Abstract
Nanostructures have played a key role in the development of different techniques to attack severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Some applications include masks, vaccines, and biosensors. The latter are of great interest for detecting diseases since some of their features allowed us to find specific markers in secretion samples such as saliva, blood, and even tears. Herein, we highlight how hierarchical nanoparticles integrated into two or more low-dimensional materials present outstanding advantages that are attractive for photonic biosensing using their nanoscale functions. The potential of nanohybrids with their superlative mechanical characteristics together with their optical and optoelectronic properties is discussed. The progress in the scientific research focused on using nanoparticles for biosensing a variety of viruses has become a medical milestone in recent years, and has laid the groundwork for future disease treatments. This perspective analyzes the crucial information about the use of hierarchical nanostructures in biosensing for the prevention, treatment, and mitigation of SARS-CoV-2 effects.
1. Introduction
Sensors based on hierarchical nanostructures in the area of nanomedicine have been meticulously investigated in order to identify different enzymes and organisms such as bacteria or viruses. Biosensors are fascinating instruments that basically serve to detect biological or chemical parameters such as those related to molecules in tissues, microorganism cultures, and nucleic or acid chains [1]. The characteristics related to biodetection like selectivity, response speed, and stability depend on the morphology and structure of the sensing materials [2].
The main types of sensors used in biodetection are electrochemical [3], thermometric [4], piezoelectric [5], magnetic [6], and optical sensors (plasmonic [7], UV-Vis/infrared spectroscopy [8], Raman and SERS [9], or attenuated total reflection [10]). Biosensors that are developed using hierarchical nanostructures can be manufactured with different nanomaterials. For example, nanohybrids can be integrated into diverse materials such as noble metals [11], graphene [12], copper, titanium [13], zinc oxide [14], and bimetallic oxide [15], among others. The biosensors can be classified into three groups according to their mechanisms: the biocatalytic group that uses enzymes, bioaffinity group that involves antibodies and nucleic acids, and microorganism group that uses microbes [16].
A strong selective control of the manufacturing parameters of noble metals is possible [17], allowing their structure to be modified [18] to improve their physicochemical properties and adjust their shape [19]. There are multiple techniques for designing nanobiosensors, but the most common ones are based on electrochemical deposition [20], electroless deposition [21], electrocatalysts [22], and physicochemical methods [23].
Besides different processing routes that have been extensively explored to improve biosensing effects, the use of the LSPR phenomenon is very attractive [24], and the development of hierarchical nanostructured biosensors can promote exceptional optical, electrical, and chemical properties based on LSPR. Some of the special characteristics exhibited by hierarchical nanostructures are derived from their ultra-high specific surface area, high flexibility, light weight, high electrical conductivity, and bio-compatibility [25,26,27,28,29,30].
The hierarchical nanostructures are replacing conventional random hybrids in counterparts thanks to their physical characteristics, stability, and efficient transfer of electronic and ionic charges [31,32]. For example, their morphologies show a high surface area with adjustable porosity or packing density. Some hierarchical assemblies serve as programmable scaffolds that provide molecule-level control over the distribution of fluorophores and nanometer-scale control over their distance. Several strategies can be used to study imperfections and to stabilize various types of nanostructures, such as hollow ones [33] or cage frames to obtain a better performance [34].
It is worth noting that hierarchical metamaterials have been reported for the development of virus-based light learning systems, in plasmonic structures for application in high-performance metamaterials, and in binary nanoparticle networks and liquid crystal arrays for sensing technologies and imaging [35]. With these procedures, diverse techniques have been demonstrated strong fluorescence intensity and mild levels of enhancement, which allows them to manipulate photonic excitation and photoemission [36].
Hierarchical nanostructures represent a potential key to the next generation of new nanomaterials. For example, a controlled structure in the agglomeration between nanoparticles can increase plasmonic effects while the stacking distance between other nanoparticles decreases; all of this can be used to develop new and effective detection methods. Some of the representative hierarchically structured shapes are nanopillars [37], nanocones [38], nanoholes [39], and gecko pillars [40], among others.
Hierarchical nanostructures can be fabricated using techniques such as nanosphere lithography [41] with multiple patterns [42], electron beam lithography [43], pattern transfer [44], and focused ionization [45].
The characterization of the morphology, structure, and stability of hierarchical nanostructures can be explored by different methods. The typical characterization techniques for hierarchical nanostructures are X-ray diffraction [46], electrical effects [47], TEM [48], energy dispersive spectroscopy (EDX) [49], AFM [50], optical interactions [51], PL [52], Brunauer–Emmett–Teller surface area analysis [53], UV–visible absorption spectroscopy [54], photovoltaic performance [55], photocatalytic processes [56], Raman spectroscopy [57], and magnetic phenomena [58].
A hierarchy in nanostructures can be developed through in situ plasmon-driven syntheses [59] or through amino acids [60] to easily detect analytes at trace levels, such as pesticides, heavy metals, explosives, proteins, pathogens, and other chemical and biological contaminants [61]. It is clear that nanomaterial sciences are essential for developing biosensors with high reliability and speed using innovative technology [62,63,64,65].
In the last two years, diverse experiments have been carried out in the development of biosensors using different hierarchical nanostructures. It is worth highlighting some examples that have been very useful in the commitment to developing biosensors with better properties.
It has been pointed out that biosensors can be used to see the effectiveness of the vaccines in healthy, convalescent, or vaccinated people [66]. They can be used to monitor diseases, observe how many antibodies exist in people’s fluids, as well as determine whether the vaccines are effective for the test subjects [67]. In the faster biosensors, it takes approximately 20 min to obtain the result. The research has sought to develop biosensors with these nanomaterials to achieve a relatively rapid response, achieving a response time of 15 min.
It has been observed that current biosensors also have some disadvantages such as not being capable of detecting analytes in samples when there are external stimuli. This has to be addressed with the development of different biosensors with the properties of nanomaterials, such as different probes, including plasmonic [68] and incorporated ones [69]. Biosensors capable of detecting pathogens with very little genetic material compared to other assays have also been developed [70]. Additionally, calorimetric strips for smartphones aimed at antibodies or antigens to combat the rapid spread of these diseases have been considered since wearable biosensors can constantly monitor patients [71].
With this motivation, this paper reviews different aspects of the cutting-edge biosensors in the detection of SARS-CoV-2, focusing in those based on hierarchical and hybrid nanoparticles. Figure 1 shows the main characteristics considered in our research.
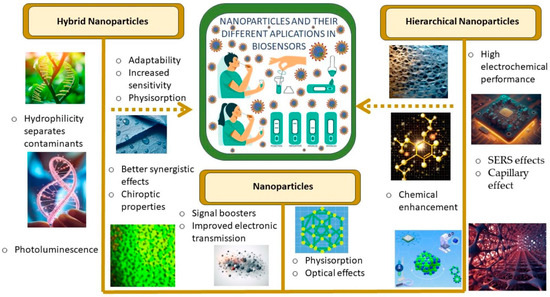
Figure 1.
Representative characteristics exhibited by different nanostructures in biosensing applications.
2. Synthesis of Hierarchical Nanostructures and Multicomponent Assemblies for Biosensors
Materials with hierarchical nanostructures have excellent mechanical properties due to the functional adaptation of their structures into different hierarchical levels. Hierarchical structures can be observed in nature, such as in bones, wood, cork, and plant stems, or in glass sponges [72]. Hierarchical nanomaterials show different architectural designs that are ordered at multiple length scales. They are grouped according to their main characteristics; in the case of porous materials, they contain interconnected pores with at least two levels of pore hierarchy from molecular (1–100 A), nano (10–100 nm), and meso (1–100 μm), to macropores [73]. It should be noted that the construction of hierarchical nanostructures requires knowledge of particular principles to avoid limitations on their properties [74]. Hierarchical materials can mimic the mechanical properties of their biological counterparts. Smart hierarchical materials can exhibit specific stimulus-response properties [75], such as self-healing and self-regeneration [76] in order to improve fracture resistance and increase strength [77]. Arrays can be constructed using proteins and microscale mechanical constraints can be used to form ordered networks within macroscopic structures [78]. The synthesis at different orders of magnitude from nanoscale to macroscale can be used to acquire outstanding characteristics through interacting with different analytes of different sizes [79], from small proteins to living cells. Different networks can be designed according to the geometry of the templates used [80]. The nanoclusters can be protected by ligands that can be prepared with atomic precision, exhibiting well-defined structures and resulting in versatile building blocks to manufacture excellent structures capable of performing certain functions [81]. For instance, nanofibers are used to construct multifunctional walkways with up to five levels of organization (depending on the method used). In the first level, there is a composite nanofiber; in the second level, a layer of composite material coated on the composite nanofiber that will result in the third level. The fourth level organizes the nanofibers to form an assembly and finally, in the last level, an assembly of nanofibers can be encapsulated within a matrix to form a massive structure by default [82]. Nanotubes are commonly used for the manufacture of hierarchical materials since they consist of molecular blocks, whose characteristics can be related to an anisotropic supramolecular self-assembly behavior at a personalized nanoscale, which allows for the creation of a percolation network at the mesoscale. They are regulated by dynamic self-assembly into four hierarchical levels of self-organization [83]. Nanosheets are composed of 2D building blocks, which have atomic or molecular thicknesses and they are considered the thinnest functional nanomaterials. They can be organized into various nanostructures or combined with a variety of materials at the nanoscale. Thanks to this, wide-range assemblies such as organic molecules, polymer gels, and inorganic nanoparticles can be designed [84].
Although hierarchical nanomaterials can be considered hybrid materials [85], nanohybrids are composed in a different way. Hybrid materials can have a variety of complex architectures with or without hierarchy. Their size varies from nanometers to several micrometers and several millimeters. Hybrid nanomaterials are combined through the synergistic mixture of two or more nanomaterials, which can be either inorganic or organic [86], that create a single material with properties that go beyond their properties as individual elements. They consist of groups of blocks with similar properties and structures with groups that cross-link the polymer into chains [87]. Their properties are determined by a combination of structure and composition at each length scale [88]. As a result, their properties are expressed in molecular length scale structures [89]. This indicates that the new mixture has superior properties compared to the original mixture. The properties to look at are the advantages derived from nanomaterials at a macroscopic level, such as energy absorption performance. The lightweight structure maximizes its functionality and improves the efficiency of the material [90,91].
There are different forms of nanohybrids such as sandwich structures, foams, reticular structures, segmented structures, zero expansion [92], and meso-structured thin films [93]. These structures serve different purposes, especially in integrated refractive and diffractive optical devices. Since these nanomaterials have a large thermal stability and better compatibility, they are typically used in the production of semiconductive devices [94]. In order to characterize organic–inorganic materials, techniques such as FTIR, Raman spectroscopy, LSPR, and various techniques based on MS are used [95].
Hybrid nanomaterials are good candidates for developing nanomaterials in the fight against bacteria and viruses thanks to their high sensitivity, good stability, and selectivity. In particular, they can detect antigens in plasma since their good electrochemical activity helps in the immobilization of the chains of different aptamers [96].
Nanomedicine, based on hybrid and hierarchical nanomaterials, has achieved great progress in the field of biosensors for the diagnosis, prevention, detection [97], and treatment of diseases in the post-pandemic period [98]. Compared to bulk materials, nanostructures are more precise, more reliable, less invasive, and easier to carry according to their chemical elements [99]. The effectiveness of nanomaterials has advanced to detect diseases at a very early stage using new technologies based on nanobiosensors [100], whose physical principles at the nanoscale level allow the biological receptors to be highly sensitive [101]. Nanobiosensors can be tailored by using different types of nanomaterials and structures [102].
Depending on their interactions, nanobiosensors can be classified into two different groups called biocatalytic or biophilic. These two groups can be classified according to recognition factors, for example, cells, organelles, tissues, enzymes, receptors, antibodies, nucleic acids, MIPs, PNAs, or aptamers.
Nanostructures are capable of obtaining information through molecular interactions in real time, and in normal and pathological biological states which provides an effective and relatively fast result. For example, in a drop of blood, an enzyme such as glucose oxidase, glucose dehydrogenase, or hexokinase can cause a reaction, which can be measure by a low-dimensional detector in a glucometer (biosensor) [103].
Because the manufacturing of biosensors has several drawbacks, efforts have been made to develop improvements in manufacturing [104,105,106,107,108]. Characteristics like adhesion ability, strong adsorption capacity, chemical catalytic efficiency, and corrosion and oxidation resistance facilitate the fabrication [109], chemical stability, and electron transfer kinetics [110]. The challenges for optimizing highly selectivity binding properties are continuously being overcome to analyze nanoscale elements of biomolecules [111].
High crystallinity with insignificant structural defects can be relevant to detecting different samples such as glucose, proteins, and nucleic acids [112]. The other main advantages of nanohybrid materials are the specific binding sites that generate a selective sensor signal, which also improves its magnitude and composition. The high surface-to-volume ratio of nanofibers can also improve the capture efficiency and it provides some surface area-related phenomena, including ion exchange and catalysis [113,114].
Heterounions in hierarchical nanomaterials can promote the selective formation of specialized structures and sensitive responses not found in other sensors [115,116,117,118]. Nanomaterials produced through molecular printing can create selectivity for specific enzymes. This method can be worked with 3D nanostructures and it is used to manufacture versatile materials for the construction of sensors to detect various analytes [119]. It has been demonstrated that these nanostructures can eliminate pathogens and better detect enzymes compared to other nanomaterials [120].
The existing improvements found when assembling nanostructures are versatile, and they open new methods for different technologies to control their structure and combine physicochemical properties [121,122,123,124,125,126,127,128,129,130,131]. On the other hand, some nanostructured systems based on organic polymers have been proposed [132], and applications for spectrochemical biosensing have been demonstrated. Biosensors based on RNA hybridization can be considered for several biological reactions and for generating analytical signals that are easily detected by different electrochemical aptasensors [133], electrochemical luminescence sensors [134], and optical transducers, among others [135]. It has been pointed out that RT-PCR [136] can be used to amplify cDNA from virus RNA [137]. This is of great interest in the studies that have to do with inhibitors that target the enzyme helicase since it is known to participate in the processes of duplication and cell reproduction [138].
Photonic nanobiosensors have been also highlighted with respect to their potential use against SARS-CoV-2. In addition to monoclonal antibody pairs, which are rapid antigen tests [139], it is important to look for more efficient ways to detect pathogens [140]. In this direction, biosensors using some promising plasmonic nanoparticles are the most powerful tools employed for the detection of viruses [141]. Moreover, polystyrene nanoparticles, graphene, and carbon nanotubes [142] present different advantages such as selectivity towards particular molecular expressions corresponding to an important challenge that requires high specificity, sensitivity, and a multiplex detection capability to offer good virus detection. The design of POC testing arrangements [143], such as LFIAs, should be mentioned as they offer fast and easy-to-use methods, as well as reliability. Each synthesis procedure can be functional, but inherent limitations in the quantitative analysis of the virus in the application of biosensors should be noted [144].
In summary, hierarchical nanostructures are formed by hybrid nanoparticles, which are good candidates for the development of nanobiosensors for pathogen detection. These nanoparticles are prepared through different synthesis methods, as it is illustrated in Figure 2.
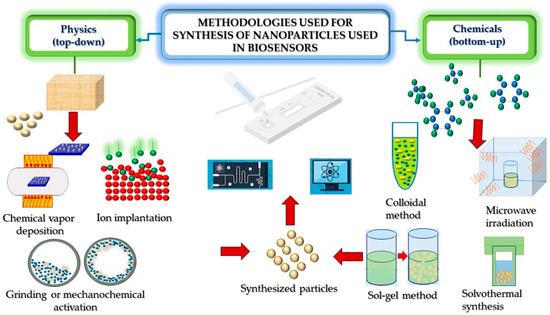
Figure 2.
Representative processing routes for the synthesis of hierarchical nanostructures used in the development of biosensors.
Hierarchical and hybrid nanostructures allow for the improvement of biosensing performance by increasing the signal intensity and enhancing a variety of energy transfer processes. Hierarchical nanostructures have larger reaction interfaces in the specific active surface area, allowing for better biomolecular recognition, catalyst charge transfer, metal ion release, and virus or bacteria capture within the nanomaterials. Sensors developed with these nanomaterials have high sensitivity, as shown in Table 1.

Table 1.
Advantages and disadvantages of hybrid nanomaterials, as well as their LOD for SARS-CoV-2 biosensing, focusing on the synthesis method employed for the preparation of the nanostructures.
3. Hierarchical Nanostructures for SARS-CoV-2 Biosensing
In particular, biosensors made from nanostructures have a good response speed, good mechanical and chemical stability, a simple manufacturing process, low cost, as well as selection of samples in situ [162]. In order to develop nanobiosensors for detecting an infection, hierarchical nanostructures can be employed to assist in the synergistic enhancement of molecular enrichment [163]. An example is polydimethylsiloxane (PDMS), a transparent and flexible substrate that can be wrapped on arbitrary surfaces and allows light to penetrate the contact surface for optical diagnosis in situ [164]. Optical and plasmonic biosensors have been investigated with different nanostructures to combat diverse types of viruses, such as those that imitate different viruses called “virus traps” and elongated nanoparticles [165,166,167]. Biosensors based on electrochemical detection have been developed for identifying different types of viruses such as nanobioconjugated nanostructures. Virus coat protein self-assemblies with nanostructures can be very symmetrical [168]; a good example is the gold-functionalized nanostructures with human ACE2 that have a detection limit below approximately 80 mL−1 copies. Another example is the nanostructured biosensing process in contaminated water, in which, the contaminant can be detected in a few minutes. Hierarchical assemblies are very promising in combating not only the SARS-CoV-2 virus, but also different pathogens. The evolution of the self-assembly pattern of the nanocomposite can alter their plasmonic response and can be used for molecular diagnoses [169]. Table 2 compares the advantages and disadvantages of representative characteristics exhibited by hierarchical nanobiosensors.

Table 2.
Advantages and disadvantages of hierarchical nanostructures, as well as their LOD for the detection of SARS-CoV-2, focusing on the biosensing method.
Hierarchical nanomaterials in detection platforms can be tailored for the construction of functional electrode nanomaterials [186]. The aim of hierarchy in biosensing is to form nanomaterials that are biomolecular self-assemblies for the enhancement of sensing [187]. Some hierarchical structures are inspired by nature since these nanostructures are functional [188] and because they contain properties that make them unique. As it can be seen in Table 2, hierarchical nanostructures are attractive for applications in the detection of pathogens. For example, biosensors using hierarchical metal nanoparticles that are monitored with SERS techniques are capable of accurately measuring and capturing unknown coronaviruses, as long as they contain the S protein and can combine with the ACE2 protein [189]. There is efficiency in the transfer of resonance energy between nanomaterials made with noble metals. These nanomaterials exhibit enhanced electrochemical conductivity when combined with hierarchical materials such as graphene, resulting in pronounced absorption peaks in the hybrid materials. For example, their narrow pore size distribution results in faster direct electron transfer and better enzyme detection [190]. Based on this, enzymatic biosensor platforms have been created, as well as highly conductive supports and nanofillers, which demonstrated the effectiveness of hierarchical nanostructures [191]. This approach results in a linear and continuous reaction that increases the uptake of RNA from the viral material. The hierarchical nanostructures of certain hybrid nanomaterials enhance their synergistic effects [192], generate hydrophobicity and colloidal stability, and can be reprogrammed to detect any protein antigen if the corresponding specific monobody exists. They can increase colorimetric fluorescence signals [193], ensuring high sensitivity and stability for detection applications. Different nanomaterials can be used to detect metals and compounds such as copper [194], nickel oxide [195], etc. The modified assemblies are good candidates for chemical adsorption, and can produce good magnetization that generates a magnetic signal to detect pathogens such as SARS-CoV-2. The hierarchical nanostructures produce molecular enrichment through the capillary effect, resulting in good biocompatibility, stability, optical effects, and promising electrical properties.
The characteristics of hierarchical nanostructures can be observed through different techniques, as illustrated in Figure 3.
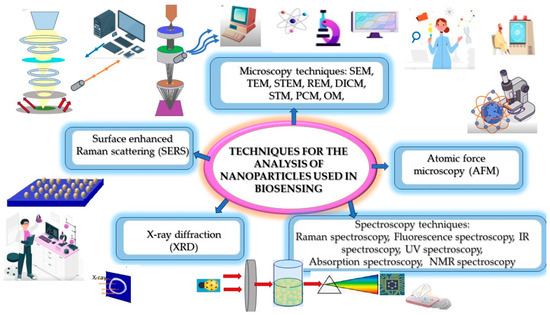
Figure 3.
Typical techniques for measurements using nanostructured biosensors.
4. Heterostructures with Different Morphologies for Biosensing
A fundamental element of nanoscience and nanotechnology are structure and morphology [196]. This is because nanostructured materials can be agonists due to their different shapes and the properties they acquire depending on their orientation. In this section, different types of nanostructures and nanomaterials are analyzed, such as nanowires, nanofibers, nanotubes, quantum dots, nanosheets, nanocomposites, and nanoparticles, and their different orientations, morphologies, and properties are discussed. Nanostructures come in a variety of shapes, sizes, structures, and origins. They can be spherical, conical, helical, cylindrical, tubular, flat, hollow, or irregular in shape. Their structures vary in size from 1 to 100 nm and are composed of elements such as carbon, metals, metal oxides, and organic or inorganic materials.
The development of biosensors using nanoparticles has become extremely important because nanostructured nanoparticles have properties that surpass other compounds such as a low detection limit, high sensitivity, biocompatibility, and a peculiar refractive index change. By being able to combine different materials to form heterostructures, it is possible to overcome the drawbacks of the individual materials and thus take advantage of their synergy [197].
The development of hierarchical nanostructures has become crucial for the tailoring of semiconductor-based nanostructures in the field of photoelectrochemical biosensing [198]. The sensors with enhanced biosensing performance include electrochemical, surface plasma resonance, photonic, immunosensors, photoelectrochemical, nucleic acid-based and enzyme-based, electrochemiluminescent, protein-based, and aptamer biosensors. Their adaptability in terms of size, shape, and composition is relevant in controlling their physicochemical properties [199] and general behavior to adapt nanostructures to biological systems [200]. Modifications of heterostructured effects such as the degree of exfoliation, crystallinity, phase, metallicity, sheet size, and decoration with metallic particles can be useful for biosensing [201]. Moreover, the arrangement of semiconductor nanostructures in biomolecules can convert this adaptation into hybrid systems to improve detection [202].
There is a goal to fabricate biosensors at a low cost in the fight against highly infectious diseases [203]. Different nanostructures have been proposed for designing multifunctional effects and nano-sculpted hybrids [204].
In this section, we will analyze the different types of heterostructures for biosensing, shown in Figure 4, which shows the particular morphologies of both hybrid materials and hierarchical structures.
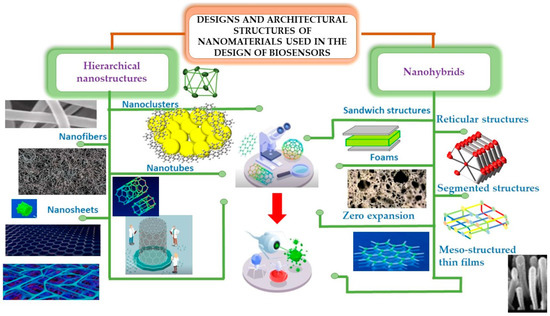
Figure 4.
Representative characteristics exhibited by different types of nanostructures.
Multidirectional networks of nanoobjects like nanocrystals or nanoflowers can im-prove their functional characteristics, which are defined by the synergy of different elements. The synthesis of heterostructures can emerge from the sequential growth of different nanoparticles or by the sequential preparation of different self-assembled building blocks. Morphologies like core–shell, multiple-shell, forest, nanoflowers, nanograss, nanopyramids, janus, and branched-tree heterostructures are typical nanostructured architectures. Table 3 presents some advantages and disadvantages of different morphologies of hierarchical nanostructured forms. The parameters related to the shape, size, distribution, and homogeneity of the building blocks are highlighted.

Table 3.
Advantages and disadvantages of the hierarchical heterostructures for biosensing, as well as their LOD for the detection of SARS-CoV-2, focusing on the morphology of the building blocks of the nanostructures.
5. Discussion
The 2020 pandemic killed millions of people around the world, increasing the need to find more effective ways to combat the disease. This has served to curb deaths as new knowledge about the virus has been quickly found, opening new windows into the age of nanotechnology. During this pandemic, various applications have emerged to combat the risks caused by SARS-CoV-2. With the aim of developing various methods for the prevention, detection, treatment, and diagnosis of diseases, diverse solutions including vaccines, drugs, and biosensors have been engineered. One of these effective methods is the use of biosensors designed with hierarchical nanostructures that have unique properties to combat COVID-19, offering reliable, effective, and fast solutions [224]. Currently, researchers are looking for ways to produce materials with a low cost, high production volume, efficient cultivation times, maximum durability, and profitability. Good candidates for meeting these objectives are hierarchical nanostructures, as they are selective and they reduce the time of detection of potentially infectious pathogens. The advantages of developing these hierarchical nanostructures are that they can develop double resonance effects or synergistic effects of the elements that are integrated into the system [225] and have a high potential for advanced photonic monitoring [226]. A previous study mentioned several options for preparing hierarchical nanostructures, since each preparation has a specific purpose [227].
The preparation of these nanostructures is through different synthetic and analytical routes, which results in remarkable applications [228]. The controlled synthesis of hierarchical nanostructures with stacked morphologies could serve as a basis for tuning effects and potentially controlling them using photonic signals [229]. By designing complexes with multiple hierarchical levels [230], different nanomaterials can be developed. These complexes are envisioned with nanohybrid nanomaterials since their recent use in biosensors has facilitated the exploration of different techniques to produce them, including recycled materials [231]. By joining nanoparticles, different properties can be obtained to obtain good biocompatibility and high covalent bio-bonds with specific proteins [232].
In addition to amplifying the detection signal, hierarchical nanostructures can be employed to label a specific sequence of a target during detection [233]. Some of these nanomaterials feature combinable virus recognition targets such as bio-AuNP hybrid nanostructures with metal oxide and carbon nanotubes [234], which have been combined by pathogen DNA hybridization [235]. These nanohybrids have improved oxidation catalytic activity, stronger signals, better colorimetry for virus detection [236], a wider linear range, a lower LOD, higher sensitivity, and a better response time [237]. All these properties significantly contribute to new rapid and cost-effective methods and have provided smart nanomaterials with great advantages in biosensor fabrication [238].
An example of hybrid nanomaterials is carbon-based biosensors that provide a good effectiveness when the active detection point has a high surface-to-volume ratio, which increases the probability of adsorption to a specific molecule [239]. Electrochemical biosensors mainly use porous carbonaceous materials, since the morphology of these biosensors, especially the pore volume and surface area, influences the electrochemical and catalytic activities. All of these properties are widely used for pathogen detection and surveillance. Biosensors with layered functionality have been developed for the detection of SARS-CoV-2. The methods used to improve performance are activation, doping, and dispersion of metal nanoparticles for the detection of various analytes, such as biomolecules, metal ions, contaminants, and food additives.
In addition to some analytical techniques to study the biosensing performance, optical interactions of hierarchical nanostructures can also be used to combat SARS-CoV-2 and other viruses, resulting in higher sensitivity [240]. This can be seen when nanostructures are used in the development of nanotechnological therapies, such as the development of antiviral drugs and nanoarchitecture-based vaccines assisted by biosensing [241]. An example of nanoparticles that contain these properties are those whose surfaces are coated with ionic nanoparticles. Metallic nanoparticles are used, which act as antiviral agents against RNA viruses because most recurrent respiratory diseases are RNA viruses that take advantage of the link between the virus and the host cell to enter the host cell [242]. From this, various functionalization and rapid viral immunodiagnosis strategies can be used [243].
The development of several hierarchical microchips with good results has been demonstrated; these microchips have a high sensitivity due to a primed surface for covalent immobilization of primary antibodies and ensuring strong binding between substrates [244].
Currently, the pharmaceutical profile of antiviral drugs has been improved and nanomaterials have been improved in terms of their virucidal properties and effectiveness against viral infections [245]. Figure 5 presents these different types of hierarchical nanostructures and how they are used for the development of nanobiosensors.
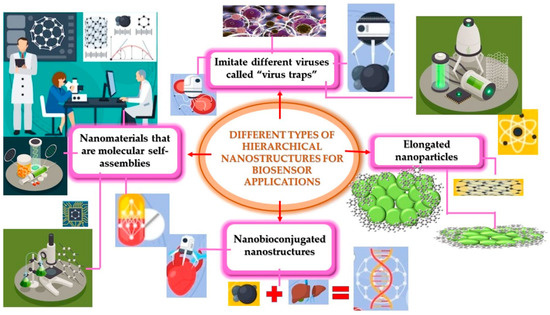
Figure 5.
Different types of hierarchical structures for the development of biosensors.
6. Conclusions
The development of hierarchical nanostructures is remarkable in the fight against SARS-CoV-2. In this paper, we reviewed different considerations for the development of hybrid nanostructures and their applications in biosensing. Nanomaterials with specific properties, such as noble metals, have become very useful for the development of vaccines and biomarkers thanks to their unique LSPR phenomena. In our analysis, we highlighted the advantages and disadvantages of the synergistic effects of the different elements integrated into nanoscale biosensors. The plasmonic properties exhibited by metallic nanoparticles together with the electronic or optoelectronic functions of carbon-based low-dimensional systems are good candidates for the advancement of biosensing technology. The use of hybrid nanomaterials for the development of biosensors for virus detection have resulted in great advantages, such as adaptability. In view of all these considerations we highlighted for hierarchical biosensing, we could obtain better results to combat the effects of some diseases using hierarchical biosensing, as was the case for COVID-19.
Author Contributions
Investigation, J.A.M.-L., I.V., M.I.S. and C.T-T.; Writing—original draft, J.A.M.-L.; Writing—review and editing, J.A.M.-L., I.V., M.I.S. and C.T.-T.; Conceptualization, C.T.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto Politécnico Nacional (SIP-2023) and Consejo Nacional de Humanidades, Ciencias y Tecnologías (CF-2023-I-2042).
Acknowledgments
The authors kindly acknowledge the financial support from the Instituto Politécnico Nacional and Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCyT-México).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| AFM | Atomic force microscopy |
| BPV | Bioinspired plasmovirus |
| CdS | Cadmium sulfide nanoparticles |
| cDNA | Copy DNA |
| COVID-19 | Coronavirus disease 19 |
| CoVNP | Nucleocapsid |
| CRISPR | Family of DNA sequences found in the genomes of prokaryotic organisms such as bacteria |
| CS | Chitosan |
| DNA | Deoxyribonucleic acid |
| DPV | Differential pulse voltammetry |
| CV | Cyclic voltammetry |
| ECL | Electrochemiluminescence |
| EDL | Electrical double layer |
| EDS | Energy dispersive spectroscopy |
| EDX | X-ray spectroscopy |
| EIS | Electrochemical impedance spectroscopy |
| FESEM | Field-emission scanning electron microscopy |
| FE-TEM | Field-emission transmission electron microscopy |
| FluA | Influenza A virus |
| FTIR, FT-IR or IR | Fourier transform infrared spectroscopy |
| GO | Graphene oxide |
| GO/AuNPs | Graphene/gold nanoparticle |
| GOD | Glucose oxidase |
| GSH | Glutathione |
| HAADF-STEM | High-angle annular dark-field scanning transmission electron microscopy |
| HCF | Hollow core fiber |
| HECF | Hollow eccentric core fiber |
| HRP | Detection based on biotinylated molecules |
| HRTEM | High-resolution transmission electron microscopy |
| ICA | Immunochromatographic assay |
| IPCF | Imprinted photonic crystal film |
| ITO | Indium tin oxide |
| LFA or LFIAs | Lateral flow immunoassay |
| LOD | Limit of detection |
| LSPR | Localized surface plasmon resonance |
| MB-CD | Methylene blue functionalized carbon |
| MINERS | Magnetically-induced nanogap-enhanced Raman scattering |
| MIP | Molecular imprinting polymer |
| MNPs | Magnetic nanoparticles |
| MOF or MOFs | Metal–organic frameworks |
| MS | Mass spectrometry |
| MUA | Mercaptoundecanoic acid |
| MWCNT | Multi-walled carbon nanotubes |
| N | Nucleocapsid |
| NGs | Nanoghosts |
| NIR | Near-infrared |
| NP | Nucleocapsid protein |
| NPs | Nanoparticles |
| NW | Nanowires |
| ORF | Open reading frame |
| PANi | Hybrid polyaniline |
| pCRISPR | Plasmid for reconstituting the CRISPR system |
| PDA | Polydopamine |
| PDMS | Polydimethylsiloxane |
| PEC | Photoelectrochemical |
| PEI | Polyethyleneimine |
| PGE | Pencil graphite electrode |
| PILs | Poly(ionic liquids) |
| PL | Photoluminescence spectroscopy |
| PMB/PILs | Provides π-π interactions with poly(methylene blue) |
| PNA | Peptide nucleic acid |
| POC | Point-of-care |
| PPT | Plasmonic photothermal |
| PPy | Platform made of polypyrrole |
| PVDF | Polyvinylidene fluoride |
| QD or QDs | Quantum dots |
| rGO | Reduced graphene oxide |
| RdRp | RNA-dependent RNA polymerase |
| rPGO | Reduced porous graphene oxide |
| RT-LAMP | Reverse transcription loop-mediated isothermal amplification |
| RT-PCR | Reverse transcription polymerase chain reaction |
| S | Spike |
| SAM | Self-assembled monolayer |
| SARS | Severe acute respiratory syndrome |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SM | Saturation magnetization |
| ssDNA | Single-stranded DNA |
| SEM | Scanning electron microscopy |
| SERS | Surface-enhanced Raman spectroscopy |
| SM | Saturation magnetization |
| SPCE | Screen-printed carbon electrode |
| SPR | Surface plasmon resonance |
| TEM | Transmission electron microscopy |
| TZ | Test zone |
| UV-Vis | Ultraviolet (UV) spectroscopy |
| X(XRD) | X-ray diffraction |
| X(XPS) | X-ray photoelectron spectroscopy |
| ZnO/rGO | Zinc oxide/reduced graphene oxide |
References
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Review—Nanostructured Materials-Based Nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- Huang, T.; Cao, W.; Elsayed-Ali, H.E.; Xu, X.-H.N. High-throughput ultrasensitive characterization of chemical, structural and plasmonic properties of EBL-fabricated single silver nanoparticles. Nanoscale 2012, 4, 380–385. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Yi, D.; Wei, Z.; Zheng, W.; Pan, Y.; Long, Y.; Zheng, H. Glucose detection based on the photothermal effect of OxTMB using a thermometer. Sens. Actuators B Chem. 2020, 323, 128691. [Google Scholar] [CrossRef]
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing using magnetic particle detection Techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef] [PubMed]
- Divya, J.; Selvendran, S.; Raja, A.S.; Sivasubramanian, A. Surface plasmon based plasmonic sensors: A review on their past, present and future. Biosens. Bioelectron. X 2022, 11, 100175. [Google Scholar] [CrossRef]
- Tahseen, A.Q.; Noshin, F.; Khasan, S.K.; Adib, I.M. A novel and stable ultraviolet and infrared intensity sensor in impedance/capacitance modes fabricated from degraded CH3NH3PbI3-xClx perovskite materials. J. Mater. Res. Technol. 2020, 9, 12795–12803. [Google Scholar]
- Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman spectroscopy for medical diagnostics—From in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 2015, 89, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-J.; Cheng, C.-C.; Yang, S.-C. Surface plasmon resonance biosensing by electro-optically modulated attenuated total reflection. Appl. Phys. B Laser Opt. 2010, 103, 701–706. [Google Scholar] [CrossRef]
- He, J.; Kunitake, T.; Nakao, A. Facile in Situ Synthesis of Noble Metal Nanoparticles in Porous Cellulose Fibers. Chem. Mater. 2003, 15, 4401–4406. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, J.; Wang, D.; Tang, Q.; Chen, T.; Rong, S.; Liu, J.; Wu, Y. Biomimetic Synthesis of hierarchical 3D Ag Butterfly wing scales arrays/graphene composites as ultrasensitive SERS substrates for efficient trace chemical detection. J. Mater. Chem. C 2018, 6, 1933–1943. [Google Scholar] [CrossRef]
- Ran, Y.; Lu, H.; Zhao, S.; Jia, L.; Guo, Q.; Gao, C.; Jiang, Z.; Wang, Z. Structural and plasmonic properties of Ti Sr N ternary nitride thin films. Appl. Surf. Sci. 2019, 476, 560–568. [Google Scholar] [CrossRef]
- Wang, M.; Luo, Q.; Hussain, S.; Liu, G.; Qiao, G.; Kim, E.J. Sharply-precipitated spherical assembly of ZnO nanosheets for low temperature H2S sensing performances. Mater. Sci. Semicond. Process. 2019, 100, 283–289. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, W.; Ren, J.; Tang, Y. Cu-Co bimetal oxide hierarchical nanostructures as high-performance electrocatalyst for oxygen evolution reaction. Mater. Today Energy 2021, 21, 100703. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Creaniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Marques-Hueso, J.; Morton, J.A.S.; Wang, X.; Bertran-Serra, E.; Desmulliez, M.P.Y. Photolithographic nano seeding method for selective synthesis of metal catalysed nanostructures. Nanotechnology 2018, 30, 015302. [Google Scholar] [CrossRef]
- Amendola, V.; Scaraamuzza, S.; Agnoli, S.; Granozzi, G.; Meneghetti, M.; Campo, G.; Bonanni, V.; Pineider, F.; Sangregorio, C.; Ghihna, P.; et al. Laser generation of iron-doped silver nano truffles with magnetic and plasmonic properties. Nano Res. 2015, 8, 4007–4023. [Google Scholar] [CrossRef]
- Hou, P.; Liu, H.; Li, J.; Yang, J. One-pot synthesis of noble metal nanoparticles with a core-shell construction. CrystEngComm 2015, 17, 1826–1832. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of nanomaterials for electrochemical sensing. Sens. J. 2019, 19, 1186. [Google Scholar] [CrossRef]
- Münch, F. Electroless plating of metal nanomaterials. Chemelectrochem 2021, 8, 2993–3012. [Google Scholar] [CrossRef]
- Lee, J.; Takemura, K.; Park, E.Y. Plasmonic nanomaterial-based Optical biosensing platforms for virus Detection. Sensors 2017, 17, 2332. [Google Scholar] [CrossRef]
- Lin, P.-C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, R.-J.; Xia, F.; Peng, Y. Current status of optical fiber biosensor based on surface plasmon resonance. Biosens. Bioelectron. 2019, 142, 111505. [Google Scholar] [CrossRef]
- Marzana, M.; Morsada, Z.; Faruk, O.; Ahmed, A.; Khan, M.A.; Jalil, M.A.; Hossain, M.; Rahman, M.M. Nanostructured Carbons: Towards Soft-Bioelectronics, Biosensing and Therapeutic Applications. Spec. Issue Recent Adv. Chem./Mater. 2022, 22, e202100319. [Google Scholar] [CrossRef]
- Yuwen, T.; Shu, D.; Zou, H.; Yang, X.; Wang, S.; Zhang, S.; Liu, Q.; Wang, X.; Wang, G.; Zhang, Y.; et al. Carbon nanotubes: A powerful bridge for conductivity and flexibility in electrochemical glucose sensors. J. Nanobiotechnol. 2023, 21, 320. [Google Scholar] [CrossRef]
- He, Y.; Hu, C.; Li, Z.; Wu, C.; Zeng, Y.; Peng, C. Multifunctional carbon nanomaterials for diagnostic applications in infectious diseases and tumors. Mater. Today Bio 2022, 14, 100231. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.; Radovic, M.; Rizzotto, F.; Vizzini, P.; Jaric, S.; Pavlovic, Z.; Radonic, V.; Nikolic, M.V.; Vidic, J. Advances in Nanomaterials-Based Electrochemical Biosensors for Foodborne Pathogen Detection. Nanomaterials 2021, 11, 2700. [Google Scholar] [CrossRef] [PubMed]
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. All-carbon multi-scale and hierarchical fibers and related structural composites: A review. Compos. Sci. Technol. 2020, 186, 107932. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Zhang, Q.; Wei, F.; Ma, J.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical NiMn Layered Double Hydroxide/Carbon Nanotubes Architecture with Superb Energy Density for Flexible Supercapacitors. Adv. Funct. Mater. 2014, 24, 2938–2946. [Google Scholar] [CrossRef]
- Cajigas, S.; Alzate, D.; Orozco, J. Gold nanoparticle/DNA-based nanobioconjugate for electrochemical detection of Zika virus. Microchim. Acta 2020, 187, 594. [Google Scholar] [CrossRef]
- Reghunath, S.; Pinheiro, D.; Kr, S.D. A review of hierarchical nanostructures of TiO2: Advances and applications. Appl. Surf. Sci. Adv. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Ding, M.; Xie, N.; Wang, C.; Kou, X.; Zhang, H.; Guo, L.; Sun, Y.; Chuai, X.; Gao, Y.; Liu, F.; et al. Enhanced NO2 gas sensing properties by Ag-doped hollow urchin-like In2O3 hierarchical nanostructures. Sens. Actuators B Chem. 2017, 252, 418–427. [Google Scholar] [CrossRef]
- Mandal, D.; Biswas, S.; Chowdhury, A.; De, D.; Tiwary, C.S.; Gupta, A.N.; Singh, T.; Chandra, A. Hierarchical cage-frame type nanostructure of CeO2 for bio sensing applications: From glucose to protein detection. Nanotechnology 2020, 32, 2. [Google Scholar]
- Wen, A.M.; Podgornik, R.; Strangi, G.; Steinmetz, N.F. Photonics and plasmonics go viral: Self-assembly of hierarchical metamaterials. Rend. Lincei 2015, 26, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Capehart, S.L.; Pal, S.; Liu, M.; Zhang, L.; Schuck, P.J.; Liu, Y.; Yan, H.; Francis, M.B.; De Yoreo, J.J. Hierarchical Assembly of Plasmonic Nanostructures Using Virus Capsid Scaffolds on DNA Origami Templates. ACS Nano 2014, 8, 7896–7904. [Google Scholar] [CrossRef]
- Greer, J.R.; Kim, J.-Y.; Burek, M.J. The In-situ Mechanical testing of nanoscale single-crystalline nanopillars. Nanomech. Test. 2009, 61, 19–25. [Google Scholar] [CrossRef]
- Bae, J.; Hong, J.-I.; Han, W.H.; Choi, Y.J.; Snyder, R.L. Superior field emission properties of ZnO nanocones synthesized by pulsed laser deposition. Chem. Phys. Lett. 2009, 475, 260–263. [Google Scholar] [CrossRef]
- Kijima, T.; Nagatomo, Y.; Takemoto, H.; Uota, M.; Fujikawa, D.; Sekiya, Y.; Kishishita, T.; Shimoda, M.; Yoshimura, T.; Kawasaki, H.; et al. Synthesis of Nanohole-Structured Single-Crystalline platinum nanosheets using surfactant-liquid-crystals and their electrochemical characterization. Adv. Funct. Mater. 2009, 19, 545–553. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, L.; Wang, Q.; Hao, P.; Zheng, X. Wettability behavior of nanodroplets on copper surfaces with hierarchical nanostructures. Colloids Surf. A 2020, 604, 125291–125297. [Google Scholar] [CrossRef]
- Cheung, C.L.; Nikolić, R.J.; Reinhardt, C.E.; Wang, T.F. Fabrication of nanopillars by nanosphere lithography. IOP Sci. 2006, 17, 1339. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wattanatorn, N.; Zhao, C.; Chiang, N.; Jonas, S.J.; Weiss, P.S. Multiple-Patterning Nanosphere Lithograhy for Fabricating periodic Three-Dimensional Hierechical Nanostrutures. ACS Nano 2017, 11, 10384–10391. [Google Scholar] [CrossRef]
- Tseng, A.A.; Chen, K.; Chen, C.D.; Ma, K.J. Electron beam lithography in nanoscale fabrication: Recent development. IEEE Trans. Electron. Packag. Manuf. 2003, 26, 141–149. [Google Scholar] [CrossRef]
- Chen, Y.F. Nanofabrication by electron beam lithography and its applications: A review. Microelectron. Eng. 2015, 135, 57–72. [Google Scholar] [CrossRef]
- Asadi, R.; Abdollahi, H.; Gharabaghi, M.; Boroumand, Z. Effective removal of Zn (II) ions from aqueous solution by the magnetic MnFe2O4 spinel ferrite nanoparticles with focuses on synthesis, characterization, adsortion, and desorption. Adv. Powder Technol. 2020, 31, 1480–1489. [Google Scholar] [CrossRef]
- Niu, C.; Liu, X.; Meng, J.; Xu, L.; Yan, M.; Wang, X.; Zhang, G.; Liu, Z.; Xu, X.; Mai, L. Three dimensional V2O5/NaV6O15 Hierarchical heterostructures: Controlled synthesisand synergistic effect investigated by in situ X-ray difraction. Nanoenergia 2016, 27, 147–156. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, P.; Zhang, H.; Zhang, D.; Sun, X.; Ma, Y. Rapid hydrothermal synthesis of hierarchical nanostructures assembled from ultrathin birnessite-type Mn = 2 nanosheets for supercapacitor applications. Electrochim. Acta 2013, 89, 523–529. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, Y.; Yan, M.; Shi, W. Construction of hierarchical FeCo2O4 MnO2 core-shell nanostructures on carbon fibers for High-performance asymmetric supercapacitor. J. Colloid Interface Sci. 2018, 512, 419–427. [Google Scholar] [CrossRef]
- Mu, C.F.; Yao, Q.Z.; Qu, X.F.; Zhou, G.T.; Li, M.L.; Fu, S.Q. Controlled synthesis of various hierarchical nanostructures of copper sulfide by a facile microwave irradation method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 14–21. [Google Scholar] [CrossRef]
- Moshnikov, V.A.; Gracheva, I.E.; Kuznezov, V.V.; Maximov, A.I.; Karpova, S.S.; Ponomareva, A.A. Hierarchical nanostructured semiconductor porous materials for gas sensonrs. J. Non-Cryst. Solids 2010, 356, 2020–2025. [Google Scholar] [CrossRef]
- Naqvi, T.K.; Bajpai, A.; Bharati, M.S.S.; Kulkarni, M.M.; Siddiqui, A.M.; Soma, V.R.; Dwivedi, P.K. Ultra-sensitive reusable SERS sensor for multiple hazardous materials detection on single platform. J. Hazard. Mater. 2020, 407, 124353. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Qian, J.; Pan, H.; Cui, Y.; Xu, M.; Tu, L.; Chai, Q.; Zhou, X. Micro-lotus constructured by Fe-doped ZnO hierarchically porous nanosheets: Preparation, characterization and gas sensing property. Sens. Actuators B Chem. B 2011, 158, 9–16. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Photocatalytic performance of novel samarium-doped spherical-like ZnO hierarchical nanostructures under visible light irradiation for 2,4-dichlorophenol degradation. J. Colloid Interface Sci. 2013, 401, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tu, J.; Li, X.; Wang, Z.; Li, Y.; Li, Q.; Wang, F. Enhanced UV-Visible light Photocatalytic activity by constructing appropriate heterostructures between mesopore TiO2 nanospheres and Sn3O4 nanoparticles. Nanomaterials 2017, 7, 336. [Google Scholar] [CrossRef]
- Wu, S.; Xu, Y.; Li, X.; Tong, R.; Chen, L.; Han, Y.; Wu, J.; Zhang, X. Controlled synthesis of porous hierarchical ZnFe2O4 Micro-/Nanostructures with multifunctional photovatalytic performance. Inorg. Chem. 2018, 57, 15481–15488. [Google Scholar] [CrossRef]
- Panmand, R.P.; Sethi, Y.A.; Kadam, S.R.; Tamboli, M.S.; Nikam, L.K.; Ambekar, J.D.; Park, C.-J.; Kale, B.B. Self-assembled hierarchical nanostrcutures of Bi2WO6 for hydrogen production and dye degradation under solar light. CrystEngComm 2015, 17, 107–115. [Google Scholar] [CrossRef]
- Tian, X.D.; Liu, B.J.; Li, J.F.; Yang, Z.L.; Ren, B.; Tian, Z.Q. Shiners and plasmonic properties of Au Core SiO2 shell nanoparticles with optimal core size and shell thickness. J. Raman Spectrosc. 2013, 44, 994–998. [Google Scholar] [CrossRef]
- Cao, S.W.; Zhu, Y.J.; Ma, M.Y.; Li, L.; Zhang, L. Hierarchically nanostructured magnetic hollow spheres of Fe3O4 and Fe2O3: Preparation and potetialapplication in drug delivery. J. Phys. Chem. 2008, 112, 1851–1856. [Google Scholar]
- Tang, X.; Cai, W.; Yang, L.; Liu, J. Monitoring plasmon-driven surface catalyzed reactions in situ using time-dependent surface-enhanced Raman spectrsocopy on single particles of hierarchical peonylike silver microflowers. Nanoscale 2014, 6, 8612–8616. [Google Scholar] [CrossRef]
- Kang, L.; Xu, P.; Chen, D.; Zhang, B.; Du, Y.; Han, X.; Li, Q.; Wang, H.-L. Amino Acid-Assisted Synthesis of hierarchical silver microspheres for single particle surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2013, 117, 10007–10012. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, L.-Y.; Zhu, L.-W.; Wan, L.-S.; Xu, Z.-K. In-Situ Immobilization of Silver Nanoparticles on Self-Assembled Honeycomb-Patterned Films Enables Surface-Enhanced Raman Scattering (SERS) Substrates. J. Phys. Chem. C 2014, 118, 11478–11484. [Google Scholar] [CrossRef]
- Butakova, M.A.; Chernov, A.V.; Kartashov, O.O.; Soldatov, A.V. Data-Centric Architecture for Self-Driving laboratories with autonomous discovery of new Nanomaterials. Nanomaterials 2021, 12, 12. [Google Scholar] [CrossRef]
- Escarpa, A.; Prodromidis, M.I. Microchimica Acta: A top analytical chemistry journal for disseminating research involving micro and nanomaterials. Microchim. Acta 2021, 188, 136. [Google Scholar] [CrossRef]
- Khomutov, G.; Gubin, S. Interfacial synthesis of noble metal nanoparticles. Mater. Sci. Eng. C 2002, 22, 141–146. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Cai, L.J.; Sui, L.; Qian, D.J.; Chen, M. Reducing properties of polymers in the Synthesis of noble metal nanoparticles. Polym. Rev. 2013, 53, 240–276. [Google Scholar] [CrossRef]
- Nunez, F.A.; Castro, A.C.; de Oliveira, V.L.; Lima, A.C.; Oliveira, J.R.; de Medeiros, G.X.; Sasahara, G.L.; Santos, K.S.; Lanfredi, A.J.C.; Alves, W.A. Electrochemical Immunosensors Based on Zinc Oxide Nanorods for Detection of Antibodies Against SARS-CoV-2 Spike Protein in Convalescent and Vaccinated Individuals. ACS Biomater. Sci. Eng. 2023, 9, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Liustrovaite, V.; Drobysh, M.; Rucinskiene, A.; Baradoke, A.; Ramanaviciene, A.; Plikusiene, I.; Samukaite-Bubniene, U.; Viter, R.; Chen, C.-F.; Ramanavicius, A. Towards an Electrochemical Immunosensor for the Detection of Antibodies against SARS-CoV-2 Spike Protein. J. Electrochem. Soc. 2022, 169, 037523. [Google Scholar] [CrossRef]
- Jeon, M.J.; Kim, S.K.; Hwang, S.H.; Lee, J.U.; Sim, S.J. Lateral flow immunoassay based on surface-enhanced Raman scattering using pH-induced phage-templated hierarchical plasmonic assembly for point-of-care diagnosis of infectious disease. Biosens. Bioelectron. 2024, 250, 116061. [Google Scholar] [CrossRef]
- Yin, Z.Z.; Liu, Z.; Zhou, M.; Yang, X.; Zheng, G.; Zhang, H.; Kong, Y. A surface molecularly imprinted electrochemical biosensor for the detection of SARS-CoV-2 spike protein by using Cu7S4-Au as built-in probe. Bioelectrochemistry 2023, 152, 108462. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Guo, M.; Wu, Y.; Liu, W.; Luo, S.; Wang, X.; Kang, H.; Chen, Y.; Dai, C.; Kong, D.; et al. Electrochemical Detection of a Few Copies of Unamplified SARS-CoV-2 Nucleic Acids by a Self-Actuated Molecular System. J. Am. Chem. Soc. 2022, 144, 13526–13537. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, D.M.; Abouzid, M.; Gaballah, M.S.; Ahmed, A.A.; Adeel, M.; Sheta, S.M. New approach in SARS-CoV-2 surveillance using biosensor technology: A review. Environ. Sci. Pollut. Res. 2022, 29, 1677–1695. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Dan, N. Synthesis of hierarchical materials. Focus 2000, 18, 370–374. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.N.; Jones, M.R.; Mirkin, C.A. The nature and implications of uniformity in the hierarchical organization of nanomaterials. Proc. Natl. Acad. Sci. USA 2016, 113, 11717–11725. [Google Scholar] [CrossRef]
- Shi, S.; Li, Y.; Ngo-Dinh, B.-N.; Markmann, J.; Weissmüller, J. Scaling behavior of stiffness and strength of hierarchical network nanomaterials. Science 2021, 371, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. Chapter 19—Hierarchical Materials. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 545–574. [Google Scholar]
- Kamada, A.; Herneke, A.; Lopez-Sanchez, P.; Harder, C.; Ornithopoulou, E.; Wu, Q.; Wei, X.; Schwartzkopf, M.; Müller-Buschbaum, P.; Roth, S.V.; et al. Hierarchical propagation of structural features in protein nanomaterials. Nanoscale 2022, 14, 2502–2510. [Google Scholar] [CrossRef]
- Baranov, O.; Belmonte, T.; Levchenko, I.; Bazaka, K.; Košiček, M.; Cvelbar, U. Hierarchical Nanomaterials by Selective Deposition of Noble Metal Nanoparticles: Insight into Control and Growth Processes. Adv. Theory Simul. 2023, 6, 2300288. [Google Scholar] [CrossRef]
- Tseng, P.; Napier, B.; Zhao, S.; Mitropoulos, A.N.; Applegate, M.B.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Directed assembly of bio-inspired hierarchical materials with controlled nanofibrillar architectures. Nat. Nanotechnol. 2017, 12, 474–480. [Google Scholar] [CrossRef]
- Hamon, C.; Novikov, S.; Scarabelli, L.; Basabe-Desmonts, L.; Liz-Marzán, L.M. Hierarchical Self-Assembly of Gold Nanoparticles into Patterned Plasmonic Nanostructures. ACS Nano 2014, 8, 10694–10703. [Google Scholar] [CrossRef]
- Bonacchi, S.; Antonello, S.; Dainese, T.; Maran, F. Atomically Precise Metal Nanoclusters: Novel Building Blocks for Hierarchical Structures. Chem. A Eur. J. 2021, 27, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Ramakrishna, S. Electrospun nanofibers as a platform for multifunctional, hierarchically organized nanocomposite. Compos. Sci. Technol. 2009, 69, 1804–1817. [Google Scholar] [CrossRef]
- Zelada-Guillén, G.A.; Escárcega-Bobadilla, M.V.; Wegrzyn, M.; Giménez, E.; Maier, G.; Kleij, A.W. Enhanced Conductivity for Carbon Nanotube Based Materials through Supramolecular Hierarchical Self-Assembly. Adv. Mater. Interfaces 2018, 5, 1701585. [Google Scholar] [CrossRef]
- Osada, M.; Sasaki, T. Nanosheet architectonics: A hierarchically structured assembly for tailored fusion materials. Polym. J. 2015, 47, 89–98. [Google Scholar] [CrossRef]
- Cameron, J.M.; Guillemot, G.; Galambos, T.; Amin, S.S.; Hampson, E.; Haidaraly, K.M.; Newton, G.N.; Izzet, G. Supramolecular assemblies of organo-functionalised hybrid polyoxometalates: From functional building blocks to hierarchical nanomaterials. R. Soc. Chem. 2022, 51, 293–328. [Google Scholar] [CrossRef] [PubMed]
- Mitzi, D.B. Thin-Film Deposition of Organic−Inorganic Hybrid Materials. Chem. Mater. 2001, 13, 3283–3298. [Google Scholar] [CrossRef]
- Schubert, U. Cluster-based inorganic–organic hybrid materials. Chem. Soc. Rev. 2011, 40, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, A.; Pietsch, T.; Mendoza, C.; Cheval, N. Functional hybrid materials. Mater. Today 2009, 12, 44–50. [Google Scholar] [CrossRef]
- Kickelbick, G. Hybrid Materials—Past, Present and Future. Hybrid Mater. 2014, 1, 39–51. [Google Scholar] [CrossRef]
- Sun, G.; Chen, D.; Zhu, G.; Li, Q. Lightweight hybrid materials and structures for energy absorption: A state-of-the-art review and outlook. Thin-Walled Struct. 2022, 172, 108760. [Google Scholar] [CrossRef]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Ashby, M. Hybrid Materials to Expand the Boundaries of Material-Property Space. J. Am. Ceram. Soc. 2011, 94, s3–s14. [Google Scholar] [CrossRef]
- Nicole, L.; Laberty-Robert, C.; Rozes, L.; Sanchez, C. Hybrid materials science: A promised land for the integrative design of multifunctional materials. Nanoscale 2014, 6, 6267–6292. [Google Scholar] [CrossRef] [PubMed]
- Houbertz, R.; Domann, G.; Cronauer, C.; Schmitt, A.; Martin, H.; Park, J.U.; Fröhlich, L.; Buestrich, R.; Popall, M.; Streppel, U.; et al. Inorganic–organic hybrid materials for application in optical devices. Thin Solid Films 2003, 442, 194–200. [Google Scholar] [CrossRef]
- Semenova, D.; Silina, Y.E. The Role of Nanoanalytics in the Development of Organic-Inorganic Nanohybrids—Seeing Nanomaterials as They Are. Nanomaterials 2019, 9, 1673. [Google Scholar] [CrossRef] [PubMed]
- Napi, M.L.M.; Sultan, S.M.; Ismail, R.; How, K.W.; Ahmad, M.K. Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials 2019, 12, 2985. [Google Scholar] [CrossRef] [PubMed]
- Kerry, R.G.; Ukhurebor, K.E.; Kumari, S.; Maurya, G.K.; Patra, S.; Panigrahi, B.; Majhi, S.; Rout, J.R.; Rodriguez-Torres, M.D.P.; Das, G.; et al. A comprehensive review on the applications of nano-biosensor-based approaches for non-communicable and communicable disease detection. Sensor 2015, 5, 14539–14568. [Google Scholar]
- Al-Ahmady, Z.S.; Ali-Boucetta, H. Nanomedicine & Nanotoxicology Future Could Be Reshaped Post-COVID-19 Pandemic. Front. Nanotechnol. 2020, 2, 610465. [Google Scholar]
- Singh, C.K.; Sodhi, K.K. The emerging significance of nanomedicine-based approaches to fighting COVID-19 variants of concern: A perspective on the nanotechnology’s role in COVID-19 diagnosis and treatment. Front. Nanotechnol. 2023, 4, 1084033. [Google Scholar] [CrossRef]
- Singh, P.; Yadava, R. Chapter 26—Nanosensors for health care. In Nanosensors for Smart Cities; Elsevier: Amsterdam, The Netherlands, 2020; pp. 433–450. [Google Scholar]
- Hulanicki, A.; Glab, S.; Ingman, F. Analytical Chemistry division commission on general aspects of analytical chemistry. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Riu, J.; Maroto, A.; Rius, F.X. Nanosensors in environmental analysis. Talanta 2006, 69, 288–301. [Google Scholar] [CrossRef]
- Tonyushkina, K.; Nichols, J.H. Glucose Meters: A Review of Technical Challenges to Obtaining Accurate Results. J. Diabetes Sci. Technol. 2009, 3, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jackman, J.A.; Yang, H.-H.; Chen, P.; Cho, N.-J.; Kim, D.-H. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80. [Google Scholar]
- Qi, H.; Yue, S.; Bi, S.; Ding, C.; Song, W. Isothermal exponential amplification techniques: From basic principles to applications in electrochemical biosensors. Biosens. Bioelectron. 2018, 110, 207–217. [Google Scholar] [CrossRef]
- Welch, E.C.; Powell, J.M.; Clevinger, T.B.; Fairman, A.E.; Shukla, A. Advances in Biosensors and Diagnostic Technologies Using Nanostructures and Nanomaterials. Adv. Funct. Mater. 2021, 31, 2104126. [Google Scholar] [CrossRef]
- Jia, Y.; Yi, X.; Li, Z.; Zhang, L.; Yu, B.; Zhang, J.; Wang, X.; Jia, X. Recent advance in biosensing applications based on two-dimensional transition metal oxide nanomaterials. Talanta 2019, 219, 121308. [Google Scholar] [CrossRef]
- Fang, L.; Liu, B.; Liu, L.; Li, Y.; Huang, K.; Zhang, Q. Direct electrochemistry of glucose oxidase immobilized on Au nanoparticles-functionalized 3D hierarchically ZnO nanostructures and its application to bioelectrochemical glucose sensor. Sens. Actuators B Chem. 2016, 222, 1096–1102. [Google Scholar] [CrossRef]
- Tripathy, N.; Kim, D.-H. Metal oxide modified ZnO nanomaterials for biosensor applications. Nano Converg. 2018, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A. Applications of carbon-based conductive nanomaterials in biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Xia, Y.; Halas, N.J. Shape-controlled synthesis and surface plasmonic properties of metallic nanostructures. MRS Bull. 2005, 30, 338–348. [Google Scholar] [CrossRef]
- Korotcenkov, G. Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef]
- Ni, J.; Liu, D.; Wang, W.; Wang, A.; Jia, J.; Tian, J.; Xing, Z. Hierarchical defect-rich flower-like BiOBr/Ag nanoparticles/ultrathin g-C3N4 with transfer channels plasmonic Z-scheme heterojunction photocatalyst for accelerated visible-light-driven photothermal-photocatalytic oxytetracycline degradation. Chem. Eng. J. 2021, 419, 129969. [Google Scholar] [CrossRef]
- Xiang, Z.; Xiong, J.; Deng, B.; Cui, E.; Yu, L.; Zeng, Q.; Pei, K.; Chen, R.; Lu, W. Rational design of 2D hierarchically laminated Fe3O4@ nanoporous carbon@rGO nanocomposites with strong magnetic coupling for excellent electromagnetic absorption applications. J. Mater. Chem. C 2020, 8, 2123–2134. [Google Scholar] [CrossRef]
- Pietá, I.S.; Rathib, A.; Piedad, P.; Nowakowski, R.; Holdynski, M.; Pisarek, M.; Kaminska, A.; Gawandeb, M.; Zborilb, R. Electrocatalytic methanol oxidation over Cu, Ni and bimetallic Cu-Ni nanoparticles supported on graphitic carbon nitride. Appl. Catal. B Environ. 2019, 244, 272–283. [Google Scholar] [CrossRef]
- Wen, F.; Zhang, W.; Wei, G.; Wang, Y.; Zhang, J.; Zhang, M.; Shi, L. Synthesis of noble metal nanoparticles embedded in the shell layer of core-shell poly(styrene-co-4-vinylpyridine) microspheres and their application in catalysis. Chem. Mater. 2008, 20, 2144–2150. [Google Scholar] [CrossRef]
- Munawar, A.; Tahir, M.A.; Shaheen, A.; Lieberzeit, P.A.; Khan, W.S.; Bajwa, S.Z. Investigating nanohybrid material based on 3D CNTs@Cu nanoparticle composite and imprinted polymer for highly selective detection of chloramphenicol. J. Hazard. Mater. 2018, 342, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zeng, G.; Xu, P.; Lai, C.; Tang, L. How Do Enzymes ‘Meet’ Nanoparticles and Nanomaterials? Trends Biochem. Sci. 2017, 42, 914–930. [Google Scholar] [CrossRef]
- Holler, R.P.M.; Dulle, M.; Thoma, S.; Mayer, M.; Steiner, A.M.; Förster, S.; Fery, A.; Kuttner, C.; Chanana, M. Protein-Assisted Assembly of modular 3D Plasmonic Raspberry-like Core/Satellite Nanoclusters: Correlation of Structure and Optical Properties. ACS Nano 2016, 6, 5740–5750. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H. Challenges and Opportunities in the Bottom-up Mechanochemical Synthesis of Noble Metal Nanoparticles. J. Mater. Chem. A Mater. Energy Sustain. 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Yan, N.; Liu, H.; Zhu, Y.; Jiang, W.; Dong, Z. Entropy-driven hierarchical nanostructures from cooperative self-assembly of gold nanoparticles/block copolymers under three-dimensional confinement. Macromolecules 2015, 48, 5980–5987. [Google Scholar] [CrossRef]
- Prominski, A.; Tomczyk, E.; Pawlak, M.; Jedrych, A.; Mieczkowki, J.; Lewandowski, W.; Wójcik, M. Size-dependent thermal- and photoresponsive plasmonic properties of liquid crystalline gold nanoparticles. Materials 2020, 13, 875. [Google Scholar] [CrossRef]
- Adesuji, E.T.; Torres-Guerrero, V.O.; Arizpe-Zapata, J.A.; Videa, M.; Sánchez-Domínguez, M.; Fuentes, K.M. Bicontinuous microemulsion as confined reaction media for the synthesis of plasmonic silver self-ass. Nanotechnology 2020, 31, 425601. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- Henry, A.-I.; Bingham, J.M.; Ringe, E.; Marks, L.D.; Schatz, G.C.; Van Duyne, R.P. Correlated Structure and Optical property Studies of plasmonic nanoparticles. J. Phys. Chem. 2011, 115, 9291–9305. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Li, Z.-Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostrutures: Engineering their plasmonic properties for biomedical application. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Sayan, J.S.; Kuruvilla, J.; Appukuttan, S. Development of Hierarchical Nanostructures for Energy Storage. In Advances in Nanocomposite Materials for Environmental and Energy Harvesting Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 663–695. [Google Scholar]
- Knebel, A.; Wulfert-Holzman, P.; Friebe, S.; Pavel, J.; Srauß, I.; Mundstock, A.; Steibach, F.; Caro, J. Hierarchical nanostructures of metal-Organic Frameworks applied in gas separating ZIF-8on-ZIF-67 membranes. Chemistry 2017, 24, 5728–5733. [Google Scholar] [CrossRef]
- Cölfen, H.; Mann, S. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Organ. Nanostruct. 2003, 42, 2350–2365. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, N.; Mishra, P.; Malhotra, B.D. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total Environ. 2021, 754, 142363. [Google Scholar] [CrossRef]
- Kurnia Sari, A. The optimization of an electrochemical aptasensor to detect RBD protein S SARS-CoV-2 as a biomarker of COVID-19 using screen-printed carbon electrode/AuNP. J. Electrochem. Sci. Eng. 2022, 12, 219–235. [Google Scholar] [CrossRef]
- Gao, Y.; Han, Y.; Wang, C.; Qiang, L.; Gao, J.; Wang, Y.; Liu, H.; Han, L.; Zhang, Y. Rapid and sensitive triple-mode detection of causative SARS-CoV-2 virus specific genes through interaction between genes and nanoparticles. Anal. Chim. Acta 2021, 1154, 338330. [Google Scholar] [CrossRef]
- Drobysh, M.; Ramanaviciene, A.; Viter, R.; Chen, C.F.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Biosensors for the Determination of SARS-CoV-2 Virus and Diagnosis of COVID-19 Infection. Int. J. Mol. Sci. 2022, 23, 666. [Google Scholar] [CrossRef]
- Wang, X.; Kong, D.; Guo, M.; Wang, L.; Gu, C.; Dai, C.; Wang, Y.; Jiang, Q.; Ai, Z.; Zhang, C.; et al. Rapid SARS-CoV-2 Nucleic Acid Testing and Pooled Assay by Tetrahedral DNA Nanostructure Transistor. Nanoletters 2021, 21, 9450–9457. [Google Scholar] [CrossRef]
- Dighe, K.; Moitra, P.; Alafeef, M.; Gunaseelan, N.; Pan, D. A rapid RNA extraction-free lateral flow assay for molecular point-of-care detection of SARS-CoV-2 augmented by chemical probes. Biosens. Bioelectron. 2022, 200, 113900. [Google Scholar] [CrossRef]
- White, A.M.; Lin, W.; Cheng, X. Discovery of COVID-19 Inhibitors Targeting the SARS-CoV-2 Nsp13 Helicase. J. Phys. Chem. Lett. 2020, 11, 9144–9151. [Google Scholar] [CrossRef]
- Salcedo, N.; Reddy, A.; Gomez, A.R.; Bosch, I.; Herrera, B.B. Monoclonal antibody pairs against SARS-CoV-2 for rapid antigen test development. PLoS Negl. Trop. Dis. 2022, 16, e0010311. [Google Scholar] [CrossRef]
- Younes, N.; Al-Sadeq, D.W.; Al-Jinghefee, H.; Younes, S.; AL-Jamal, O.; Daas, H.I.; Yassine, H.M.; Nasrallah, G. Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2. Viruses 2020, 12, 582. [Google Scholar] [CrossRef]
- Wang, J.; Drelich, A.J.; Hopkins, C.M.; Mecozzi, S.; Li, L.; Know, G.; Hong, S. Gold nanoparticles in virus detection: Recent advances and potential considerations for SARS-CoV-2 testing development. WIREs Nanomed. Nanobiotechnol. 2021, 14, e1754. [Google Scholar] [CrossRef]
- Özmen, E.N.; Kartal, E.; Turan, M.B.; Yaziciglu, A.; Niazi, J.H.; Qureshi, A. Graphene and carbon nanotubes interfaced electrochemical nanobiosensors for the detection of SARS-CoV-2 (COVID-19) and other respiratory viral infections: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112356. [Google Scholar] [CrossRef]
- Mattioli, I.A.; Hassan, A.; Oliveira, O.N.; Crespilho, F.N. On the Challenges for the Diagnosis of SARS-CoV-2 Based on a Review of Current Methodologies. ACS Sens. 2020, 5, 3655–3677. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 in Clinical Samples. ACS Appl. Bio Mater. 2021, 4, 2974–2995. [Google Scholar] [CrossRef]
- Avelino, K.Y.S.; Santos, G.S.D.; Frías, I.A.M.; Silva-Junior, A.G.; Pereira, M.C.; Pitta, M.G.R.; de Araújo, B.C.; Errachid, A.; Oliveira, M.D.; Andrade, C.A.S. Nanostructured sensor platform based on organic polymer conjugated to metallic nanoparticle for the impedimetric detection of SARS-CoV-2 at various stages of viral infection. J. Pharm. Biomed. Anal. 2021, 206, 114392. [Google Scholar] [CrossRef]
- Fan, Z.; Yao, B.; Ding, Y.; Xu, D.; Zhao, J.; Zhang, K. Rational engineering the DNA tetrahedrons of dual wavelength ratiometric electrochemiluminescence biosensor for high efficient detection of SARS-CoV-2 RdRp gene by using entropy-driven and bipedal DNA walker amplification strategy. Chem. Eng. J. 2022, 427, 131686. [Google Scholar] [CrossRef]
- Adeel, M.; Asif, K.; Alshabouna, F.; Canzonieri, V.; Rahman, M.M.; Ansari, S.A.; Güder, F.; Rizzolio, F.; Daniele, S. Label-free electrochemical aptasensor for the detection of SARS-CoV-2 spike protein based on carbon cloth sputtered gold nanoparticles. Biosens. Bioelectron. X 2022, 12, 100256. [Google Scholar] [CrossRef]
- Cajigas, S.; Alzate, D.; Fernández, M.; Muskus, C.; Orozco, J. Electrochemical genosensor for the specific detection of SARS-CoV-2. Talanta 2022, 245, 123482. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.-C.; Yang, J.O.; Jin, L.; Li, H.; Zhao, C.-Y.; Zhang, L.; Wei, J.; et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators B Chem. 2021, 327, 128899. [Google Scholar] [CrossRef]
- Park, Y.; Ryu, B.; Ki, S.J.; Chen, M.; Liang, X.; Kurabayashi, K. Bioinspired Plasmo-virus for Point-of-Care SARS-CoV-2 Detection. Nanoletters 2023, 23, 98–106. [Google Scholar] [CrossRef]
- Rabiee, N.; Akhavan, O. CaZnO-based nanoghosts for the detection of ssDNA, pCRISPR and recombinant SARS-CoV-2 spike antigen and targeted delivery of doxorubicin. Chemosphere 2022, 306, 135578. [Google Scholar] [CrossRef]
- Villa-Manso, A.M.; Guerrero-Esteban, T.; Pariente, F.; Toyos-Rodríguez, C.; de la Escosura-Muñiz, A.; Revenga-Parra, M. Gutiérrez-Sánchez and E. Lorenzo. Bifunctional Au@Pt/Au nanoparticles as electrochemiluminescence signaling probes for SARS-CoV-2 detection. Talanta 2023, 260, 124614. [Google Scholar] [CrossRef]
- Babadi, A.A.; Rahmati, S.; Fakhlaei, R.; Heidari, R.; Baradaran, S.; Akbariqomi, M.; Wang, S.; Tavoosidana, G.; Doherty, W.; Ostrikov, K. SARS-CoV-2 detection by targeting four loci of viral genome using graphene oxide and gold nanoparticle DNA biosensor. Sci. Rep. 2022, 12, 19416. [Google Scholar] [CrossRef]
- Liang, P.; Guo, Q.; Zhao, T.; Wen, C.-Y.; Tian, Z.; Shang, Y.; Xing, J.; Jiang, Y.; Zeng, J. Ag Nanoparticles with Ultrathin Au Shell-Based Lateral Flow Immunoassay for Colorimetric and SERS Dual-Mode Detection of SARS-CoV-2 IgG. Anal. Chem. 2022, 94, 8466–8473. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Feng, S.; Pei, F.; Xia, M.; Hao, Q.; Liu, B.; Tong, Z.; Wang, J.J.; Lei, W.; Mu, X. Magnetic/fluorescent dual-modal lateral flow immunoassay based on multifunctional nanobeads for rapid and accurate SARS-CoV-2 nucleocapsid protein detection. Anal. Chim. Acta 2022, 1233, 340486. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, X.; Liu, L.; Zhang, X.; Yang, X.; Zheng, S.Z.; Rong, Z.; Shengqi, W. Ultrasensitive and Simultaneous Detection of Two Specific SARS-CoV-2 Antigens in Human Specimens Using Direct/Enrichment Dual-Mode Fluorescence Lateral Flow Immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 40342–40353. [Google Scholar] [CrossRef]
- Haghayegh, F.; Salahandish, R.; Hassani, M.; Sanati-Nezhad, A. Highly Stable Buffer-Based Zinc Oxide/Reduced Graphene Oxide Nanosurface Chemistry for Rapid Immunosensing of SARS-CoV-2 Antigens. ACS Appl. Mater. Interfaces 2022, 14, 10844–10855. [Google Scholar] [CrossRef]
- Sarwar, S.; Lin, M.-C.; Amezaga, C.; Wei, Z.; Iyayi, E.; Polk, H.; Wang, R.; Wang, H.; Zhang, X. Ultrasensitive electrochemical biosensors based on zinc sulfide/graphene hybrid for rapid detection of SARS-CoV-2. Adv. Compos. Hybrid Mater. 2023, 6, 49. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Rong, Z.; Tu, Z.; Zhang, X.; Gu, B.; Wang, C.; Wang, S. Introduction of graphene oxide-supported multilayer-quantum dots nanofilm into multiplex lateral flow immunoassay: A rapid and ultrasensitive point-of-care testing technique for multiple respiratory viruses. Nano Res. 2023, 16, 3062–3073. [Google Scholar] [CrossRef]
- Dahiya, U.R.; Gupt, G.D.; Dhaka, R.S.; Kalyanasundaram, D. Functionalized Co2FeAl Nanoparticles for Detection of SARS-CoV-2 Based on Reverse Transcriptase Loop-Mediated Isothermal Amplification. ACS Appl. Nano Mater. 2021, 4, 5871–5882. [Google Scholar] [CrossRef]
- Chen, W.Y.; Lin, H.; Barui, A.K.; Gomez, A.M.U.; Wendt, M.K.; Stanciu, L.A. DNA-Functionalized Ti3C2Tx MXenes for Selective and Rapid Detection of SARS-CoV-2 Nucleocapsid Gene. ACS Appl. Nano Mater. 2022, 5, 1902–1910. [Google Scholar] [CrossRef]
- Mei, J.; Wang, D.; Zhang, Y.; Wu, D.; Cui, J.; Gan, M.; Liu, P. Portable Paper-Based Nucleic Acid Enrichment for Field Testing. Adv. Sci. 2023, 10, 2205217. [Google Scholar] [CrossRef]
- Karuppaiah, G.; Vashist, A.; Nair, M.; Veerapandian, M.; Manickam, P. Emerging trends in point-of-care biosensing strategies for molecular architectures and antibodies of SARS-CoV-2. Biosens. Bioelectron. X 2023, 13, 100324. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Z.; Liu, H.; Perea-López, N.; Ranasinghe, J.C.; Bepete, G.; Minns, A.M.; Rossi, R.M.; Lindner, S.E.; Huan, S.X.; et al. Understanding the Excitation Wavelength Dependence and Thermal Stability of the SARS-CoV-2 Receptor-Binding Domain Using Surface-Enhanced Raman Scattering and Machine Learning. ACS Photonics 2022, 9, 2963–2972. [Google Scholar] [CrossRef]
- Ni, R.; Chau, Y. Structural Mimics of Viruses Through Peptide/DNA Co-Assembly. J. Am. Chem. Soc. 2014, 136, 17902–17905. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, Y.; Zhao, W.; Qi, R.; Han, Y.; Wu, R.; Wang, Y.; Xu, H. Peptide-Induced DNA Condensation into Virus-Mimicking Nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 24349–24360. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.A.; van Doremalen, N.; Greaney, A.J.; Andersen, H.; Sharma, A.; Starr, T.N.; Keeffe, J.R.; Fan, C.; Schulz, J.E.; Gnanapragasam, P.N.P.; et al. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science 2022, 377, eabq0839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, K.; Wang, Q. Tailoring the Self-Assembly Behaviors of Recombinant Tobacco Mosaic Virus by Rationally Introducing Covalent Bonding at the Protein-Protein Interface. Small 2016, 12, 4955–4959. [Google Scholar] [CrossRef]
- Moitra, P.; Iftesum, M.; Skrodzki, D.; Paul, P.; Sheikh, E.; Gray, J.L.; Dighe, K.; Sheffield, Z.; Gartia, M.R.; Pan, D. Nucleotide-Driven Molecular Sensing of Monkeypox Virus Through Hierarchical Self-Assembly of 2D Hafnium Disulfide Nanoplatelets and Gold Nanospheres. Adv. Funct. Mater. 2023, 33, 2212569. [Google Scholar] [CrossRef]
- Ali, A.; Hu, C.; Zhang, F.; Jahan, S.; Yuan, B.; Saleh, M.S.; Gao, S.-J.; Panat, R. N protein-based ultrasensitive SARS-CoV-2 antibody detection in seconds via 3D nanoprinted, microarchitected array electrodes. Med. Virol. 2022, 94, 2067–2078. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Lin, C.; Long, L.; Hu, J.; He, J.; Zeng, H.; Huang, Z.; Li, Z.-Y.; Tanemura, M.; et al. Human ACE2-Functionalized Gold “Virus-Trap” Nanostructures for Accurate Capture of SARS-CoV-2 and Single-Virus SERS Detection. Nano-Micro Lett. 2021, 13, 109. [Google Scholar] [CrossRef]
- El-Said, W.A.; Al-Bogami, A.S.; Alshitari, W. Synthesis of gold nanoparticles@reduced porous graphene-modified ITO electrode for spectroelectrochemical detection of SARS-CoV-2 spike protein. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120237. [Google Scholar] [CrossRef]
- Palanisamy, S.; Lee, L.-Y.; Kao, C.-F.; Chen, W.-L.; Wang, H.-C.; Shen, S.-T.; Jian, J.-W.; Yuan, S.-S.F.; Kung, Y.-A.; Wang, Y.-M. One-step-one-pot hydrothermally derived metal-organic-framework-nanohybrids for integrated point-of-care diagnostics of SARS-CoV-2 viral antigen/pseudovirus utilizing electrochemical biosensor chip. Sens. Actuators B Chem. 2023, 390, 133960. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, C.; Hu, Y.; Zan, H.; Xu, X.; Sun, C.; Zou, H.; Li, Y. Sensitive fluorescence biosensor for SARS-CoV-2 nucleocapsid protein detection in cold-chain food products based on DNA circuit and g-CNQDs@Zn-MOF. LWT-Food Sci. Technol. 2022, 169, 114032. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Zheng, S.; Cheng, X.; Xiao, R.; Li, Q.; Wang, W.; Liu, X.; Wang, S. Development of an ultrasensitive fluorescent immunochromatographic assay based on multilayer quantum dot nanobead for simultaneous detection of SARS-CoV-2 antigen and influenza A virus. Sens. Actuators B Chem. 2021, 345, 130372. [Google Scholar] [CrossRef]
- Kim, J.; Mayorga-Martinez, C.C.; Vyskočil, J.; Ruzek, D.; Pumera, M. Plasmonic-magnetic nanorobots for SARS-CoV-2 RNA detection through electronic readout. Appl. Mater. Today 2022, 27, 101402. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, J.; Fan, Z.; Ding, Y.; Zhou, B.; Yang, R.; Zhao, J.; Zhang, K. Rational Engineering of the DNA Walker Amplification Strategy by Using a Au@Ti3C2@PEI-Ru(bpy)32+ Nanocomposite Biosensor for Detection of the SARS-CoV-2 RdRp Gene. ACS Appl. Mater. Interfaces 2021, 13, 19816–19824. [Google Scholar] [CrossRef]
- Tan, Q.; Wu, S.; Liu, Z.; Wu, X.; Forsberg, E.; He, S. High sensitivity detection of SARS-CoV-2 by an optofluidic hollow eccentric core fiber. Biomed. Opt. Express 2022, 13, 4592–4605. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.J.; Le, T.N.; Hsiao WW, W.; Tung, K.L.; Ostrikov, K.K.; Chiang, W.H. Plasmonic nanostructure-enhanced Raman scattering for detection of SARS-CoV-2 nucleocapsid protein and spike protein variants. Anal. Chim. Acta 2023, 1239, 340651. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, W.; Chena, F.; Ren, P. Highly sensitive and selective surface plasmon resonance biosensor for the detection of SARS-CoV-2 spike S1 protein. Analyst 2022, 147, 2809–2818. [Google Scholar] [CrossRef]
- Chang, H.; Jiang, M.; Zhu, Q.; Liu, A.; Wua, Y.; Li, C.; Jib, X.; Gongb, L.; Li, S.; Chen, Z.; et al. A novel photoelectrochemical immunosensor based on TiO2@Bi2WO6 hollow microspheres and Ag2S for sensitive detection of SARS-CoV-2 nucleocapsid protein. Microchem. J. 2022, 182, 107866. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, Z.R.; Roushani, M.; Hosseini, H.; Choobin, H. Label-free electrochemical aptasensor for rapid detection of SARS-CoV-2 spike glycoprotein based on the composite of Cu(OH)2 nanorods arrays as a high-performance surface substrate. Bioelectrochemistry 2022, 146, 108106. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M. SARS-CoV-2 virus label-free electrochemical nanohybrid MIP-aptasensor based on Ni3(BTC)2 MOF as a high-performance surface substrate. Microchim. Acta 2022, 189, 287. [Google Scholar] [CrossRef]
- Liu, H.; Xue, D.; Zhang, J.; Gu, C.; Wei, G.; Jiang, T. Quantitative monitoring of SARS-CoV-2 mediated by the intrinsic Raman signal of silicon nanoparticles and SiC@RP composite semiconductor SERS substrate. Sens. Actuators B Chem. 2023, 394, 134404. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, C.; Li, Y.; Gao, Y.; Wang, J.; He, J.; Huang, Z.; Liu, J.; Luo, X.; Yang, Y. Identifying infectiousness of SARS-CoV-2 by ultra-sensitive SnS2 SERS biosensors with capillary effect. Matter 2022, 5, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Gong, C.; Sun, S.; Zhang, Y.; Sun, L.; Su, Z.; Wu, A.; Wei, G. Hierarchical nanomaterials via biomolecular self-assembly and bioinspiration for energy and environmental applications. Nanoscale 2019, 11, 4147–4182. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Puente-Santiago, A.R.; Rodríguez-Padrón, D.; Muñoz-Batista, M.J.; Ahsan, M.A.; Noveron, J.C.; Luque, R. Nature-inspired hierarchical materials for sensing and energy storage applications. Chem. Soc. Rev. 2021, 50, 4856–4871. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, X.; Sun, Y.; Yang, S.; Song, X.; Yang, Z. Hierarchical CuO nanoflowers: Water-required synthesis and their application in a nonenzymatic glucose biosensor. Phys. Chem. Chem. Phys. 2013, 15, 10904–10913. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Ding, S.; Yuan, J.; Lou, X.W.; Kim, D.H. Hierarchically Structured One-Dimensional TiO2 for Protein Immobilization, Direct Electrochemistry, and Mediator-Free Glucose Sensing. ACS Nano 2011, 5, 7617–7626. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Y.; Zhao, W.; Wu, Q.; Chen, Y.; Huang, X.; Sun, Z.; Zhu, Y.; Liu, X. Hierarchical 0D-2D bio-composite film based on enzyme-loaded polymeric nanoparticles decorating graphene nanosheets as a high-performance bio-sensing platform. Biosens. Bioelectron. 2020, 156, 112134. [Google Scholar] [CrossRef]
- Ren, Z.; Guo, Y.; Liu, C.-H.; Gao, P.-X. Hierarchically nanostructured materials for sustainable environmental applications. Front. Chem. 2013, 1, 18. [Google Scholar] [CrossRef]
- Yao, T.; Dong, G.; Qian, S.; Cui, Y.; Chen, X.; Tan, T.; Li, L. Persistent luminescence nanoparticles/hierarchical porous ZIF-8 nanohybrids for autoluminescence-free detection of dopamine. Sens. Actuators B Chem. 2022, 357, 131470. [Google Scholar] [CrossRef]
- Sanko, V.; Şenocak, A.; Tümay, S.O.; Çamurcu, T.; Demirbas, E. Core-shell Hierarchical Enzymatic Biosensor Based on Hyaluronic Acid Capped Copper Ferrite Nanoparticles for Determination of Endocrine-disrupting Bisphenol A. Electroanalysis 2022, 34, 561–572. [Google Scholar] [CrossRef]
- Song, J.; Xu, L.; Xing, R.; Qin, W.; Dai, Q.; Song, H. Ag nanoparticles coated NiO nanowires hierarchical nanocomposites electrode for nonenzymatic glucose biosensing. Sens. Actuators B Chem. 2013, 182, 675–681. [Google Scholar] [CrossRef]
- Zhao, K.; Yan, X.; Gu, Y.; Kang, Z.; Bai, Z.; Cao, S.; Liu, Y.; Zhang, X.; Zhang, Y. Self-Powered Photoelectrochemical Biosensor Based on CdS/RGO/ZnO Nanowire Array Heterostructure. Small 2016, 12, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Anichini, C.; Samorì, P.; Criado, A.; Prato, M. 2D Van der Waals Heterostructures for Chemical Sensing. Adv. Funct. Mater. 2022, 32, 2207065. [Google Scholar] [CrossRef]
- Wang, B.; Cao, J.-T.; Liu, Y.-M. Recent progress of heterostructure-based photoelectrodes in photoelectrochemical biosensing: A mini review. Analyst 2020, 145, 1121–1128. [Google Scholar] [CrossRef]
- Shinde, P.V.; Saxena, M.; Singh, M.K. Chapter 11—Recent Developments in Graphene-Based Two-Dimensional Heterostructures for Sensing Applications. In Fundamentals and Sensing Applications of 2D Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 407–436. [Google Scholar]
- Martín-Palma, R.J.; Manso, M.; Torres-Costa, V. Optical Biosensors Based on Semiconductor Nanostructures. Sensors 2009, 9, 5149–5172. [Google Scholar] [CrossRef]
- Sakthivel, R.; Keerthi, M.; Chung, R.-J.; He, J.-H. Heterostructures of 2D materials and their applications in biosensing. Prog. Mater. Sci. 2023, 132, 101024. [Google Scholar] [CrossRef]
- Li, J.; Xiong, P.; Tang, J.; Liu, L.; Gao, S.; Zeng, Z.; Xie, H.; Tang, D.; Zhuang, J. Biocatalysis-induced formation of BiOBr/Bi2S3 semiconductor heterostructures: A highly efficient strategy for establishing sensitive photoelectrochemical sensing system for organophosphorus pesticide detection. Sens. Actuators B Chem. 2021, 331, 129451. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajjadi, M.; Sajadi, S.M.; Atarod, M. Chapter 2—Types of Nanostructures. Interface Sci. Technol. 2019, 28, 29–80. [Google Scholar]
- Haes, A.J.; Van Duyne, R.P. A Nanoscale Optical Biosensor: Sensitivity and Selectivity of an Approach Based on the Localized Surface Plasmon Resonance Spectroscopy of Triangular Silver Nanoparticle. J. Am. Chem. Soc. 2002, 124, 10597–10604. [Google Scholar] [CrossRef]
- Pina-Coronado, C.; Martínez-Sobrino, Á.; Gutiérrez-Gálvez, L.; Del Caño, R.; Martínez-Periñán, E.; García-Nieto, D.; Rodríguez-Peña, M.; Luna, M.; Milán-Rois, P.; Castellanos, M.; et al. Methylene Blue functionalized carbon nanodots combined with different shape gold nanostructures for sensitive and selective SARS-CoV-2 sensing. Sens. Actuators B Chem. 2022, 369, 132217. [Google Scholar] [CrossRef]
- Achadu, O.J.; Nwaji, N.; Lee, D.; Lee, J.L.; Akinoglu, E.M.; Giersig, M.; Park, E.Y. 3D hierarchically porous magnetic molybdenum trioxide@gold nanospheres as a nanogap-enhanced Raman scattering biosensor for SARS-CoV-2. Nanoscale Adv. 2022, 4, 871–883. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, W.; Xu, L.; Li, G.; Wan, Y.; Li, H. Nanobody-based label-free photoelectrochemical immunoassay for highly sensitive detection of SARS-CoV-2 spike protein. Anal. Chim. Acta 2022, 1211, 339904. [Google Scholar] [CrossRef]
- Panferov, V.G.; Byzova, N.A.; Biketov, S.F.; Zherdev, A.V.; Dzantiev, B.B. Comparative Study of In Situ Techniques to Enlarge Gold Nanoparticles for Highly Sensitive Lateral Flow Immunoassay of SARS-CoV-2. Biosensor 2021, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Jo, E.-J.; Jung, C.; Kim, M.-G. Absorption-Modulated SiO2@Au Core-Satellite Nanoparticles for Highly Sensitive Detection of SARS-CoV-2 Nucleocapsid Protein in Lateral Flow Immunosensors. ACS Appl. Mater. Interfaces 2022, 14, 45189–45200. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yin, Z.-Z.; Zheng, G.; Zhou, M.; Zhang, H.; Li, J.; Cai, W.; Kong, Y. Molecularly imprinted miniature electrochemical biosensor for SARS-CoV-2 spike protein based on Au nanoparticles and reduced graphene oxide modified acupuncture needle. Bioelectrochemistry 2023, 151, 108375. [Google Scholar] [CrossRef] [PubMed]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 2020, 14, 6359–7674. [Google Scholar] [CrossRef]
- Li, Y.; Lin, C.; Peng, Y.; He, J.; Yang, Y. High-sensitivity and point-of-care detection of SARS-CoV-2 from nasal and throat swabs by magnetic SERS biosensor. Sens. Actuators B Chem. 2022, 365, 131974. [Google Scholar] [CrossRef]
- Guo, A.; Pei, F.; Feng, S.S.; Hu, W.; Zhang, P.; Xia, M.; Mu, X.; Tong, Z.; Wang, F.; Liu, B. A photoelectrochemical immunosensor based on magnetic all-solid-state Z-scheme heterojunction for SARS-CoV-2 nucleocapsid protein detection. Sens. Actuators B Chem. 2023, 374, 132800. [Google Scholar] [CrossRef]
- Liu, X.; Bai, L.; Cao, X.C.; Wu, F.; Yin, T.; Lu, W. Rapid determination of SARS-CoV-2 nucleocapsid proteins based on 2D/2D MXene/P–BiOCl/Ru(bpy)32+ heterojunction composites to enhance electrochemiluminescence performanc. Anal. Chim. Acta 2022, 1234, 340522. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Highly selective and sensitive sandwich immunosensor platform modified with MUA-capped GNPs for detection of spike Receptor Binding Domain protein: A precious marker of COVID 19 infection. Sens. Actuators B Chem. 2021, 345, 130355. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Jia, X.; Zhou, J.; Li, H.; Wang, X.; Chen, Y.; Deng, J.; Jin, Z.; Wang, G. Directly and ultrasensitivity detecting SARS-CoV-2 spike protein in pharyngeal swab solution by using SERS-based biosensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 303, 123275. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. An electrochemical immunosensor using SARS-CoV-2 spike protein-nickel hydroxide nanoparticles bio-conjugate modified SPCE for ultrasensitive detection of SARS-CoV-2 antibodies. Microchem. J. 2021, 170, 106718. [Google Scholar] [CrossRef]
- Kawasaki, D.; Yamada, H.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Imprinted Photonic Crystal-Film-Based Smartphone-Compatible Label-Free Optical Sensor for SARS-CoV-2 Testing. Biosensors 2022, 12, 200. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Wu, T.; Fu, Z.; Zhang, W.; Lian, Z.; Cai, S.; Yang, R. Dual ligand-induced photoelectrochemical sensing by integrating Pt/MoS2 heterostructure and Au polyhedra for sensitive detection of SARS-CoV-2. Sens. Actuators B Chem. 2023, 376, 132970. [Google Scholar] [CrossRef]
- Vadlamani, B.S.; Uppal, T.; Verma, S.C.; Misra, M. Functionalized TiO2 Nanotube-Based Electrochemical Biosensor for Rapid Detection of SARS-CoV-2. Sensors 2020, 20, 5871. [Google Scholar] [CrossRef]
- Rabiee, N.; Fatahi, Y.; Ahmadi, S.; Abbariki, N.; Ojaghi, A.; Rabiee, M.; Radmanesh, F.; Dinarvand, R.; Bagherzadeh, M.; Mostafavi, E.; et al. Bioactive hybrid metal-organic framework (MOF)-based nanosensors for optical detection of recombinant SARS-CoV-2 spike antigen. Sci. Total Environ. 2022, 825, 153902. [Google Scholar] [CrossRef]
- Daoudi, K.; Ramachandran, K.; Alawadhi, H.; Boukherroub, R.; Dogheche, E.; El Khakani, M.A.; Gaidi, M. Ultra-sensitive and fast optical detection of the spike protein of the SARS-CoV-2 using AgNPs/SiNWs nanohybrid based sensors. Surf. Interfaces 2021, 27, 101454. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, H.; Tai, S.; Pedowitz, M.; Rzasa, J.R.; Pennachio, D.J.; Hajzus, J.R.; Milton, D.K.; Myers-Ward, R.; Daniels, K.M. Real-time ultra-sensitive detection of SARS-CoV-2 by quasi-freestanding epitaxial graphene-based biosenso. Biosens. Bioelectron. 2022, 197, 113803. [Google Scholar] [CrossRef]
- Martin-Martinez, F.J.; Jin, K.; Barreiro, D.L.; Buehler, M.J. The Rise of Hierarchical Nanostructured Materials from Renewable Sources: Learning from Nature. ACS Nano 2018, 12, 7425–7433. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.M.; Karumuri, A.; Barney, I.T. Hierarchical nanostructures by nanotube grafting on porous cellular surfaces. J. Phys. D Appl. Phys. 2009, 42, 195503. [Google Scholar] [CrossRef]
- Passoni, L.; Criante, L.; Fumagalli, F.; Scotognella, F.; Lanzani, G.; Di Fonzo, F. Self-Assembled Hierarchical Nanostructures for High-Efficiency Porous Photonic Crystals. ACS Nano 2014, 8, 12167–12174. [Google Scholar] [CrossRef]
- Tao, Y.; Zohar, H.; Olsen, B.D.; Segalman, R.A. Hierarchical Nanostructure Control in Rod−Coil Block Copolymers with Magnetic Fields. Nano Lett. 2007, 7, 2742–2746. [Google Scholar] [CrossRef]
- Cheng, B.; Niu, Q.; Cui, Y.; Jiang, W.; Zhao, Y.; Kong, L. Effects of different hierarchical hybrid micro/nanostructure surfaces on implant osseointegration. Clin. Implant Dent. Relat. Res. 2017, 19, 539–548. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Xu, H.; Sun, S.; Shang, M. Bi2O3 Hierarchical Nanostructures: Controllable Synthesis, Growth Mechanism, and their Application in Photocatalysis. Chem. A Eur. J. 2009, 15, 1776–1782. [Google Scholar] [CrossRef]
- Faul, C.F.J. Ionic Self-Assembly for Functional Hierarchical Nanostructured Materials. Acc. Chem. Res. 2014, 47, 3428–3438. [Google Scholar] [CrossRef]
- Ferreira, L.M.C.; Reis, I.F.; Martins, P.R.; Marcolino-Junior, L.H.; Bergamini, M.F.; Camargo, J.R.J.R.; Janegitz, B.C.; Vicentini, F.C. Using low-cost disposable immunosensor based on flexible PET screen-printed electrode modified with carbon black and gold nanoparticles for sensitive detection of SARS-CoV-2. Talanta Open 2023, 7, 100201. [Google Scholar] [CrossRef]
- Gosselin, B.; Retout, M.; Dutour, R.; Troian-Gautier, L.; Bevernaegie, R.; Herens, S.; Lefèvre, P.; Denis, O.; Bruylants, G.; Jabin, I. Ultrastable Silver Nanoparticles for Rapid Serology Detection of Anti-SARS-CoV-2 Immunoglobulins G. Anal. Chem. 2022, 94, 7383–7390. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chuang, Y.-C.; Lu, Y.-C.; Lin, H.-C.; Yang, Y.-L.; Lin, C.-S. A method of layer-by-layer gold nanoparticle hybridization in a quartz crystal microbalance DNA sensing system used to detect dengue virus. Nanotechnology 2009, 20, 215501. [Google Scholar] [CrossRef]
- Draz, M.S.; Shafiee, H. Applications of gold nanoparticles in virus detection. Theranostics 2018, 8, 1985–2017. [Google Scholar] [CrossRef]
- Lee, J.; Morita, M.; Takemura, K.; Park, E.Y. A multi-functional gold/iron-oxide nanoparticle-CNT hybrid nanomaterial as virus DNA sensing platform. Biosens. Bioelectron. 2018, 102, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Kim, J.; Suzuki, T.; Lee, J.; Park, E.Y. Enhanced catalytic activity of gold nanoparticle-carbon nanotube hybrids for influenza virus detection. Biosens. Bioelectron. 2016, 85, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; John, A.T.; Nagabooshanam, S.; Mathur, A.; Narang, J. Graphene quantum dot-gold hybrid nanoparticles integrated aptasensor for ultra-sensitive detection of vitamin D3 towards point-of-care application. Appl. Surf. Sci. 2020, 521, 146427. [Google Scholar] [CrossRef]
- Singh, K.R.; Rathee, S.; Nagpure, G.; Singh, J.; Singh, R.P. Smart and emerging nanomaterials-based biosensor for SARS-CoV-2 detection. Mater. Lett. 2022, 307, 131092. [Google Scholar] [CrossRef]
- Mendes, R.G.; Wróbel, P.S.; Bachmatiuk, A.; Sun, J.; Gemming, T.; Liu, Z.; Rümmeli, M.H. Carbon Nanostructures as a Multi-Functional Platform for Sensing Applications. Chemosensors 2018, 6, 60. [Google Scholar] [CrossRef]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Aplied Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- Kiremitler, N.B.; Kiremitler, N.B.; Kemerli, M.Z.; Kayaci, N.; Karagoz, S.; Sami, P.; Sarp, G.; Sanduvac, S.; Onses, M.S.; Yilmaz, E. Nanostructures for the Prevention, Diagnosis, and Treatment of SARS-CoV-2: A Review. ACS Appl. Nano Mater. 2022, 5, 6029–6054. [Google Scholar] [CrossRef]
- Mukherjee, S.; Manna, S.; Som, N.; Dhara, S. Organic–Inorganic Hybrid Nanocomposites for Nanotheranostics: Special Focus on Preventing Emerging Variants of SARS-CoV-2. Biomed. Mater. Devices 2023, 10, 633–647. [Google Scholar] [CrossRef]
- Ionescu, R.E. Updates on the Biofunctionalization of Gold Nanoparticles for the Rapid and Sensitive Multiplatform Diagnosis of SARS-CoV-2 Virus and Its Proteins: From Computational Models to Validation in Human Samples. Int. J. Mol. Sci. 2023, 24, 9249. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, J.; Petrochenko, P.; Zheng, J.; Hewlett, I. Sensitive detection of influenza viruses with Europium nanoparticles on an epoxy silica sol-gel functionalized polycarbonate-polydimethylsiloxane hybrid microchip. Biosens. Bioelectron. 2016, 86, 150–155. [Google Scholar] [CrossRef]
- Zhou, J.; Krishnan, N.; Jiang, Y.; Fang, R.H.; Zhang, L. Nanotechnology for virus treatment. Nano Today 2020, 36, 101031. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).