Photocell-Based Optofluidic Device for Clogging-Free Cell Transit Time Measurements
Abstract
1. Introduction
2. Materials and Methods
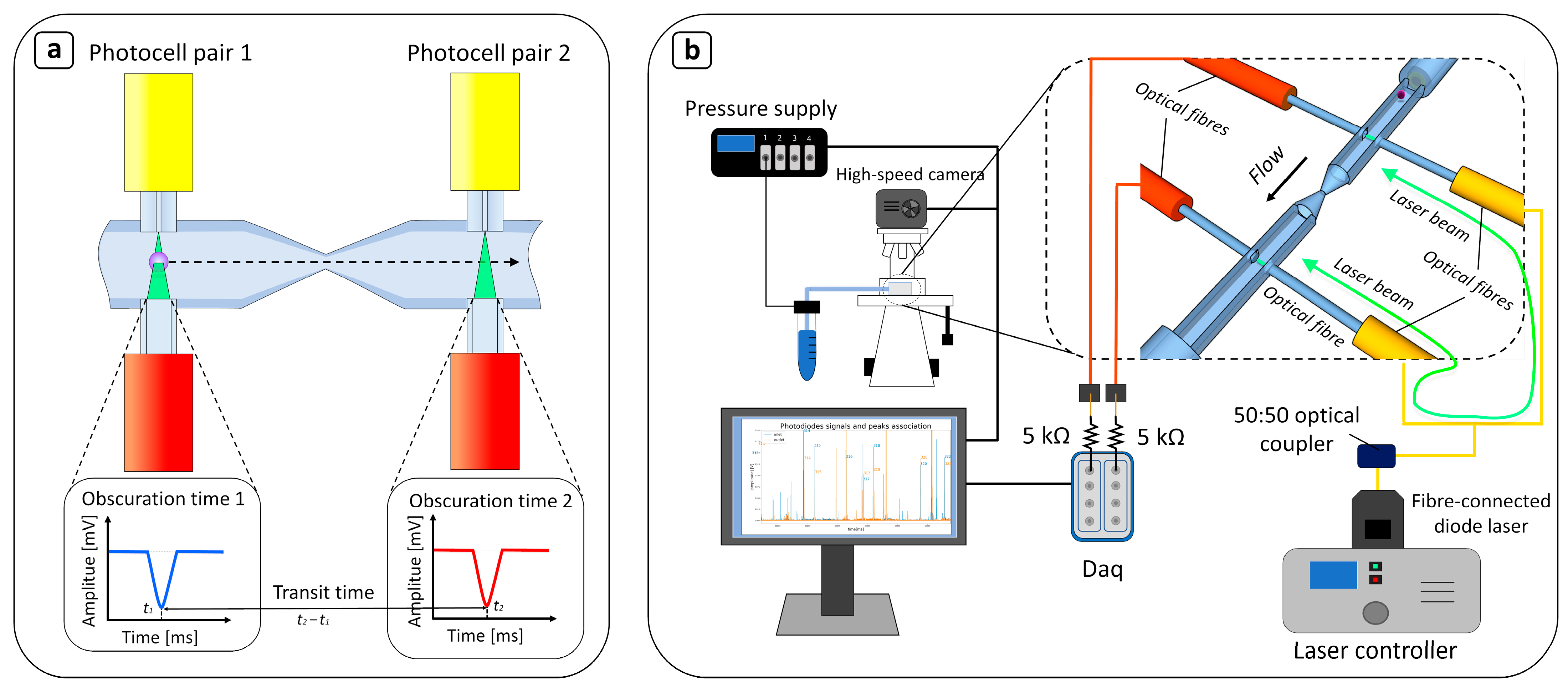
2.1. Device Design
2.2. Device Fabrication
2.3. Device Characterization
2.4. Cell Preparation
2.5. Data Analysis
3. Results and Discussion
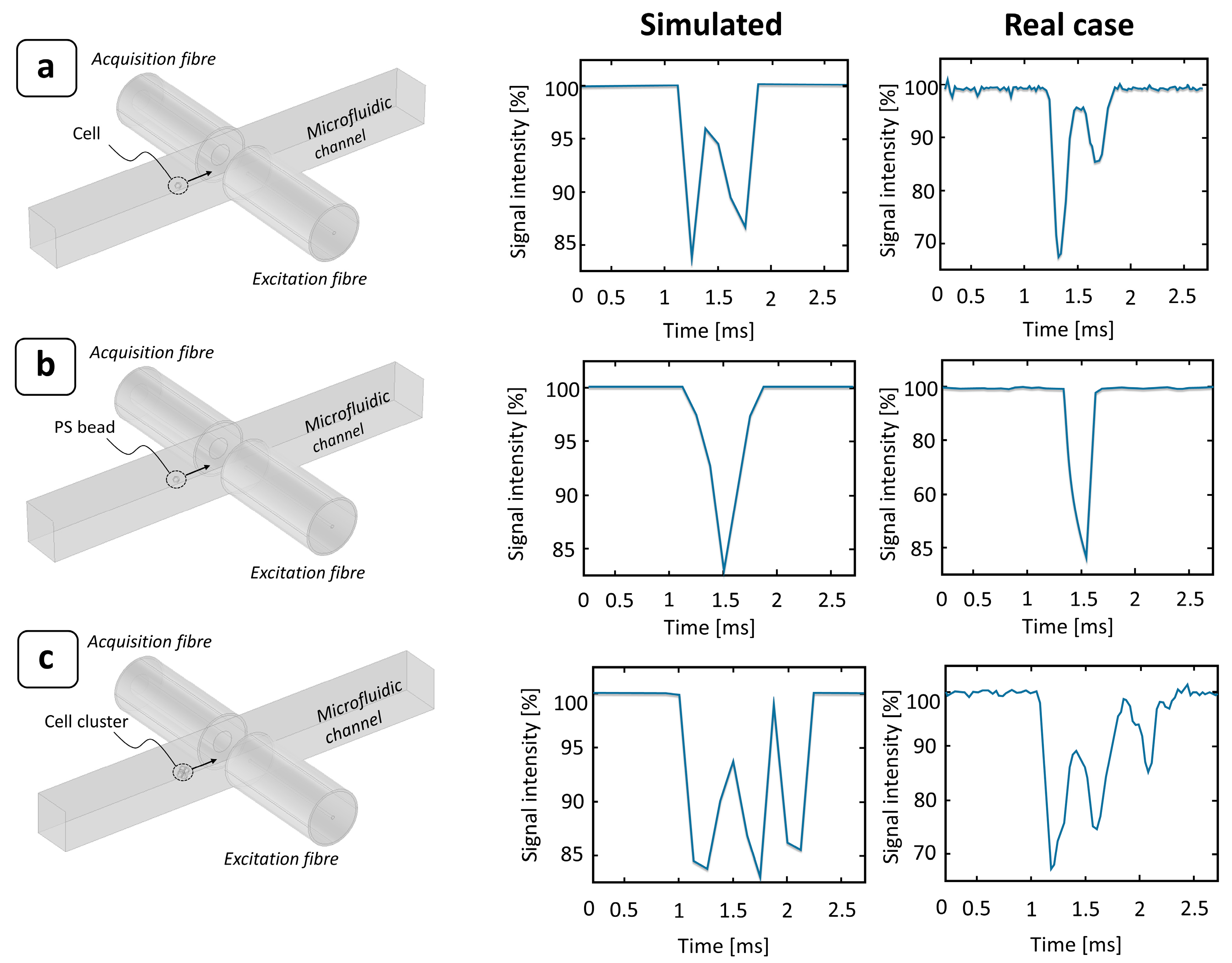
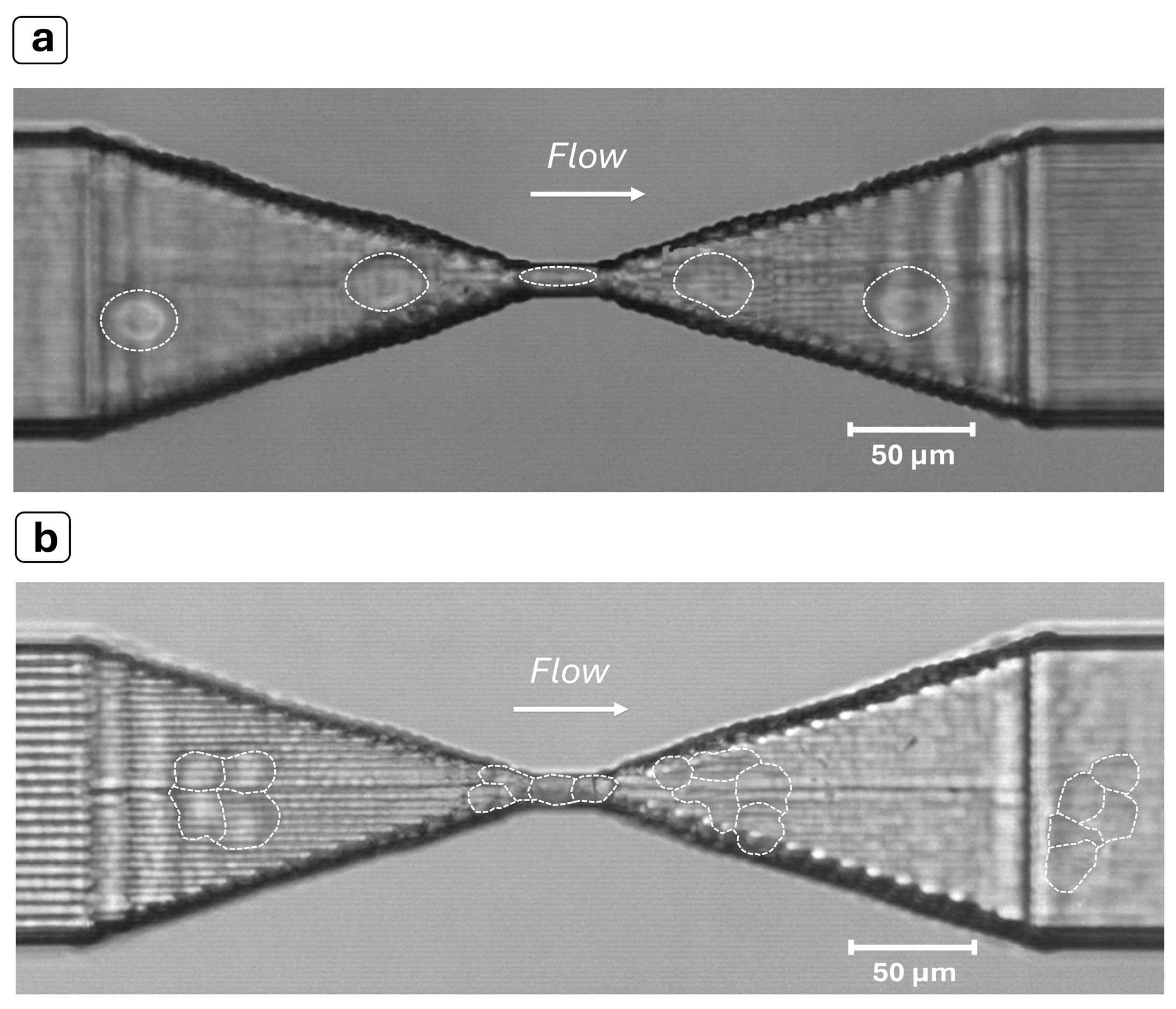
3.1. Polystyrene (PS) Particles
3.2. MCF-7 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Carlo, D. A Mechanical Biomarker of Cell State in Medicine. J. Lab. Autom. 2012, 17, 32–42. [Google Scholar] [CrossRef]
- Agrawal, R.; Smart, T.; Nobre-Cardoso, J.; Richards, C.; Bhatnagar, R.; Tufail, A.; Shima, D.; Jones, P.H.; Pavesio, C. Assessment of Red Blood Cell Deformability in Type 2 Diabetes Mellitus and Diabetic Retinopathy by Dual Optical Tweezers Stretching Technique. Sci. Rep. 2016, 6, srep15873. [Google Scholar] [CrossRef]
- Donadello, K.; Piagnerelli, M.; Reggiori, G.; Gottin, L.; Scolletta, S.; Occhipinti, G.; Zouaoui Boudjeltia, K.; Vincent, J.L. Reduced Red Blood Cell Deformability over Time Is Associated with a Poor Outcome in Septic Patients. Microvasc. Res. 2015, 101, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Reiling, S.J.; Rohrbach, P.; Ma, H. Microfluidic Biomechanical Assay for Red Blood Cells Parasitized by Plasmodium Falciparum. Lab. Chip 2012, 12, 1143–1150. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Miao, J.; Cui, L.; Guan, W. High-Throughput and Label-Free Parasitemia Quantification and Stage Differentiation for Malaria-Infected Red Blood Cells. Biosens. Bioelectron. 2017, 98, 408–414. [Google Scholar] [CrossRef]
- Runel, G.; Lopez-ramirez, N.; Chlasta, J.; Masse, I. Biomechanical Properties of Cancer Cells. Cells 2021, 10, 887. [Google Scholar] [CrossRef]
- Darling, E.M.; Di Carlo, D. High-Throughput Assessment of Cellular Mechanical Properties. Annu. Rev. Biomed. Eng. 2015, 17, 35–62. [Google Scholar] [CrossRef]
- Harris, A.R.; Charras, G.T. Experimental Validation of Atomic Force Microscopy-Based Cell Elasticity Measurements. Nanotechnology 2011, 22, 345102. [Google Scholar] [CrossRef] [PubMed]
- Guck, J.; Ananthakrishnan, R.; Mahmood, H.; Moon, T.J.; Cunningham, C.C.; Käs, J. The Optical Stretcher: A Novel Laser Tool to Micromanipulate Cells. Biophys. J. 2001, 81, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.; Lim, C.T.; Suresh, S. Mechanics of the Human Red Blood Cell Deformed by Optical Tweezers. J. Mech. Phys. Solids 2003, 51, 2259–2280. [Google Scholar] [CrossRef]
- Kollmannsberger, P.; Fabry, B. High-Force Magnetic Tweezers with Force Feedback for Biological Applications. Rev. Sci. Instrum. 2007, 78, 114301. [Google Scholar] [CrossRef]
- Guo, Q.; Park, S.; Ma, H. Microfluidic Micropipette Aspiration for Measuring the Deformability of Single Cells. Lab. Chip 2012, 12, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, G.F.; Feng, X.Q.; Yu, S.W. Micropipette Aspiration Method for Characterizing Biological Materials with Surface Energy. J. Biomech. 2018, 80, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Cheng, S.; Tanaka, Y.; Hosokawa, Y.; Yalikun, Y.; Li, M. Mechanical Properties of Single Cells: Measurement Methods and Applications. Biotechnol. Adv. 2020, 45, 107648. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhou, Y.; Zhou, Y.; Han, J.; Ai, Y. Biophysical Phenotyping of Single Cells Using a Differential Multiconstriction Microfluidic Device with Self-Aligned 3D Electrodes. Biosens. Bioelectron. 2019, 133, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, M.; Muñoz, H.E.; Shaw Bagnall, J.; Otto, O.; Manalis, S.R.; Di Carlo, D.; Guck, J. A Comparison of Microfluidic Methods for High-Throughput Cell Deformability Measurements. Nat. Methods 2020, 17, 587–593. [Google Scholar] [CrossRef]
- Otto, O.; Rosendahl, P.; Mietke, A.; Golfier, S.; Herold, C.; Klaue, D.; Girardo, S.; Pagliara, S.; Ekpenyong, A.; Jacobi, A.; et al. Real-Time Deformability Cytometry: On-the-Fly Cell Mechanical Phenotyping. Nat. Methods 2015, 12, 199–202. [Google Scholar] [CrossRef]
- Gossett, D.R.; Tse, H.T.K.; Lee, S.A.; Ying, Y.; Lindgren, A.G.; Yang, O.O.; Rao, J.; Clark, A.T.; Di Carlo, D. Hydrodynamic Stretching of Single Cells for Large Population Mechanical Phenotyping. Proc. Natl. Acad. Sci. USA 2012, 109, 7630–7635. [Google Scholar] [CrossRef]
- Ai, Y.; Liang, M.; Yang, D.; Zhou, Y.; Li, P.; Zhong, J. Single-Cell Stretching in Viscoelastic Fluids with Electronically Triggered Imaging for Cellular Mechanical Phenotyping. Anal. Chem. 2021, 93, 4567–4575. [Google Scholar] [CrossRef]
- Mietke, A.; Otto, O.; Girardo, S.; Rosendahl, P.; Taubenberger, A.; Golfier, S.; Ulbricht, E.; Aland, S.; Guck, J.; Fischer-Friedrich, E. Extracting Cell Stiffness from Real-Time Deformability Cytometry: Theory and Experiment. Biophys. J. 2015, 109, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, M.; Mokbel, D.; Mietke, A.; Träber, N.; Girardo, S.; Otto, O.; Guck, J.; Aland, S. Numerical Simulation of Real-Time Deformability Cytometry to Extract Cell Mechanical Properties. ACS Biomater. Sci. Eng. 2017, 3, 2962–2973. [Google Scholar] [CrossRef]
- Wyss, H.M. Cell Mechanics: Combining Speed with Precision. Biophys. J. 2015, 109, 1997–1998. [Google Scholar] [CrossRef][Green Version]
- Byun, S.; Son, S.; Amodei, D.; Cermak, N.; Shaw, J.; Kang, J.H.; Hecht, V.C.; Winslow, M.M.; Jacks, T.; Mallick, P.; et al. Characterizing Deformability and Surface Friction of Cancer Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7580–7585. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Zhou, Y.; Khoo, B.L.; Han, J.; Ai, Y. Characterizing Deformability and Electrical Impedance of Cancer Cells in a Microfluidic Device. Anal. Chem. 2018, 90, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Tadakuma, K.; Higashimori, M.; Arai, T.; Kaneko, M.; Iitsuka, R.; Yamanishi, Y.; Arai, F. A New Stiffness Evaluation toward High Speed Cell Sorter. In Proceedings of the 2010 IEEE International Conference on Robotics and Automation (ICRA 2010), Anchorage, Alaska, 3–8 May 2010; pp. 4113–4118. [Google Scholar] [CrossRef]
- Lange, J.R.; Steinwachs, J.; Kolb, T.; Lautscham, L.A.; Harder, I.; Whyte, G.; Fabry, B. Microconstriction Arrays for High-Throughput Quantitative Measurements of Cell Mechanical Properties. Biophys. J. 2015, 109, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, K.D.; Hu, K.H.; Kleinman, S.H.; Khismatullin, D.B.; Butte, M.J.; Rowat, A.C. Quantitative Deformability Cytometry: Rapid, Calibrated Measurements of Cell Mechanical Properties. Biophys. J. 2017, 113, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, X.; Kojima, M.; Huang, Q.; Arai, T. Automated Cell Mechanical Characterization by On-Chip Sequential Squeezing: From Static to Dynamic. Langmuir 2021, 37, 8083–8094. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, Y.; Tan, Q.; Shojaei-Baghini, E.; Zhang, Y.L.; Li, J.; Prasad, P.; You, L.; Wu, X.Y.; Sun, Y. Classification of Cell Types Using a Microfluidic Device for Mechanical and Electrical Measurement on Single Cells. Lab. Chip 2011, 11, 3174–3181. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.; Sharei, A.; Adamo, L.; Lee, B.; Mao, S.; Jensen, K.F. Microfluidics-Based Assessment of Cell Deformability. Anal. Chem. 2012, 84, 6438–6443. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shojaei-Baghini, E.; Azad, A.; Wang, C.; Sun, Y. High-Throughput Biophysical Measurement of Human Red Blood Cells. Lab. Chip 2012, 12, 2560–2567. [Google Scholar] [CrossRef]
- Honrado, C.; Bisegna, P.; Swami, N.S.; Caselli, F. Single-Cell Microfluidic Impedance Cytometry: From Raw Signals to Cell Phenotypes Using Data Analytics. Lab. Chip 2021, 21, 22–54. [Google Scholar] [CrossRef]
- Sano, M.; Kaji, N.; Rowat, A.C.; Yasaki, H.; Shao, L.; Odaka, H.; Yasui, T.; Higashiyama, T.; Baba, Y. Microfluidic Mechanotyping of a Single Cell with Two Consecutive Constrictions of Different Sizes and an Electrical Detection System. Anal. Chem. 2019, 91, 12890–12899. [Google Scholar] [CrossRef]
- Hou, H.W.; Li, Q.S.; Lee, G.Y.H.; Kumar, A.P.; Ong, C.N.; Lim, C.T. Deformability Study of Breast Cancer Cells Using Microfluidics. Biomed. Microdevices 2009, 11, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Gattass, R.R.; Mazur, E. Femtosecond Laser Micromachining in Transparent Materials. Nat. Photonics 2008, 2, 219–225. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Femtosecond Laser Processing for Optofluidic Fabrication. Lab. Chip 2012, 12, 3576–3589. [Google Scholar] [CrossRef] [PubMed]
- Osellame, R.; Cerullo, G.; Ramponi, R. Femtosecond Laser Micromachining: Photonic and Microfluidic Devices in Transparent Materials. In Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2012; Volume 123. [Google Scholar] [CrossRef]
- Paiè, P.; Bragheri, F.; Martinez Vazquez, R.; Osellame, R. Straightforward 3D hydrodynamic focusing in femtosecond laser fabricated microfluidic channels. Lab Chip 2014, 14, 1826–1833. [Google Scholar] [CrossRef]
- Zorzi, F.; Bonfadini, S.; Aloisio, L.; Moschetta, M.; Storti, F.; Simoni, F.; Lanzani, G.; Criante, L. Optofluidic Flow Cytometer with In-Plane Spherical Mirror for Signal Enhancement. Sensors 2023, 23, 9191. [Google Scholar] [CrossRef]
- Hnatovsky, C.; Taylor, R.S.; Simova, E.; Bhardwaj, V.R.; Rayner, D.M.; Corkum, P.B. Polarization-Selective Etching in Femtosecond Laser-Assisted Microfluidic Channel Fabrication in Fused Silica. Opt. Lett. 2005, 30, 1867. [Google Scholar] [CrossRef]
- Sommer, C.; Quint, S.; Spang, P.; Walther, T.; Baßler, M. The Equilibrium Velocity of Spherical Particles in Rectangular Microfluidic Channels for Size Measurement. Lab. Chip 2014, 14, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.J.; Liu, A.Q.; Lim, C.S.; Ayi, T.C.; Yap, P.H. Determining Refractive Index of Single Living Cell Using an Integrated Microchip. Sens. Actuators A Phys. 2007, 133, 349–354. [Google Scholar] [CrossRef]
- Sultanova, N.G.; Kasarova, S.N.; Nikolov, I.D. Characterization of Optical Properties of Optical Polymers. Opt. Quantum Electron. 2013, 45, 221–232. [Google Scholar] [CrossRef]
- Hedde, P.N.; Malacrida, L.; Ahrar, S.; Siryaporn, A.; Gratton, E. SideSPIM—Selective Plane Illumination Based on a Conventional Inverted Microscope. Biomed. Opt. Express 2017, 8, 3918. [Google Scholar] [CrossRef] [PubMed]
- Storti, F.; Bonfadini, S.; Criante, L. Simplified 3D Hydrodynamic Flow Focusing for Lab-on-Chip Single Particle Study. Sci. Rep. 2023, 13, 14671. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storti, F.; Bonfadini, S.; Bondelli, G.; Vurro, V.; Lanzani, G.; Criante, L. Photocell-Based Optofluidic Device for Clogging-Free Cell Transit Time Measurements. Biosensors 2024, 14, 154. https://doi.org/10.3390/bios14040154
Storti F, Bonfadini S, Bondelli G, Vurro V, Lanzani G, Criante L. Photocell-Based Optofluidic Device for Clogging-Free Cell Transit Time Measurements. Biosensors. 2024; 14(4):154. https://doi.org/10.3390/bios14040154
Chicago/Turabian StyleStorti, Filippo, Silvio Bonfadini, Gaia Bondelli, Vito Vurro, Guglielmo Lanzani, and Luigino Criante. 2024. "Photocell-Based Optofluidic Device for Clogging-Free Cell Transit Time Measurements" Biosensors 14, no. 4: 154. https://doi.org/10.3390/bios14040154
APA StyleStorti, F., Bonfadini, S., Bondelli, G., Vurro, V., Lanzani, G., & Criante, L. (2024). Photocell-Based Optofluidic Device for Clogging-Free Cell Transit Time Measurements. Biosensors, 14(4), 154. https://doi.org/10.3390/bios14040154





