Hybrid Nanomaterials: A Brief Overview of Versatile Solutions for Sensor Technology in Healthcare and Environmental Applications
Abstract
1. Introduction
2. Nanomaterials for Sensor Applications
3. Use of Hybrid Nanomaterials in Sensors for the Environmental Applications
3.1. Nanomaterials with Applications in Environmental Sensors
Environmental Monitoring
| Nanomaterial | Application | References |
|---|---|---|
| Carbon nanotubes, carbon nanofibers, C 60 fullerene | electrode materials for detecting heavy metal ions | [83,84,85,86,87] |
| Carbon dots | fluorescent sensing (gas molecules, pH, ions, and biological analytes) | [42,88,89] |
| Graphene, graphene oxide | electrode materials for detecting heavy metal ions | [24,90,91] |
| Reduced graphene oxide | gas sensing, biosensing, environmental monitoring | [74,80,81,92,93,94] |
| Graphene quantum dot | electrochemical biosensing | [95] |
| Mxene, Functionalized Mxene | VOC sensing device, healthcare sensors, Internet of Things | [22,23,35,36,37,38,96] |
| Metal nanoparticles (MeNPs) | copper, iron, zinc, silver, gold, nickel—electrode materials for detecting heavy metal ions; Au, Ag, Pt-optical sensing, electrochemical sensing | [66,79,97] |
| Magnetic nanoparticles + functionalization | biosensors, drug delivery, system MRI | [98] |
| Semiconductor nanomaterials | optical and electrochemical sensors | [42] |
| QD | Fluorescent-based sensing | [42] |
| Metal oxides-NPs | VOCs | [25] |
| 2D materials like MoS2, WS2, black phosphorus | gas sensing, chemicals, biomolecules | [45] |
| Polymers: [99] phenol-formaldehyde resin, resorcinol, poly-methyl methacrylate, chitosan, polyvinylacohol, poly(acrylic acid) | electrode materials for detecting heavy metal ions | [66,78,100,101,102] |
| MOF, Nanocellulose-MOF | sensor technologies | [47] |
| Hybrid nanomaterials: carbon nanomaterials + MeNPs | PMs sensors | [62,73] |
| Me oxides + carbon nanomaterials or MeNPs | Gas sensors, VOCs, env. pollutants | [81,103] |
| Graphene oxide + Me NPs or Me oxides | water quality monitoring, heavy metals, org. pollutants, pathogens | [16,69] |
| Hybrid nanomaterials–Nanocomposites: Carbon nanomaterials + Me oxides NPs + polymer | VOCs detection | [104,105] |
| QDs + org. dyes or plasmonic NPs | optical sensors for environmental monitoring | [43,68,106] |
| Nanomaterials + biomolecules | biosensors for environmental monitoring | [17,107] |
3.2. Nanomaterials in Sensor Technology
Mxene Based Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bodor, K.; Szp, R.; Bodor, Z. Examination of air pollutants and their risk for human health in urban and suburban environments for two Romanian cities: Brasov and Iasi. Heliyon 2023, 9, e21810. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Pandiaraj, S.; Ramasubburayan, R.; Gaur, A.; Sekar, M.; Viswanathan, D.; Govindasamy, R. Investigating the ecological implications of nanomaterials: Unveiling plants’ notable responses to nano-pollution. Plant Physiol. Biochem. 2024, 206, 108261. [Google Scholar] [CrossRef] [PubMed]
- Rovira, J.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Environmental impact and human health risks of air pollutants near a large chemical/petrochemical complex: Case study in Tarragona, Spain. Sci. Total Environ. 2021, 787, 147550. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, Y.; Sun, Z.; Su, J.; Liu, Q.S.; Zhou, Q.; Jiang, G. Environmental pollution, a hidden culprit for health issues. Eco-Environ. Health 2022, 1, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, K.; Jia, P. Impact of environmental regulations on environmental quality and public health in China: Empirical analysis with panel data approach. Sustainability 2020, 12, 623. [Google Scholar] [CrossRef]
- European Council. Regulation (EU) 2019/1021 of the European Parliament and of the Council of 20 June 2019 on persistent organic pollutants (recast). Off. J. Eur. Union 2019, L169, 45–77. [Google Scholar]
- Samriti; Rumyantseva, M.; Sun, S.; Kuznetsov, A.; Prakash, J. Emerging nanomaterials in the detection and degradation of air pollutants. Curr. Opin. Environ. Sci. Health 2023, 35, 100497. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Q.; Zheng, S.; Li, J.; Wu, T.; Wang, S.; Wang, C.; Gu, B. Ultrasensitive and simultaneous monitoring of multiple small-molecule pollutants on an immunochromatographic strip with multilayered film-like fluorescent tags. Sci. Total Environ. 2023, 878, 162968. [Google Scholar] [CrossRef]
- Prajapati, S.; Padhan, B.; Amulyasai, B.; Sarkar, A. Nanotechnology-Based Sensors; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 237–262. [Google Scholar] [CrossRef]
- Sahu, M.K.; Yadav, R. Recent advances in nanotechnology. Int. J. Nanomater. Nanotechnol. Nanomed. 2023, 9, 15–23. [Google Scholar]
- Cross, E.S.; Williams, L.R.; Lewis, D.K.; Magoon, G.R.; Onasch, T.B.; Kaminsky, M.L.; Worsnop, D.R.; Jayne, J.T. Use of electrochemical sensors for measurement of air pollution: Correcting interference response and validating measurements. Atmos. Meas. Tech. 2017, 10, 3575–3588. [Google Scholar] [CrossRef]
- Tovar-Lopez, F.J. Recent Progress in Micro- and Nanotechnology-Enabled Sensors for Biomedical and Environmental Challenges. Sensors 2023, 23, 5406. [Google Scholar] [CrossRef]
- Ramya, M.; Senthil Kumar, P.; Rangasamy, G.; Uma Shankar, V.; Rajesh, G.; Nirmala, K.; Saravanan, A.; Krishnapandi, A. A recent advancement on the applications of nanomaterials in electrochemical sensors and biosensors. Chemosphere 2022, 308, 136416. [Google Scholar] [CrossRef]
- Ali, Z.; Ullah, R.; Tuzen, M.; Ullah, S.; Rahim, A.; Saleh, T.A. Colorimetric sensing of heavy metals on metal doped metal oxide nanocomposites: A review. Trends Environ. Anal. Chem. 2023, 37, e00187. [Google Scholar] [CrossRef]
- Negahdary, M.; Akira Ameku, W.; Gomes Santos, B.; dos Santos Lima, I.; Gomes de Oliveira, T.; Carvalho França, M.; Angnes, L. Recent electrochemical sensors and biosensors for toxic agents based on screen-printed electrodes equipped with nanomaterials. Microchem. J. 2023, 185, 108281. [Google Scholar] [CrossRef]
- Hashem, A.; Hossain, M.A.M.; Marlinda, A.R.; Mamun, M.A.; Simarani, K.; Johan, M.R. Nanomaterials based electrochemical nucleic acid biosensors for environmental monitoring: A review. Appl. Surf. Sci. Adv. 2021, 4, 100064. [Google Scholar] [CrossRef]
- Laad, M.; Ghule, B. Removal of toxic contaminants from drinking water using biosensors: A systematic review. Groundw. Sustain. Dev. 2023, 20, 100888. [Google Scholar] [CrossRef]
- Gaviria-Arroyave, M.I.; Cano, J.B.; Peñuela, G.A. Nanomaterial-based fluorescent biosensors for monitoring environmental pollutants: A critical review. Talanta Open 2020, 2, 100006. [Google Scholar] [CrossRef]
- Mohamed, E.F.; Awad, G. Development of Nano-Sensor and Biosensor as an Air Pollution Detection Technique for the Foreseeable Future, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2022; Volume 99, pp. 163–188. [Google Scholar]
- Adeniji, T.M.; Stine, K.J. Nanostructure Modified Electrodes for Electrochemical Detection of Contaminants of Emerging Concern. Coatings 2023, 13, 381. [Google Scholar] [CrossRef]
- Chen, W.Y.; Sullivan, C.D.; Lai, S.N.; Yen, C.C.; Jiang, X.; Peroulis, D.; Stanciu, L.A. Noble-Nanoparticle-Decorated Ti3C2TxMXenes for Highly Sensitive Volatile Organic Compound Detection. ACS Omega 2022, 7, 29195–29203. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Khanna, V.; Ahmed Awan, H.T.; Singh, K.; Khalid, M.; Mishra, Y.K.; Bhansali, S.; Li, C.Z.; Kaushik, A. Towards hospital-on-chip supported by 2D MXenes-based 5th generation intelligent biosensors. Biosens. Bioelectron. 2023, 220, 114847. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, N.; Palani, Y.; Jonnalagadda, R.R.; Shanmugam, E. Covalently dual functionalized graphene oxide-based multiplex electrochemical sensor for Hg(II) and Cr(VI) detection. Sens. Actuators B Chem. 2022, 367, 132165. [Google Scholar] [CrossRef]
- Ali, A.; Yucheng, W.; Fatima, Q.; Raza, A.; Zhong, L.; Chen, H.; Rutendo, C.; Ghani, F. Synthesis and characterization of TiO2 nanomaterials for sensing environmental volatile compounds (VOCs): A review. Trends Anal. Chem. 2024, 170, 117454. [Google Scholar]
- Khanmohammadi, A.; Jalili Ghazizadeh, A.; Hashemi, P.; Afkhami, A.; Arduini, F.; Bagheri, H. An overview to electrochemical biosensors and sensors for the detection of environmental contaminants. J. Iran. Chem. Soc. 2020, 17, 2429–2447. [Google Scholar] [CrossRef]
- Navrotskaya, A.G.; Aleksandrova, D.D.; Krivoshapkina, E.F.; Sillanpää, M.; Krivoshapkin, P.V. Hybrid Materials Based on Carbon Nanotubes and Nanofibers for Environmental Applications; Frontiers Media S.A.: Lausanne, Switzerland, 2020; Volume 8. [Google Scholar] [CrossRef]
- Hassan, M.H.; Khan, R.; Andreescu, S. Advances in electrochemical detection methods for measuring contaminants of emerging concerns. Electrochem. Sci. Adv. 2022, 2, e2100184. [Google Scholar] [CrossRef]
- Barhoum, A.; Hamimed, S.; Slimi, H.; Othmani, A.; Abdel-Haleem, F.M.; Bechelany, M. Modern designs of electrochemical sensor platforms for environmental analyses: Principles, nanofabrication opportunities, and challenges. Trends Environ. Anal. Chem. 2023, 38, e00199. [Google Scholar] [CrossRef]
- Polyakov, M.; Ivanova, V.; Klyamer, D.; Köksoy, B.; Şenocak, A.; Demirbaş, E.; Durmuş, M.; Basova, T. A hybrid nanomaterial based on single walled carbon nanotubes cross-linked via axially substituted silicon (IV) phthalocyanine for chemiresistive sensors. Molecules 2020, 25, 2073. [Google Scholar] [CrossRef]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chapter 19—Innovative Progress in Functionalized Carbon Nanomaterials, Their Hybrids, and Nanocomposites: Fabrication, Antibacterial, Biomedical, Bioactivity, and Biosensor Applications. In Functionalized Carbon Nanomaterials for Theranostic Applications; Mallakpour, S., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 439–479. [Google Scholar] [CrossRef]
- Potes-Lesoinne, H.A.; Ramirez-Alvarez, F.; Perez-Gonzalez, V.H.; Martinez-Chapa, S.O.; Gallo-Villanueva, R.C. Nanomaterials for electrochemical detection of pollutants in water: A review. Electrophoresis 2022, 43, 249–262. [Google Scholar] [CrossRef]
- Ehtesabi, H. Carbon nanomaterials for salivary-based biosensors: A review. Mater. Today Chem. 2020, 17, 100342. [Google Scholar] [CrossRef]
- Tian, S.; Wang, M.; Fornasiero, P.; Yang, X. Recent advances in MXenes-based glucose biosensors. Chin. Chem. Lett. 2023, 34, 108241. [Google Scholar] [CrossRef]
- Wang, Q.; Han, N.; Shen, Z.; Li, X.; Chen, Z.; Cao, Y.; Si, W.; Wang, F.; Ni, B.J.; Thakur, V.K. MXene-based electrochemical (bio) sensors for sustainable applications: Roadmap for future advanced materials. Nano Mater. Sci. 2023, 5, 39–52. [Google Scholar] [CrossRef]
- Noh, S.; Lee, H.; Kim, J.; Jang, H.; An, J.; Park, C.; Lee, M.H.; Lee, T. Rapid electrochemical dual-target biosensor composed of an Aptamer/MXene hybrid on Au microgap electrodes for cytokines detection. Biosens. Bioelectron. 2022, 207, 114159. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xu, Y.; Yang, J.; Song, Y.; Yin, F.; Yuan, W. Stretchable and wearable conductometric VOC sensors based on microstructured MXene/polyurethane core-sheath fibers. Sens. Actuators B Chem. 2021, 346, 130500. [Google Scholar] [CrossRef]
- Yadav, N.; Chaudhary, P.; Dey, K.K. Non-functionalized Au nanoparticles can act as high-performing humidity sensor. J. Mater. Sci. Mater. Electron. 2020, 31, 17843–17854. [Google Scholar] [CrossRef]
- Beck, F.; Loessl, M.; Baeumner, A.J. Signaling strategies of silver nanoparticles in optical and electrochemical biosensors: Considering their potential for the point-of-care. Microchim. Acta 2023, 190, 91. [Google Scholar] [CrossRef]
- Sio, A.F.; Graphene, R.; Spheres, O.; Ma, D.; Su, Y.; Tian, T.; Yin, H.; Zou, C.; Huo, T.; Hu, N.; et al. Multichannel Room-Temperature Gas Sensors Based on Magnetic-Field Multichannel Room-Temperature Gas Sensors Based on Magnetic-Field-Aligned 3D Fe3O4@SiO2@Reduced Graphene Oxide Spheres. ACS Appl. Mater. Interfaces 2020, 12, 37418–37426. [Google Scholar] [CrossRef]
- Ozyurt, D.; Kobaisi, M.A.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Kadam, V.V.; Balakrishnan, R.M.a. Sensing of p-nitrophenol in aqueous solution using zinc oxide quantum dots coated with APTES. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100474. [Google Scholar] [CrossRef]
- Pham, X.H.; Park, S.M.; Ham, K.M.; Kyeong, S.; Son, B.S.; Kim, J.; Hahm, E.; Kim, Y.H.; Bock, S.; Kim, W.; et al. Synthesis and Application of Silica-Coated Quantum Dots in Biomedicine. Int. J. Mol. Sci. 2021, 22, 10116. [Google Scholar] [CrossRef]
- Filipovic, L.; Selberherr, S. Application of Two-Dimensional Materials towards CMOS-Integrated Gas Sensors. Nanomaterials 2022, 12, 3651. [Google Scholar] [CrossRef]
- Silva, R.R.; Raymundo-Pereira, P.A.; Campos, A.M.; Wilson, D.; Otoni, C.G.; Barud, H.S.; Costa, C.A.R.; Domeneguetti, R.R.; Balogh, D.T.; Ribeiro, S.J.L.A. Microbial nanocellulose adherent to human skin used in electrochemical sensors to detect metal ions and biomarkers in sweat. Talanta 2020, 218, 121153. [Google Scholar] [CrossRef]
- Mai, T.; Li, D.D.; Chen, L.; Ma, M.G. Collaboration of two-star nanomaterials: The applications of nanocellulose-based metal organic frameworks composites. Carbohydr. Polym. 2023, 302, 120359. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology Roadmap for Flexible Sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Ali, I.; Kousar, S.; Ahmed, S. The environmental impact of industrialization and foreign direct investment: Empirical evidence from Asia-Pacific region. Environ. Sci. Pollut. Res. 2022, 29, 29778–29792. [Google Scholar] [CrossRef]
- Fino, A. Air Quality Legislation, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–11. [Google Scholar] [CrossRef]
- Ahmed, J.; Thakur, A.; Goyal, A. Industrial Wastewater and Its Toxic Effects. In Biological Treatment of Industrial Wastewater; Shah, M.P., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, J.; Wang, C.; Liu, Y.; Li, J. Research progress on the magnetite nanoparticles in the fields of water pollution control and detection. Chemosphere 2023, 336, 139220. [Google Scholar] [CrossRef]
- Afreen, S.; Chauhan, D.; Talreja, N.; Ashfaq, M. Polymer/Metal/Carbon-Based Hybrid Materials for the Detection of Heavy Metal Ions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–353. [Google Scholar] [CrossRef]
- Sasidharan, V.; Sachan, D.; Chauhan, D.; Talreja, N.; Ashfaq, M. Three-dimensional (3D) polymer—Metal–carbon framework for efficient removal of chemical and biological contaminants. Sci. Rep. 2021, 11, 7708. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Hu, H.; Zhou, X.; You, F.; Yao, C.; Liu, F.J.; Yu, P.; Wu, D.; Yao, J.; et al. Advanced Metal–Organic Frameworks-Based Catalysts in Electrochemical Sensors. Front. Chem. 2022, 10, 881172. [Google Scholar] [CrossRef] [PubMed]
- Motaghedifard, M.H.; Pourmortazavi, S.M.; Mirsadeghi, S. Selective and sensitive detection of Cr(VI) pollution in waste water via polyaniline/sulfated zirconium dioxide/multi walled carbon nanotubes nanocomposite based electrochemical sensor. Sens. Actuators B Chem. 2021, 327, 128882. [Google Scholar] [CrossRef]
- Soto, D.; Orozco, J. Hybrid Nanobioengineered Nanomaterial-Based Electrochemical Biosensors. Molecules 2022, 27, 3841. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, G.; Tonello, S. Wearable Biosensor Standardization: How to Make Them Smarter. Standards 2022, 2, 366–384. [Google Scholar] [CrossRef]
- Saleh, H.M. Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices. Sustainability 2023, 15, 10891. [Google Scholar] [CrossRef]
- Dubey, R.; Kumar, A.; Joshi, J.; Blankenberg, D.; Sekhara, S.; Kolluru, R.; Madhu, B.; Raval, S. Evaluation of low-cost particulate matter sensors OPC N2 and PM Nova for aerosol monitoring. Atmos. Pollut. Res. 2022, 13, 101335. [Google Scholar] [CrossRef]
- Lowther, S.D.; Jones, K.C.; Wang, X.; Whyatt, J.D.; Wild, O.; Booker, D. Particulate Matter Measurement Indoors: A Review of Metrics, Sensors, Needs, and Applications. Environ. Sci. Technol. 2019, 53, 11644–11656. [Google Scholar] [CrossRef]
- Maryse, F.; Pierre, B.; Gabriel, J.; Adrien, M.; Salim, B.; Julien, M.; Pierre, L.; Sergio, N.; Laurent, D. A miniaturized optical sensor for particulate matter detection. Proc. SPIE 2020, 1128717. [Google Scholar] [CrossRef]
- Adotey, E.K.; Amouei Torkmahalleh, M.; Tastanova, L.; Bekeshev, A.; Shah, D.; Hopke, P.K.; Lee, W.; Balanay, M.P. Ultrasensitive fluorescent carbon dot sensor for quantification of soluble and insoluble Cr(VI) in particulate matter. J. Hazard. Mater. 2024, 462, 132671. [Google Scholar] [CrossRef]
- Dutta, S.; Kumar, S.; Yu, Y.; Rubahn, H.-G.; Kumar, Y.; Awasthi, K. Functional nanomaterials in flexible gas sensors: Recent progress and future prospects. Mater. Today Chem. 2023, 29, 101428. [Google Scholar] [CrossRef]
- Chávez-Lara, J.; Galicia, M.; Carrasco-Urrutia, K.; Torres-Pérez, J. Sensitive detection of chromium (VI) using Au-NPs/MWCNT/chitosan composite via electrochemical approach. Int. J. Electrochem. Sci. 2023, 18, 100161. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano Biosensors: Properties, applications and electrochemical techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Pavlicek, A.; Ehmoser, E.-K.; Part, F. Applications of Fluorescent quantum dots for medical and environmental science applications. Nano Trust Doss. 2023, 59en, 1–6. [Google Scholar]
- Xu, Y.; Zhang, W.; Huang, X.; Shi, J.; Zou, X.; Li, Z.; Cui, X. Adsorptive stripping voltammetry determination of hexavalent chromium by a pyridine functionalized gold nanoparticles/three-dimensional graphene electrode. Microchem. J. 2019, 149, 104022. [Google Scholar] [CrossRef]
- Khan, M.A.; Qazi, F.; Hussain, Z.; Idrees, M.U.; Soomro, S. Recent Trends in Electrochemical Detection of NH3, H2S and NOx Gases. Int. J. Electrochem. Sci. 2017, 12, 1711–1733. [Google Scholar] [CrossRef]
- Ramaiyan, K.P.; Mukundan, R. Electrochemical Sensors for Air Quality Monitoring. Electrochem. Soc. Interface 2019, 28, 59. [Google Scholar] [CrossRef]
- Han, P.; Mei, H.; Liu, D.; Zeng, N.; Tang, X.; Wang, Y.; Pan, Y. Calibrations of Low-Cost Air Pollution Monitoring Sensors for CO, NO2, O3, and SO2. Sensors 2021, 21, 256. [Google Scholar] [CrossRef] [PubMed]
- Alfano, B.; Barretta, L.; Giudice, A.D.; Vito, S.D.; Francia, G.D.; Esposito, E.; Formisano, F.; Massera, E.; Miglietta, M.L.; Polichetti, T. A Review of Low-Cost Particulate Matter Sensors from the Developers’ Perspectives. Sensors 2020, 20, 6819. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, U.; Ramgir, N.; Late, D.; Jagtap, S.; Debnath, A.K.; Muthe, K.P. Selective detection of Cd (II) and Cr (VI) ions using rGO functionalized metal doped SnO2 nanocomposites. Microchem. J. 2023, 190, 108728. [Google Scholar] [CrossRef]
- Filik, H.; Aslıhan Avan, A. Neutral red interlinked gold nanoparticles/multiwalled carbon nanotubes modified electrochemical sensor for simultaneous speciation and detection of chromium (VI) and vanadium (V) in water samples. Microchem. J. 2020, 158, 105242. [Google Scholar] [CrossRef]
- Inam, A.K.M.S.; Angeli, M.A.C.; Shkodra, B.; Douaki, A.; Avancini, E.; Magagnin, L.; Petti, L.; Lugli, P. Flexible Screen-Printed Electrochemical Sensors Functionalized with Electrodeposited Copper for Nitrate Detection in Water. ACS Omega 2021, 6, 33523–33532. [Google Scholar] [CrossRef] [PubMed]
- Karthika, A.; Nikhil, S.; Suganthi, A.; Rajarajan, M. A facile sonochemical approach based on graphene carbon nitride doped silver molybdate immobilized nafion for selective and sensitive electrochemical detection of chromium (VI) in real sample. Adv. Powder Technol. 2020, 31, 1879–1890. [Google Scholar] [CrossRef]
- Kaya, İ.; Yeldir, E.K.; Kolcu, F.; Erdener, D. Synthesis of pyrene and pyrrole-appended fluorescent turn-off sensor toward Cr (VI) detection: Chemical oxidative and electrochemical polymerization of carboxamide. J. Photochem. Photobiol. A Chem. 2023, 449, 115386. [Google Scholar] [CrossRef]
- Shahbakhsh, M.; Noroozifar, M. Ultra-trace determination of hexavalent chromium by novel two dimensional biphenol-biphenoquinone nanoribbons/silver nanoparticles. Sens. Actuators B Chem. 2019, 281, 1023–1033. [Google Scholar] [CrossRef]
- Li, H.; Guo, S.; Wang, L. Voltammetric sensor detection of bisphenol AP in industrial wastewater. Int. J. Environ. Anal. Chem. 2021, 101, 1220–1230. [Google Scholar] [CrossRef]
- Al-hamry, A.; Lu, T.; Bai, J.; Adiraju, A.; Ega, T.K.; Paterno, L.G.; Pa, I.A.; Kanoun, O. Versatile sensing capabilities of layer-by-layer deposited polyaniline-reduced graphene oxide composite-based sensors. Sens. Actuators B Chem. 2023, 390, 133988. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS EST Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Gupta, P.; Rahm, C.E.; Jiang, D.; Gupta, V.K.; Heineman, W.R.; Justin, G.; Alvarez, N.T. Parts per trillion detection of heavy metals in as-is tap water using carbon nanotube microelectrodes. Anal. Chim. Acta 2021, 1155, 338353. [Google Scholar] [CrossRef] [PubMed]
- Venkateswara, C.; Hwan, C.; Mohana, G.; Manju, V.; Umapathi, R.; Suk, Y.; Pil, J. Emerging insights into the use of carbon-based nanomaterials for the electrochemical detection of heavy metal ions. Coord. Chem. Rev. 2023, 476, 214920. [Google Scholar] [CrossRef]
- Tapia, A.; Clara, P.; Oliveira, F.M.; Gusm, R.; Serrano, N. Antimonene-Modified Screen-Printed Carbon Nanofibers Electrode for Enhanced Electroanalytical Response of Metal Ions. Chemosensors 2023, 11, 219. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.; Wang, L. Application of Carbon Based Material for the Electrochemical Detection of Heavy Metal Ions in Water Environment. Int. J. Electrochem. Sci. 2020, 15, 4252–4263. [Google Scholar] [CrossRef]
- Han, X.; Meng, Z.; Zhang, H.; Zheng, J. Fullerene-based anodic stripping voltammetry for simultaneous determination of Hg(II), Cu(II), Pb(II) and Cd(II) in foodstuff. Microchim. Acta 2018, 185, 274. [Google Scholar] [CrossRef]
- Safari, M.; Moghaddam, A.; Salehi Moghaddam, A.; Absalan, M.; Kruppke, B.; Ruckdäschel, H.; Khonakdar, H.A. Carbon-based biosensors from graphene family to carbon dots: A viewpoint in cancer detection. Talanta 2023, 258, 124399. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; He, D.; Wang, X.; Shi, Y.; Pan, L. Recent progress on performances and mechanisms of carbon dots for gas sensing. Lumin. J. Biol. Chem. Lumin. 2023, 38, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.R.; Miranda Ribeiro Júnior, E.J. Graphene-Based electrochemical sensors for detection of environmental pollutants. Curr. Opin. Environ. Sci. Health 2022, 29, 100381. [Google Scholar] [CrossRef]
- Wang, X.; Karaman, C.; Zhang, Y.; Xia, C. Graphene oxide/cellulose nanofibril composite: A high-performance catalyst for the fabrication of an electrochemical sensor for quantification of p-nitrophenol, a hazardous water pollutant. Chemosphere 2023, 331, 138813. [Google Scholar] [CrossRef] [PubMed]
- Kiranakumar, H.V.; Thejas, R.; Naveen, C.S.; Khan, M.I.; Prasanna, G.D.; Reddy, S.; Oreijah, M.; Guedri, K.; Bafakeeh, O.T.; Jameel, M. A review on electrical and gas-sensing properties of reduced graphene oxide-metal oxide nanocomposites. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Dorosti, N.; Hossein, S.; Karami, N.; Taheri-anganeh, M.; Mahhengam, N.; Rajabvand, N.; Asadi, P.; Movahedpour, A.; Ghasemi, H. Clinica Chimica Acta MicroRNA biosensors for detection of gastrointestinal cancer. Clin. Chim. Acta 2023, 541, 117245. [Google Scholar] [CrossRef]
- Kasturi, S.; Eom, Y.; Torati, S.R.; Kim, C.G. Highly sensitive electrochemical biosensor based on naturally reduced rGO/Au nanocomposite for the detection of miRNA-122 biomarker. J. Ind. Eng. Chem. 2021, 93, 186–195. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hayat, H.; Abbas, A.; Narayan, R.J. Graphene quantum dot–based electrochemical biosensing for early cancer detection. Curr. Opin. Electrochem. 2021, 30, 100786. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Aich, S. Recent progress in surface and heterointerface engineering of 2D MXenes for gas sensing applications. Coord. Chem. Rev. 2024, 500, 215542. [Google Scholar] [CrossRef]
- Sedgi, I.; Lerner, N.; Lerner, A.; Zeiri, O. Mixed-Ligand gold nanoparticles based optical sensor array for the recognition and quantification of seven toxic metals. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2022, 277, 121241. [Google Scholar] [CrossRef]
- Yannick Ouedraogo, S.; Zhou, X.; Chen, H.; Chen, F.; Ma, C. Recent advances in biosensors and sequencing technologies for the detection of mutations. Microchem. J. 2023, 185, 108306. [Google Scholar] [CrossRef]
- Fotia, A.; Frontera, P.; Bonaccorsi, L.; Sciacca, B.; Malara, A. Encapsulation of Disodium-EDTA in Electrospun Polymeric Fibers for the Detection of Heavy Metals. Adv. Sustain. Syst. 2023, 2300463. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, P.; Liu, S.; Lv, Y.; Zhang, D. Catalyst-free synthesis of phenolic-resin-based carbon nanospheres for simultaneous electrochemical detection of Cu (II) and Hg (II). Diam. Relat. Mater. 2021, 111, 108170. [Google Scholar] [CrossRef]
- Niazy, B.; Ghasemzadeh, H.; Keshtkar Vanashi, A.; Afraz, S. Polyvinyl alcohol/polyacrylamide hydrogel-based sensor for lead (II) ion sensing by resonance Rayleigh scattering. React. Funct. Polym. 2022, 175, 105266. [Google Scholar] [CrossRef]
- Zhumanazar, N.; Korolkov, I.V.; Yeszhanov, A.B.; Shlimas, D.I.; Zdorovets, M.V. Electrochemical detection of lead and cadmium ions in water by sensors based on modified track-etched membranes. Sens. Actuators A Phys. 2023, 354, 114094. [Google Scholar] [CrossRef]
- Hung, C.M.; Phuong, H.V.; Van Thinh, V.; Hong, L.T.; Thang, N.T.; Hanh, N.H.; Dich, N.Q.; Van Duy, N.; Van Hieu, N.; Hoa, N.D. Au doped ZnO/SnO2 composite nanofibers for enhanced H2S gas sensing performance. Sens. Actuators A Phys. 2021, 317, 112454. [Google Scholar] [CrossRef]
- Khan, A.A.; Bahadar, A.; Hussain, M.; Ullah, F.; Ullah, A.; Rasheed, S. Analytical evaluation of polymeric CNTs/CuO nanocomposite electrode for the room temperature detection of volatile organic compounds (VOCs). Results Chem. 2023, 5, 100928. [Google Scholar] [CrossRef]
- Miah, M.R.; Yang, M.; Khandaker, S.; Bashar, M.M.; Alsukaibi, A.K.D.; Hassan, H.M.A.; Znad, H.; Awual, M.R. Polypyrrole-based sensors for volatile organic compounds (VOCs) sensing and capturing: A comprehensive review. Sens. Actuators A Phys. 2022, 347, 113933. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Li, T.; Tao, T. ZnO quantum dots for fluorescent detection of environmental contaminants. J. Environ. Chem. Eng. 2021, 9, 106800. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2022, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, T.; Zhou, H.; Zhang, Z.; Lee, C. Artificial intelligence enhanced sensors—Enabling technologies to next-generation healthcare and biomedical platform. Bioelectron. Med. 2023, 9, 17. [Google Scholar] [CrossRef]
- Rubino, A.; Queirós, R. Electrochemical determination of heavy metal ions applying screen-printed electrodes based sensors. A review on water and environmental samples analysis. Talanta Open 2023, 7, 100203. [Google Scholar] [CrossRef]
- Zhao, K.; Ge, L.; Wong, T.I.; Zhou, X.; Lisak, G. Gold-silver nanoparticles modified electrochemical sensor array for simultaneous determination of chromium(III) and chromium(VI) in wastewater samples. Chemosphere 2021, 281, 130880. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.O.; Orimolade, B.; Dennany, L.; Mamba, B.; Azizi, S.; Kaviyarasu, K.; Maaza, M. A Review on Monitoring of Organic Pollutants in Wastewater Using Electrochemical Approach. Electrocatalysis 2023, 14, 659–687. [Google Scholar] [CrossRef]
- Hajivand, P.; Jansen, J.C.; Pardo, E.; Armentano, D.; Mastropietro, T.F.; Azadmehr, A. Application of metal-organic frameworks for sensing of VOCs and other volatile biomarkers. Coord. Chem. Rev. 2024, 501, 215558. [Google Scholar] [CrossRef]
- Lei, Z.L.; Guo, B. 2D Material-Based Optical Biosensor: Status and Prospect; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2022; Volume 9. [Google Scholar]
- Mahmood Khan, I.; Niazi, S.; Akhtar, W.; Yue, L.; Pasha, I.; Khan, M.K.I.; Mohsin, A.; Waheed Iqbal, M.; Zhang, Y.; Wang, Z. Surface functionalized AuNCs optical biosensor as an emerging food safety indicator: Fundamental mechanism to future prospects. Coord. Chem. Rev. 2023, 474, 214842. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, J.; Liu, Z.; Chu, Z.; Jin, W. Nanomaterial-based electrochemical enzymatic biosensors for recognizing phenolic compounds in aqueous effluents. Environ. Res. 2022, 214, 113858. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.; Lin, G.; Feng, Y. Recent progress in the fabrication of flexible materials for wearable sensors. Biomater. Sci. 2022, 10, 614–632. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Arduini, F.; Mirasoli, M.; Zangheri, M.; Fabiani, L.; Colozza, N.; Marchegiani, E.; Simoni, P.; Moscone, D. A challenge in biosensors: Is it better to measure a photon or an electron for ultrasensitive detection? Biosens. Bioelectron. 2020, 155, 112093. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, F.; Xu, Y.; Mauk, M.G.; Qiu, X.; Tian, Z.; Zhang, L. Recent progress in terahertz biosensors based on artificial electromagnetic subwavelength structure. TrAC—Trends Anal. Chem. 2023, 158, 116888. [Google Scholar] [CrossRef]
- Dimitrievska, I.; Paunovic, P.; Grozdanov, A. Recent advancements in nano sensors for air and water pollution control. Mater. Sci. Eng. 2023, 7, 113–128. [Google Scholar] [CrossRef]
- Kumunda, C.; Adekunle, A.S.; Mamba, B.B.; Hlongwa, N.W.; Nkambule, T.T.I. Electrochemical Detection of Environmental Pollutants Based on Graphene Derivatives: A Review. Front. Mater. 2021, 7, 616787. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; de Faria, L.V.; Nascimento, A.B.; Germscheidt, R.L.; Patra, S.; Hernández-Saravia, L.P.; Bonacin, J.A.; Munoz, R.A.A.; Angnes, L. Sensing performances of spinel ferrites MFe2O4 (M = Mg, Ni, Co, Mn, Cu and Zn) based electrochemical sensors: A review. Anal. Chim. Acta 2022, 1233, 340362. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Zhao, H. Understanding signal amplification strategies of nanostructured electrochemical sensors for environmental pollutants. Curr. Opin. Electrochem. 2019, 17, 56–64. [Google Scholar] [CrossRef]
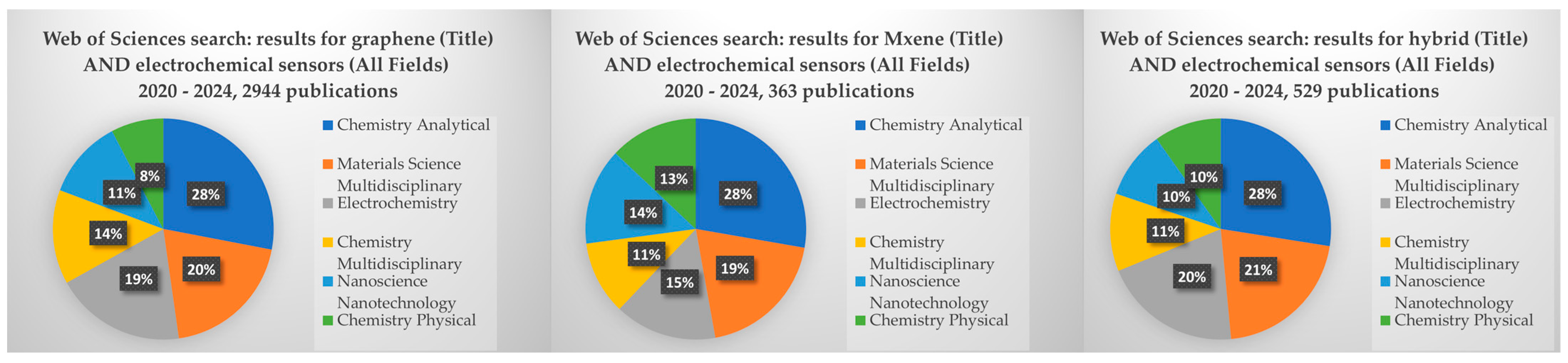

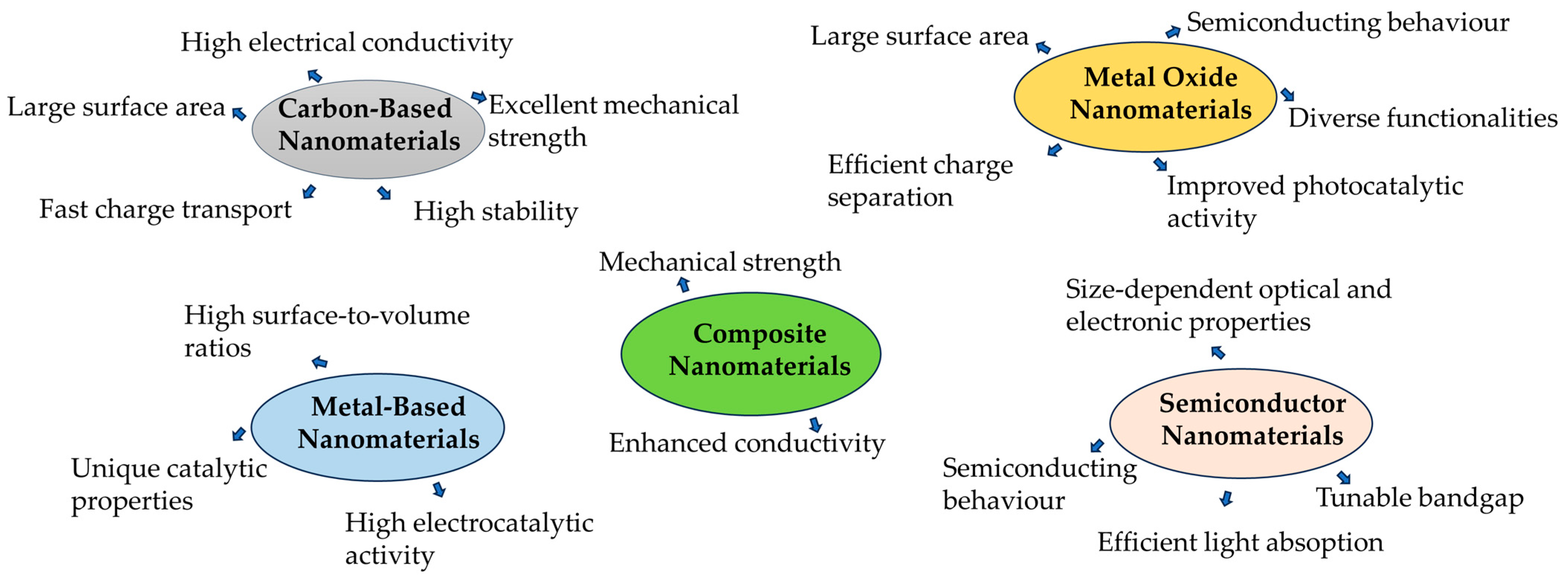
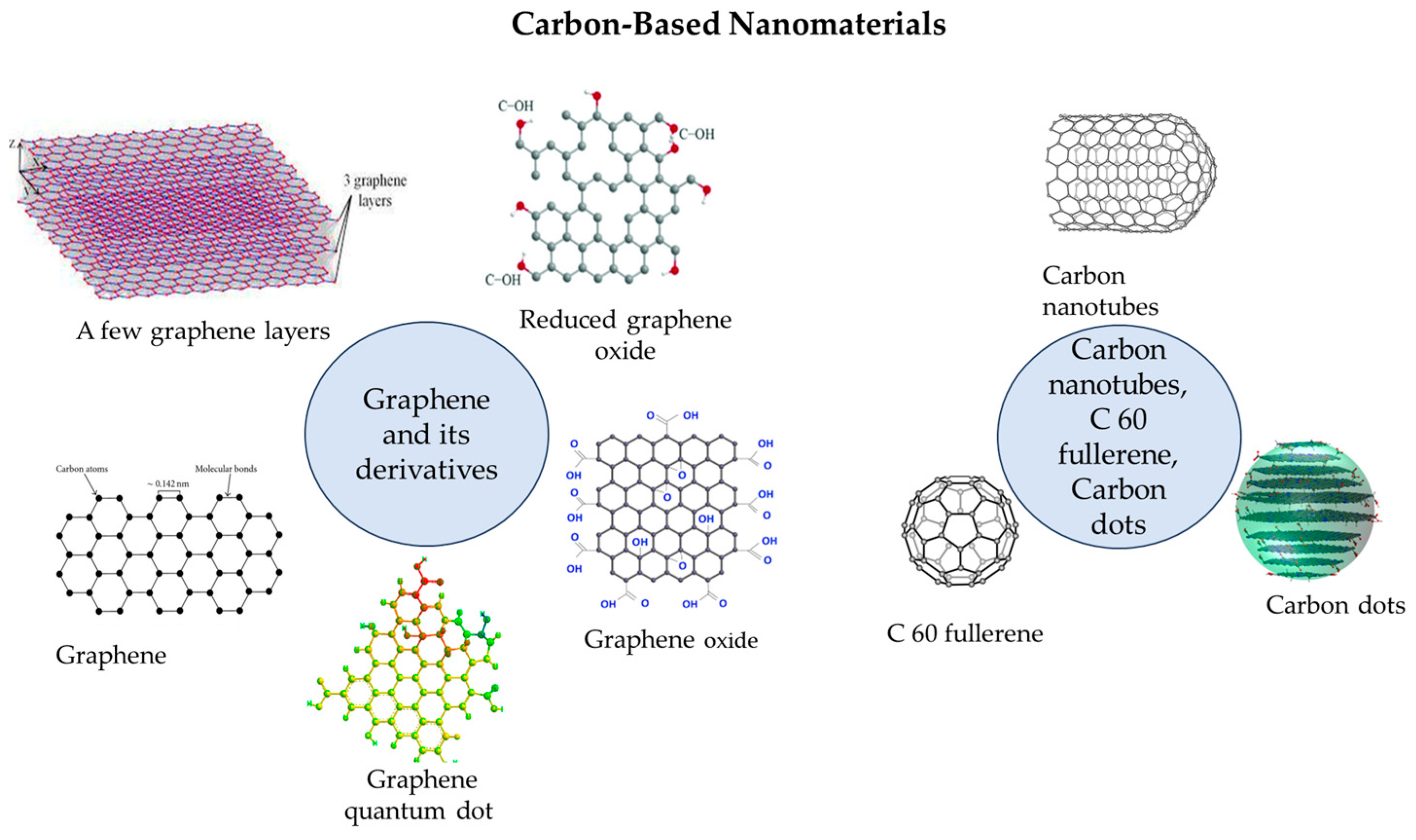
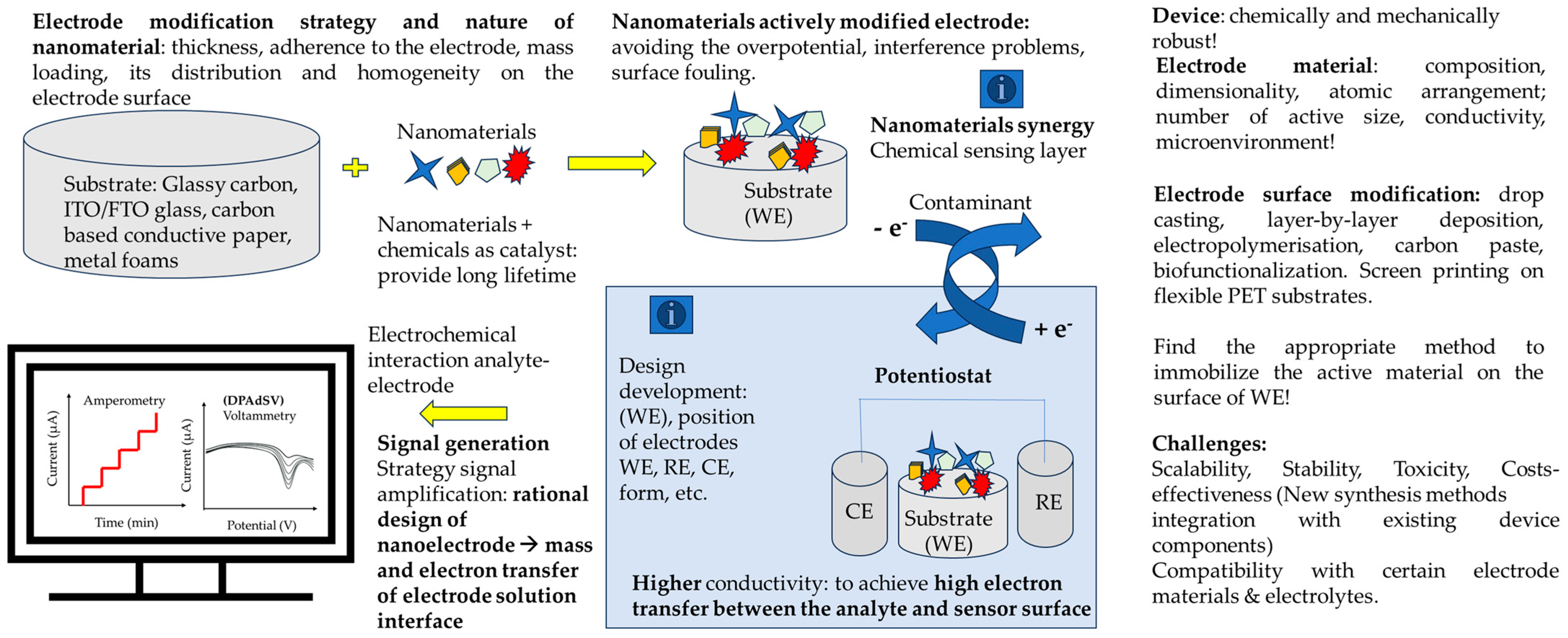

| Pollutants | Detection | |
|---|---|---|
| Air pollutants | ||
| (CO), nitrogen dioxide (NO2), ammonia (NH3), sulfur dioxide (SO2), and nitrogen monoxide (NO) Second, secondary gases are formed through the interaction of pollutants from the first group, including ozone (O3), sulfur trioxide (SO3), ammonium (NH4) Particulate matter (PM10, PM2.5) Volatile organic compounds (VOCs) | Electrochemical sensors Detection: amperometry, potentiometry, conductometry, impedance spectroscopy. Semiconductor sensors Detection: changes in resistance, capacitance, or other electrical properties are monitored when the semiconductor material interacts with the target analyte. Gas resistance sensors Detection: the electrical resistance of the sensor material is measured, and changes in resistance due to gas interactions indicate the presence and concentration of the target gas. Optical sensors, resonant mass sensors. Semiconductor sensors Electrochemical sensors | [22,25,30,47,63,65,70,104,112] |
| Water pollutants | ||
| Heavy metals (e.g., lead, cadmium, mercury, chromium (VI), vanadium) Organic substances (e.g., pesticides, industrial chemicals): | Electrochemical sensors: Differential pulse voltammetry (DPV), Differential pulse adsorptive stripping voltammetry (DPAdSV), Direct linear sweep voltammetry (LSV). Ion-selective electrode sensors Detection: The membrane potential changes in response to the concentration of the specific ion being measured. This change in potential is measured and used to determine the ion concentration. Optical spectroscopy. Detection: by measuring the absorption, emission, or scattering of light, optical spectroscopy allows for the identification and quantification of specific molecules or chemical compounds Biosensor-based sensors The choice of bioreceptor depends on the target analyte, and the transducer converts the biological response into an electrical, optical, or other measurable signals. Optical sensors utilize the interaction between light and matter to detect and quantify analytes. The optical properties of the sensing material change in response to the presence of the target analyte, and these changes are measured using various optical techniques. | [57,66,69,74,75,77,78,79,82,97,113,114] |
| Electrode/Material/Modifier | Target Analyte | Linear Range | Sensitivity | Techniques | Limit of Detection (LOD) | References |
|---|---|---|---|---|---|---|
| rGO functionalized metal-doped SnO2 nanocomposites | Selective detection of Cd (II) and Cr (VI) ions | Cd: 0.1–50 ppb | (~1.4 µA/ppb (Co doping); ~2.6 µA/ppb (Fe doping)). 1 wt% dopping of Co and Fe into SnO2. ((3.2 µA/ppb) for Cd(II) and 9.4 µA/ppb) for Cr(VI) for rGO/Me/SnO2). | CV | 0.07 ppb Cd(II) 0.04 ppb Cr(VI) | [74] |
| Pyridine functionalized AuNPs/3D rGO/GCE | Cr (VI) ions | 25–200 µg/L | 1.01 × 10−2 (μA/(μg/L) | DPAdSV | 1.16 µg/L | [69] |
| Two-dimensional biphenol-biphenoquinone nanoribbons/silver nanoparticles (AgNPs-BP-BPQ NRs) | Cr (VI) ions | 52–5200 µg/L | - | DPV | 2.0 × 10−12 M | [79] |
| g-C3N4/AgM/Nf/GCE (g-C3N4: graphene carbon nitride; AgM: silver molybdate; Nf: Nafion) | Cr (VI) ions | 0.1–0.7 µM | 65.8 µAµM−1cm−2 | Amperometry | 0.0016 µM | [77] |
| Au-NPs/MWCNT/chitosan | Cr (VI) ions | - | Quantification limit of 0.02 µg L−1 | DPV | 0.007 μg L−1 | [66] |
| A carboxylic amide compound containing pyrrole and pyrene groups | Cr(VI) ions | - | - | Fluorescence | 0.106 µM. | [78] |
| Multi-walled carbon nanotube-neutral red-gold nanoparticles (MWCNTs-NR-AuNPs) modified commercially available screen-printed carbon electrode (SPCE). | Cr(VI) and V(V) ions | Cr(VI) 0.4–80 µM V(V) 3–200 µM | Cr(VI): 0.5137 µA/µM V(V): 0.0688 µA/µM | LSV | Cr(VI): 0.025 µM V(V): 0.42 µM (S/N = 3) | [75] |
| Screen printed electrodes, Thymine -GO- Carbohydrazide | Cr(VI) and Hg(II) ions | for Hg(II) and Cr(VI) in above 5 ppb | - | SWV | Hg(II) and Cr(VI) were estimated to be one ppb and 20 ppb, respectively. | [24] |
| GCE/PZrS nanocomposite | Cr(VI) ions | 0.55–39.5 µmol/L | - | DPV | 64.3 nmol L−1 | [57] |
| Ni-Co manganate supported on rGO | Bisphenol-A | 0.005–7 µM | LSV | 2nM | [80] | |
| Au NPS decorated Ti3C2Tx MXenes | Formaldehyde | - | - | Electrical signals-wireless sensor | 92 ppb at RT | [22] |
| (PANI/rGO) | Acetone | 1 to 60 ppm | - | - | 1 ppm | [81] |
| SWCNT/SiPc (Silicon (IV) Phthalocyanine) | NH3 | 0.5–50 ppm | - | Chemiresistivity | 0.5 ppm 25–80 °C | [30] |
| SWCNT/SiPc | H2 | 70–1000 ppm | - | Chemiresistivity | 70 ppm | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godja, N.-C.; Munteanu, F.-D. Hybrid Nanomaterials: A Brief Overview of Versatile Solutions for Sensor Technology in Healthcare and Environmental Applications. Biosensors 2024, 14, 67. https://doi.org/10.3390/bios14020067
Godja N-C, Munteanu F-D. Hybrid Nanomaterials: A Brief Overview of Versatile Solutions for Sensor Technology in Healthcare and Environmental Applications. Biosensors. 2024; 14(2):67. https://doi.org/10.3390/bios14020067
Chicago/Turabian StyleGodja, Norica-Carmen, and Florentina-Daniela Munteanu. 2024. "Hybrid Nanomaterials: A Brief Overview of Versatile Solutions for Sensor Technology in Healthcare and Environmental Applications" Biosensors 14, no. 2: 67. https://doi.org/10.3390/bios14020067
APA StyleGodja, N.-C., & Munteanu, F.-D. (2024). Hybrid Nanomaterials: A Brief Overview of Versatile Solutions for Sensor Technology in Healthcare and Environmental Applications. Biosensors, 14(2), 67. https://doi.org/10.3390/bios14020067






