Critical Issues on the Surface Functionalization of Plasmonic Au-Ag/TiO2 Thin Films with Thiolated Oligonucleotide-Based Biorecognition Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Plasmonic Thin Films Containing Noble Metal NPs (Au, Ag) Dispersed in TiO2
2.1.1. Thin-Film Deposition by DC Reactive Magnetron Sputtering
2.1.2. In-Vacuum Thermal Annealing Treatment to NPs Growth
2.1.3. Chemical, Morphological, and Optical Characterization of Nanoplasmonic Thin Films
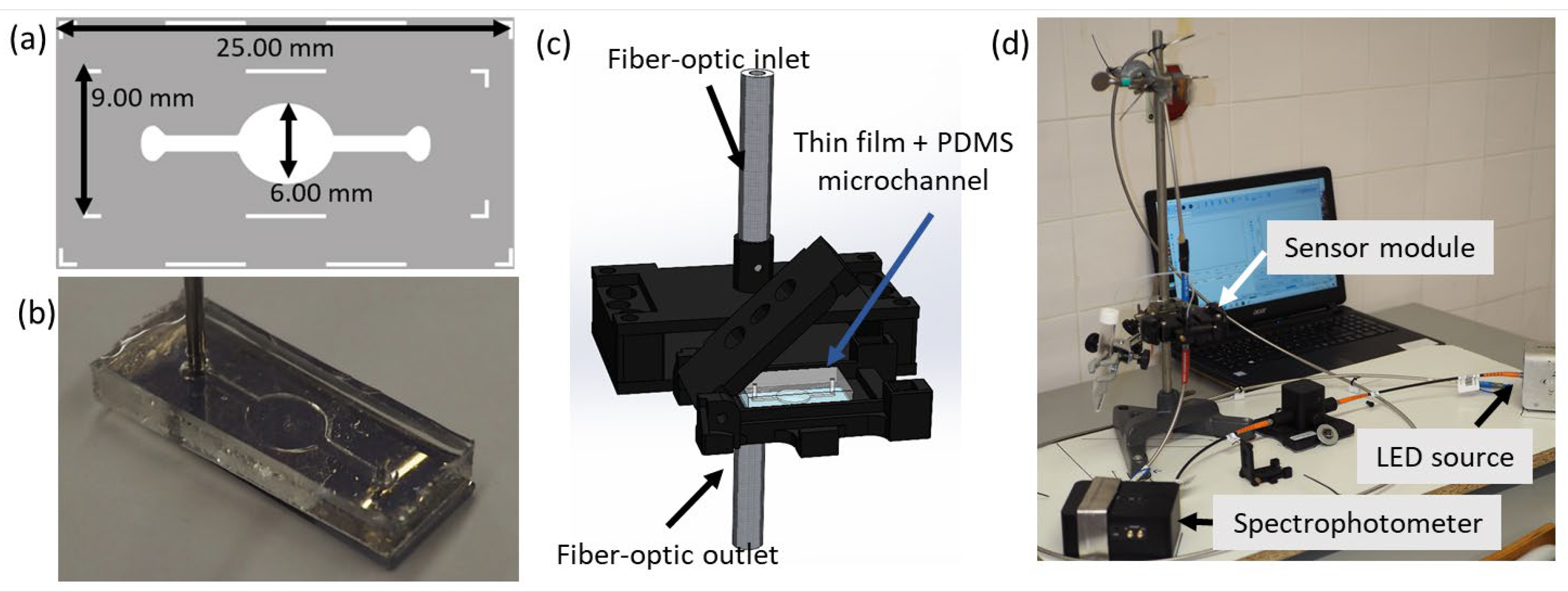
2.2. Fabrication of the Microfluidic Device and Connection to LSPR Spectroscopy System
2.2.1. Microfluidic Channel Development
2.2.2. Microfluidic Module Development and Alignment with LSPR Spectroscopy System
2.2.3. Transmittance LSPR Spectroscopy System (Hardware and Software)
2.3. Immobilization of Thiolated Oligonucleotide on Nanoplasmonic Thin Films
2.3.1. Probe Selection
2.3.2. Optimization of Immobilization Conditions Using LSPR Sensing Response
2.3.3. Hybridization Events Studies with Complementary Oligonucleotide Probes
3. Results
3.1. Nanoplasmonic Thin Film Characterization
3.2. Thin Film’s Surface Functionalization with SH-Oligonucleotide
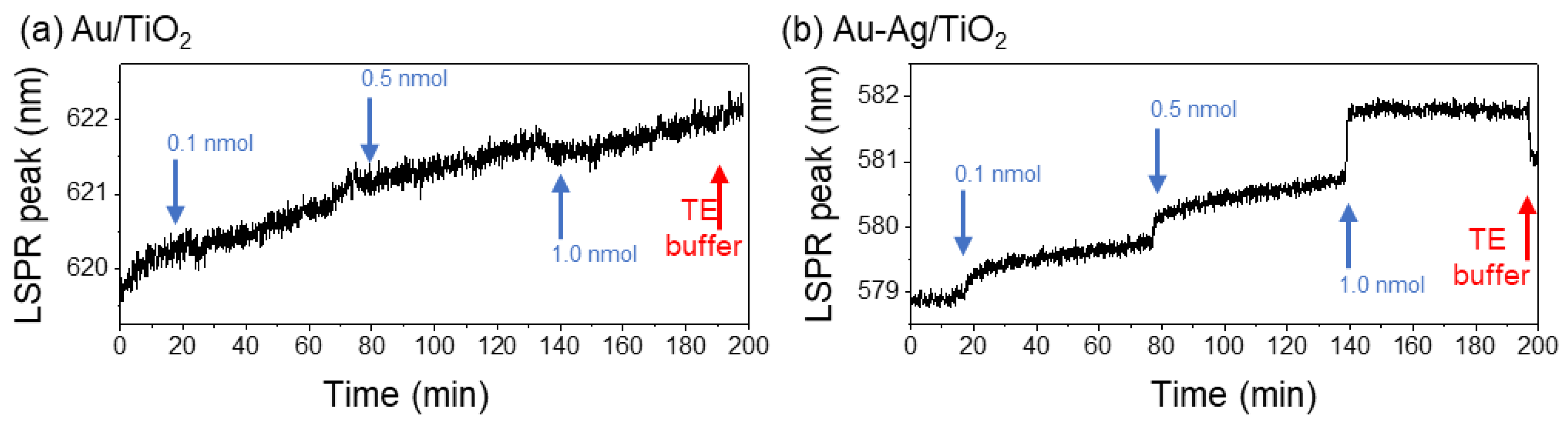
3.2.1. LSPR Sensing Response of Au/TiO2 and Au-Ag/TiO2 Thin Films during Probe Immobilization
3.2.2. Optimizing SH-Oligonucleotide Immobilization as a Biorecognition Element
3.2.3. Detection of Complementary DNA Sequence
3.2.4. Hypothesizing the Performance of LSPR Biosensor Observed during Detection of Target-Oligonucleotide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willett, D.R.; Chumanov, G. LSPR Sensor Combining Sharp Resonance and Differential Optical Measurements. Plasmonics 2014, 9, 1391–1396. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Zayats, A.V.; Smolyaninov, I.I. Near-Field Photonics: Surface Plasmon Polaritons and Localized Surface Plasmons. J. Opt. A Pure Appl. Opt. 2003, 5, S16–S50. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Yonzon, C.R.; Haes, A.J.; Van Duyne, R.P. Localized Surface Plasmon Resonance Biosensors. Nanomedicine 2006, 1, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Krull, U.J. Localized Surface Plasmon Resonance: Nanostructures, Bioassays and Biosensing—A Review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Haes, A.J.; Van Duyne, R.P. A Unified View of Propagating and Localized Surface Plasmon Resonance Biosensors. Anal. Bioanal. Chem. 2004, 379, 920–930. [Google Scholar] [CrossRef]
- Verellen, N.; Van Dorpe, P.; Huang, C.; Lodewijks, K.; Vandenbosch, G.A.E.; Lagae, L.; Moshchalkov, V.V. Plasmon Line Shaping Using Nanocrosses for High Sensitivity Localized Surface Plasmon Resonance Sensing. Nano Lett. 2011, 11, 391–397. [Google Scholar] [CrossRef]
- Zhang, L.M.; Uttamchandani, D. Optical Chemical Sensing Employing Surface Plasmon Resonance. Electron. Lett. 1988, 24, 1469–1470. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Yin, Z.; Liu, Y.; Zhao, J.; Quan, Z. Ordered Mesoporous Silver Superstructures with SERS Hot Spots. Chem. Commun. 2019, 55, 7982–7985. [Google Scholar] [CrossRef]
- Hyungsoon Im, C.; Bantz, K.C.; Hoon Lee, S.; Johnson, T.W.; Haynes, C.L.; Oh, S.-H.; Im, H.; Johnson, T.W.; Oh, S.; Bantz, K.C.; et al. Self-Assembled Plasmonic Nanoring Cavity Arrays for SERS and LSPR Biosensing. Adv. Mater. 2013, 25, 2678–2685. [Google Scholar] [CrossRef]
- Rao, V.K.; Radhakrishnan, T.P. Tuning the SERS Response with Ag-Au Nanoparticle-Embedded Polymer Thin Film Substrates. ACS Appl. Mater. Interfaces 2015, 7, 12767–12773. [Google Scholar] [CrossRef]
- West, J.L.; Halas, N.J. Engineered Nanomaterials for Biophotonics Applications: Improving Sensing, Imaging, and Therapeutics. Annu. Rev. Biomed. Eng. 2003, 5, 285–292. [Google Scholar] [CrossRef]
- Peng, Y.; Xiong, B.; Peng, L.; Li, H.; He, Y.; Yeung, E.S. Recent Advances in Optical Imaging with Anisotropic Plasmonic Nanoparticles. Anal. Chem. 2015, 87, 200–215. [Google Scholar] [CrossRef]
- Fazal, S.; Jayasree, A.; Sasidharan, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Green Synthesis of Anisotropic Gold Nanoparticles for Photothermal Therapy of Cancer. ACS Appl. Mater. Interfaces 2014, 6, 8080–8089. [Google Scholar] [CrossRef]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Cancer Targeting with Biomolecules: A Comparative Study of Photodynamic Therapy Efficacy Using Antibody or Lectin Conjugated Phthalocyanine-PEG Gold Nanoparticles. Photochem. Photobiol. Sci. 2015, 14, 737–747. [Google Scholar] [CrossRef]
- Bido, A.T.; Ember, K.J.I.; Trudel, D.; Durand, M.; Leblond, F.; Brolo, A.G. Detection of SARS-CoV-2 in Saliva by a Low-Cost LSPR-Based Sensor. Anal. Methods 2023, 15, 3955–3966. [Google Scholar] [CrossRef]
- Ning, W.; Hu, S.; Zhou, C.; Luo, J.; Li, Y.; Zhang, C.; Luo, Z.; Li, Y. An Ultrasensitive J-Shaped Optical Fiber LSPR Aptasensor for the Detection of Helicobacter Pylori. Anal. Chim. Acta 2023, 1278, 341733. [Google Scholar] [CrossRef]
- Nan, M.; Darmawan, B.A.; Go, G.; Zheng, S.; Lee, J.; Kim, S.; Lee, T.; Choi, E.; Park, J.O.; Bang, D. Wearable Localized Surface Plasmon Resonance-Based Biosensor with Highly Sensitive and Direct Detection of Cortisol in Human Sweat. Biosensors 2023, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M.; Today, N.; Angelom, P.C. LSPR-Based Nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Sagle, L.B.; Ruvuna, L.K.; Ruemmele, J.A.; Van Duyne, R.P. Advances in Localized Surface Plasmon Resonance Spectroscopy Biosensing. Nanomedicine 2011, 6, 1447–1462. [Google Scholar] [CrossRef]
- Bhattacharjee, K.; Prasad, B.L.V. Surface Functionalization of Inorganic Nanoparticles with Ligands: A Necessary Step for Their Utility. Chem. Soc. Rev. 2023, 52, 2573–2595. [Google Scholar] [CrossRef]
- Mujica, M.L.; Tamborelli, A.; Vaschetti, V.; Gallay, P.; Perrachione, F.; Reartes, D.; Delpino, R.; Rodríguez, M.; Rubianes, M.D.; Dalmasso, P.; et al. Biorecognition Elements in Biosensors. In Biosensors; CRC Press: Boca Raton, FL, USA, 2022; pp. 107–122. [Google Scholar] [CrossRef]
- Häkkinen, H. The Gold–Sulfur Interface at the Nanoscale. Nat. Chem. 2012, 4, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; Sim, S.J. Nanoplasmonic Biosensor: Detection and Amplification of Dual Bio-Signatures of Circulating Tumor DNA. Biosens. Bioelectron. 2015, 67, 443–449. [Google Scholar] [CrossRef]
- Reimers, J.R.; Ford, M.J.; Marcuccio, S.M.; Ulstrup, J.; Hush, N.S. Competition of van Der Waals and Chemical Forces on Gold–Sulfur Surfaces and Nanoparticles. Nat. Rev. Chem. 2017, 1, 17. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef]
- Bechelany, M.; Maeder, X.; Riesterer, J.; Hankache, J.; Lerose, D.; Christiansen, S.; Michler, J.; Philippe, L. Synthesis Mechanisms of Organized Gold Nanoparticles: Influence of Annealing Temperature and Atmosphere. Cryst. Growth Des. 2010, 10, 587–596. [Google Scholar] [CrossRef]
- Daniel, M.-C.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Laser Ablation Synthesis of Gold Nanoparticles in Organic Solvents. J. Phys. Chem. B 2006, 110, 7232–7237. [Google Scholar] [CrossRef]
- Wender, H.; De Oliveira, L.F.; Feil, A.F.; Lissner, E.; Migowski, P.; Meneghetti, M.R.; Teixeira, S.R.; Dupont, J. Synthesis of Gold Nanoparticles in a Biocompatible Fluid from Sputtering Deposition onto Castor Oil. Chem. Commun. 2010, 46, 7019–7021. [Google Scholar] [CrossRef]
- Takele, H.; Schürmann, U.; Greve, H.; Paretkar, D.; Zaporojtchenko, V.; Faupel, F. Controlled Growth of Au Nanoparticles in Co-Evaporated Metal/Polymer Composite Films and Their Optical and Electrical Properties. Eur. Phys. J. Appl. Phys. 2006, 33, 83–89. [Google Scholar] [CrossRef]
- Costa, D.; Rodrigues, M.S.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. Tuning the Refractive Index Sensitivity of LSPR Transducers Based on Nanocomposite Thin Films Composed of Noble Metal Nanoparticles Dispersed in TiO2. Materials 2023, 16, 7355. [Google Scholar] [CrossRef]
- Costa, D.; Rodrigues, M.S.; Roiban, L.; Aouine, M.; Epicier, T.; Steyer, P.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. In-Situ Annealing Transmission Electron Microscopy of Plasmonic Thin Films Composed of Bimetallic Au–Ag Nanoparticles Dispersed in a TiO2 Matrix. Vacuum 2021, 193, 110511. [Google Scholar] [CrossRef]
- Pereira-Silva, P.; Meira, D.I.; Costa-Barbosa, A.; Costa, D.; Rodrigues, M.S.; Borges, J.; Machado, A.V.; Cavaleiro, A.; Sampaio, P.; Vaz, F. Immobilization of Streptavidin on a Plasmonic Au-TiO2 Thin Film towards an LSPR Biosensing Platform. Nanomaterials 2022, 12, 1526. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Luo, Z.Q. Cell Biology of Infection by Legionella pneumophila. Microbes Infect. 2013, 15, 157–167. [Google Scholar] [CrossRef]
- Assaidi, A.; Ellouali, M.; Latrache, H.; Mabrouki, M.; Timinouni, M.; Zahir, H.; Tankiouine, S.; Barguigua, A.; Mliji, E.M. Adhesion of Legionella pneumophila on Glass and Plumbing Materials Commonly Used in Domestic Water Systems. Int. J. Environ. Health Res. 2018, 28, 125–133. [Google Scholar] [CrossRef]
- Totaro, M.; Valentini, P.; Costa, A.L.; Giorgi, S.; Casini, B.; Baggiani, A. Rate of Legionella pneumophila Colonization in Hospital Hot Water Network after Time Flow Taps Installation. J. Hosp. Infect. 2018, 98, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Newton, H.J.; Ang, D.K.Y.; Van Driel, I.R.; Hartland, E.L. Molecular Pathogenesis of Infections Caused by Legionella pneumophila. Clin. Microbiol. Rev. 2010, 23, 274–298. [Google Scholar] [CrossRef]
- Samba-Louaka, A. Legionella pneumophila-Induced Cell Death: Two Hosts, Two Responses. Virulence 2017, 9, 17–19. [Google Scholar] [CrossRef]
- Hase, R.; Miyoshi, K.; Matsuura, Y.; Endo, Y.; Nakamura, M.; Otsuka, Y. Legionella Pneumonia Appeared during Hospitalization in a Patient with Hematological Malignancy Confirmed by Sputum Culture after Negative Urine Antigen Test. J. Infect. Chemother. 2018, 24, 10–13. [Google Scholar] [CrossRef]
- Párraga-Niño, N.; Quero, S.; Uria, N.; Castillo-Fernandez, O.; Jimenez-Ezenarro, J.; Muñoz, F.X.; Sabrià, M.; Garcia-Nuñez, M. Antibody Test for Legionella pneumophila Detection. Diagn. Microbiol. Infect. Dis. 2018, 90, 85–89. [Google Scholar] [CrossRef]
- Rolando, M.; Buchrieser, C. Legionella Effectors Explored with INSeq: New Functional Insights. Trends Microbiol. 2018, 26, 169–170. [Google Scholar] [CrossRef]
- Barradas, N.P.P.; Jeynes, C.; Webb, R.P.P.; Kreissig, U.; Grötzschel, R. Unambiguous Automatic Evaluation of Multiple Ion Beam Analysis Data with Simulated Annealing. Nucl. Instrum. Methods Phys. Res. B 1999, 149, 233–237. [Google Scholar] [CrossRef]
- Barradas, N.P.; Pascual-Izarra, C. Double Scattering in RBS Analysis of PtSi Thin Films on Si. In Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions with Materials and Atoms, Proceedings of the Seventh International Conference on Computer Simulation of Radiation Effects in Solids, Helsinki, Finland, 28 June–2 July 2004; Elsevier: Amsterdam, The Netherlands, 2005; Volume 228, pp. 378–382. [Google Scholar] [CrossRef]
- Barradas, N.P.; Reis, M.A. Accurate Calculation of Pileup Effects in PIXE Spectra from First Principles. X-Ray Spectrom. 2006, 35, 232–237. [Google Scholar] [CrossRef]
- Pinto, V.; Sousa, P.; Cardoso, V.; Minas, G. Optimized SU-8 Processing for Low-Cost Microstructures Fabrication without Cleanroom Facilities. Micromachines 2014, 5, 738–755. [Google Scholar] [CrossRef]
- Kriebel, J.; Gonçalves, I.M.; Baptista, V.; Veiga, M.I.; Minas, G.; Lima, R.; Catarino, S.O. Extensional Flow for Assessing the Effect of Nanocarriers on the Mechanical Deformability of Red Blood Cells. Exp. Therm. Fluid. Sci. 2023, 146, 110931. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Pereira, R.M.S.; Vasilevskiy, M.I.; Borges, J.; Vaz, F. NANOPTICS: In-Depth Analysis of NANomaterials for OPTICal Localized Surface Plasmon Resonance Sensing. SoftwareX 2020, 12, 100522. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Liang, Y.; Liu, Y.; Yu, L.; Li, C.; Liu, Q.; Chen, L. Development of a DNA Microarray Assay for Rapid Detection of Fifteen Bacterial Pathogens in Pneumonia. BMC Microbiol. 2020, 20, 177. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Yen-Lieberman, B.; Reischl, U.; Gordon, S.M.; Procop, G.W. Detection of Legionella pneumophila by Real-Time PCR for the Mip Gene. J. Clin. Microbiol. 2003, 41, 3327–3330. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.S.; Costa, D.; Domingues, R.P.; Apreutesei, M.; Pedrosa, P.; Martin, N.; Correlo, V.M.; Reis, R.L.; Alves, E.; Barradas, N.P.; et al. Optimization of Nanocomposite Au/TiO2 Thin Films towards LSPR Optical-Sensing. Appl. Surf. Sci. 2018, 438, 74–83. [Google Scholar] [CrossRef]
- Costa-Barbosa, A.; Ferreira, D.; Pacheco, M.I.; Casal, M.; Duarte, H.O.; Gomes, C.; Barbosa, A.M.; Torrado, E.; Sampaio, P.; Collins, T. Candida Albicans Chitinase 3 with Potential as a Vaccine Antigen: Production, Purification, and Characterisation. Biotechnol. J. 2023, 19, 2300219. [Google Scholar] [CrossRef]
- Scholl, J.A.; Koh, A.L.; Dionne, J.A. Quantum Plasmon Resonances of Individual Metallic Nanoparticles. Nature 2012, 483, 421–427. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, J.; Wang, Y.; Chen, J.; Li, Y.; Duan, Y. An Aptamer Based Method for Small Molecules Detection through Monitoring Salt-Induced AuNPs Aggregation and Surface Plasmon Resonance (SPR) Detection. Sens. Actuators B Chem. 2016, 236, 474–479. [Google Scholar] [CrossRef]
- Ning, W.; Zhang, C.; Tian, Z.; Wu, M.; Luo, Z.; Hu, S.; Pan, H.; Li, Y. Ω-Shaped Fiber Optic LSPR Biosensor Based on Mismatched Hybridization Chain Reaction and Gold Nanoparticles for Detection of Circulating Cell-Free DNA. Biosens. Bioelectron. 2023, 228, 115175. [Google Scholar] [CrossRef]
- Han, X.; Wang, E.; Cui, Y.; Lin, Y.; Chen, H.; An, R.; Liang, X.; Komiyama, M. The Staining Efficiency of Cyanine Dyes for Single-Stranded DNA Is Enormously Dependent on Nucleotide Composition. Electrophoresis 2019, 40, 1708–1714. [Google Scholar] [CrossRef]
- Elhadj, S.; Singh, G.; Saraf, R.F. Optical Properties of an Immobilized DNA Monolayer from 255 to 700 Nm. Langmuir 2004, 20, 5539–5543. [Google Scholar] [CrossRef]
- Singh, R.; Priye, V.; Chack, D. Highly Sensitive Refractive Index-Based Sensor for DNA Hybridization Using Subwavelength Grating Waveguide. IETE Tech. Rev. 2022, 39, 1463–1472. [Google Scholar] [CrossRef]
- Xu, F.; Pellino, A.M.; Knoll, W. Electrostatic Repulsion and Steric Hindrance Effects of Surface Probe Density on Deoxyribonucleic Acid (DNA)/Peptide Nucleic Acid (PNA) Hybridization. Thin Solid. Films 2008, 516, 8634–8639. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, D.; Pereira-Silva, P.; Sousa, P.; Pinto, V.; Borges, J.; Vaz, F.; Minas, G.; Sampaio, P. Critical Issues on the Surface Functionalization of Plasmonic Au-Ag/TiO2 Thin Films with Thiolated Oligonucleotide-Based Biorecognition Elements. Biosensors 2024, 14, 159. https://doi.org/10.3390/bios14040159
Costa D, Pereira-Silva P, Sousa P, Pinto V, Borges J, Vaz F, Minas G, Sampaio P. Critical Issues on the Surface Functionalization of Plasmonic Au-Ag/TiO2 Thin Films with Thiolated Oligonucleotide-Based Biorecognition Elements. Biosensors. 2024; 14(4):159. https://doi.org/10.3390/bios14040159
Chicago/Turabian StyleCosta, Diogo, Patrícia Pereira-Silva, Paulo Sousa, Vânia Pinto, Joel Borges, Filipe Vaz, Graça Minas, and Paula Sampaio. 2024. "Critical Issues on the Surface Functionalization of Plasmonic Au-Ag/TiO2 Thin Films with Thiolated Oligonucleotide-Based Biorecognition Elements" Biosensors 14, no. 4: 159. https://doi.org/10.3390/bios14040159
APA StyleCosta, D., Pereira-Silva, P., Sousa, P., Pinto, V., Borges, J., Vaz, F., Minas, G., & Sampaio, P. (2024). Critical Issues on the Surface Functionalization of Plasmonic Au-Ag/TiO2 Thin Films with Thiolated Oligonucleotide-Based Biorecognition Elements. Biosensors, 14(4), 159. https://doi.org/10.3390/bios14040159










