Assessment of Microvascular Function Based on Flowmotion Monitored by the Flow-Mediated Skin Fluorescence Technique
Abstract
1. Introduction
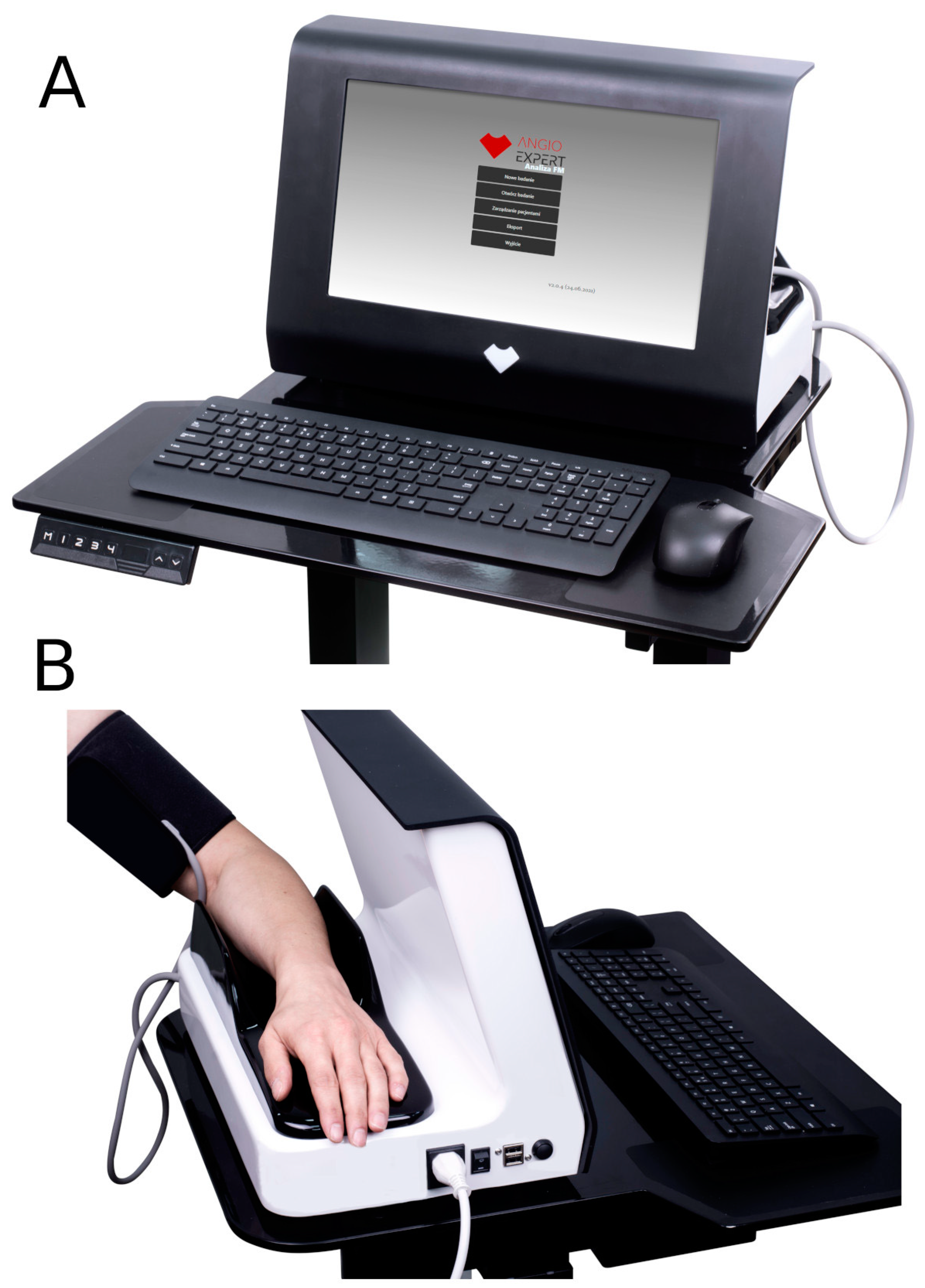
2. Precise Monitoring of Microcirculatory Oscillations Using the FMSF Technique
3. Selected Examples of Impaired Microcirculatory Oscillations at Normoxia
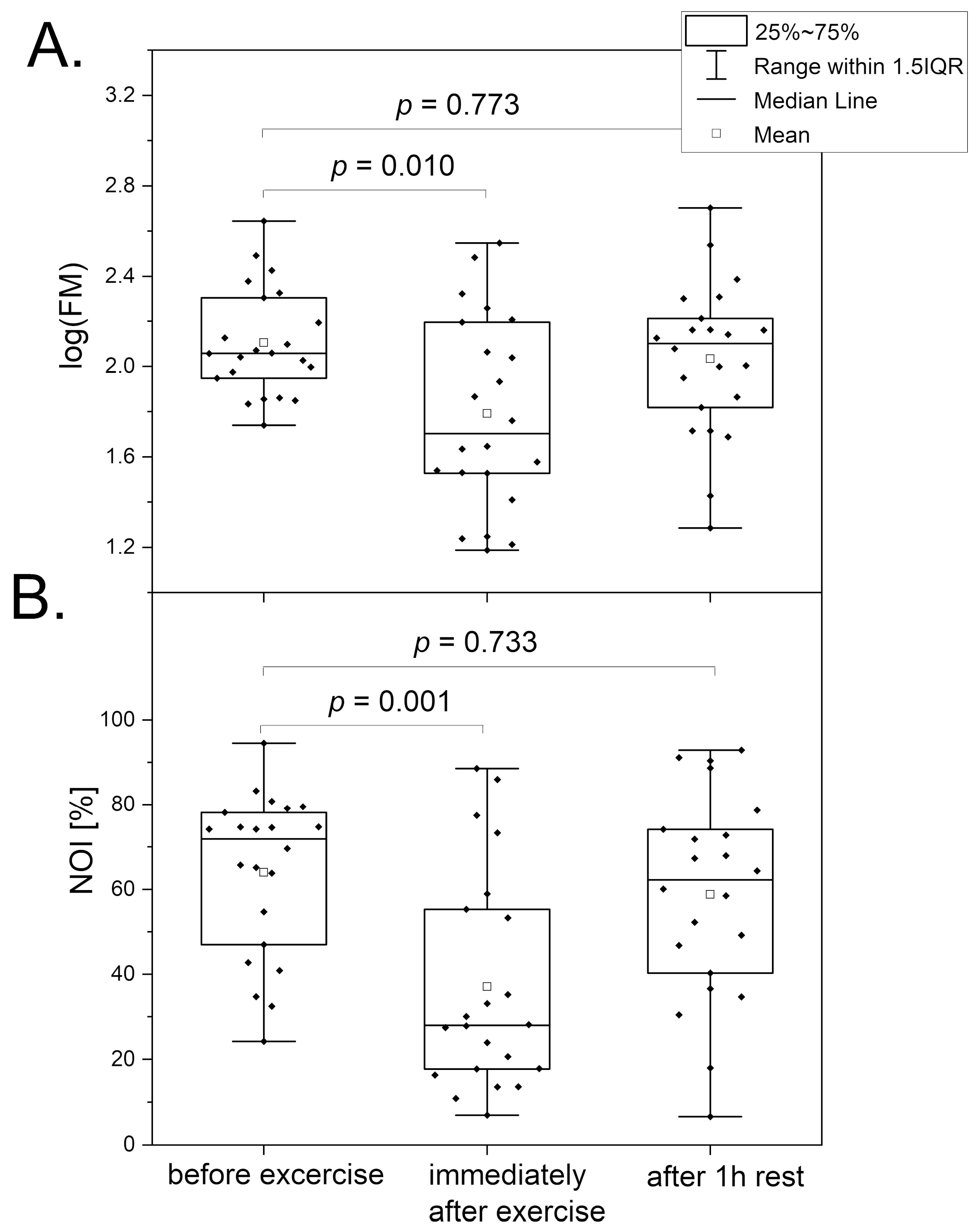
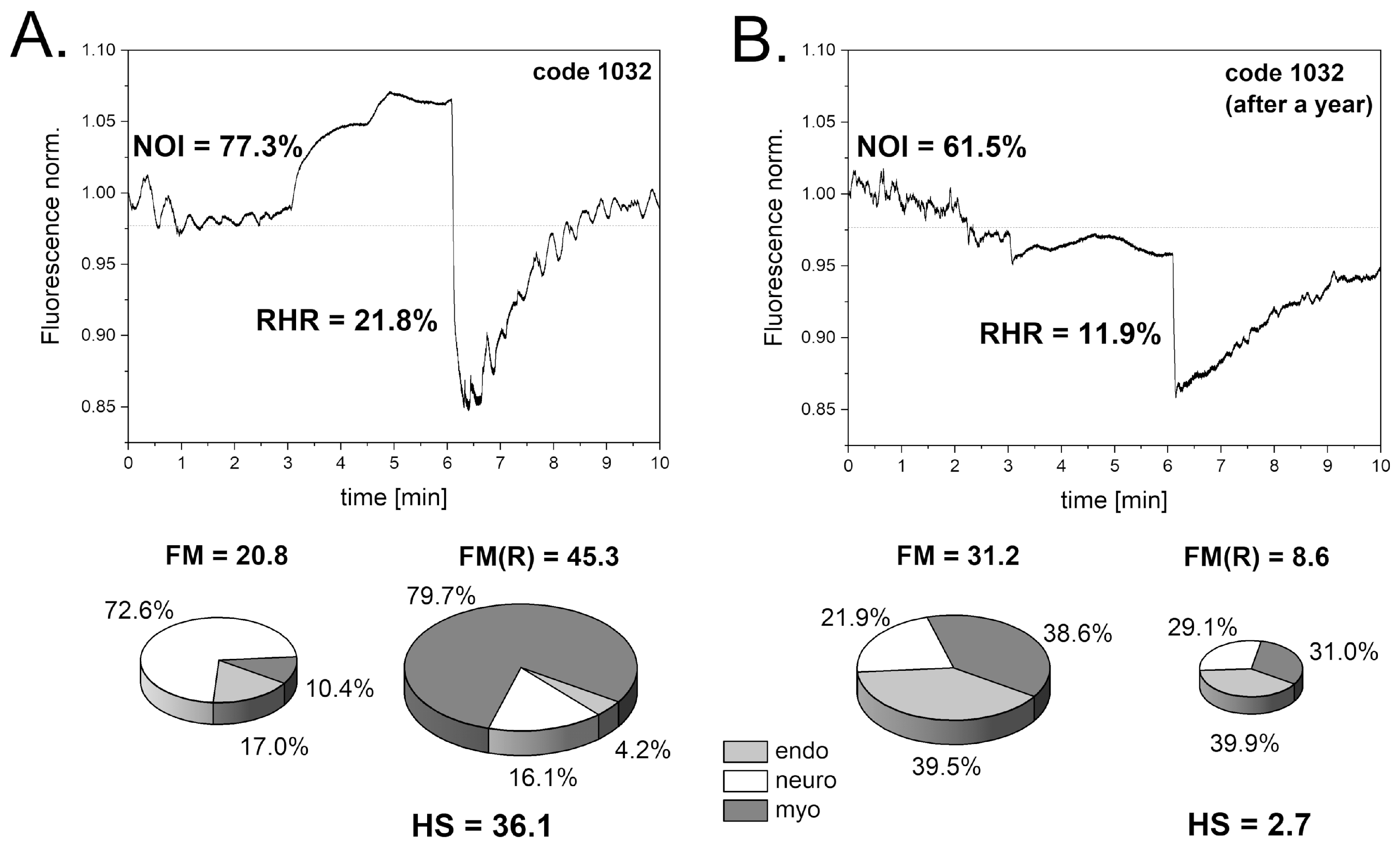
3.1. Intense Physical Exercise
3.2. Post-COVID Syndrome
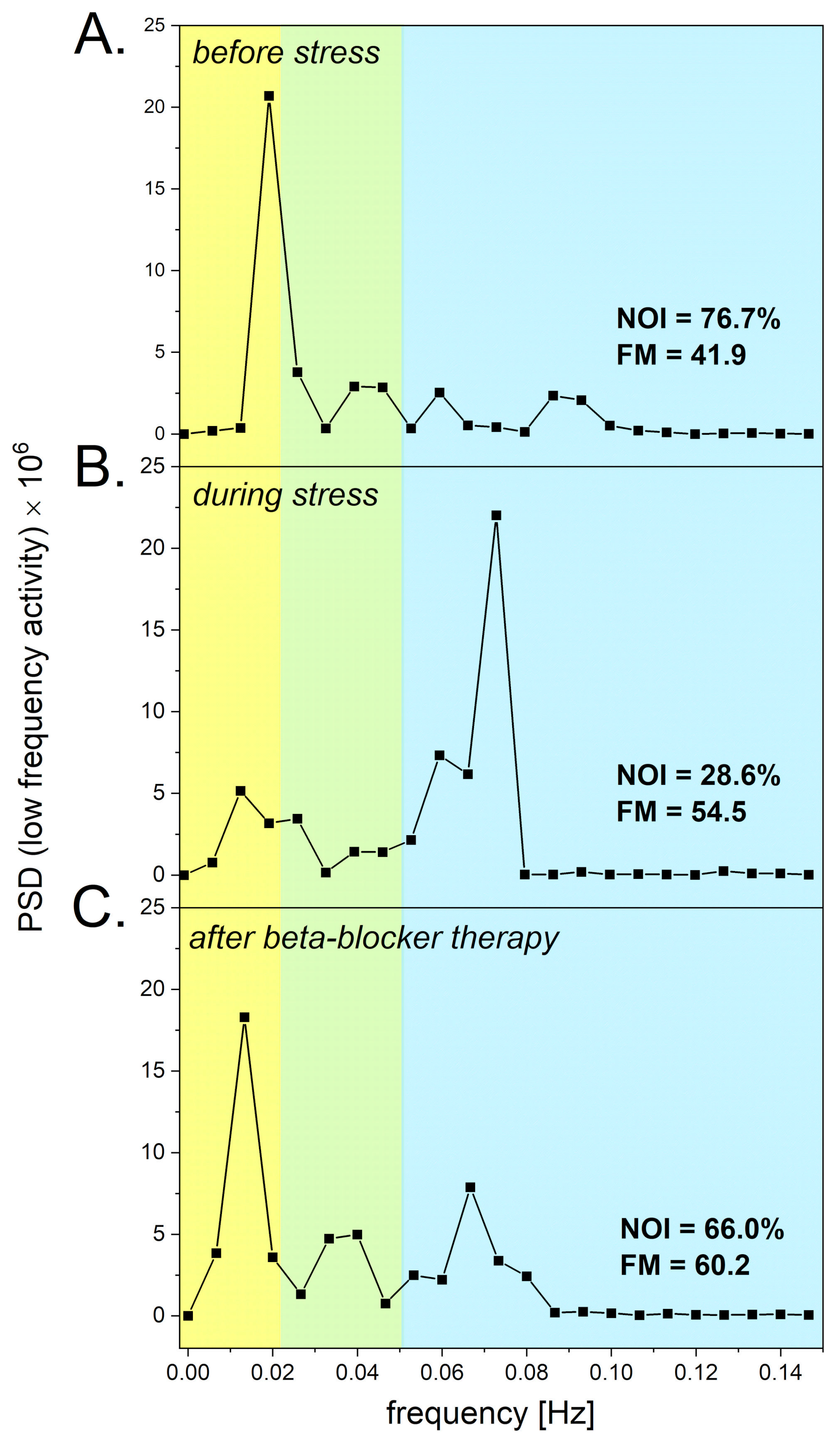
3.3. Psychological Stress
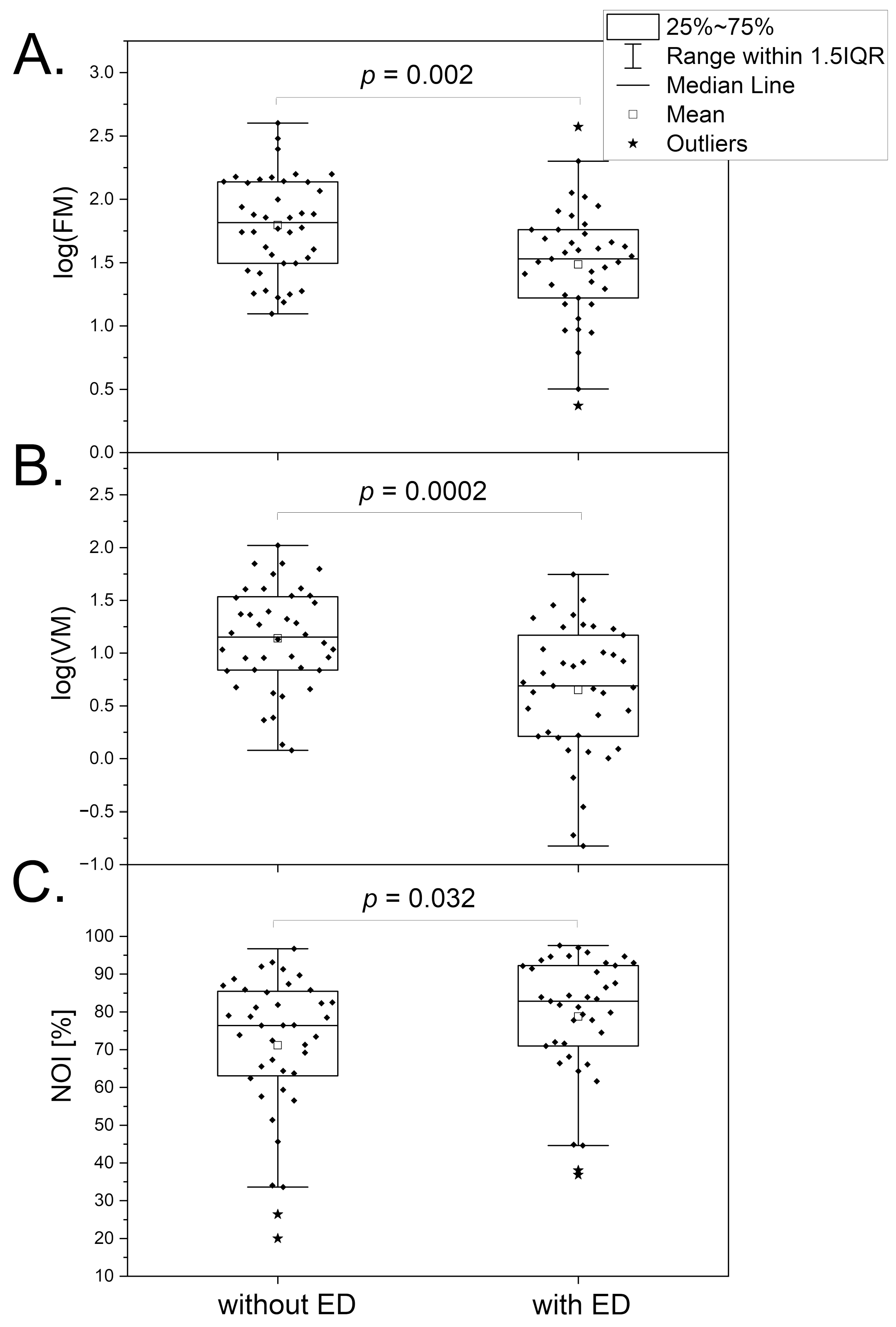
3.4. Erectile Dysfunction
4. Evaluation of Microvascular Complications in Diabetes Based on Response of Microcirculatory Oscillations to Hypoxia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Nilsson, H.; Aalkjaer, C. Vasomotion: Mechanisms and Physiological Importance. Mol. Interv. 2003, 3, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Carpi, A.; Galetta, F.; Franzoni, F.; Santoro, G. The Investigation of Skin Blood Flowmotion: A New Approach to Study the Microcirculatory Impairment in Vascular Diseases? Biomed. Pharmacother. 2006, 60, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Aalkjær, C.; Boedtkjer, D.; Matchkov, V. Vasomotion—What Is Currently Thought? Acta Physiol. 2011, 202, 253–269. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, J.Y.; Kim, S.M.; Son, S.-M.; Choi, S.-Y.; Koo, B.; Rah, C.-S.; Nam, J.H.; Ju, M.J.; Lee, J.S.; et al. Vasomotion in Human Arteries and Their Regulations Based on Ion Channel Regulations: 10 Years Study. J. Cell. Physiol. 2023, 238, 2076–2089. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Borgström, P.; Schmidt-Lucke, J.A. Low Frequency Flowmotion/(Vasomotion) during Patho-Physiological Conditions. Life Sci. 2002, 71, 2713–2728. [Google Scholar] [CrossRef] [PubMed]
- Bari, F.; Tóth-Szűki, V.; Domoki, F.; Kálmán, J. Flow Motion Pattern Differences in the Forehead and Forearm Skin: Age-Dependent Alterations Are Not Specific for Alzheimer’s Disease. Microvasc. Res. 2005, 70, 121–128. [Google Scholar] [CrossRef]
- Rossi, M.; Matteucci, E.; Pesce, M.; Consani, C.; Galetta, F.; Giampietro, O.; Santoro, G. Study of Skin Vasomotion in Type 1 Diabetic Patients and of Its Possible Relationship with Clinical and Laboratory Variables. Clin. Hemorheol. Microcirc. 2013, 53, 357–367. [Google Scholar] [CrossRef]
- Bruning, R.S.; Kenney, W.L.; Alexander, L.M. Altered Skin Flowmotion in Hypertensive Humans. Microvasc. Res. 2015, 97, 81–87. [Google Scholar] [CrossRef]
- Tikhonova, I.V.; Kosyakova, N.I.; Tankanag, A.V.; Chemeris, N.K. Oscillations of Skin Microvascular Blood Flow in Patients with Asthma. Microcirculation 2016, 23, 33–43. [Google Scholar] [CrossRef]
- Mizeva, I.; Makovik, I.; Dunaev, A.; Krupatkin, A.; Meglinski, I. Analysis of Skin Blood Microflow Oscillations in Patients with Rheumatic Diseases. J. Biomed. Opt. 2017, 22, 070501. [Google Scholar] [CrossRef]
- Pedanekar, T.; Kedare, R.; Sengupta, A. Monitoring Tumor Progression by Mapping Skin Microcirculation with Laser Doppler Flowmetry. Lasers Med. Sci. 2019, 34, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Sorelli, M.; Francia, P.; Bocchi, L.; De Bellis, A.; Anichini, R. Assessment of Cutaneous Microcirculation by Laser Doppler Flowmetry in Type 1 Diabetes. Microvasc. Res. 2019, 124, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Zhang, D.; Wang, W.; Xie, H.; Ruan, J.; Jin, Y.; Li, T.; Li, X.; Zhao, B.; et al. Ca2+ Oscillation in Vascular Smooth Muscle Cells Control Myogenic Spontaneous Vasomotion and Counteract Post-Ischemic No-Reflow. Commun. Biol. 2024, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Stefanovska, A.; Bracic, M.; Kvernmo, H.D. Wavelet Analysis of Oscillations in the Peripheral Blood Circulation Measured by Laser Doppler Technique. IEEE Trans. Biomed. Eng. 1999, 46, 1230–1239. [Google Scholar] [CrossRef]
- Kvandal, P.; Landsverk, S.A.; Bernjak, A.; Stefanovska, A.; Kvernmo, H.D.; Kirkebøen, K.A. Low-Frequency Oscillations of the Laser Doppler Perfusion Signal in Human Skin. Microvasc. Res. 2006, 72, 120–127. [Google Scholar] [CrossRef]
- Holowatz, L.A.; Thompson-Torgerson, C.S.; Kenney, W.L. The Human Cutaneous Circulation as a Model of Generalized Microvascular Function. J. Appl. Physiol. 2008, 105, 370–372. [Google Scholar] [CrossRef]
- Hellmann, M.; Roustit, M.; Cracowski, J.L. Skin Microvascular Endothelial Function as a Biomarker in Cardiovascular Diseases? Pharmacol. Rep. 2015, 67, 803–810. [Google Scholar] [CrossRef]
- Bernjak, A.; Clarkson, P.B.M.; McClintock, P.V.E.; Stefanovska, A. Low-Frequency Blood Flow Oscillations in Congestive Heart Failure and after Beta1-Blockade Treatment. Microvasc. Res. 2008, 76, 224–232. [Google Scholar] [CrossRef]
- Clough, G.F.; Kuliga, K.Z.; Chipperfield, A.J. Flow Motion Dynamics of Microvascular Blood Flow and Oxygenation: Evidence of Adaptive Changes in Obesity and Type 2 Diabetes Mellitus/Insulin Resistance. Microcirculation 2017, 24, e12331. [Google Scholar] [CrossRef]
- Marcinek, A.; Katarzynska, J.; Sieron, L.; Skokowski, R.; Zielinski, J.; Gebicki, J. Non-Invasive Assessment of Vascular Circulation Based on Flow Mediated Skin Fluorescence (FMSF). Biology 2023, 12, 385. [Google Scholar] [CrossRef]
- Marcinek, A.; Katarzynska, J.; Gebicki, J. A New Approach to Vascular Screening: Identification of Impaired Vascular Function Using the FMSF Technique. Sensors 2024, 24, 1721. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.; Katarzynska, J.; Cholewinski, T.; Sieron, L.; Marcinek, A. Flowmotion Monitored by Flow Mediated Skin Fluorescence (FMSF): A Tool for Characterization of Microcirculatory Status. Front. Physiol. 2020, 11, 702. [Google Scholar] [CrossRef]
- Gebicki, J.; Marcinek, A.; Zielinski, J. Assessment of Microcirculatory Status Based on Stimulation of Myogenic Oscillations by Transient Ischemia: From Health to Disease. Vasc. Health Risk Manag. 2021, 17, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Bugaj, O.; Zieliński, J.; Kusy, K.; Kantanista, A.; Wieliński, D.; Guzik, P. The Effect of Exercise on the Skin Content of the Reduced Form of NAD and Its Response to Transient Ischemia and Reperfusion in Highly Trained Athletes. Front. Physiol. 2019, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Bugaj, O.; Kusy, K.; Kantanista, A.; Korman, P.; Wieliński, D.; Zieliński, J. The Effect of a 7-Week Training Period on Changes in Skin NADH Fluorescence in Highly Trained Athletes. Appl. Sci. 2020, 10, 5133. [Google Scholar] [CrossRef]
- Henderson, S.A.; Graham, H.K.; Mollan, R.A.B.; Riddoch, C.; Sheridan, B.; Johnston, H. Calcium Homeostasis and Exercise. Int. Orthop. 1989, 13, 69–73. [Google Scholar] [CrossRef]
- Maïmoun, L.; Sultan, C. Effect of Physical Activity on Calcium Homeostasis and Calciotropic Hormones: A Review. Calcif. Tissue Int. 2009, 85, 277–286. [Google Scholar] [CrossRef]
- Chudzik, M.; Cender, A.; Mordaka, R.; Zieliński, J.; Katarzyńska, J.; Marcinek, A.; Gebicki, J. Chronic Fatigue Associated with Post-COVID Syndrome versus Transient Fatigue Caused by High-Intensity Exercise: Are They Comparable in Terms of Vascular Effects? Vasc. Health Risk Manag. 2022, 18, 711–719. [Google Scholar] [CrossRef]
- Wirth, K.J.; Scheibenbogen, C. Dyspnea in Post-COVID Syndrome Following Mild Acute COVID-19 Infections: Potential Causes and Consequences for a Therapeutic Approach. Medicina 2022, 58, 419. [Google Scholar] [CrossRef]
- Nguyen, T.; Johnston, S.; Clarke, L.; Smith, P.; Staines, D.; Marshall-Gradisnik, S. Impaired Calcium Mobilization in Natural Killer Cells from Chronic Fatigue Syndrome/Myalgic Encephalomyelitis Patients Is Associated with Transient Receptor Potential Melastatin 3 Ion Channels. Clin. Exp. Immunol. 2017, 187, 284–293. [Google Scholar] [CrossRef]
- Romanowska-Kocejko, M.; Jędrzejewska, A.; Braczko, A.; Stawarska, K.; Król, O.; Frańczak, M.; Harasim, G.; Smoleński, R.T.; Hellmann, M.; Kutryb-Zając, B. Red Blood Cell Adenylate Energetics Is Related to Endothelial and Microvascular Function in Long COVID. Biomedicines 2024, 12, 554. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.S.; Toya, T.; Ahmad, A.; Clark, M.M.; Gilliam, W.P.; Lerman, L.O.; Lerman, A. Mental Stress and Its Effects on Vascular Health. Mayo Clin. Proc. 2022, 97, 951–990. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.; Katarzynska, J.; Marcinek, A. Effect of Psychological Stress on Microcirculation Oscillations: Diagnostic Aspects. Vasc. Health Risk Manag. 2023, 19, 79–82. [Google Scholar] [CrossRef]
- MacCormack, J.K.; Armstrong-Carter, E.L.; Gaudier-Diaz, M.M.; Meltzer-Brody, S.; Sloan, E.K.; Lindquist, K.A.; Muscatell, K.A. β-Adrenergic Contributions to Emotion and Physiology During an Acute Psychosocial Stressor. Psychosom. Med. 2021, 83, 959–968. [Google Scholar] [CrossRef]
- Brotons, F.B.; Campos, J.C.; Gonzalez-Correales, R.; Martín-Morales, A.; Moncada, I.; Pomerol, J.M. Core Document on Erectile Dysfunction: Key Aspects in the Care of a Patient with Erectile Dysfunction. Int. J. Impot. Res. 2004, 16, S26–S39. [Google Scholar] [CrossRef] [PubMed]
- Montorsi, F.; Briganti, A.; Salonia, A.; Rigatti, P.; Margonato, A.; Macchi, A.; Galli, S.; Ravagnani, P.M.; Montorsi, P. Erectile Dysfunction Prevalence, Time of Onset and Association with Risk Factors in 300 Consecutive Patients with Acute Chest Pain and Angiographically Documented Coronary Artery Disease. Eur. Urol. 2003, 44, 360–365. [Google Scholar] [CrossRef]
- Guo, W.; Liao, C.; Zou, Y.; Li, F.; Li, T.; Zhou, Q.; Cao, Y.; Mao, X. Erectile Dysfunction and Risk of Clinical Cardiovascular Events: A Meta-Analysis of Seven Cohort Studies. J. Sex. Med. 2010, 7, 2805–2816. [Google Scholar] [CrossRef]
- Alberti, L.; Torlasco, C.; Lauretta, L.; Loffi, M.; Maranta, F.; Salonia, A.; Margonato, A.; Montorsi, F.; Fragasso, G. Erectile Dysfunction in Heart Failure Patients: A Critical Reappraisal. Andrology 2013, 1, 177–191. [Google Scholar] [CrossRef]
- Yannas, D.; Frizza, F.; Vignozzi, L.; Corona, G.; Maggi, M.; Rastrelli, G. Erectile Dysfunction Is a Hallmark of Cardiovascular Disease: Unavoidable Matter of Fact or Opportunity to Improve Men’s Health? J. Clin. Med. 2021, 10, 2221. [Google Scholar] [CrossRef]
- Corona, G.; Monami, M.; Boddi, V.; Cameron-Smith, M.; Fisher, A.D.; De Vita, G.; Melani, C.; Balzi, D.; Sforza, A.; Forti, G.; et al. Low Testosterone Is Associated with an Increased Risk of MACE Lethality in Subjects with Erectile Dysfunction. J. Sex. Med. 2010, 7, 1557–1564. [Google Scholar] [CrossRef]
- Colin, O.; Bergh, A.; Damber, J.-E.; Widmark, A. Control of Testicular Vasomotion by Testosterone and Tubular Factors in Rats. J. Reprod. Fertil. 1993, 97, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.; Sharpe, R.M.; Moffat, L.; Atanassova, N.; Saunders, P.T.K.; Kilter, S.; Bergh, A.; Smith, L.B. Androgen Action via Testicular Arteriole Smooth Muscle Cells Is Important for Leydig Cell Function, Vasomotion and Testicular Fluid Dynamics. PLoS ONE 2010, 5, e13632. [Google Scholar] [CrossRef]
- Slowikowska-Hilczer, J.; Walczak-Jedrzejowska, R.; Adamczewska, D.; Byczkiewicz, P.; Marchlewska, K.; Katarzynska, J.; Gebicki, J. A New Approach to the Assessment of Erectile Dysfunction Based on Vasomotion Monitored by the Flow-Mediated Skin Fluorescence (FMSF) Technique—A Preliminary Study. J. Clin. Med. 2024, 13, 3210. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.J.; Wen, J.; Jiang, W.H.; Lin, J.; Hong, Y.; Zhu, Y.S. Androgen Actions on Endothelium Functions and Cardiovascular Diseases. J. Geriatr. Cardiol. 2016, 13, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Er, F.; Michels, G.; Brandt, M.C.; Khan, I.; Haase, H.; Eicks, M.; Lindner, M.; Hoppe, U.C. Impact of Testosterone on Cardiac L-Type Calcium Channels and Ca2+ Sparks: Acute Actions Antagonize Chronic Effects. Cell Calcium 2007, 41, 467–477. [Google Scholar] [CrossRef]
- Zito, S.; Nosari, G.; Pigoni, A.; Moltrasio, C.; Delvecchio, G. Association between Testosterone Levels and Mood Disorders: A Minireview. J. Affect. Disord. 2023, 330, 48–56. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Q.; Guo, C.; Dong, G.; Liu, Y.; Tang, C.; Dong, Z. Hypoxia, HIF, and Associated Signaling Networks in Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18, 950. [Google Scholar] [CrossRef]
- Catrina, S.-B.; Zheng, X. Disturbed Hypoxic Responses as a Pathogenic Mechanism of Diabetic Foot Ulcers. Diabetes Metab. Res. Rev. 2016, 32, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.B.; Zheng, X. Hypoxia and Hypoxia-Inducible Factors in Diabetes and Its Complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef]
- Dei Cas, A.; Gnudi, L. VEGF and Angiopoietins in Diabetic Glomerulopathy: How Far for a New Treatment? Metabolism 2012, 61, 1666–1673. [Google Scholar] [CrossRef]
- Thangarajah, H.; Yao, D.; Chang, E.I.; Shi, Y.; Jazayeri, L.; Vial, I.N.; Galiano, R.D.; Du, X.-L.; Grogan, R.; Galvez, M.G.; et al. The Molecular Basis for Impaired Hypoxia-Induced VEGF Expression in Diabetic Tissues. Proc. Natl. Acad. Sci. USA 2009, 106, 13505–13510. [Google Scholar] [CrossRef] [PubMed]
- Los-Stegienta, A.; Borkowska, A.; Cypryk, K. Assessment of Microvascular Function Using a Novel Technique Flow Mediated Skin Fluorescence (FMSF) in Patients with Diabetic Kidney Disease: A Preliminary Study. Microvasc. Res. 2022, 144, 104417. [Google Scholar] [CrossRef] [PubMed]
- Los-Stegienta, A.; Katarzynska, J.; Borkowska, A.; Marcinek, A.; Cypryk, K.; Gebicki, J. Differentiation of Diabetic Foot Ulcers Based on Stimulation of Myogenic Oscillations by Transient Ischemia. Vasc. Health Risk Manag. 2021, 17, 145–152. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinek, A.; Katarzynska, J.; Cypryk, K.; Los-Stegienta, A.; Slowikowska-Hilczer, J.; Walczak-Jedrzejowska, R.; Zielinski, J.; Gebicki, J. Assessment of Microvascular Function Based on Flowmotion Monitored by the Flow-Mediated Skin Fluorescence Technique. Biosensors 2024, 14, 459. https://doi.org/10.3390/bios14100459
Marcinek A, Katarzynska J, Cypryk K, Los-Stegienta A, Slowikowska-Hilczer J, Walczak-Jedrzejowska R, Zielinski J, Gebicki J. Assessment of Microvascular Function Based on Flowmotion Monitored by the Flow-Mediated Skin Fluorescence Technique. Biosensors. 2024; 14(10):459. https://doi.org/10.3390/bios14100459
Chicago/Turabian StyleMarcinek, Andrzej, Joanna Katarzynska, Katarzyna Cypryk, Agnieszka Los-Stegienta, Jolanta Slowikowska-Hilczer, Renata Walczak-Jedrzejowska, Jacek Zielinski, and Jerzy Gebicki. 2024. "Assessment of Microvascular Function Based on Flowmotion Monitored by the Flow-Mediated Skin Fluorescence Technique" Biosensors 14, no. 10: 459. https://doi.org/10.3390/bios14100459
APA StyleMarcinek, A., Katarzynska, J., Cypryk, K., Los-Stegienta, A., Slowikowska-Hilczer, J., Walczak-Jedrzejowska, R., Zielinski, J., & Gebicki, J. (2024). Assessment of Microvascular Function Based on Flowmotion Monitored by the Flow-Mediated Skin Fluorescence Technique. Biosensors, 14(10), 459. https://doi.org/10.3390/bios14100459







