozymes
2.1. Design Principle of COF Nanozymes
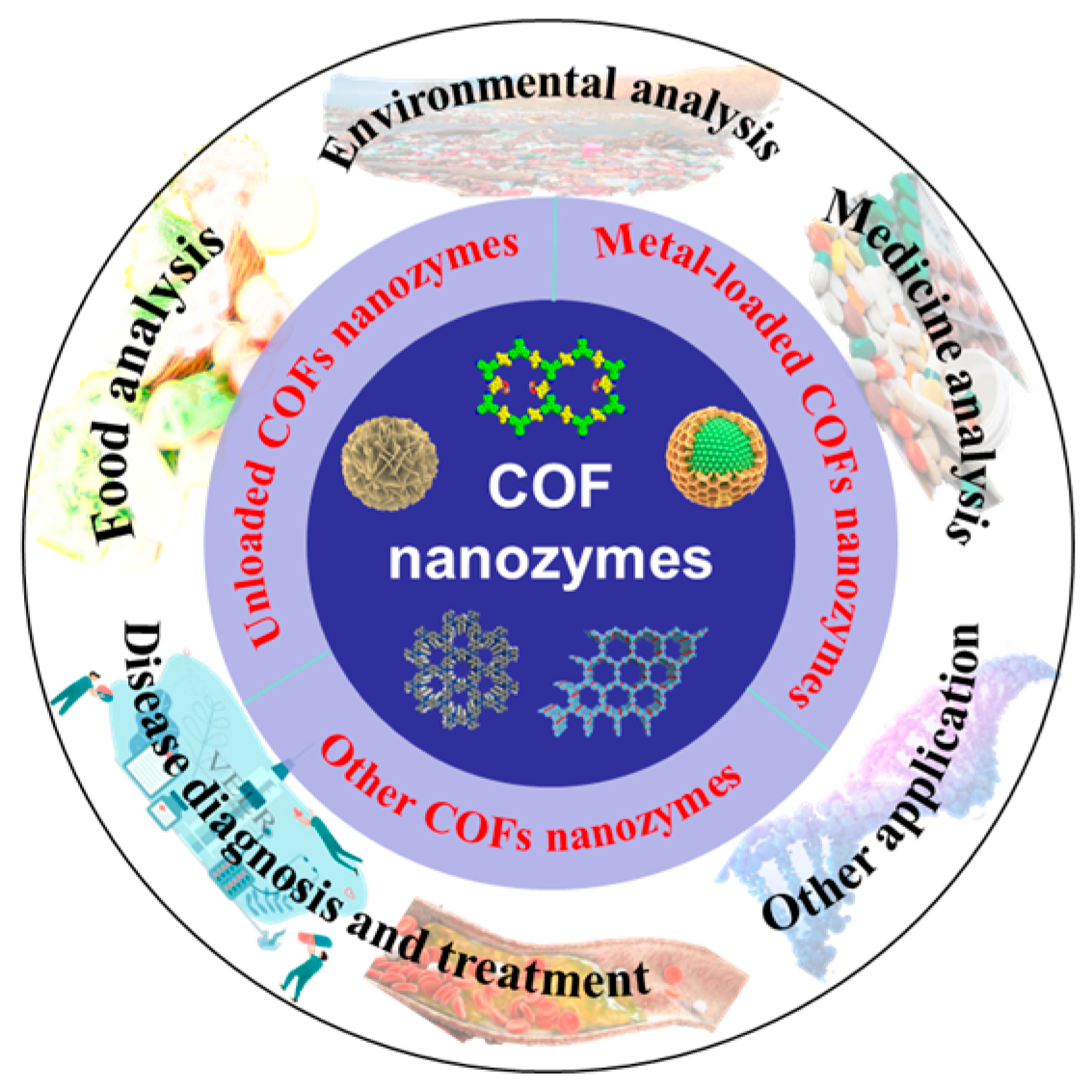
2.2. Types and Characteristics of COF Nanozymes
2.2.1. Unloaded COF Nanozymes
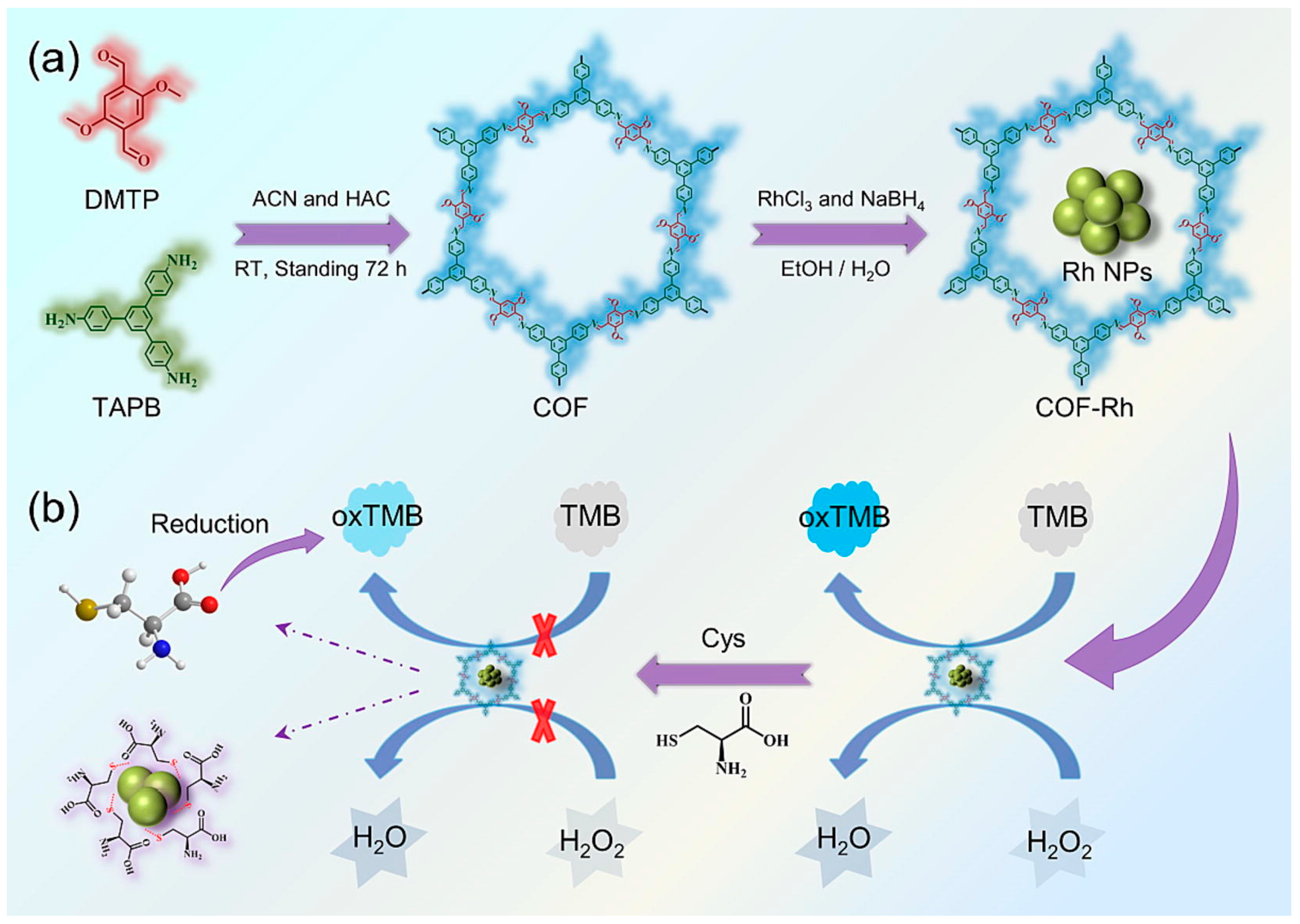
2.2.2. Metal-Loaded COF Nanozymes
2.2.3. Other COF Nanozymes
2.3. Synthesis Method of COF Nanozymes
3. Application of COF Nanozymes in Analytical Chemistry
3.1. Environmental Analysis
3.2. Food Analysis
3.3. Medicine Analysis
3.4. Disease Diagnosis and Treatment
3.5. Others
4. Potential Catalytic Mechanism of COF Nanozymes
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Viloca, M.; Gao, J.; Karplus, M.; Truhlar, D.G. How enzymes work: Analysis by modern rate theory and computer simulations. Science 2004, 303, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Wolfenden, R.; Snider, M.J. The depth of chemical time and the power of enzymes as catalysts. Acc. Chem. Res. 2001, 34, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R. Biomimetic chemistry and artificial enzymes: Catalysis by Design. Acc. Chem. Res. 1995, 28, 146–153. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lou, R.; Pan, W.; Li, N.; Tang, B. Nanoenzymes in disease diagnosis and therapy. Chem. Commun. 2020, 56, 15513–15524. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Jv, Y.; Li, B.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef]
- Zhang, X.; He, S.; Chen, Z.; Huang, Y. CoFe2O4 nanoparticles as oxidase mimic-mediated chemiluminescence of aqueous luminol for sulfite in white wines. J. Agric. Food Chem. 2013, 61, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Zhao, C.; Qu, K.; Ren, J.; Qu, X. Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chem.-Eur. J. 2010, 16, 3617–3621. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, L.; Li, M.; Wang, M.; Liu, G.; Ping, J. Nanozyme-based biosensor for organophosphorus pesticide monitoring: Functional design, biosensing strategy, and detection application. TrAC-Trend. Anal. Chem. 2023, 165, 117152. [Google Scholar] [CrossRef]
- Hou, H.; Wang, L.; Gao, Y.; Ping, J.; Zhao, F. Recent advances in metal-organic framework-based nanozymes and their enabled optical biosensors for food safety analysis. TrAC-Trend. Anal. Chem. 2024, 173, 117602. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, T.; Li, J.; Zhang, F.; Lou, H.; Zhang, J.; Zhang, W.; Zhang, W.; Zhou, B. Nanosized porous artificial enzyme as a pH-sensitive doxorubicin delivery system for joint enzymatic and chemotherapy towards tumor treatment. New J. Chem. 2022, 46, 14565–14577. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, Y.; Wang, L.; Chen, S.; Du, Y.; Song, Y. Electrochemical sensors based on metal-porous carbon nanozymes for dopamine, uric acid and furazolidone. Chemosensors 2022, 10, 458. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Lien, C.W.; Chu, H.W.; Huang, C.C. A review on metal nanozyme-based sensing of heavy metal ions: Challenges and future perspectives. J. Hazard. Mater. 2021, 401, 123397. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Gottschling, K.; Savasci, G.; Ochsenfeld, C.; Lotsch, B.V. H2 evolution with covalent organic framework photocatalysts. ACS Energy Lett. 2018, 3, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
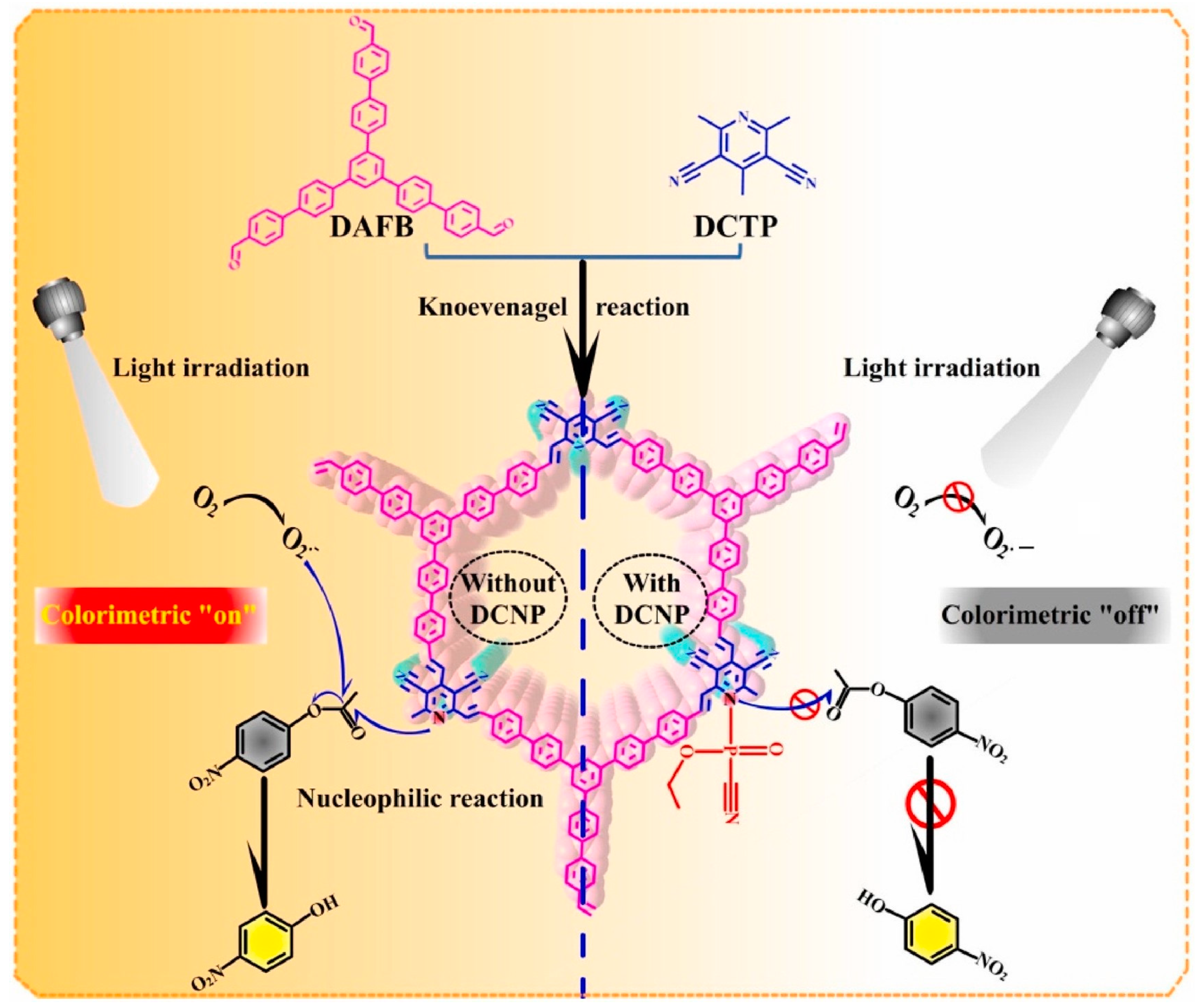
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Wang, H.; Yi, H.; Li, B.; Liu, S.; Zhang, M.; et al. Recent advances in covalent organic frameworks (COFs) as a smart sensing material. Chem. Soc. Rev. 2019, 48, 5266–5302. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, M.H.; Qi, M.L.; Wang, L.; Yang, Y.W. Nanoplatforms based on covalent organic frameworks (COFs) for biomedical applications. Chem. Mater. 2023, 35, 8353–8370. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, F.; Jiang, C.; Ye, S.; Tong, J.; Dramou, P.; He, H. Recent progress in the construction and applications of metal-organic frameworks and covalent-organic frameworks-based nanozymes. Coord. Chem. Rev. 2022, 471, 214760. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhao, Y. Covalent organic frameworks for energy conversion in photocatalysis. Angew. Chem. Int. Edit. 2023, 62, 202303086. [Google Scholar] [CrossRef]
- Phan, P.T.; Ta, Q.T.H.; Nguyen, P.K.T. Designed synthesis of three-dimensional covalent organic frameworks: A mini review. Polymers 2023, 15, 887. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, H.M.; Dong, J.H.; Lin, J.S.; Lin, Z. Super-hydrophilic sulfonate-modified covalent organic framework nanosheets for efficient separation and enrichment of glycopeptides. J. Chromatogr. A 2023, 1699, 464020. [Google Scholar] [CrossRef]
- Pang, Z.-F.; Xu, S.-Q.; Zhou, T.-Y.; Liang, R.-R.; Zhan, T.-G.; Zhao, X. Construction of covalent organic frameworks bearing three different kinds of pores through the heterostructural mixed linker strategy. J. Am. Chem. Soc. 2016, 138, 4710–4713. [Google Scholar] [CrossRef]
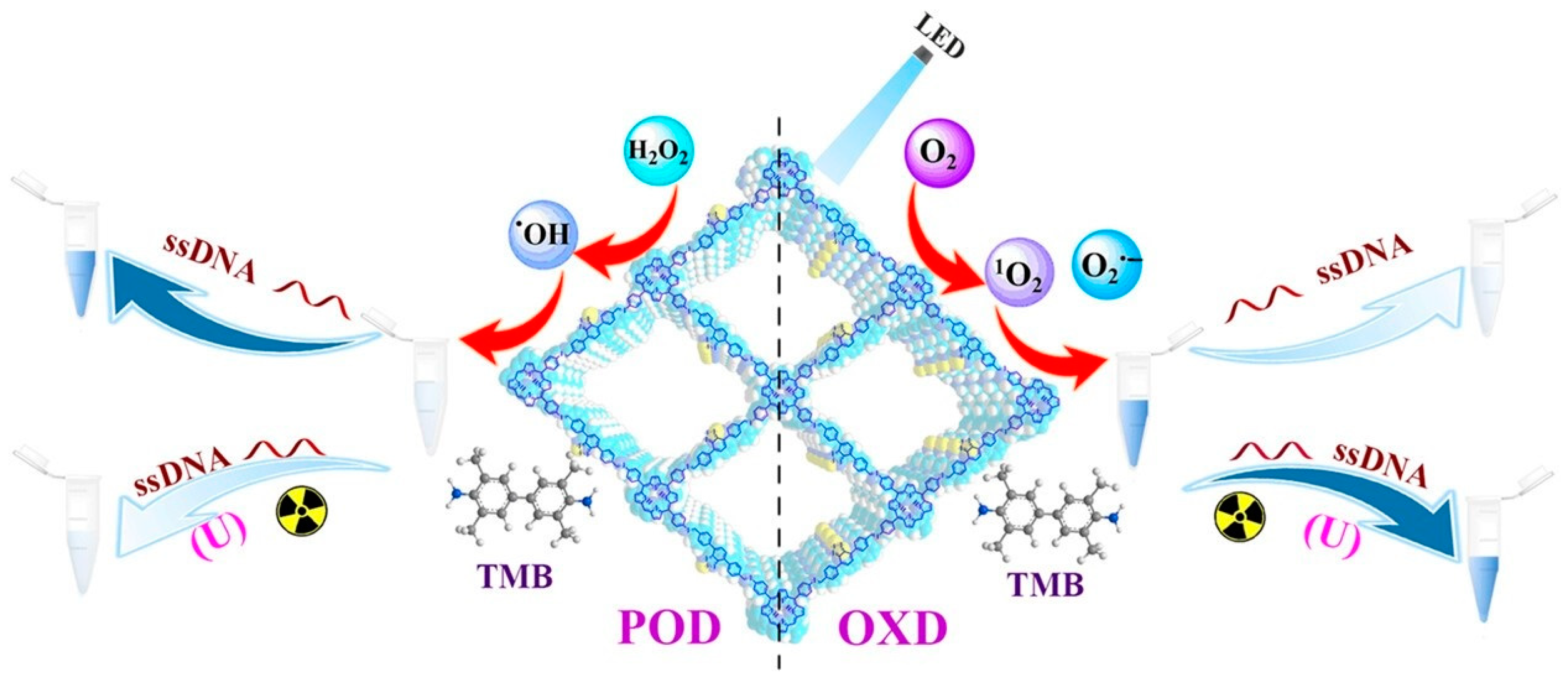
- Zhang, L.; Tan, Q.G.; Xiao, S.J.; Yang, G.P.; Zheng, Q.Q.; Sun, C.; Mao, X.L.; Fan, J.Q.; Liang, R.P.; Qiu, J.D. Reversed regulation effects of ssDNA on the mimetic oxidase and peroxidase activities of covalent organic frameworks. Small 2023, 19, 2207798. [Google Scholar] [CrossRef]
- Zhong, C.; Hu, C.; Ouyang, D.; Dan, A.; Zhong, Y.; Cai, Z.; Lin, Z. Bioinspired construction of histidine-doped porphyrin covalent organic framework nanozyme with enhanced peroxidase-like activity for sensitive uric acid detection. Chem. Eng. J. 2023, 477, 146979. [Google Scholar] [CrossRef]
- Su, Y.; Wu, D.; Chen, J.; Chen, G.; Hu, N.; Wang, H.; Wang, P.; Han, H.; Li, G.; Wu, Y. Ratiometric surface enhanced raman scattering immunosorbent assay of allergenic proteins via covalent organic framework composite material based nanozyme tag triggered Raman signal “turn-on” and amplification. Anal. Chem. 2019, 91, 11687–11695. [Google Scholar] [CrossRef] [PubMed]
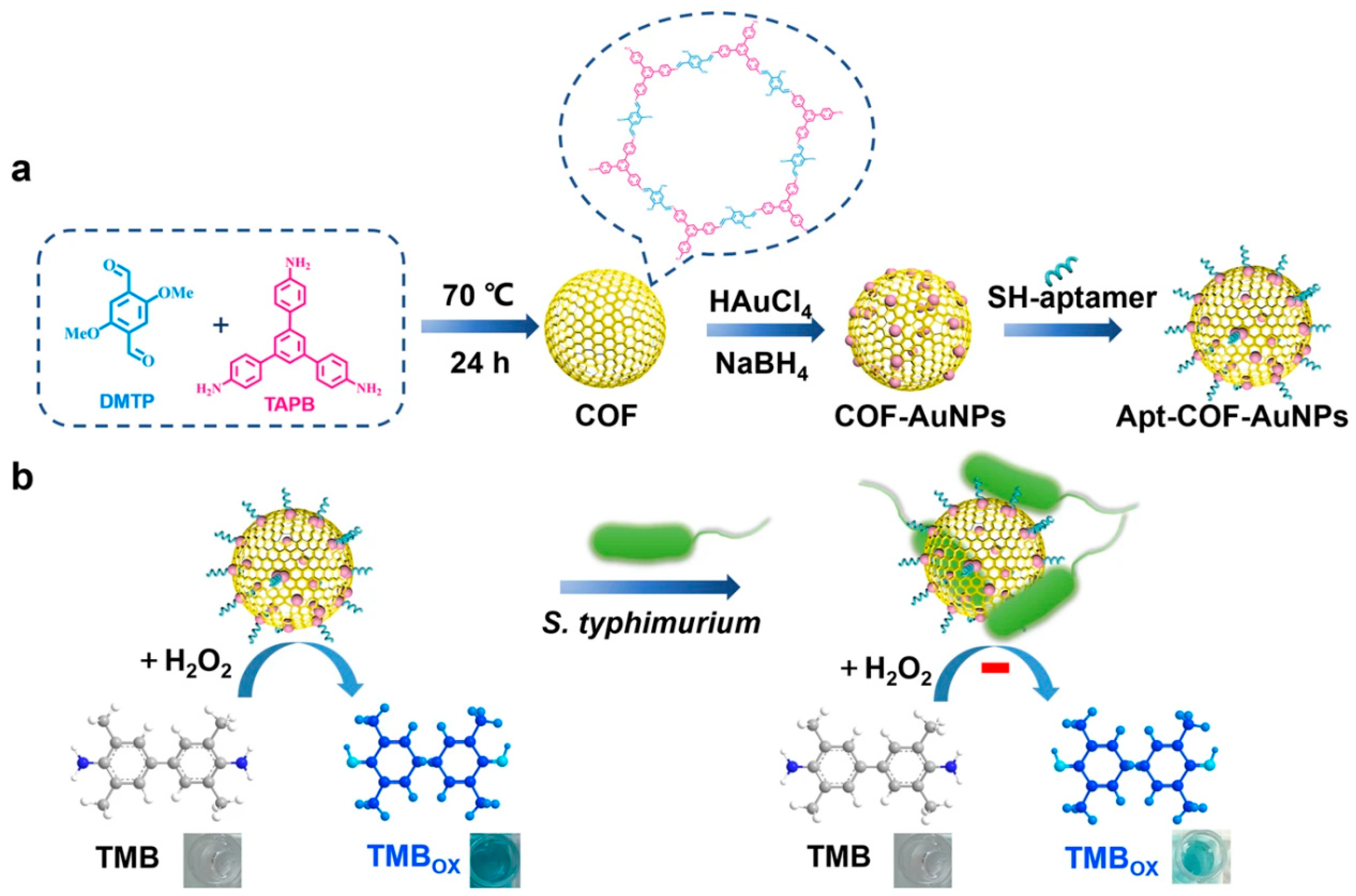
- Li, H.; Xu, H.; Yao, S.; Wei, S.; Shi, X.; Zhao, C.; Li, J.; Wang, J. Colorimetry/fluorescence dual-mode detection of Salmonella typhimurium based on self-assembly of MCOF with Au NPs nanozyme coupled AIEgen. Talanta 2024, 270, 125505. [Google Scholar] [CrossRef]
- Zhou, S.; Tian, T.; Meng, T.; Wu, J.; Hu, D.; Liao, Q.; Zhuang, J.; Wang, H.; Zhang, G. Tumor-derived covalent organic framework nanozymes for targeted chemo-photothermal combination therapy. iScience 2023, 26, 107348. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Puthiaraj, P.; Lee, Y.-R.; Zhang, S.; Ahn, W.-S. Triazine-based covalent organic polymers: Design, synthesis and applications in heterogeneous catalysis. J. Mater. Chem. A 2016, 4, 16288–16311. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Gong, Z.T.; Rong, Q.; Liu, J.M.; Yao, L.F.; Cheng, F.X.; Liu, J.J.; Xia, S.B.; Guo, H. Improved and stable triazine-based covalent organic framework for lithium storage. Appl. Surf. Sci. 2022, 594, 153481. [Google Scholar] [CrossRef]
- Yu, S.Y.; Mahmood, J.; Noh, H.J.; Seo, J.M.; Jung, S.M.; Shin, S.H.; Im, Y.K.; Jeon, I.Y.; Baek, J.B. Direct synthesis of a covalent triazine-based framework from aromatic amides. Angew. Chem. Int. Edit. 2018, 57, 8438–8442. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Edit. 2008, 47, 3450–3453. [Google Scholar] [CrossRef]
- Yuan, M.Y.; Xiao, S.J.; Wu, Y.N.; Qiu, A.T.; Guo, J.; Zhong, Z.Q.; Zhang, L. Visual detection of captopril based on the light activated oxidase-mimic activity of covalent organic framework. Microchem. J. 2022, 175, 107080. [Google Scholar] [CrossRef]
- Keller, N.; Bein, T. Optoelectronic processes in covalent organic frameworks. Chem. Soc. Rev. 2021, 50, 1813–1845. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, C.; Bi, S.; Wang, W.; He, Y.; Wu, D.; Liang, Q.; Wang, X.; Zhang, F. Vinylene-linked covalent organic frameworks (COFs) with symmetry-tuned polarity and photocatalytic activity. Angew. Chem. Int. Edit. 2020, 59, 23845–23853. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zou, W.; Ou, Z.; Zhang, W.; Liang, Z.; Du, L. Stabilization of 2D imine-linked covalent organic frameworks: From linkage chemistry to interlayer interaction. Chem. Eur. J. 2023, 29, 202203610. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.-P.; Xiao, S.J.; Tan, Q.G.; Zheng, Q.Q.; Liang, R.P.; Qiu, J.D. Facile construction of covalent organic framework nanozyme for colorimetric detection of uranium. Small 2021, 17, 2102944. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Ma, L. Recent advances in covalent organic frameworks for catalysis. Chem. Asian J. 2020, 15, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.J.; Yuan, M.Y.; Shi, Y.D.; Wang, M.P.; Li, H.H.; Zhang, L.; Qiu, J.D. Construction of covalent organic framework nanozymes with photo-enhanced hydrolase activities for colorimetric sensing of organophosphorus nerve agents. Anal. Chim. Acta 2023, 1278, 341706. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.S.; Bein, T. Covalent organic frameworks: Structures, synthesis, and applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef]
- Xu, M.; Luo, X.; Zhang, G.; Zhao, B.; Li, S.; Xiao, Z.; Wu, Q.; Wang, Z.; Wang, C. Construction of imine-linked covalent organic framework as advanced adsorbent for the sensitive determination of chlorophenols. J. Chromatogr. A 2021, 1658, 462610. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, W.; Li, Y.; Wei, Z.; Li, F.; Li, C. Imine-linked covalent organic frameworks with controllable morphology. Mater. Chem. Phys. 2023, 301, 127645. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, H.; Chen, Y.; Song, Z.; Ouyang, S.; Lian, S.; Tao, J.; Song, Y.; Zhao, P. Imine-linked covalent organic framework modulates oxidative stress in alzheimer’s disease. ACS Appl. Mater. Interfaces 2023, 15, 4947–4958. [Google Scholar] [PubMed]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef] [PubMed]
- Kandambeth, S.; Shinde, D.B.; Panda, M.K.; Lukose, B.; Heine, T.; Banerjee, R. Enhancement of chemical stability and crystallinity in porphyrin-containing covalent organic frameworks by intramolecular hydrogen bonds. Angew. Chem. Int. Ed. 2013, 52, 13052–13056. [Google Scholar]
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klöck, C.; O’Keeffe, M.; Yaghi, O.M. A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework. J. Am. Chem. Soc. 2009, 131, 4570–4571. [Google Scholar] [CrossRef]
- Ding, S.Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.G.; Su, C.Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in suzuki–miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Shu, Y.; Wen, G.; Liang, A.; Jiang, Z. A novel difunctional COF-loaded peptide Au nanocluster probe and its application to molecular spectral determination of trace Cd2+. Microchem. J. 2023, 193, 109067. [Google Scholar] [CrossRef]
- Li, C.; He, X.; Li, H.; Xiao, Y.; Xu, X.; Jiang, C.; Wen, G.; Jiang, Z. A new COF@AuNC catalytic amplification-aptamer SERS quantitative analysis method for trace estradiol with nanoreaction of HAuCl4-sulfite. Microchem. J. 2023, 191, 108920. [Google Scholar] [CrossRef]
- Wei, S.; Su, Z.; Bu, X.; Shi, X.; Pang, B.; Zhang, L.; Li, J.; Zhao, C. On-site colorimetric detection of Salmonella typhimurium. npj Sci. Food 2022, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Jiang, S.; Li, W.; Jalil Shah, S.; Zhao, Z.; Pan, L.; Zhao, Z. Confined construction of COF@Cu-nanozyme with high activity and stability as laccase biomimetic catalyst for the efficient degradation of phenolic pollutants. Chem. Eng. J. 2022, 448, 137701. [Google Scholar] [CrossRef]
- Wang, L.; Liang, H.; Xu, M.; Wang, L.; Xie, Y.; Song, Y. Ratiometric electrochemical biosensing based on double-enzymes loaded on two-dimensional dual-pore COFETTA-TPAL. Sens. Actuators B Chem. 2019, 298, 126859. [Google Scholar] [CrossRef]
- Li, D.; Luo, L.; Pang, Z.; Ding, L.; Wang, Q.; Ke, H.; Huang, F.; Wei, Q. Novel phenolic biosensor based on a magnetic polydopamine-laccase-nickel nanoparticle loaded carbon nanofiber composite. ACS Appl. Mater. Interfaces 2014, 6, 5144–5151. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhao, X.; Wang, X.; Huang, T.; Ding, Y.; Zhang, J.; Zhang, Z.; Wang, Z.L.; Li, L. Bioinspired electron polarization of nanozymes with a human self-generated electric field for cancer catalytic therapy. Adv. Mater. 2022, 34, 2109568. [Google Scholar] [CrossRef]
- Yu, S.; Guo, Z.; Zhou, Y.; Li, C. Research progress of MOFs/carbon nanocomposites on promoting ORR in microbial fuel cell cathodes. Environ. Sci. Pollut. Res. 2023, 30, 93422–93434. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, Y.; Lin, H.; Liu, C.j. Nanoparticle/metal–organic framework composites for catalytic applications: Current status and perspective. Molecules 2017, 22, 2103. [Google Scholar] [CrossRef]
- Ma, X.; Liu, F.; Helian, Y.; Li, C.; Wu, Z.; Li, H.; Chu, H.; Wang, Y.; Wang, Y.; Lu, W.; et al. Current application of MOFs based heterogeneous catalysts in catalyzing transesterification/esterification for biodiesel production: A review. Energy Convers. Manag. 2021, 229, 113760. [Google Scholar] [CrossRef]
- Cui, K.; Zhong, W.; Li, L.; Zhuang, Z.; Li, L.; Bi, J.; Yu, Y. Well-defined metal nanoparticles@covalent organic framework yolk–shell nanocages by ZIF-8 template as catalytic nanoreactors. Small 2019, 15, 1804419. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Cheng, D.; Zou, S.; Gao, J.; Wang, J.; Wang, Y. Rational design of magnetic MOFs-COFs hybrid nanozyme for the colorimetric detection of phenol. J. Environ. Chem. Eng. 2023, 11, 110914. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, C.; Wen, Z.; Ding, L.; Wei, J.; Qian, J.; Hao, N.; Wang, K. Simulation design of a binding-pocket structure of natural enzymes in MOFs for enhanced catalytic activity. Chem. Commun. 2022, 58, 6745–6748. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, Y.; Liu, S.; Wu, D.; Su, Z.; Chen, G.; Liu, J.; Li, G. Recent advances in enzyme immobilization based on novel porous framework materials and its applications in biosensing. Coord. Chem. Rev. 2022, 459, 214414. [Google Scholar] [CrossRef]
- Nikkhoo, E.; Mallakpour, S.; Hussain, C.M. Design, synthesis, and application of covalent organic frameworks as catalysts. New J. Chem. 2023, 47, 6765–6788. [Google Scholar] [CrossRef]
- Xiong, Y.; Su, L.; He, X.; Duan, Z.; Zhang, Z.; Chen, Z.; Xie, W.; Zhu, D.; Luo, Y. Colorimetric determination of copper ions based on regulation of the enzyme-mimicking activity of covalent triazine frameworks. Sens. Actuators B Chem. 2017, 253, 384–391. [Google Scholar] [CrossRef]
- Wan, X.; Yin, J.; Yan, Q.; Hu, H.; Zheng, T.; Chai, Y.; Pan, W.; Gao, Y.; Li, N.; Tang, B. Sustained-release nanocapsule based on a 3D COF for long-term enzyme prodrug therapy of cancer. Chem. Commun. 2022, 58, 5877–5880. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zang, L.; Yu, Z.; Xu, Z.; Zhang, Y.; Liu, X.; Sun, L. Solvothermal synthesis of uniform covalent organic framework microspheres enabling high-loading palladium for oxygen reduction reaction. Macromolecules 2023, 56, 4482–4490. [Google Scholar] [CrossRef]
- Xiong, S.; Guo, J.; Lv, F.; Zhao, X.; Zhang, W.; Wang, X.; Hua, C.; Zhang, R.; Chu, J.; Wang, C.; et al. Solvothermal synthesis and supercapacitive properties of highly electrochemical stable covalent organic frameworks with triazine building block. J. Appl. Polym. Sci. 2023, 140, e54538. [Google Scholar] [CrossRef]
- Rodríguez-Carríllo, C.; Benítez, M.; El Haskouri, J.; Amorós, P.; Ros-Lis, J.V. Novel microwave-assisted synthesis of COFs: 2020-2022. Molecules 2023, 28, 3112. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Y.; Xu, X.; Sun, Y.; Li, Y.; Du, J. A covalent triazine framework as an oxidase mimetic in the luminol chemiluminescence system: Application to the determination of the antioxidant rutin. Microchim. Acta 2019, 187, 42. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, F.; Hu, J.; Wang, S.; Hou, X.; Long, Z. Covalent triazine framework-1: A novel oxidase and peroxidase mimic. Microchem. J. 2017, 135, 91–99. [Google Scholar] [CrossRef]
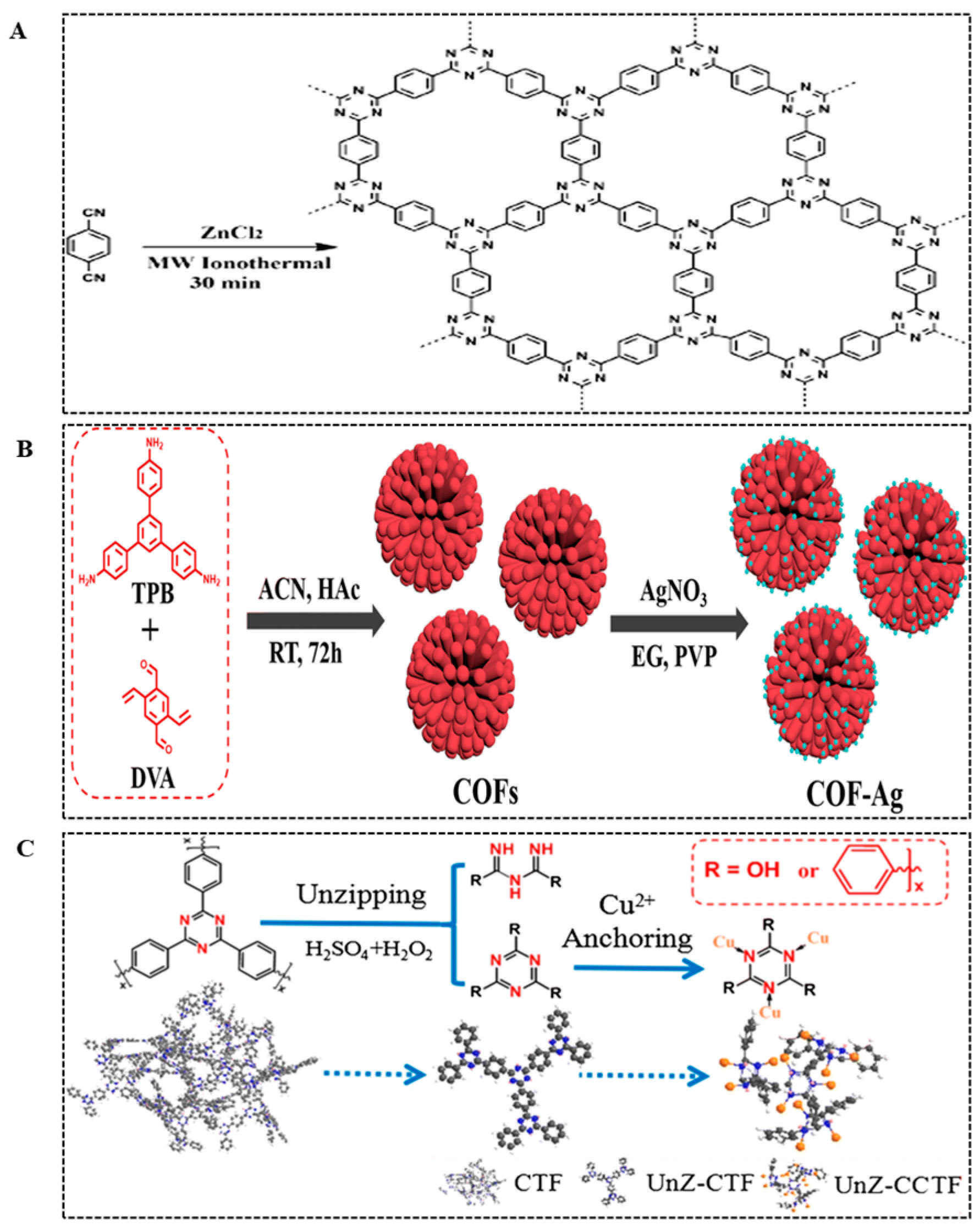
- Liu, Q.; Xu, C.; Chu, S.; Li, S.; Wang, F.; Si, Y.; Mao, G.; Wu, C.; Wang, H. Covalent organic framework-loaded silver nanoparticles as robust mimetic oxidase for highly sensitive and selective colorimetric detection of mercury in blood. J. Mater. Chem. B 2022, 10, 10075–10082. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, S.; Pang, C.; Xiong, Y.; Li, J. A Cu(II)-anchored unzipped covalent triazine framework with peroxidase-mimicking properties for molecular imprinting–based electrochemiluminescent detection of sulfaquinoxaline. Microchim. Acta 2018, 185, 546. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Qin, Y.; Su, L.; Ye, F. Bioinspired synthesis of Cu2+-modified covalent triazine framework: A new highly efficient and promising peroxidase mimic. Chem. Eur. J. 2017, 23, 11037–11045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tan, Q.G.; Xiao, S.J.; Yang, G.P.; Liu, X.; Zheng, Q.Q.; Fan, J.Q.; Liang, R.P.; Qiu, J.D. DNAzyme-derived aptamer reversely regulates the two types of enzymatic activities of covalent–organic frameworks for the colorimetric analysis of uranium. Anal. Chem. 2023, 95, 4703–4711. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, J.; Chen, X. Visible light-responsive sulfone-based covalent organic framework as metal-free nanoenzyme for visual colorimetric determination of uranium. Chemosensors 2022, 10, 248. [Google Scholar] [CrossRef]
- Jin, P.; Niu, X.; Zhang, F.; Dong, K.; Dai, H.; Zhang, H.; Wang, W.; Chen, H.; Chen, X. Stable and reusable light-responsive reduced covalent organic framework (COF-300-AR) as a oxidase-mimicking catalyst for GSH detection in cell lysate. ACS Appl. Mater. Interfaces 2020, 12, 20414–20422. [Google Scholar] [CrossRef]
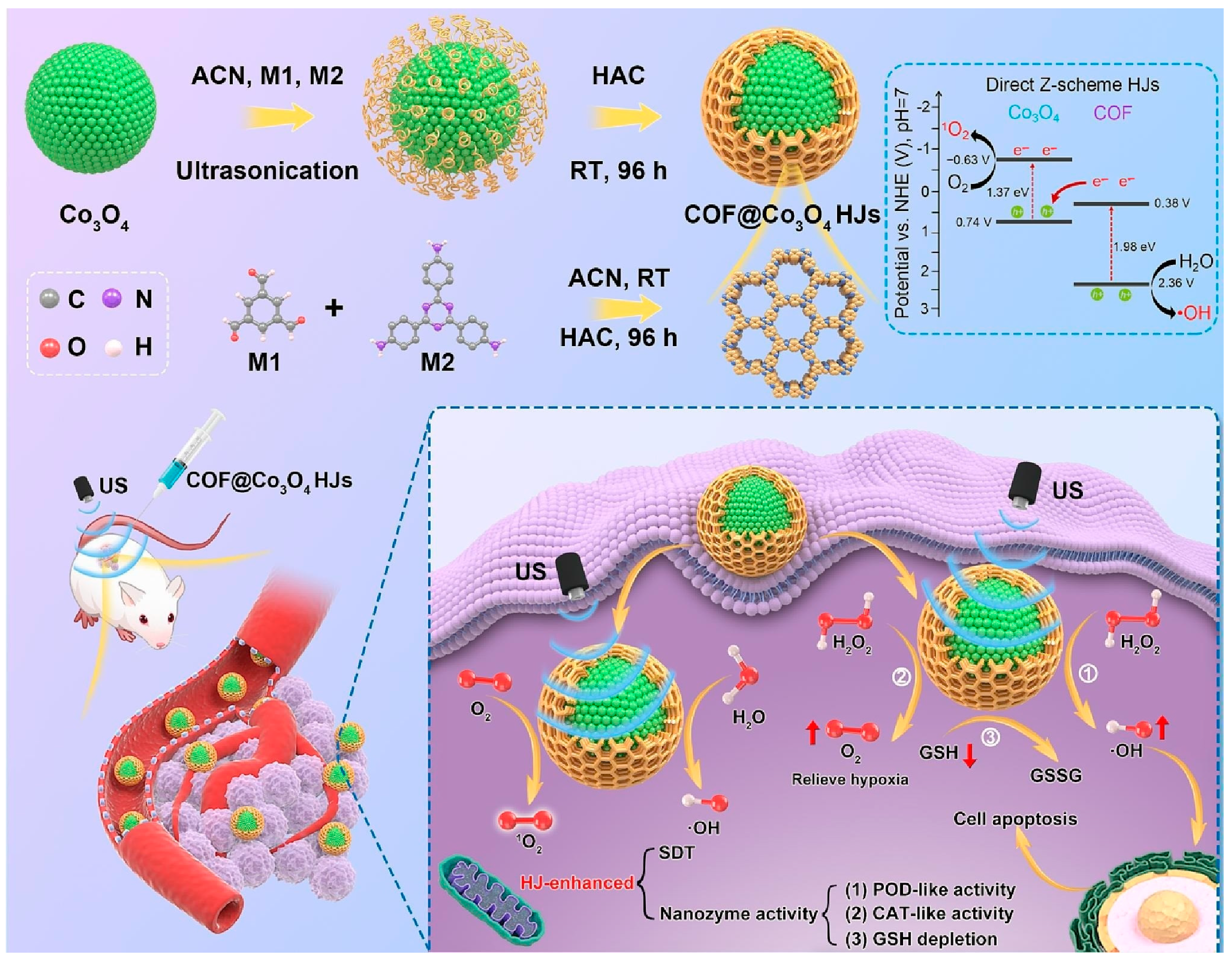
- Feng, C.; Hu, J.; Xiao, C.; Yang, J.; Xin, B.; Jia, Z.; Zhang, S.; Tian, G.; Zhang, D.; Geng, L.; et al. Shell-core COF@Co3O4 Z-scheme heterojunctions for triple amplification of oxidative stress to enhance nanocatalytic-sonodynamic tumor therapy. Chem. Eng. J. 2023, 460, 141874. [Google Scholar] [CrossRef]
- Li, J.; Gao, M.; Xia, X.; Cen, Y.; Wei, F.; Yang, J.; Wang, L.; Hu, Q.; Xu, G. Spherical hydrogel sensor based on PB@Fe-COF@Au nanoparticles with triplet peroxidase-like activity and multiple capture sites for effective detection of organophosphorus pesticides. ACS Appl. Mater. Interfaces 2023, 15, 6473–6485. [Google Scholar] [CrossRef]
- Sun, P.; Hai, J.; Sun, S.; Lu, S.; Liu, S.; Liu, H.; Chen, F.; Wang, B. Aqueous stable Pd nanoparticles/covalent organic framework nanocomposite: An efficient nanoenzyme for colorimetric detection and multicolor imaging of cancer cells. Nanoscale 2020, 12, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Niu, X.; Gao, Z.; Xue, X.; Zhang, F.; Cheng, W.; Ren, C.; Du, H.; Manyande, A.; Chen, H. Ultrafine platinum nanoparticles supported on covalent organic frameworks as stable and reusable oxidase-like catalysts for cellular glutathione detection. ACS Appl. Nano Mater. 2021, 4, 5834–5841. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Nie, Y.; Wang, Y.; Zhang, P. Covalent organic framework-supported ultrasmall Rh nanoparticles as peroxidase mimics for colorimetric sensing of cysteine. J. Colloid Interf. Sci. 2023, 636, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Deng, Q.; Sang, Y.; Dong, K.; Ren, J.; Qu, X. Nature-inspired construction of MOF@COF nanozyme with active sites in tailored microenvironment and pseudopodia-like surface for enhanced bacterial inhibition. Angew. Chem. Int. Edit. 2021, 60, 3469–3474. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zheng, M.; Wang, S.; Huang, T.; Wang, Z.; Zhao, Y.; Yuan, W.; Li, Z.; Wang, Z.L.; Li, L. Self-driven electrical stimulation promotes cancer catalytic therapy based on fully conjugated covalent organic framework nanocages. Adv. Funct. Mater. 2022, 32, 2209142. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y. COF-300-AR@CRL as a two-in-one nanocatalyst for one-step chemiluminescent detection of diphenyl ether herbicide residues in vegetable and fruit samples. Microchim. Acta 2023, 190, 492. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.L.; Zhu, J.L.; Wu, Z.; Wen, W.; Zhang, X.H.; Chen, M.M.; Wang, S.F. High-efficient Pt@COF nanospheres-based electrochemical-chemical-chemical redox cycling for ultrasensitive microRNAs biosensing. Sens. Actuators B Chem. 2023, 392, 134074. [Google Scholar] [CrossRef]
- Li, T.T.; Deng, D.L.; Tan, D.D.; Chen, S.Q.; Ji, Y.B.; Li, R.J. Immobilized glucose oxidase on hierarchically porous COFs and integrated nanozymes: A cascade reaction strategy for ratiometric fluorescence sensors. Anal. Bioanal. Chem. 2022, 414, 6247–6257. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xie, Y.; Chu, H.; Long, M.; Zhang, M.; Wang, Y.; Hu, X. A highly sensitive electrochemical biosensor for the detection of hydroquinone based on a magnetic covalent organic framework and enzyme for signal amplification. New J. Chem. 2022, 46, 11902–11909. [Google Scholar] [CrossRef]
- Cui, W.-R.; Li, Y.-J.; Jiang, Q.-Q.; Wu, Q.; Luo, Q.-X.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. Covalent organic frameworks as advanced uranyl electrochemiluminescence monitoring platforms. Anal. Chem. 2021, 93, 16149–16157. [Google Scholar] [CrossRef]
- Liang, L.; Jiang, Y.; Liu, F.; Wu, J.; Tian, L.; Zhao, S.; Ye, F. Smartphone flashlight-triggered covalent organic framework nanozyme activity: A universal scheme for visual point-of-care testing. Sens. Actuators B Chem. 2023, 381, 133422. [Google Scholar] [CrossRef]
- Li, J.; Shu, Y.; Li, C.; Jiang, Z. Highly catalytic nanoenzyme of covalent organic framework loaded starch- surface-enhanced Raman scattering/absorption bi-mode peptide as biosensor for ultratrace determination of cadmium. Front. Nutr. 2023, 9, 1075296. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, F.; Mantawy, E.M.; Azab, S.S.; El-Demerdash, E. The angiotensin converting enzyme inhibitor captopril attenuates testosterone-induced benign prostatic hyperplasia in rats; a mechanistic approach. Eur. J. Pharmacol. 2019, 865, 172729. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pang, C.; Ma, X.; Wu, Y.; Wang, M.; Xu, Z.; Luo, J. Aggregation-induced electrochemiluminescence and molecularly imprinted polymer based sensor with Fe3O4@Pt nanoparticle amplification for ultrasensitive ciprofloxacin detection. Microchem. J. 2022, 178, 107345. [Google Scholar] [CrossRef]
- Su, L.; Zhang, Z.; Xiong, Y. Water dispersed two-dimensional ultrathin Fe(iii)-modified covalent triazine framework nanosheets: Peroxidase like activity and colorimetric biosensing applications. Nanoscale 2018, 10, 20120–20125. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Meng, Y.; Zhao, L.; Shi, W.; Wang, X.; He, Z.; Chao, J.; Li, C. Electrochemical/visual microfluidic detection with a covalent organic framework supported platinum nanozyme-based device for early diagnosis of pheochromocytoma. Biosens. Bioelectron. 2022, 207, 114208. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.O.; Evangelopoulos, M.; Brozovich, A.A.; Bauza, G.; Molinaro, R.; Corbo, C.; Liu, X.; Taraballi, F.; Tasciotti, E. Mesenchymal stromal cell-mediated treatment of local and systemic inflammation through the triggering of an anti-tnflammatory response. Adv. Funct. Mater. 2021, 31, 2002997. [Google Scholar] [CrossRef]
- Zhang, G.; Li, X.; Liao, Q.; Liu, Y.; Xi, K.; Huang, W.; Jia, X. Water-dispersible PEG-curcumin/amine-functionalized covalent organic framework nanocomposites as smart carriers for in vivo drug delivery. Nat. Commun. 2018, 9, 2785. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.L.; Guan, Q.; Zhou, W.; Kan, J.L.; Teng, K.; Hu, M.; Dong, Y.B. A multifunctional covalent organic framework nanozyme for promoting ferroptotic radiotherapy against esophageal cancer. ACS Nano 2023, 17, 20445–20461. [Google Scholar] [CrossRef]
- Rong, M.; Liu, J.; Lu, L. Self-assembly of 2D polyphthalocyanine in lysosome enables multienzyme activity enhancement to induce tumor ferroptosis. Adv. Healthc. Mater. 2024, 2400325. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, M.; Chen, L.; Gong, C.; Li, N.; Huang, Y.; Cheng, C. Ultrathin covalent organic framework nanosheet-based photoregulated metal-free oxidase-like nanozyme. Nano Res. 2022, 15, 8783–8790. [Google Scholar] [CrossRef]
- Li, G.; Ma, W.; Yang, Y.; Zhong, C.; Huang, H.; Ouyang, D.; He, Y.; Tian, W.; Lin, J.; Lin, Z. Nanoscale covalent organic frameworks with donor–acceptor structures as highly efficient light-responsive oxidase-like mimics for colorimetric detection of glutathione. ACS Appl. Mater. Interfaces 2021, 13, 49482–49489. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Li, Z.; Li, J.; Tuo, K.; Zhang, D.; Fan, C.; Liu, G.; Deng, Y.; Pu, S. Construction of core–shell MOF@COF hybrid materials as highly efficient light-responsive oxidase-like nanozymes for colorimetric detection of glutathione. Microchem. J. 2023, 193, 109214. [Google Scholar] [CrossRef]
- He, N.; Zhu, X.; Liu, F.; Yu, R.; Xue, Z.; Liu, X. Rational design of FeS2-encapsulated covalent organic frameworks as stable and reusable nanozyme for dual-signal detection glutathione in cell lysates. Chem. Eng. J. 2022, 445, 136543. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Ren, J.; Qu, X. A chiral covalent organic framework (COF) nanozyme with ultrahigh enzymatic activity. Mater. Horiz. 2020, 7, 3291–3297. [Google Scholar] [CrossRef]
- Li, G.; Tian, W.; Zhong, C.; Yang, Y.; Lin, Z. Construction of donor–acceptor heteroporous covalent organic frameworks as photoregulated oxidase-like nanozymes for sensing signal amplification. ACS Appl. Mater. Interfaces 2022, 14, 21750–21757. [Google Scholar] [CrossRef]
- Liang, L.; Jiang, Y.; Liu, F.; Li, S.; Wu, J.; Zhao, S.; Ye, F. Three-in-one covalent organic framework nanozyme: Self-reporting, self-correcting and light-responsive for fluorescence sensing 3-nitrotyrosine. Biosens. Bioelectron. 2023, 237, 115542. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, L.; Chi, W.; Bai, Y.; Gao, W.; Zhu, P.; Yu, J. Porphyrin-based covalent organic frameworks with donor-acceptor structure for enhanced peroxidase-like activity as a colorimetric biosensing platform. Biosensors 2023, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Dashtian, K.; Zare-Dorabei, R.; Ghafuri, H.; Mahdavi, M.; Amourizi, F. Photo-responsive oxidase-like nanozyme based on a vanadium-docked porphyrinic covalent organic framework for colorimetric L-Arginine sensing. Anal. Chim. Acta 2023, 1247, 340924. [Google Scholar] [CrossRef]
- Gao, Z.X.; Zhu, A.L.; Wu, M.F.; Du, Y.L.; Zhang, Y.; Zhang, H.G.; Ren, C.L.; Chen, H.L. Colorimetric detection of alkaline phosphatase based on the off-on effect of light-responsive oxidase mimicking activity of covalent organic framework (Cu-TpBpy-COF) under near-neutral condition. Microchim. Acta 2024, 191, 93. [Google Scholar] [CrossRef]
- Yang, L.; Dong, S.M.; Gai, S.L.; Yang, D.; Ding, H.; Feng, L.L.; Yang, G.X.; Rehman, Z.; Yang, P.P. Deep insight of design, mechanism, and cancer theranostic strategy of nanozymes. Nano-Micro Lett. 2024, 16, 28. [Google Scholar] [CrossRef] [PubMed]


| Type of COF Nanozyme | COF Nanozyme | Type of COF | Loaded Particles | Load Way | Synthesis Method | Temperature (°C)/Time | Type of Mimic Enzyme | Characteristics | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Unloaded COF nanozymes | TTA-Tp COF | Triazine-based COF | —— | —— | Solvothermal synthesis | 120 °C/72 h | Mimic oxidase | Excellent catalytic ability, good stability | [36] |
| Tph-BDP | Imine-linked COF | Solvothermal synthesis | 120 °C/120 h | Mimic oxidase | Sensitive and selective | [40] | |||
| DAFB-DCTP COF | Vinylidene-linked COF | Solvothermal synthesis | 180 °C/72 h | Mimic hydrolase | Reliability and simplicity | [42] | |||
| CTF-1 | Triazine-based COF | Microwave-assisted synthesis | —/30 min | Mimic oxidase and peroxidase | Low cost, easy preparation | [72] | |||
| Tph-BT | Imine-linked COF | Solvothermal synthesis | 150 °C/72 h | Mimic oxidase and peroxidase | Sensitive and selective | [76] | |||
| TAS-COF | Imine-linked COF | Solvothermal synthesis | 120 °C/72 h | Mimic oxidase | Simple and fast | [77] | |||
| COF-300-AR | Imine-linked COF | NaBH4 -reducing method | 0 °C/1 h | Mimic oxidase | Easy, light control and good reusability | [78] | |||
| Metal-loaded COF nanozymes | TPE-s COF-Au@Cisplatin | Imine-linked COF | AuNPs | Au-SH bond | NaBH4-reducing method | 0 °C/8 h | Mimic glucose oxidase | Facile | [30] |
| COF-AuNPs | Imine-linked COF | AuNPs | Coordination of unsaturated amino groups | Citrate-reducing method | Room temperature/- | Mimic peroxidase | Good accuracy and sensitivity | [53] | |
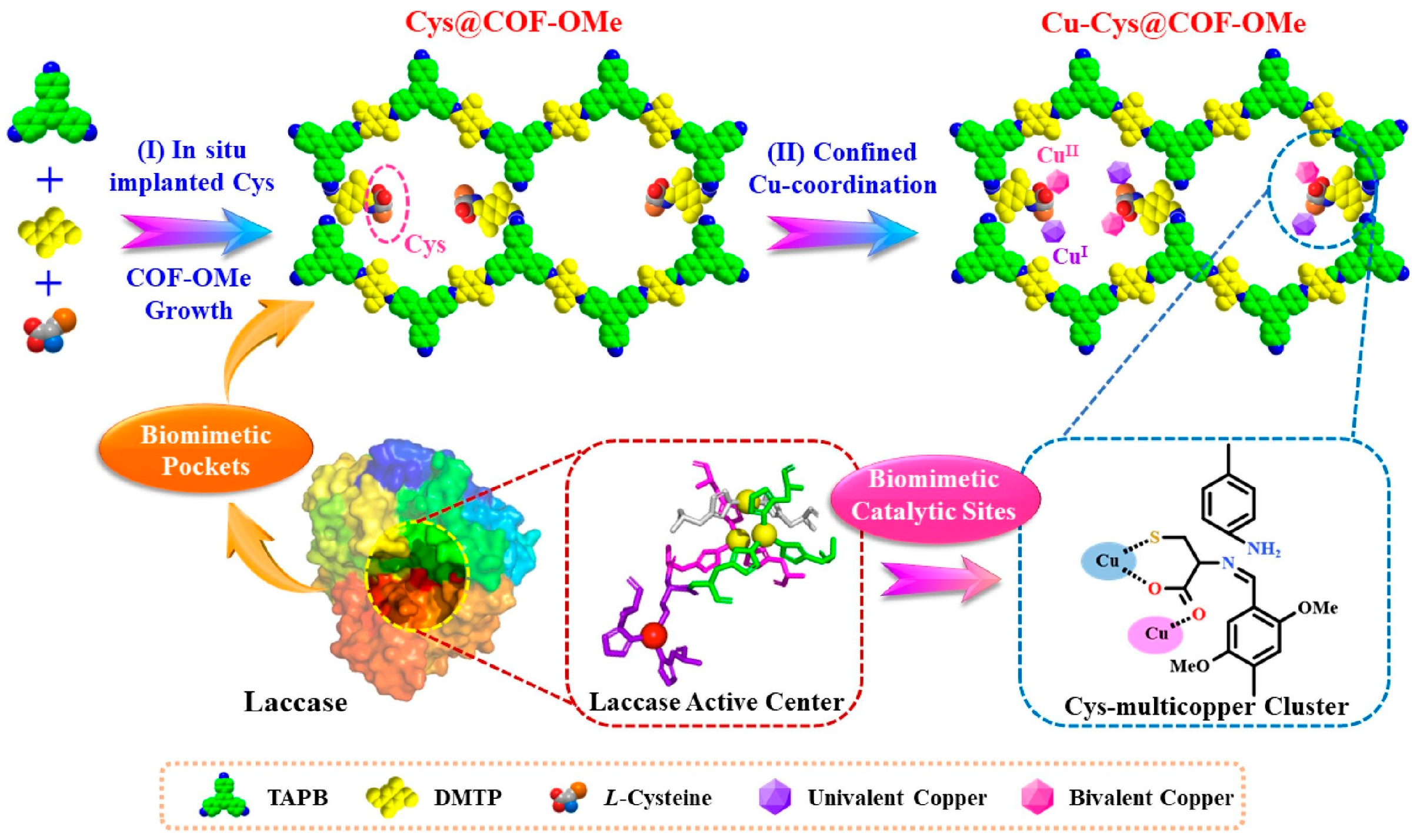
| Cu-Cys@COF-OMe | Imine-linked COF | Cu+/Cu2+ | Cu-coordination bond | Coordination | 150 °C/5 h | Mimic laccase | Excellent stability | [54] | |
| COF@Co3O4 | Imine-linked COF | Co3O4 | Electrostatic force | Sonochemical synthesis | Room temperature/96 h | Mimic peroxidase, catalase, and glutathione peroxidase | Complete eradication of tumors | [79] | |
| PB@Fe-COF@Au | Imine-linked COF | AuNPs | Au–SH bond | Citrate-reducing method | Room temperature/30 min | Mimic peroxidase | Affordable, sensitive, and selective | [80] | |
| PdNPs/CMC-COF-LZU1 | Imine-linked COF | PdNPs | In situ coordination | Reflux | 78 °C/3 h | —— | Accurate and sensitive | [81] | |
| Pt NPs/COF-300-AR | Imine-linked COF | PtNPs | Nitrogen atom affinity | NaBH4-reducing method | Room temperature/3 h | Mimic oxidase | Good stability and high reusability | [82] | |
| COF-Rh | Imine-linked COF | RhNPs | Electrostatic force | NaBH4-reducing method | Room temperature/15 h | Mimic peroxidase | Good catalytic properties | [83] | |
| Other COF nanozymes | GOD-MP-11/COFETTA-TPAL | Imine-linked COF | Glucose oxidase and microperoxidase-11 | Hydrogen bonds | Doping | 4 °C/10 h | Mimic glucose oxidase and microperoxidase-11 | Large surface area | [55] |
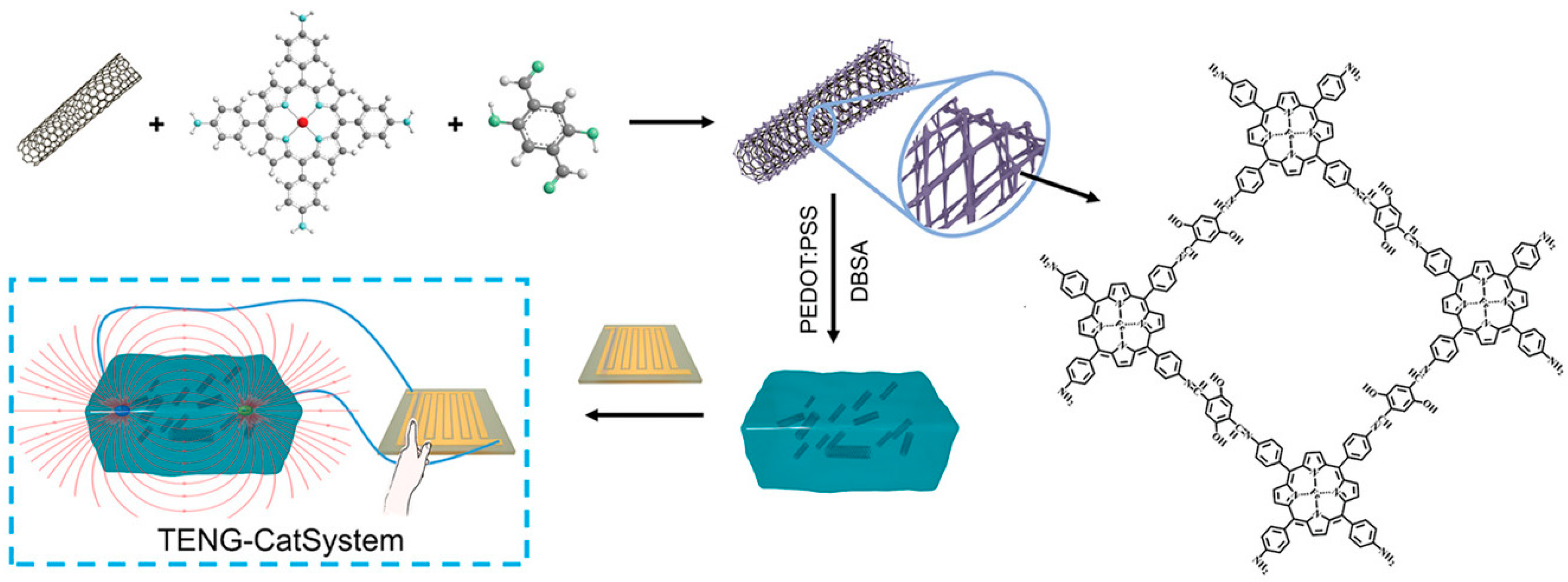
| COF-CNT | Imine-linked COF | Nanotube | Doping | Solvothermal synthesis | 70 °C/24 h | Mimic peroxidase | Good catalytic properties | [57] | |
| MOFs-COFs | Imine-linked COF | MOFs | Affinity | Solvothermal synthesis | 80 °C/12 h | Mimic peroxidase | High selectivity and excellent stability | [62] | |
| MOF@COF | Imine-linked COF | MOFs | Schiff base reaction | Solvothermal synthesis | 110 °C/48 h | Mimic peroxidase | Good treatment effect | [84] | |
| hCOF | Imine-linked COF | ZIF-8 | π-stacking | Room temperature etching | Room temperature/1 h | Mimic oxidase | Good catalytic effect | [85] | |
| COF-300-AR@CRL | Imine-linked COF | Candida rugosa lipase | Electrostatic force | Self-assembly strategy | Room temperature/12 h | Mimic oxidase | Dual enzymatic catalytic activities | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, D.; Xia, L.; Li, G. Research Progress on the Application of Covalent Organic Framework Nanozymes in Analytical Chemistry. Biosensors 2024, 14, 163. https://doi.org/10.3390/bios14040163
Yao D, Xia L, Li G. Research Progress on the Application of Covalent Organic Framework Nanozymes in Analytical Chemistry. Biosensors. 2024; 14(4):163. https://doi.org/10.3390/bios14040163
Chicago/Turabian StyleYao, Dongmei, Ling Xia, and Gongke Li. 2024. "Research Progress on the Application of Covalent Organic Framework Nanozymes in Analytical Chemistry" Biosensors 14, no. 4: 163. https://doi.org/10.3390/bios14040163
APA StyleYao, D., Xia, L., & Li, G. (2024). Research Progress on the Application of Covalent Organic Framework Nanozymes in Analytical Chemistry. Biosensors, 14(4), 163. https://doi.org/10.3390/bios14040163






