Abstract
NO2 is a toxic gas that can damage the lungs with prolonged exposure and contribute to health conditions, such as asthma in children. Detecting NO2 is therefore crucial for maintaining a healthy environment. Carbon nanotubes (CNTs) are promising materials for NO2 gas sensors due to their excellent electronic properties and high adsorption energy for NO2 molecules. However, conventional CNT-based sensors face challenges, including low responses at room temperature (RT) and slow recovery times. This study introduces a memristor-based NO2 gas sensor comprising CNT/ZnO/ITO decorated with an N-[3-(trimethoxysilyl)propyl] ethylene diamine (en-APTAS) membrane to enhance room-temperature-sensing performance. The amine groups in the en-APTAS membrane increase adsorption sites and boost charge transfer interactions between NO2 and the CNT surface. This modification improves the sensor’s response by 60% at 20 ppm compared to the undecorated counterpart. However, the high adsorption energy of NO2 slows the recovery process. To overcome this, a pulse-recovery method was implemented, applying a −2.5 V pulse with a 1 ms width, enabling the sensor to return to its baseline within 1 ms. These findings highlight the effectiveness of en-APTAS decoration and pulse-recovery techniques in improving the sensitivity, response, and recovery of CNT-based gas sensors.
1. Introduction
The ability to detect specific gas types is crucial across various sectors, including food safety, healthcare, pollution monitoring, and industrial process management. Thus, researchers are working on highly sensitive and selective gas sensors to detect these gases. NO2 is a toxic pollutant that is produced as a byproduct in industry and emitted from vehicle exhausts, causing serious health issues such as asthma and bronchitis [1]. Therefore, the detection of NO2 gas is required so that its concentration can be evaluated in an effort to maintain a healthy habitat. Recently, there have been various studies and efforts to fabricate a NO2 gas sensor based on metal oxides [2], conducting polymers [3], and carbon-based materials [4]. Among these carbon materials, carbon nanotubes (CNTs) have been extensively studied for their superlative properties such as high specific surface area, high carrier mobility, and high detection sensitivity to NO2 gas at room temperature (RT) [5]. Despite this, the CNTs-based gas sensor still faces issues related to stability due to surface poisoning or high gas diffusion of NO2 gas into CNTs. This high diffusion energy results in the irreversible change in the resistance state and a very slow recovery process [6]. To address these challenges, various methods and techniques have been employed, utilizing external energy sources to modify the operating conditions of CNT-based gas sensors. These techniques include the use of ultraviolet light [7], visible light [8], and external heaters [9]. However, the use of an external light source, such as UV light or a visible-light-emitting diodes (LEDs), requires integrating additional components into the sensor system. This integration increases the device’s overall energy consumption and adds complexity to its design and operation [10]. Furthermore, external heating systems are required to attain the high operational temperature for traditional gas sensors to attain maximum efficiency. Thus, the elevated operational temperature leads to high power consumption [11]. Therefore, it is important to further look for possible solutions to develop systems that can improve gas sensor technology.
Memristors offer a range of advantages regarding gas-sensing applications, combining sensitivity, efficiency, and versatility. Memristors retain their state without constant energy input, unlike conventional sensors, which often require a continuous supply of power in order to maintain their operation [12,13]. Memristor-based gas sensors exhibit exceptional sensitivity by detecting traces of gas concentrations with precision, and their nanoscale dimensions enable miniaturization and integration into advanced devices. Memristor-based gas sensors also provide rapid response and recovery, which ensure real-time monitoring in dynamic environments [13]. Their compatibility with diverse materials allows for the tailored sensing of specific gases, whereas their robustness ensures reliable performance under extreme conditions. Memristors also integrate sensing, data processing, and storage, which eliminate the need for external circuitry and support intelligent functionalities, such as pattern recognition [14]. Cost-effective fabrication further enhances their viability, making memristors a superior alternative to conventional gas sensors for modern applications.
On the other hand, the high sensitivity of gas sensors is crucial to the fulfillment of the requirements of various systems. Various approaches have been utilized to enhance the sensitivity of gas sensors, such as the development of heterostructure-based gas sensors [15], surface functionalization [16], composite material [17], and doping [18]. Therefore, the functionalization of the gas sensor surface with an en-APTAS membrane is reported to be an effective approach to improving sensitivity towards NOx gases. Chae et al. has reported the improved gas-sensing response of a CNTs gasistor towards NO gas by functionalizing the CNTs surface with en-APTAS [19]. Furthermore, Namsoo et al. reported an en-APTAS-decorated CNT gas sensor with improved gas-sensing response characteristics towards NO gas. However, the study did not cover the selectivity properties of the proposed gas sensor [20].
We propose an en-APTAS-decorated CNTs/ZnO/ITO-based memristor gas sensor in this study for the detection of NO₂ gas, as shown in Figure 1a. Here, en-APTAS is used for the functionalization of the CNTs surface in order to obtain enhanced sensitivity of a CNTs/ZnO/ITO memristor-based gas sensor. It is consequently observed that the en-APTAS-decorated CNTs/ZnO/ITO gas sensor showed an enhanced gas-sensing response to NO2 gas at 20–0.5 ppm compared to a bare CNTs/ZnO/ITO gas sensor. The sensing voltage (Vsensing) is set to 0.5 V in order to observe the gas-sensing characteristics at RT, which was optimized in our previous study [21]. Furthermore, ZnO is introduced as a conduction filament heater (CFH), providing thermal energy to activate the CNTs-based gas sensor. The CFH acts as a nano-heater, which works in the high-resistance state (HRS). The HRS of the CFH is obtained by applying the reset operation, which is driven by the Joule heating effect. The CFH works as a nano-heater due to power dissipation across the gap of the ruptured CF when a Vsensing of 0.5 V is applied to the proposed gas sensor, as shown in Figure 1b. Moreover, a pulse-recovery process is employed using the CFH, wherein a high-voltage pulse of −2.5 V with a pulse width of 1 ms is applied to the proposed gas sensor. The high voltage pulse generated a sufficient energy to enable the rapid desorption of NO₂ gas from the CNTs’ surface, and the proposed gas sensor attains the initial current level, as displayed in Figure 1c.
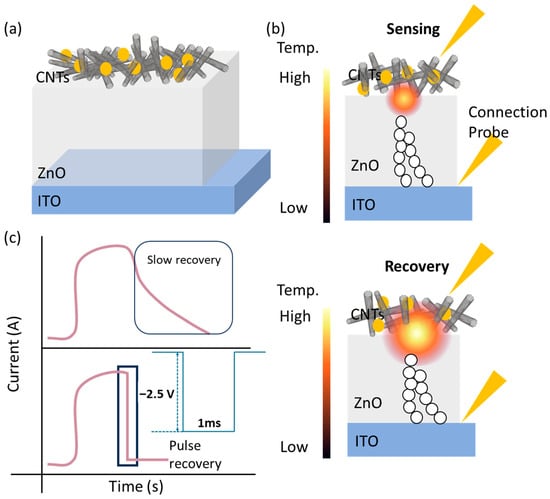
Figure 1.
Schematic illustration of (a) en-APTAS-decorated CNTs-CFH gas sensor; (b) sensing mode in HRS; (c) pulse-recovery process. The pink line shows the schematics of transient response curve; the blue line shows a pulse with an amplitude of −2.5 V with a pulse width of 1 ms and recovery mode in HRS.
2. Materials and Methods
2.1. Dispersion of CNTs
CNTs are used as sensing material and deposited on the top of CFH. Prior to the deposition of CNTs, a dispersion of CNTs was prepared by mixing 0.2 mg of CNTs into 10 mL of benzene (SAMCHUM 99.0%, SAMCHUN Pure Chemical Co., Ltd., Seoul, Republic of Korea). The dispersion was then treated in an ultrasonic bath for 5 h, maintaining a sonication power of 70%, to achieve a uniform dispersion.
2.2. Fabrication of CNTs-CFH Gas Sensor
Prior to the fabrication process, ITO-coated glass substrates were cleaned using the standard cleaning process. Then, 100 nm of ZnO layer was deposited using radio frequency (RF) sputtering (KVS-2000L, vacuum system, and components were obtained from KOREA VACUUM TECH., Paju, Republic of Korea). Before the deposition process, the initial pressure of 1 uTorr was maintained. During the deposition process, 20 sccm Ar gas was utilized as a carrier gas and a working pressure was maintained at 5 mTorr. The photolithography process was performed using a chrome mask with a diameter of 200 µm to obtain the patterning for the top electrode (TE). CNTs were deposited as the TE onto the ZnO layer using the optimal dipping process previously reported in the literature [22]. Lift-off was performed using acetone to remove the photoresist and obtain the patterned CNTs. The deposited CNTs appeared as accumulated black dots on the top of the ZnO layer.
2.3. Fabrication of en-APTAS-Decorated CNTs-CFH Gas Sensor
Prior to the decoration process, an en-APTAS membrane (Sigma Aldrich 97%, Dow Coming Corp., Washington, DC, USA) solution was prepared in ethanol (SAMCHUM 99.5%, SAMCHUN Pure Chemical Co., Ltd., Republic of Korea) by adding 1 wt% en-APTAS into 99 wt% ethanol followed by a 15 min ultrasonication process to obtain a homogeneous solution. To perform the decoration process, the CNTs-coated CFH gas sensor was immersed in the solution for 10 min. Afterwards, the en-APTAS-decorated CNTs-CFH gas sensor was dried with N2 gas to remove any remaining traces of ethanol.
2.4. Measurements of CNTs-CFH Gas Sensor
Firstly, electrical measurements of CNTs-CFH were performed to obtain the RS characteristics using a Keithley 2400 SCS (Tektronix, I.V Solutions, Seoul, Republic of Korea. Gas sensing measurements were performed to obtain the response characteristics towards NO2 gas. All the measurements were performed at room temperature and a relative humidity of 30%. The gas sensing measurements were performed by injecting a constant flow of 500 sccm gas into the testing chamber. The gas flow was maintained using a microflow meter (MFC: DFPC1000). Dry air was used as a mixture of gas to obtain a constant flow of gas during the gas sensing measurements.
The proposed gas sensors were exposed to base gas for 50 s to maintain the baseline current. The target gas was injected for 150 s to measure the gas sensing characteristics. To calculate the response percentage of the CNTs-CFH gas sensor, the following equation was used [23].
Here, Ig is the current in the target gas environment and Ia is the current in the base gas environment.
2.5. COMSOL Multiphysics Simulation
To gain a deeper insight into the gas sensing performance of the CFH-based CNT gas sensor, the heat distribution within the proposed CFH-based CNT gas sensor was analyzed using COMSOL Multiphysics 6.2. A Joule heating module was employed in the thermal field simulation to model both the thermal and electrical behaviors of the device. In the simulation, a bias voltage of 0.5 V was applied to the top electrode (TE), while the bottom electrode was grounded to calculate the thermal distribution. For accurate simulation results, the following continuity equations were applied:
Here, J represents the current density, Qj, v denotes the heat source, V is the applied voltage, E refers to the electric field, and σ signifies the electrical conductivity. The material parameters utilized for these simulations are provided in Table 1. This analysis highlights the thermal dynamics essential for understanding and optimizing the device’s performance.

Table 1.
Material parameters used in the model [24,25].
3. Results and Discussions
3.1. CFH-CNT Gas Sensor Simulations via COMSOL Multiphysics
To evaluate the heating characteristics of the proposed CFH, heating profile was initially examined by using COMSOL Multiphysics 6.2 simulations. Heat generation in the CFH occurs in the HRS, where the maximum power dissipation occurs across the gap of ruptured CF due to the applied voltage. It is important to note that the CF is formed within the CFH through DC voltage sweeping in the positive bias region. Conversely, during the reverse operation, the CF ruptures due to Joule heating, particularly at the edge of the CF. This ruptured CF was utilized as the heat source in the COMSOL Multiphysics 6.2 simulations involving the CFH. The majority of the electric field is concentrated within the thin ZnO gap between the ruptured CF and the top electrode (TE), illustrating the electric field distribution when a Vsensing of 0.5 V is applied. This is one of the key outcomes of the simulation. Figure 2a,b present the simulated temperature distribution for the HRS of the CNTs-CFH gas sensor and the en-APTAS-coated CNTs-CFH gas sensor, respectively. In both cases, heat generated has the same value of 579 K. The reason to obtain the same temperature is due to the constant Vsensing of 0.5 V. Hence, the enhanced gas sensing response observed is mainly due to decoration of en-APTAS membrane on the surface of CNTs. In conclusion, the heating temperature generated in the CFH acts as a nano-heater for CNT gas sensors.
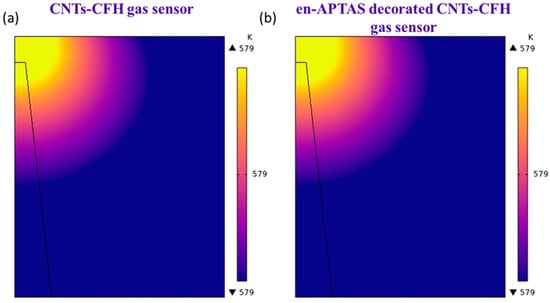
Figure 2.
(a) COMSOL simulation of CNTs-CFH gas sensor; (b) en-APTAS-decorated CNTs-CFH gas sensor.
3.2. Material Characteristics
Material characterizations are performed to evaluate the structure and morphological properties of CNTs and ZnO. Field-enhanced scanning electron microscopy (FESEM) was performed to observe the stack morphology of the sensors. Figure 3a shows a cross-sectional FESEM image of the CNTs-CFH gas sensor. CNTs appear to be well deposited on the top of the 50 nm thick ZnO layer. X-ray photoelectron spectroscopy (XPS) analysis was performed to study the elemental composition of ZnO and CNTs. The chemical states and compositions were analyzed by using XPS in which C1s (284.8 eV) was utilized to calibrate the binding energies of ZnO and CNTs. The high-resolution spectrum of XPS for Zn 2p, as shown in Figure 3b, indicates that the two prominent peaks at 1021.53 eV and 1044.63 eV correspond to Zn 2p3/2 and Zn 2p1/2, respectively. The difference in binding energies between these two peaks is calculated to be ~23 eV, and this corresponds to the Zn2+ valance state [26,27]. Moreover, Figure 3c shows the high-resolution O1s spectra, which are deconvoluted into three distinct peaks. The peak that fitted at 494.45 eV refers to the lattice oxygen present in the form of O2− in Zn-O bonding. The peak fitted at 531.13 eV refers to the oxygen vacancies, whereas the peaks located at high binding energy of 532.06 eV correspond to the chemisorbed oxygen due to hydroxyl groups. These results are consistent with the previously reported results [28]. The N1s peak of the CNTs and en-APTAS-decorated samples showed significant changes in peak intensity, as shown in Figure 3d. The XPS spectra of N1s peak of en-APTAS-decorated CNTs appeared to be intense as compared to those for bare CNTs. These results demonstrate the successful decoration of the nitrogen-based space group on the surface of the CNTs [20]. Figure 3e,f show the high-resolution XPS spectra of C1s of the CNTs and en-APTAS-decorated CNTs. In the case of bare CNTs, the C1s peaks are deconvoluted into two distinct peaks, corresponding to sp2 C-C graphitic carbon at 287.75 eV and the hydroxyl group at 285.83 eV. Similarly, in the case of en-APTAS-decorated CNTs, the dominant peak of graphitic carbon appeared at 287.72 and the hydroxyl group appeared at 285.67. Thus, C1s peaks of the bare CNTs and en-APTAS-decorated CNTs do not show any distinct differences in the binding energy. However, the peak intensity of C1s of en-APTAS-decorated CNTs-CFH gas sensor is observed to be decreased as compared to that of the CNTs-CFH gas sensor, which is consistent with the previously reported results [29]. To further confirm the successful coating of en-APTAS onto the surface of CNTs, the atomic percentages of C1s, O1s, and N1s were analyzed for both bare CNTs and en-APTAS-decorated CNTs. For the bare CNTs, the atomic percentages of C1s and O1s were determined to be 96.55% and 3.45%, respectively, with no detectable N1s peak. In contrast, for en-APTAS-decorated CNTs, the atomic percentages of C1s, O1s, and N1s were measured at 90.56%, 3.40%, and 6.04%, respectively, indicating successful functionalization and showing consistency with previously reported results [20].
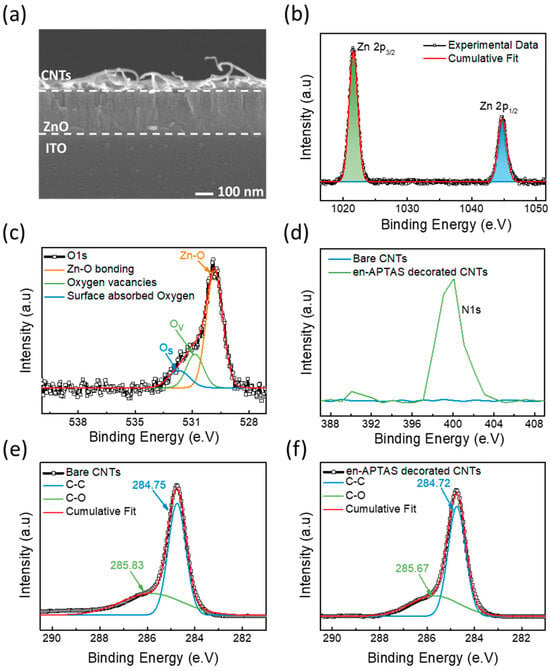
Figure 3.
(a) Cross-sectional FE-SEM image of device. High-resolution XPS spectra of (b) Zn 2P and (c) O 1s from ZnO film. (d) XPS spectra of N1s peak with bare CNTs and en-APTAS-decorated CNTs; (e) XPS spectra of C 1s peaks of bare CNTs sample and (f) en-APTAS-decorated CNTs.
3.3. Electrical Measurements
Prior to performing the gas sensing measurements, electrical measurements were performed to determine the formation and rupture of the CF and evaluate its properties as a heat source. Figure 4a,b show the typical RS characteristics of the proposed CNTs-CFH and en-APTAS-decorated CNTs-CFH gas sensors. First, a high voltage was applied to observe the initial forming process in which migration of oxygen vacancies formed the CF under the influence of the applied voltage. The forming process was observed at 11.5 and 10.2 V for CNTs-CFH and en-APTAS-decorated CNTs-CFH, respectively. The formation of CF changed the resistance state of the device from a high-resistance state (HRS) to a low-resistance state (LRS). After the initial forming process, a negative voltage was applied to rupture the CF to obtain the HRS at reset voltages of −1.51 V and −1.53 V for CNTs-CFH and en-APTAS-decorated CNTs-CFH gas sensors, respectively. To again observe the LRS, positive voltages of 2.99 V and 2.40 V were applied to CNTs-CFH and en-APTAS-decorated CNTs-CFH gas sensors, respectively. The RS characteristics of the proposed gas sensors provide a novel approach to addressing the issue of long recovery times. Figure 4c presents a comparison between real-time recovery and pulse recovery. When CNTs interact with NO₂ gas, the current level increases due to gas reaction. Once the gas is turned off, NO₂ begins to desorb, and the current level decreases as a result of this desorption. However, this recovery process is slow, taking more than 200 s to completely recover. To achieve faster recovery, a negative pulse of −2.5 V was applied to the CFH, which accelerated the desorption of the gas and allowed the sensor to rapidly return to its initial current level. The sensor stability of both of the gas sensors was measured at Vsensing of 0.5 V in the ambient environment, as shown in Figure 4d. Both gas sensors showed a stable current, which remained level till 4000 s, confirming the stable initial state of the gas sensors. To ensure the reliability of the en-APTAS-decorated CNTs-CFH gas sensor, an endurance test comprising 200 DC cycles was performed at a Vread of 0.5 V, as shown in Figure 4e. The resistance state stability of the en-APTAS-decorated CFH-CNT gas sensor over a longer period of time was evaluated by performing the retention measurement for 10,000 s under both HRS and LRS, as shown in Figure 4f, indicating the superior stability of the sensors.

Figure 4.
I-V curve of the gasistor with (a) CNTs-CFH gas sensor and (b) en-APTAS-decorated CNTs-CFH gas sensor. (c) Schematics of pulse recovery; (d) sensor stabilities of CNTs-CFH gas sensor and en-APTAS-decorated CNTs-CFH-based gas sensor in ambient environment under constant Vsensing; (e) endurance of the en-APTAS-decorated CNTs-CFH gas sensor; (f) retention of 104 s of the en-APTAS-decorated CNTs-CFH gas sensor.
3.4. Gas Sensing Measurements
Measuring the gas sensing of the proposed gas sensors was performed by exposing the devices to the target gas while monitoring the change in the current value at a Vsensing of 0.5 V. The gas sensing measurements were performed in the HRS of both gas sensors. The HRS of the gas sensor corresponds to the high resistance state, which results in a high temperature inside the CFH. This high temperature will be used as a nano-heater to observe the enhanced gas sensor characteristics at room temperature. The gas sensing measurements were performed to evaluate the sensitivity of the proposed gas sensors towards different concentrations (20–0.5 ppm) of the NO2 gas. Figure 5a,b show the transient curve of the CNTs-CFH and en-APTAS-decorated CNTs-CFH gas sensors. The gas sensing results show the instant change in the current level as the target gas is injected into the chamber. The change in the current level resulted from the reaction between the surface of the CNTs and the NO2 gas. The change in the current levels of both the gas sensors showed a direct relation with the concentration of NO2 gas. Equation (1) is used to calculate the response percentage of the gas sensor. The calculated response percentage of the CNTs-CFH gas sensor and the en-APTAS-decorated gas sensor is shown in Figure 5c. The response of the en-APTAS-decorated CNTs-CFH gas sensor is observed to be higher than that of the CNTs-CFH gas sensor at all concentrations of NO2 gas. Figure 5d shows the response time plot of CNTs-CFH and en-APTAS-decorated CNTs-CFH gas sensors for all the concentrations of NO2 gas. The response time increases with the decrease in the concentration of NO2 gas, which is due to the low diffusion rate and slow reaction at low concentrations of NO2 gas with the CNTs. The response time of the en-APTAS-decorated CNTs-CFH gas sensor is observed to be faster than that of the pure CNTs-CFH gas sensor, which again confirms that the modified CNTs surface offers more reaction sites and high reactivity towards NO2 gas.
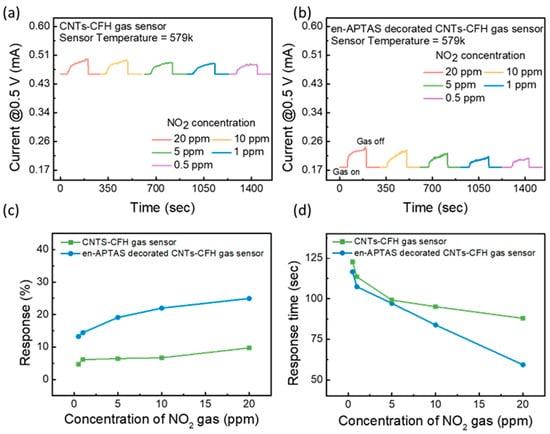
Figure 5.
Gas sensing characteristics of (a) CNTs-CFH gas sensor and (b) en-APTAS-decorated CNTs-CFH gas sensor. (c) Evaluated gas sensing response % of CNTs-CFH and en-APTAS-decorated CNTs-CFH gas sensors. (d) Evaluated gas sensing response time of CNTs-CFH and en-APTAS-decorated CNTs-CFH gas sensors.
To further check the superiority of the proposed en-APTAS-decorated CNTs-CFH gas sensor, selectivity measurements were performed towards NO and C2H6 gases, as shown in Figure 6a,b. In addition to NO, C2H6 gas was chosen for the selectivity measurements because it is a representative gas of the hydrocarbon group. C2H6 is extensively used in industries for the development of plastic materials [30] and it is also present in exhaled human breath [31]. Thus, detection of C2H6 gas extends the detection capabilities of the proposed en-APTAS-decorated CNTs-CFH gas sensor. Selectivity measurements were performed with 20–0.5 ppm of the target gases. Furthermore, a comparison of response percentages for NO2, NO, and C2H6 is shown in Figure 6c. The en-APTAS-decorated CNTs-CFH gas sensor showed the lowest response towards C2H6 gas due to its low adsorption energy. CNTs-based gas sensors have frequently been utilized for the detection of NO gas. However, the en-APTAS-decorated CNTs-CFH gas sensor showed a significantly high response towards NO2 compared to NO gas. The increase in the response towards NO2 gas is related to the larger kinetic diameter of NO2 gas, which is reported to be 3.83 Å [32,33]. Due to the larger kinetic diameter of NO₂ gas, it tends to have stronger interactions with both CNTs and en-APTAS. These results show the superiority of the selectivity measurements of the sensor towards NO2 gas. The effect of relative humidity (RH) was evaluated by increasing the RH level. Figure 6d shows the transient gas sensing curves of the en-APTAS-decorated CNTs-CFH gas sensor at 20 ppm of NO2 gas by maintaining an RH range of 50–90%. The response percentages at different RH levels are plotted in the inset of Figure 6d, indicating a 39% degradation in the response percentage at 90% RH as compared to the response percentage at 30% RH. The en-APTAS-decorated CNTs-CFH gas sensor showed fairly stable response characteristics due to the heating provided by the CFH at a Vsensing of 0.5 V at an elevated RH level. These results suggest that heating the CNTs using CFH provided resistance in the adsorption of water molecules and validated the stability of the en-APTAS-decorated gas sensor towards a high RH level.
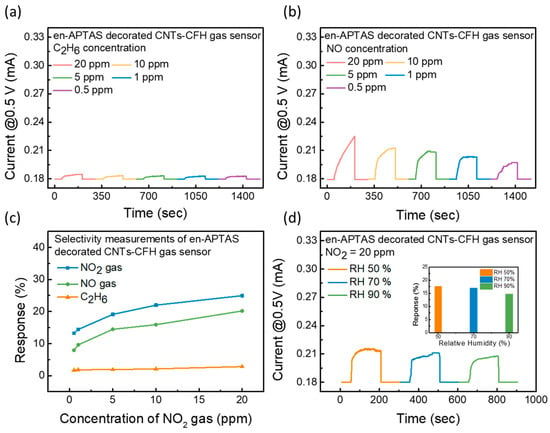
Figure 6.
(a) Transient response of gas sensing measurements of C2H6 gas and (b) NO gas to evaluate the selectivity of the gas sensors; (c) evaluated response percentages comparison with response percentage of NO2 gas; (d) transient response curves of en-APTAS-decorated CNTs-CFH gas sensor at relative humidity levels of 50%, 70%, and 90%. Inset shows the evaluation of response degradation due to relative humidity effect.
Table 2 presents a comparative analysis of this study’s findings with previous research on gas sensors utilizing CNTs materials. Despite these advantages, ongoing efforts are directed toward improving their ability to detect a wider range of gases, often through the integration of supplementary devices like heaters. In this study, a CNT gas sensor was combined with a CFH. Unlike conventional heaters, CFH enhance the sensing capabilities of the proposed CNT gas sensors. Consequently, the proposed gas sensor demonstrates a competitive performance compared to those presented in prior studies, achieving a response of 24.94 at 20 ppm for the en-APTAS-decorated CNTs-CFH gas sensor and 9.72 for the bare CNTs-CFH gas sensor. Additionally, the incorporation of a CFH significantly improved the recovery time, reducing it to just 1 millisecond. This addresses the challenge of slow recovery and enables CNTs to effectively return to their initial state.

Table 2.
Comparison of CNT-based gas sensors for NO2.
3.5. Gas Sensing Mechanism
The gas sensing mechanism of the CNTs-CFH gas sensor operates through charge transfer when NO₂ molecules are adsorbed and desorbed onto the sensor’s surface. Prior to performing the gas sensing measurements, the HRS of the CFH was achieved by rupturing the CF. When a negative voltage is applied, the CF ruptures due to the Joule heating effect. At the HRS, two types of resistances appear inside the CFH, i.e., (i) resistance of the ruptured CF (RCF) and (ii) resistance of the bulk (RB), which appeared at the gap of the ruptured CF. On the application of a sensing voltage of 0.5 V, maximum power dissipation occurs at the bulk resistance (RB), resulting in an increase in the heat. This generation of heat is used as a nano-heater to observe the gas sensing characteristics at RT. Figure 7a,b show the interaction of the NO2 gas with the CNTs and the en-APTAS-decorated CNTs. Due to their natural abundance of holes, CNTs function as p-type semiconductors, while NO2, being an excellent oxidizing agent, tends to capture electrons [39]. In ambient conditions, oxygen interacts with the CNT surface, capturing an electron and creating an depletion layer on the CNT surface. The removal of electrons from the CNT surface increases the number of holes, which facilitates electric current conduction. When the CNTs are exposed to NO₂ gas, the chemisorbed ions react with NO₂ gas molecules, leading to the formation of numerous species. These reactions can typically be described as follows [40]:
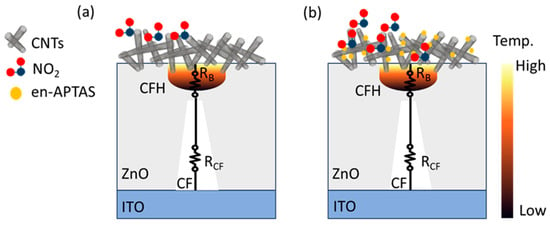
Figure 7.
Schematics of interaction of NO2 gas with (a) CNTs and (b) en-APTAS-decorated CNTs.
The increase in the gas sensing response of the en-APTAS-decorated CNTs-CFH gas sensor is due to increased adsorption energy of the functionalized surface of CNTs. Furthermore, the amine group attached to the surface of CNTs results in the occurrence of electron-abundant adsorption sides, which resulted in the enhanced gas sensing response. Thus, in conclusion, these findings suggest that functionalization of the CNTs surface is an adoptable method to improve the gas sensing response towards NO2 gas.
4. Conclusions
CNT gas sensors are considered to be potential candidates for the detection of NO2 gas. However, CNTs still face various issues related to sensitivity and slow recovery. The proposed gas sensor is decorated with an en-APTAS membrane and embedded with a CFH as a heater to resolve these issues. An enhanced gas sensing response of 24.9% was achieved for the en-APTAS-decorated CNTs-CFH gas sensor, which is 60% higher than the undecorated CNTs-CFH gas sensor. Furthermore, fast recovery is achieved by applying a pulse of −2.5 V, with a pulse width of 1 ms, to the CFH. The application of high voltage resulted in the quick desorption of NO2 gas due to the occurrence of an elevated temperature inside the CFH. Moreover, CFH also improved the effect of RH levels of the proposed gas sensor, as the en-APTAS-decorated CNTs-CFH gas sensor showed a degradation of 39% in the response value at the 90% RH level. Thus, the proposed methods offer a possible solution to resolve the drawbacks of CNT gas sensors.
Author Contributions
Conceptualization, I.A., M.A. and H.-D.K.; methodology, I.A., M.A. and H.-D.K.; validation, I.A. and M.A.; formal analysis, I.A.; investigation, I.A. and M.A.; resources, H.-D.K.; data curation, I.A. and M.A.; writing—original draft preparation, I.A. and M.A.; writing—review and editing, I.A. and H.-D.K.; visualization, I.A. and H.-D.K.; supervision, H.-D.K.; project administration, H.-D.K.; funding acquisition, H.-D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2024-00419201).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Ahmad, I.; Lee, D.; Mehmood, S.; Chae, M.; Ali, A.; Aftab, J.; Bhatti, A.S.; Karamat, S.; Kim, H.-D. Comprehensive analysis of reaction mechanisms in reduced graphene oxide and hematite heterostructure gas sensors. J. Alloys Compd. 2023, 967, 171698. [Google Scholar] [CrossRef]
- Kumar, R.; Mamta; Kumari, R.; Singh, V.N. SnO2-based NO2 gas sensor with outstanding sensing performance at room temperature. Micromachines 2023, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Navale, S.; Mane, A.; Chougule, M.; Sakhare, R.; Nalage, S.; Patil, V. Highly selective and sensitive room temperature NO2 gas sensor based on polypyrrole thin films. Synth. Met. 2014, 189, 94–99. [Google Scholar] [CrossRef]
- Tian, T.; Yin, H.; Zhang, L.; Zhu, M.; Ma, D.; Shao, F.; Hu, N.; Yang, Z.; Zhang, Y.; Su, Y. Gas sensing performance and charge-transfer mechanism of semiconducting single-walled carbon nanotubes. Appl. Surf. Sci. 2023, 609, 155357. [Google Scholar] [CrossRef]
- Wang, Y.; Yeow, J.T. A review of carbon nanotubes-based gas sensors. J. Sens. 2009, 1, 493904. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Zhang, W.-H. Carbon nanotubes as active components for gas sensors. J. Sens. 2009, 1, 160698. [Google Scholar] [CrossRef]
- Chen, G.; Paronyan, T.M.; Pigos, E.M.; Harutyunyan, A.R. Enhanced gas sensing in pristine carbon nanotubes under continuous ultraviolet light illumination. Sci. Rep. 2012, 2, 343. [Google Scholar] [CrossRef] [PubMed]
- Elakia, M.; Gobinath, M.; Sivalingam, Y.; Palani, E.; Ghosh, S.; Nutalapati, V.; Surya, V.J. Investigation on visible light assisted gas sensing ability of multi-walled carbon nanotubes coated with pyrene based organic molecules. Phys. E Low-Dimens. Syst. Nanostructures 2020, 124, 114232. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Lee, E.-B.; Kim, S.-J.; Choi, J.-K.; Cha, J.-H.; Lee, H.-J.; Ju, B.-K.; Lee, J.-H. Gas sensing properties of SnO2 nanowires on micro-heater. Sens. Actuators B Chem. 2011, 154, 295–300. [Google Scholar] [CrossRef]
- Simon, I.; Bârsan, N.; Bauer, M.; Weimar, U. Micromachined metal oxide gas sensors: Opportunities to improve sensor performance. Sens. Actuators B Chem. 2001, 73, 1–26. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Nam, M.-S.; Kim, H.W.; Kim, S.S. Room-temperature detection of acetone gas by PANI/NiO-loaded TiO2 nanoparticles under UV irradiation. Sens. Actuators B Chem. 2023, 374, 132850. [Google Scholar] [CrossRef]
- Ali, M.; Lee, D.; Chae, M.; Ahmad, I.; Kim, H.-D. Advances in MXene-based Synaptic Devices and Sensors. Mater. Today Phys. 2024, 45, 101456. [Google Scholar] [CrossRef]
- Chae, M.; Lee, D.; Kim, H.D. Dynamic Response and Swift Recovery of Filament Heater-Integrated Low-Power Transparent CNT Gas Sensor. Adv. Funct. Mater. 2024, 34, 2405260. [Google Scholar] [CrossRef]
- Sangwan, V.K.; Hersam, M.C. Neuromorphic nanoelectronic materials. Nat. Nanotechnol. 2020, 15, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, N.; Xu, J.; Jin, Q.; San, X.; Wang, X. Co3O4/In2O3 pn heterostructures based gas sensor for efficient structure-driven trimethylamine detection. Ceram. Int. 2023, 49, 17354–17362. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, M.Y.; Mirzaei, A.; Kim, H.-S.; Kim, S.-i.; Baek, S.-H.; Chun, D.W.; Jin, C.; Lee, K.H. Selective, sensitive, and stable NO2 gas sensor based on porous ZnO nanosheets. Appl. Surf. Sci. 2021, 568, 150910. [Google Scholar] [CrossRef]
- Ying, Z.; Zhang, T.; Feng, C.; Wen, F.; Li, L.; Zheng, X.; Zheng, P.; Wang, G. UV-enhanced NO2 gas sensors based on In2O3/ZnO composite material modified by polypeptides. Nanotechnology 2022, 33, 155501. [Google Scholar] [CrossRef]
- Dong, Z.; Hu, Q.; Liu, H.; Wu, Y.; Ma, Z.; Fan, Y.; Li, R.; Xu, J.; Wang, X. 3D flower-like Ni doped CeO2 based gas sensor for H2S detection and its sensitive mechanism. Sens. Actuators B Chem. 2022, 357, 131227. [Google Scholar] [CrossRef]
- Chae, M.; Lee, D.; Kim, H.-D. Influence of en-APTAS membrane on NO gas selectivity of HfO2-based memristor gas sensors. Jpn. J. Appl. Phys. 2024, 63, 03SP07. [Google Scholar] [CrossRef]
- Lim, N.; Kim, K.H.; Byun, Y.T. Preparation of defected SWCNTs decorated with en-APTAS for application in high-performance nitric oxide gas detection. Nanoscale 2021, 13, 6538–6544. [Google Scholar] [CrossRef]
- Ahmad, I.; Lee, D.; Chae, M.; Kim, H.-D. Advanced recovery and enhanced humidity tolerance of CNTs gas sensor using a filament heater. Chem. Eng. J. 2024, 496, 154014. [Google Scholar] [CrossRef]
- Chae, M.; Lee, D.; Jung, J.; Kim, H.-D. Enhanced memristor-based gas sensor for fast detection using a porous carbon nanotube top electrode with membrane. Cell Rep. Phys. Sci. 2023, 4, 11. [Google Scholar] [CrossRef]
- Kim, T.; Lee, D.; Chae, M.; Kim, K.-H.; Kim, H.-D. Enhancing the Resistive Switching Properties of Transparent HfO2-Based Memristor Devices for Reliable Gasistor Applications. Sensors 2024, 24, 6382. [Google Scholar] [CrossRef] [PubMed]
- Bature, U.I.; Nawi, I.M.; Khir, M.H.M.; Zahoor, F.; Algamili, A.S.; Hashwan, S.S.B.; Zakariya, M.A. Statistical simulation of the switching mechanism in ZnO-based RRAM devices. Materials 2022, 15, 1205. [Google Scholar] [CrossRef] [PubMed]
- Bature, U.I.; Nawi, I.M.; Khir, M.H.M.; Zahoor, F.; Hashwan, S.S.B.; Algamili, A.S.; Abbas, H. Analysis of thermodynamic resistive switching in ZnO-based RRAM device. Phys. Scr. 2023, 98, 035020. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Chang, Q.-Q.; Cui, Y.-W.; Zhang, H.-H.; Chang, F.; Zhu, B.-H.; Yu, S.-Y. C-doped ZnO decorated with Au nanoparticles constructed from the metal–organic framework ZIF-8 for photodegradation of organic dyes. RSC Adv. 2019, 9, 12689–12695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, R.; Huo, Y.; Li, H.; Wang, L. Formation, detection, and function of oxygen vacancy in metal oxides for solar energy conversion. Adv. Funct. Mater. 2022, 32, 2109503. [Google Scholar] [CrossRef]
- Bourlier, Y.; Bouttemy, M.; Patard, O.; Gamarra, P.; Piotrowicz, S.; Vigneron, J.; Aubry, R.; Delage, S.; Etcheberry, A. Investigation of InAlN layers surface reactivity after thermal annealings: A complete XPS study for HEMT. ECS J. Solid State Sci. Technol. 2018, 7, P329. [Google Scholar] [CrossRef]
- Sun, J.; Chang, J.; Zhang, Y.; Wei, Y.; Zhang, Q.; Wang, F.; Lin, S.; Wang, Z.; Mao, M. CH4/C2H6 dual gas sensing system using a single mid-infrared laser. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 291, 122368. [Google Scholar] [CrossRef] [PubMed]
- Winkowski, M.; Stacewicz, T. Detection of ethane, methane, formaldehyde and water vapor in the 3.33 μm range. Metrol. Meas. Syst. 2022, 29, 271–282. [Google Scholar] [CrossRef]
- Chen, B.; Xu, R.; Zhang, R.; Liu, N. Economical way to synthesize SSZ-13 with abundant ion-exchanged Cu+ for an extraordinary performance in selective catalytic reduction (SCR) of NOX by ammonia. Environ. Sci. Technol. 2014, 48, 13909–13916. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H. Structure–activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Leghrib, R.; Felten, A.; Pireaux, J.; Llobet, E. Gas sensors based on doped-CNT/SnO2 composites for NO2 detection at room temperature. Thin Solid Film. 2011, 520, 966–970. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhang, Y.; Zhang, C.; Zhang, T. High performance room temperature NO2 sensors based on reduced graphene oxide-multiwalled carbon nanotubes-tin oxide nanoparticles hybrids. Sens. Actuators B Chem. 2015, 211, 318–324. [Google Scholar] [CrossRef]
- Cho, W.-S.; Moon, S.-I.; Paek, K.-K.; Lee, Y.-H.; Park, J.-H.; Ju, B.-K. Patterned multiwall carbon nanotube films as materials of NO2 gas sensors. Sens. Actuators B Chem. 2006, 119, 180–185. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Yuan, Z.; Jiang, Y.; Su, Y.; Ma, J.; Tai, H. A flexible NO2 gas sensor based on polypyrrole/nitrogen-doped multiwall carbon nanotube operating at room temperature. Sens. Actuators B Chem. 2019, 295, 86–92. [Google Scholar] [CrossRef]
- Wei, B.-Y.; Hsu, M.-C.; Su, P.-G.; Lin, H.-M.; Wu, R.-J.; Lai, H.-J. A novel SnO2 gas sensor doped with carbon nanotubes operating at room temperature. Sens. Actuators B Chem. 2004, 101, 81–89. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, W.; Hong, Y.; Lee, G.; Yoon, D.S. Recent advances in carbon material-based NO2 gas sensors. Sens. Actuators B Chem. 2018, 255, 1788–1804. [Google Scholar] [CrossRef]
- Ma, D.; Su, Y.; Tian, T.; Yin, H.; Huo, T.; Shao, F.; Yang, Z.; Hu, N.; Zhang, Y. Highly sensitive room-temperature NO2 gas sensors based on three-dimensional multiwalled carbon nanotube networks on SiO2 nanospheres. ACS Sustain. Chem. Eng. 2020, 8, 13915–13923. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).