Terahertz Metamaterials for Biosensing Applications: A Review
Abstract
:1. Introduction
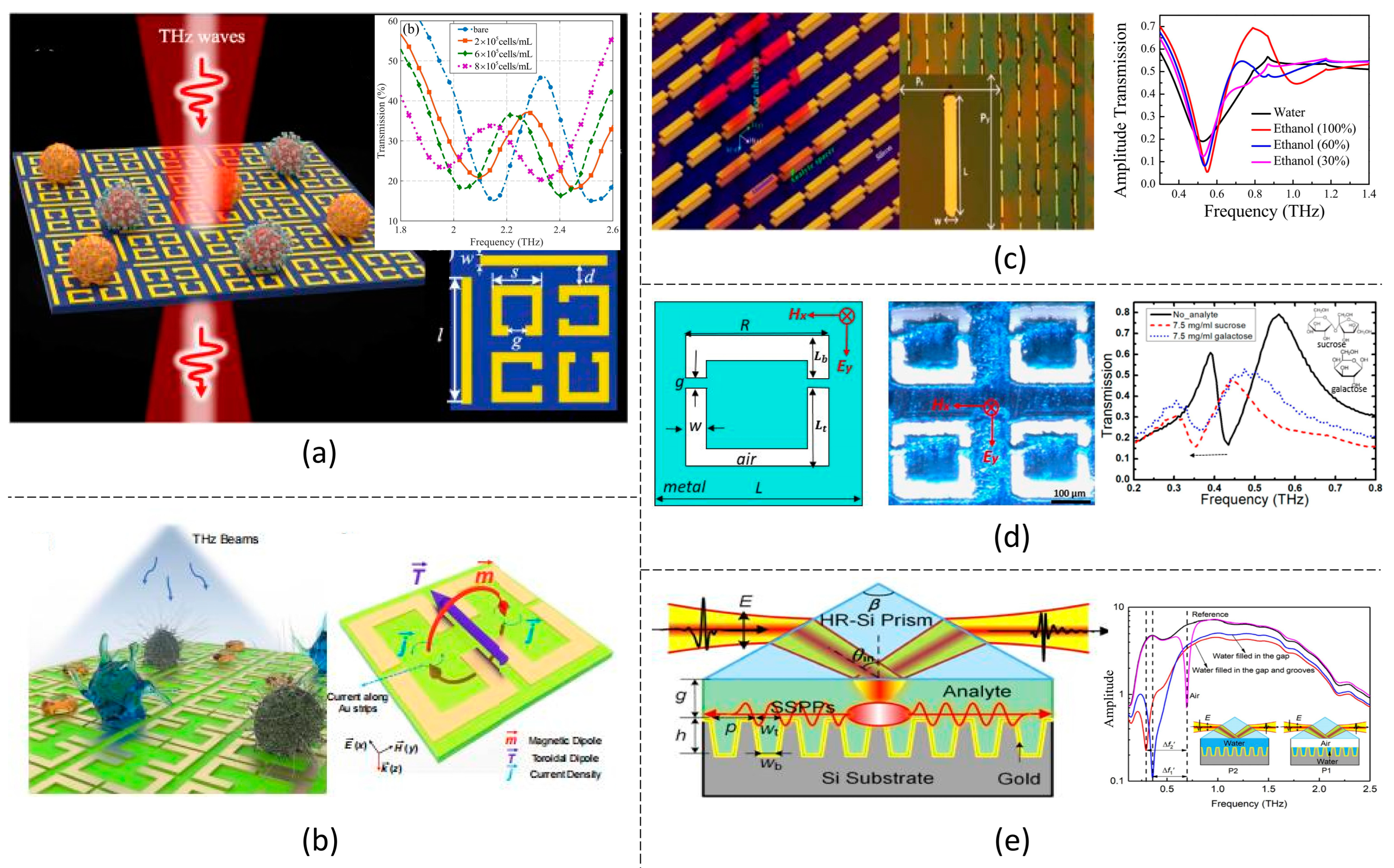
2. THz Wave Sensing Principles
2.1. Transmission Dip Shift
2.2. Absorption Peak Shift
2.3. Polarization and Chirality
2.4. Transmission Imaging

3. Different Metamaterial Resonances
3.1. EIT-like Resonances
3.2. Fano Resonances
3.3. Toroidal Resonances
3.4. SPP Resonances
3.5. Sensing Based on Quasi-BIC
4. Non-Conventional Materials
4.1. All-Dielectric Metamaterials
4.2. Carbon-Assisted Metamaterials
4.3. NW and NP Metamaterials
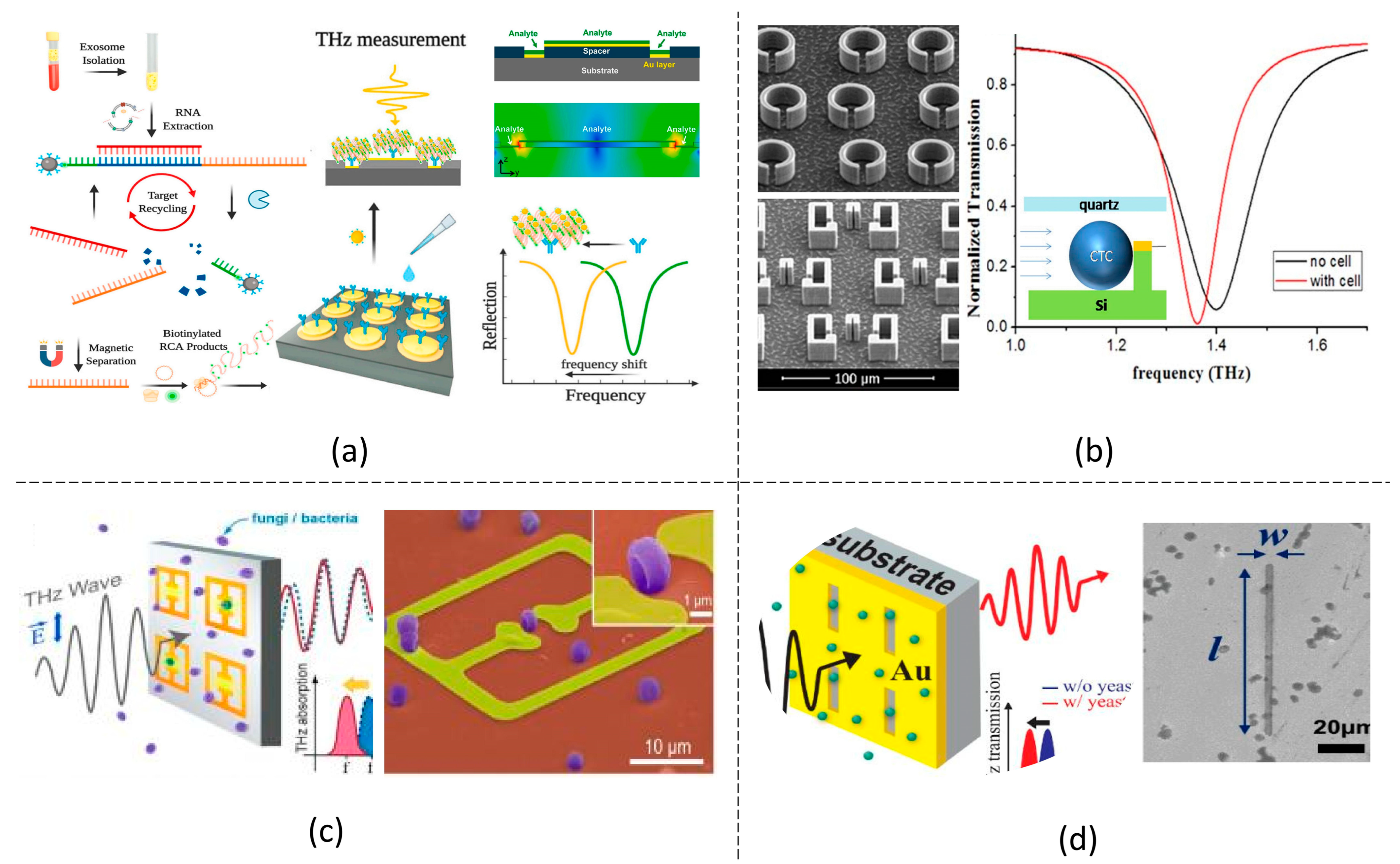
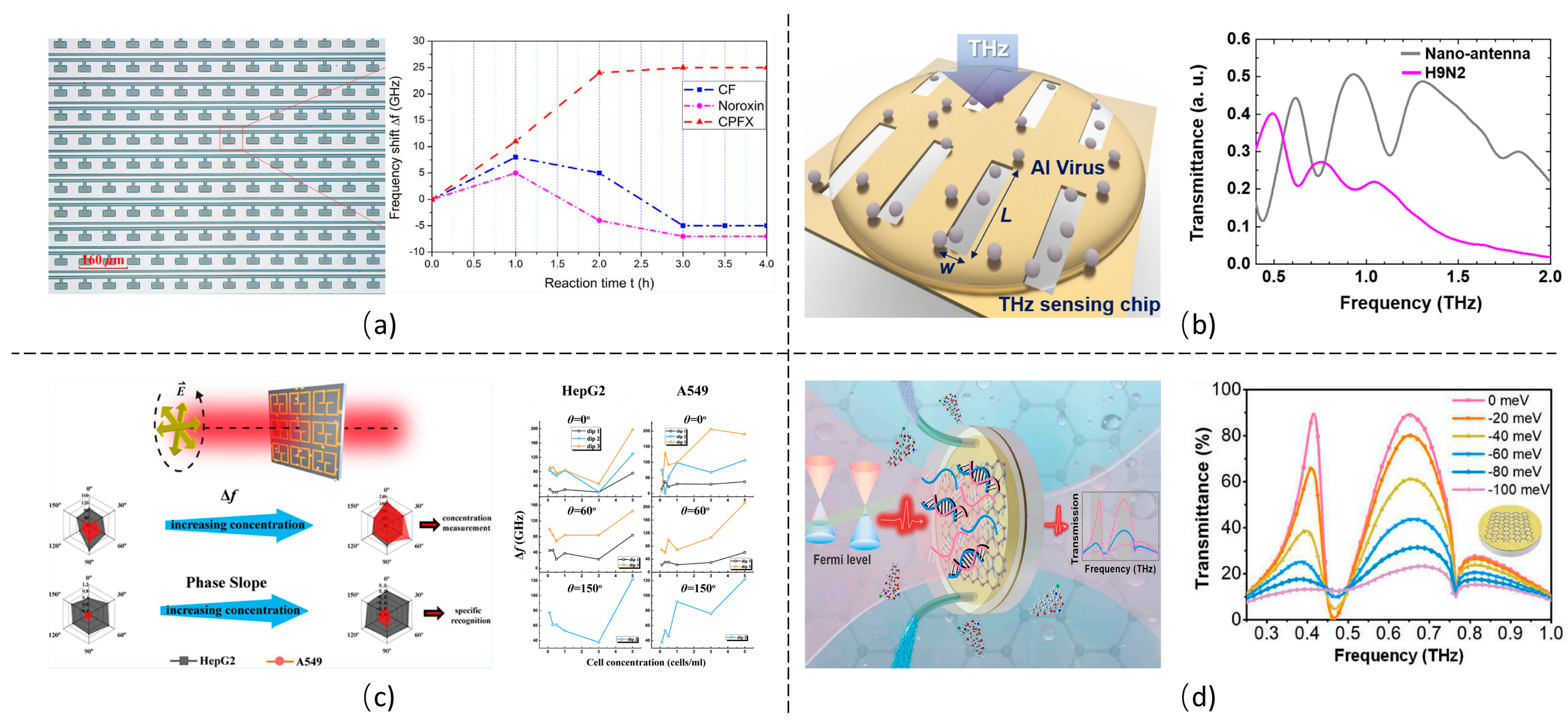
5. THz Metamaterial Biosensors Targeting Different Analytes
5.1. Chemical Reagents
5.2. Biomolecules
5.3. Microorganisms and Cells
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chalklen, T.; Jing, Q.; Kar-Narayan, S. Biosensors Based on Mechanical and Electrical Detection Techniques. Sensors 2020, 20, 5605. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Wang, L.H.; Zhao, J.Y.; Zhu, Y.F.; Yang, J.L.; Yang, F.H. Enhanced Binding Efficiency of Microcantilever Biosensor for the Detection of Yersinia. Sensors 2019, 19, 3326. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, F.; Qualtieri, A.; Dattoma, T.; Epifani, G.; De Vittorio, M. Biomimetics of underwater hair cell sensing. Microelectron. Eng. 2015, 132, 90–97. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.T.; Quan, A.J.; Qiu, C.H.; Cao, W.G.; Fu, C.; Fu, Y.Q. Highly selective and label-free Love-mode surface acoustic wave biosensor for carcinoembryonic antigen detection using a self-assembled monolayer bioreceptor. Appl. Surf. Sci. 2020, 518, 146061. [Google Scholar] [CrossRef]
- Nahid, M.A.; Campbell, C.E.; Fong, K.S.K.; Barnhill, J.C.; Washington, M.A. An evaluation of the impact of clinical bacterial isolates on epithelial cell monolayer integrity by the electric Cell-Substrate Impedance Sensing (ECIS) method. J. Microbiol. Methods 2020, 169, 105833. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Anand, R.K. Cellular dielectrophoresis coupled with single-cell analysis. Anal. Bioanal. Chem. 2018, 410, 2499–2515. [Google Scholar] [CrossRef] [PubMed]
- Presnova, G.; Presnov, D.; Krupenin, V.; Grigorenko, V.; Trifonov, A.; Andreeva, I.; Ignatenko, O.; Egorov, A.; Rubtsova, M. Biosensor based on a silicon nanowire field-effect transistor functionalized by gold nanoparticles for the highly sensitive determination of prostate specific antigen. Biosens. Bioelectron. 2017, 88, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Walser, R.M. Electromagnetic metamaterials. In Complex Mediums II: Beyond Linear Isotropic Dielectrics; SPIE: Pontoise, France, 2001; pp. 1–15. [Google Scholar]
- Marqués, R.; Martel, J.; Mesa, F.; Medina, F. A new experiment. Microw. Opt. Technol. Lett. 2002, 35, 405–408. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, X.; Chang, L.; Wang, C.; Zhao, D. High-efficiency and multi-frequency polarization converters based on graphene metasurface with twisting double L-shaped unit structure array. Opt. Commun. 2017, 394, 50–55. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, J.; Fang, X.; Lin, Y.; Wang, K.; Zhang, M. An Active Electromagnetically Induced Transparency (EIT) Metamaterial Based on Conductive Coupling. Materials 2022, 15, 7371. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Yan, Z.; Zhao, X.; Cheng, J.; Liu, A.Q. Single mode to dual mode switch through a THz reconfigurable metamaterial. Appl. Phys. Lett. 2017, 111, 241106. [Google Scholar] [CrossRef]
- Cao, T.; Wei, C.-W.; Simpson, R.E.; Zhang, L.; Cryan, M.J. Broadband Polarization-Independent Perfect Absorber Using a Phase-Change Metamaterial at Visible Frequencies. Sci. Rep. 2014, 4, 3955. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Padilla, W.J.; Vier, D.C.; Nemat-Nasser, S.C.; Schultz, S. Composite Medium with Simultaneously Negative Permeability and Permittivity. Phys. Rev. Lett. 2000, 84, 4184–4187. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.; Enkrich, C.; Wegener, M.; Zhou, J.; Koschny, T.; Soukoulis, C.M. Magnetic response of metamaterials at 100 terahertz. Science 2004, 306, 1351–1353. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, A.Q.; Zhu, W.M.; Li, E.P.; Tanoto, H.; Wu, Q.Y.; Teng, J.H.; Zhang, X.H.; Tsai, M.L.J.; Lo, G.Q.; et al. Micromachined switchable metamaterial with dual resonance. Appl. Phys. Lett. 2012, 101, 151902. [Google Scholar] [CrossRef]
- Valentine, J.; Zhang, S.; Zentgraf, T.; Ulin-Avila, E.; Genov, D.A.; Bartal, G.; Zhang, X. Three-dimensional optical metamaterial with a negative refractive index. Nature 2008, 455, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Kafesaki, M.; Tsiapa, I.; Katsarakis, N.; Koschny, T.; Soukoulis, C.M.; Economou, E.N. Left-handed metamaterials: The fishnet structure and its variations. Phys. Rev. B 2007, 75, 235114. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, W.M.; Chia, E.E.M.; Shen, Z.X.; Cai, H.; Gu, Y.D.; Ser, W.; Liu, A.Q. A pseudo-planar metasurface for a polarization rotator. Opt. Express 2014, 22, 10446–10454. [Google Scholar] [CrossRef]
- Shelby, R.A.; Smith, D.R.; Schultz, S. Experimental verification of a negative index of refraction. Science 2001, 292, 77–79. [Google Scholar] [CrossRef]
- Shalaev, V.M. Optical negative-index metamaterials. Nat. Photon. 2007, 1, 41–488. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, W.; Wu, P.C.; Zhu, W.; Shen, Z.X.; Chong, P.H.J.; Liang, Q.X.; Yang, Z.C.; Hao, Y.L.; Cai, H.; et al. Water-Resonator-Based Metasurface: An Ultrabroadband and Near-Unity Absorption. Adv. Opt. Mater. 2017, 5, 1601103. [Google Scholar] [CrossRef]
- He, L.; Yi, Y.; Zhang, J.; Xu, X.; Tang, B.; Li, G.; Zeng, L.; Chen, J.; Sun, T.; Yi, Z. A four-narrowband terahertz tunable absorber with perfect absorption and high sensitivity. Mater. Res. Bull. 2024, 170, 112572. [Google Scholar] [CrossRef]
- Plum, E.; Liu, X.X.; Fedotov, V.A.; Chen, Y.; Tsai, D.P.; Zheludev, N.I. Metamaterials: Optical activity without chirality. Phys. Rev. Lett. 2009, 102, 113902. [Google Scholar] [CrossRef] [PubMed]
- Valev, V.K.; Baumberg, J.J.; Sibilia, C.; Verbiest, T. Chirality and chiroptical effects in plasmonic nanostructures: Fundamentals, recent progress, and outlook. Adv. Mater. 2013, 25, 2517–2534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, B.; Fang, X.; Cheng, K.; Chen, W.; Wang, Z.; Hong, D.; Zhang, M. Microfluid-based soft metasurface for tunable optical activity in THz wave. Opt. Express 2021, 29, 8786–8795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Genov, D.A.; Wang, Y.; Liu, M.; Zhang, X. Plasmon-induced transparency in metamaterials. Phys. Rev. Lett. 2008, 101, 047401. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Langguth, L.; Weiss, T.; Kästel, J.; Fleischhauer, M.; Pfau, T.; Giessen, H. Plasmonic analogue of electromagnetically induced transparency at the Drude damping limit. Nat. Mater. 2009, 8, 758–762. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Ling, L.; Sheng, Z.; Yi, Z.; Zhou, Z.; Yang, Y.; Tang, B.; Zeng, Q.; Sun, T. Multifunctional terahertz device with active switching between bimodal perfect absorption and plasmon-induced transparency. Mater. Res. Bull. 2024, 171, 112635. [Google Scholar] [CrossRef]
- Schurig, D.; Mock, J.J.; Justice, B.J.; Cummer, S.A.; Pendry, J.B.; Starr, A.F.; Smith, D.R. Metamaterial electromagnetic cloak at microwave frequencies. Science 2006, 314, 977–980. [Google Scholar] [CrossRef]
- Cai, W.; Chettiar, U.K.; Kildishev, A.V.; Shalaev, V.M. Optical cloaking with metamaterials. Nat. Photonics 2007, 1, 224–227. [Google Scholar] [CrossRef]
- Valentine, J.; Li, J.; Zentgraf, T.; Bartal, G.; Zhang, X. An optical cloak made of dielectrics. Nat. Mater. 2009, 8, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Z. Superlenses to overcome the diffraction limit. Nat. Mater. 2008, 7, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Jacob, Z.; Alekseyev, L.V.; Narimanov, E. Optical hyperlens: Far-field imaging beyond the diffraction limit. Opt. Express 2006, 14, 8247–8256. [Google Scholar] [CrossRef] [PubMed]
- Poddubny, A.; Iorsh, I.; Belov, P.; Kivshar, Y. Hyperbolic metamaterials. Nat. Photonics 2013, 7, 958–967. [Google Scholar] [CrossRef]
- Grady, N.K.; Heyes, J.E.; Chowdhury, D.R.; Zeng, Y.; Reiten, M.T.; Azad, A.K.; Taylor, A.J.; Dalvit, D.A.R.; Chen, H.T. Terahertz metamaterials for linear polarization conversion and anomalous refraction. Science 2013, 340, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Gansel, J.K.; Thiel, M.; Rill, M.S.; Decker, M.; Bade, K.; Saile, V.; Von Freymann, G.; Linden, S.; Wegener, M. Gold helix photonic metamaterial as broadband circular polarizer. Science 2009, 325, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, W.; Liu, A.Q.; Li, F.C.; Lan, C.F. Tunable Polarization Conversion and Rotation based on a Reconfigurable Metasurface. Sci. Rep. 2017, 7, 12068. [Google Scholar] [CrossRef]
- Aieta, F.; Genevet, P.; Kats, M.A.; Yu, N.; Blanchard, R.; Gaburro, Z.; Capasso, F. Aberration-free ultrathin flat lenses and axicons at telecom wavelengths based on plasmonic metasurfaces. Nano Lett. 2012, 12, 4932–4936. [Google Scholar] [CrossRef]
- Khorasaninejad, M.; Chen, W.T.; Devlin, R.C.; Oh, J.; Zhu, A.Y.; Capasso, F. Metalenses at visible wavelengths: Diffraction-limited focusing and subwavelength resolution imaging. Science 2016, 352, 1190–1194. [Google Scholar] [CrossRef]
- Zhang, M.; Li, D.; Lin, J.; Zhang, B.; Tao, J.; Zhao, Z.; Zeng, Y.; Shang, W.; Zhang, W. A reflective metasurface for broadband OAM vortex wave generation. Front. Phys. 2022, 10, 1042024. [Google Scholar] [CrossRef]
- Zheng, G.; Mühlenbernd, H.; Kenney, M.; Li, G.; Zentgraf, T.; Zhang, S. Metasurface holograms reaching 80% efficiency. Nat. Nanotechnol. 2015, 10, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Kildishev, A.V.; Shalaev, V.M. Metasurface holograms for visible light. Nat. Commun. 2013, 4, 2807. [Google Scholar] [CrossRef]
- Kawata, S.; Ono, A.; Verma, P. Subwavelength colour imaging with a metallic nanolens. Nat. Photonics 2008, 2, 438–442. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, X.F.; Liang, L.J.; Yao, J.Q. Research Progress in the Application of Biosensors by Using Metamaterial in Terahertz Wave. Spectrosc. Spectr. Anal. 2014, 34, 2365–2371. [Google Scholar]
- Yang, J.; Qi, L.M.; Wu, L.Q.; Lan, F.; Lan, C.W.; Tao, X.; Liu, Z.Y. Research Progress of Terahertz Metamaterial Biosensors. Spectrosc. Spectr. Anal. 2021, 41, 1669–1677. [Google Scholar]
- Wang, Q.; Chen, Y.; Mao, J.; Yang, F.; Wang, N. Metasurface-Assisted Terahertz Sensing. Sensors 2023, 23, 5902. [Google Scholar] [CrossRef]
- Wang, S.; Xia, L.; Mao, H.; Jiang, X.; Yan, S.; Wang, H.; Wei, D.; Cui, H.; Du, C. Terahertz biosensing based on a polarization-insensitive metamaterial. IEEE Photonics Technol. Lett. 2016, 28, 986–989. [Google Scholar] [CrossRef]
- Zhao, R.; Zou, B.; Zhang, G.; Xu, D.; Yang, Y. High-sensitivity identification of aflatoxin B1 and B2 using terahertz time-domain spectroscopy and metamaterial-based terahertz biosensor. J. Phys. D Appl. Phys. 2020, 53, 195401. [Google Scholar] [CrossRef]
- Hu, F.; Guo, E.; Xu, X.; Li, P.; Xu, X.; Yin, S.; Wang, Y.; Chen, T.; Yin, X.; Zhang, W. Real-timely monitoring the interaction between bovine serum albumin and drugs in aqueous with terahertz metamaterial biosensor. Opt. Commun. 2017, 388, 62–67. [Google Scholar] [CrossRef]
- Lee, D.K.; Kang, J.H.; Kwon, J.; Lee, J.S.; Lee, S.; Woo, D.H.; Kim, J.H.; Song, C.S.; Park, Q.H.; Seo, M. Nano metamaterials for ultrasensitive Terahertz biosensing. Sci. Rep. 2017, 7, 8146. [Google Scholar] [CrossRef]
- Ou, H.; Lu, F.; Xu, Z.; Lin, Y.-S. Terahertz Metamaterial with Multiple Resonances for Biosensor Application. In Proceedings of the 25th Opto-Electronics and Communications Conference (OECC), Taipei, Taiwan, 4–8 October 2020. [Google Scholar]
- Zhang, Z.; Yang, M.; Yan, X.; Guo, X.; Li, J.; Yang, Y.; Wei, D.; Liu, L.; Xie, J.; Liu, Y.; et al. The Antibody-Free Recognition of Cancer Cells Using Plasmonic Biosensor Platforms with the Anisotropic Resonant Metasurfaces. ACS Appl. Mater. Interfaces 2020, 12, 11388–11396. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, C.; Huang, Y.; Huang, K.; Wang, Y.; Xu, W.; Xie, L.; Ying, Y. Label-free terahertz microfluidic biosensor for sensitive DNA detection using graphene-metasurface hybrid structures. Biosens. Bioelectron. 2021, 188, 113336. [Google Scholar] [CrossRef]
- Liu, J. High-sensitivity detection method for organochlorine pesticide residues based on loop-shaped absorber. Mater. Chem. Phys. 2020, 242, 122542. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, C.; Hu, D.; Li, D.; Hui, X.; Zhang, F.; Chen, M.; Mu, X. Terahertz biosensing based on bi-layer metamaterial absorbers toward ultra-high sensitivity and simple fabrication. Appl. Phys. Lett. 2019, 115, 143507. [Google Scholar] [CrossRef]
- Hu, X.; Xu, G.; Wen, L.; Wang, H.; Zhao, Y.; Zhang, Y.; Cumming, D.R.S.; Chen, Q. Metamaterial absorber integrated microfluidic terahertz sensors. Laser Photonics Rev. 2016, 10, 962–969. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Z.; Zhu, D.; Wang, X.; Chen, S.; Nie, P. Multiband terahertz absorber and selective sensing performance. Opt. Express 2019, 27, 14133–14143. [Google Scholar] [CrossRef]
- Zhong, Y.; Du, L.; Liu, Q.; Zhu, L.; Zhang, B. Metasurface-enhanced ATR sensor for aqueous solution in the terahertz range. Opt. Commun. 2020, 465, 125508. [Google Scholar] [CrossRef]
- Liu, L.; Li, T.; Liu, Z.; Fan, F.; Yuan, H.; Zhang, Z.; Chang, S.; Zhang, X. Terahertz polarization sensing based on metasurface microsensor display anti-proliferation of tumor cells with aspirin. Biomed. Opt. Express 2020, 11, 2416–2430. [Google Scholar] [CrossRef]
- Asgari, S.; Granpayeh, N.; Fabritius, T. Controllable terahertz cross-shaped three-dimensional graphene intrinsically chiral metastructure and its biosensing application. Opt. Commun. 2020, 474, 126080. [Google Scholar] [CrossRef]
- Amin, M.; Siddiqui, O.; Abutarboush, H.; Farhat, M.; Ramzan, R. A THz graphene metasurface for polarization selective virus sensing. Carbon N. Y. 2021, 176, 580–591. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Fan, F.; Li, T.-F.; Ji, Y.-Y.; Chang, S.-J. Terahertz polarization conversion and sensing with double-layer chiral metasurface. Chin. Phys. B 2020, 29, 078707. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, F.; Shi, W.; Zhang, T.; Chang, S. Terahertz circular polarization sensing for protein denaturation based on a twisted dual-layer metasurface. Biomed. Opt. Express 2022, 13, 209–221. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, C.; Fan, F.; Liu, G.; Chang, S. Terahertz polarization and chirality sensing for amino acid solution based on chiral metasurface sensor. Sens. Actuators B Chem. 2020, 330, 129315. [Google Scholar] [CrossRef]
- Lee, D.-K.; Kim, G.; Kim, C.; Jhon, Y.M.; Kim, J.H.; Lee, T.; Son, J.-H.; Seo, M. Ultrasensitive Detection of Residual Pesticides Using THz Near-Field Enhancement. IEEE Trans. Terahertz Sci. Technol. 2016, 6, 389–395. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, S.; Roh, Y.; Oh, S.J.; Lee, S.H.; Song, H.S.; Ryu, Y.-S.; Kim, Y.K.; Seo, M. Label-free brain tissue imaging using large-area terahertz metamaterials. Biosens. Bioelectron. 2020, 170, 112663. [Google Scholar] [CrossRef]
- Jahani, Y.; Arvelo, E.R.; Yesilkoy, F.; Koshelev, K.; Cianciaruso, C.; De Palma, M.; Kivshar, Y.; Altug, H. Imaging-based spectrometer-less optofluidic biosensors based on dielectric metasurfaces for detecting extracellular vesicles. Nat. Commun. 2021, 12, 3246. [Google Scholar] [CrossRef]
- Yan, X.; Yang, M.; Zhang, Z.; Liang, L.; Wei, D.; Wang, M.; Zhang, M.; Wang, T.; Liu, L.; Xie, J.; et al. The terahertz electromagnetically induced transparency-like metamaterials for sensitive biosensors in the detection of cancer cells. Biosens. Bioelectron. 2019, 126, 485–492. [Google Scholar] [CrossRef]
- Yang, M.; Liang, L.; Zhang, Z.; Xin, Y.; Wei, D.; Song, X.; Zhang, H.; Lu, Y.; Wang, M.; Zhang, M.; et al. Electromagnetically induced transparency-like metamaterials for detection of lung cancer cells. Opt. Express 2019, 27, 19520–19529. [Google Scholar] [CrossRef]
- Zhang, J.; Mu, N.; Liu, L.; Xie, J.; Feng, H.; Yao, J.; Chen, T.; Zhu, W. Highly sensitive detection of malignant glioma cells using metamaterial-inspired THz biosensor based on electromagnetically induced transparency. Biosens. Bioelectron. 2021, 185, 113241. [Google Scholar] [CrossRef]
- Chen, M.; Singh, L.; Xu, N.; Singh, R.; Zhang, W.; Xie, L. Terahertz sensing of highly absorptive water-methanol mixtures with multiple resonances in metamaterials. Opt. Express 2017, 25, 14089–14097. [Google Scholar] [CrossRef]
- Li, F.; He, K.; Tang, T.; Mao, Y.; Wang, R.; Li, C.; Shen, J. The terahertz metamaterials for sensitive biosensors in the detection of ethanol solutions. Opt. Commun. 2020, 475, 126287. [Google Scholar] [CrossRef]
- Cheng, R.; Xu, L.; Yu, X.; Zou, L.; Shen, Y.; Deng, X. High-sensitivity biosensor for identification of protein based on terahertz Fano resonance metasurfaces. Opt. Commun. 2020, 473, 125850. [Google Scholar] [CrossRef]
- Taleb, F.; Al-Naib, I.; Koch, M. Free-Standing Complementary Asymmetric Metasurface for Terahertz Sensing Applications. Sensors 2020, 20, 2265. [Google Scholar] [CrossRef]
- Karmakar, S.; Kumar, D.; Varshney, R.K.; Chowdhury, D.R. Strong terahertz matter interaction induced ultrasensitive sensing in Fano cavity based stacked metamaterials. J. Phys. D Appl. Phys. 2020, 53, 415101. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Manickam, P.; Kaushik, A.; Bhansali, S.; Nair, M.; Pala, N. Rapid Detection of Infectious Envelope Proteins by Magnetoplasmonic Toroidal Metasensors. ACS Sens. 2017, 2, 1359–1368. [Google Scholar] [CrossRef]
- Xu, J.; Liao, D.; Gupta, M.; Zhu, Y.; Zhuang, S.; Singh, R.; Chen, L. Terahertz Microfluidic Sensing with Dual-Torus Toroidal Metasurfaces. Adv. Opt. Mater. 2021, 9, 2100024. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, T.; Zhang, J.; Liu, L.; Xie, J.; Wang, G.; Yao, J.; Zhu, W.; Ye, X. Terahertz toroidal metasurface biosensor for sensitive distinction of lung cancer cells. Nanophotonics 2021, 11, 101–109. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, R. Toroidal versus Fano Resonances in High Q planar THz Metamaterials. Adv. Opt. Mater. 2016, 4, 2119–2125. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Ramezani, Z.; Kaushik, A.; Manickam, P.; Ghoreishi, S.A. Functionalized terahertz plasmonic metasensors: Femtomolar-level detection of SARS-CoV-2 spike proteins. Biosens. Bioelectron. 2021, 177, 112971. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Tomitaka, A.; Manickam, P.; Kaushik, A.; Bhansali, S.; Nair, M.; Pala, N. Extreme sensitive metasensor for targeted biomarkers identification using colloidal nanoparticles-integrated plasmonic unit cells. Biomed. Opt. Express 2018, 9, 373–386. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, S.; Shi, T.; Shen, Y.C.; Cui, D. HR-Si prism coupled tightly confined spoof surface plasmon polaritons mode for terahertz sensing. Opt. Express 2019, 27, 34067–34078. [Google Scholar] [CrossRef]
- Williams, C.R.; Andrews, S.R.; Maier, S.A.; Fernández-Domínguez, A.I.; Martín-Moreno, L.; García-Vidal, F.J. Highly confined guiding of terahertz surface plasmon polaritons on structured metal surfaces. Nat. Photonics 2008, 2, 175–179. [Google Scholar] [CrossRef]
- Miyamaru, F.; Hayashi, S.; Otani, C.; Kawase, K.; Ogawa, Y.; Yoshida, H.; Kato, E. Terahertz surface-wave resonant sensor with a metal hole array. Opt. Lett. 2006, 31, 1118–1120. [Google Scholar] [CrossRef]
- Tian, Z.; Han, J.; Lu, X.; Gu, J.; Xing, Q.; Zhang, W. Surface plasmon enhanced terahertz spectroscopic distinguishing between isotopes. Chem. Phys. Lett. 2009, 475, 132–134. [Google Scholar] [CrossRef]
- Hasebe, T.; Yamada, Y.; Tabata, H. Label-free THz sensing of living body-related molecular binding using a metallic mesh. Biochem. Biophys. Res. Commun. 2011, 414, 192–198. [Google Scholar] [CrossRef]
- Ng, B.; Wu, J.; Hanham, S.M.; Fernández-Domínguez, A.I.; Klein, N.; Liew, Y.F.; Breese, M.B.H.; Hong, M.; Maier, S.A. Spoof Plasmon Surfaces: A Novel Platform for THz Sensing. Adv. Opt. Mater. 2013, 1, 543–548. [Google Scholar] [CrossRef]
- Ng, B.; Hanham, S.M.; Wu, J.; Fernandez-Dominguez, A.I.; Klein, N.; Liew, Y.F.; Breese, M.B.H.; Hong, M.; Maier, S.A. Broadband Terahertz Sensing on Spoof Plasmon Surfaces. ACS Photonics 2014, 1, 1059–1067. [Google Scholar] [CrossRef]
- Dias, E.J.C.; Peres, N.M.R. Controlling Spoof Plasmons in a Metal Grating Using Graphene Surface Plasmons. ACS Photonics 2017, 4, 3071–3080. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Z. Spoof surface plasmon based planar antennas for the realization of Terahertz hotspots. Sci. Rep. 2015, 5, 18606. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, W. Ultrasensitive terahertz metamaterial sensor based on spoof surface plasmon. Sci. Rep. 2017, 7, 2092. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; He, X.; Huang, X.; Zhang, B.; Liu, G.; Shu, G.; Fang, C.; Wang, J.; Luo, Y. Terahertz biosensing metamaterial absorber for virus detection based on spoof surface plasmon polaritons, International Journal of RF and Microwave Computer-Aided Engineering. Int. J. RF Microw. Comput. Eng. 2018, 28, e21448. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, B.; Liu, G.; Wang, J.; Luo, Y. Terahertz ultrasensitive biosensing metamaterial and metasurface based on spoof surface plasmon polaritons. Int. J. Numer. Model. Electron. Netw. Devices Fields 2018, 33, e2529. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, W.; Gu, J.; Cong, L.; Chen, X.; Wang, K.; Xu, Q.; Han, J.; Zhang, W. Terahertz metasurface with multiple BICs/QBICs based on a split ring resonator. Opt. Express 2022, 30, 29088–29098. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, L.; Wang, J.; Sun, L.; Jiao, Y.; Meng, Y.; Chen, S.; Chang, C.; Fan, C. Electric Fano resonance-based terahertz metasensors. Nanoscale 2021, 13, 18467–18472. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, F.; Li, Y.; Tang, T.; Liao, Y.; Lu, Y.; Wen, Q. Terahertz metasurfaces based on bound states in the continuum (BIC) for high-sensitivity refractive index sensing. Optik 2022, 261, 169248. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, Y.; Xu, S.; Dai, X. Ultrasensitive terahertz sensing in all-dielectric asymmetric metasurfaces based on quasi-BIC. J. Opt. Soc. Am. B Opt. Phys. 2022, 39, 286–291. [Google Scholar] [CrossRef]
- Srivastava, Y.K.; Ako, R.T.; Gupta, M.; Bhaskaran, M.; Sriram, S.; Singh, R. Terahertz sensing of 7 nm dielectric film with bound states in the continuum metasurfaces. Appl. Phys. Lett. 2019, 115, 151105. [Google Scholar] [CrossRef]
- Wang, R.; Xu, L.; Huang, L.; Zhang, X.; Ruan, H.; Yang, X.; Lou, J.; Chang, C.; Du, X. Ultrasensitive Terahertz Biodetection Enabled by Quasi-BIC-Based Metasensors. Small 2023, 19, e2301165. [Google Scholar] [CrossRef]
- Nie, P.; Zhu, D.; Cui, Z.; Qu, F.; Lin, L.; Wang, Y. Sensitive detection of chlorpyrifos pesticide using an all-dielectric broadband terahertz metamaterial absorber. Sens. Actuators B Chem. 2020, 307, 127642. [Google Scholar] [CrossRef]
- Niharika, N.; Singh, S.; Kumar, P. Bicontrollable all dielectric metasurface absorber for chemical and biosensing applications. Photonics Nanostruct. Fundam. Appl. 2023, 54, 101116. [Google Scholar] [CrossRef]
- Yue, L.; Wang, Y.; Cui, Z.; Zhang, X.; Zhu, Y.; Zhang, X.; Chen, S.; Wang, X.; Zhang, K. Multi-band terahertz resonant absorption based on an all-dielectric grating metasurface for chlorpyrifos sensing. Opt. Express 2021, 29, 13563–13575. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Cui, Z.; Yue, L.; Wang, X.; Yang, C.; Zhang, K.; Wang, Y. A silicon-based metasurface for terahertz sensing. Opt. Commun. 2022, 506, 127572. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, D.; Cui, Z.; Yue, L.; Zhang, X.; Hou, L.; Zhang, K.; Hu, H. Properties and Sensing Performance of All-Dielectric Metasurface THz Absorbers. IEEE Trans. Terahertz Sci. Technol. 2020, 10, 599–605. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Qin, J. A terahertz metasurface sensor with fingerprint enhancement in a wide spectrum band for thin film detection. Nanoscale Adv. 2023, 5, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Du, L.; Liu, Q.; Zhu, L.; Meng, K.; Zou, Y.; Zhang, B. Ultrasensitive specific sensor based on all-dielectric metasurfaces in the terahertz range. RSC Adv. 2020, 10, 33018–33025. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, W.; Chen, D.; Zhou, R.; Wang, Q.; Gao, W.; Kono, J.; Xie, L.; Ying, Y. Ultrahigh-Sensitivity Molecular Sensing with Carbon Nanotube Terahertz Metamaterials. ACS Appl. Mater. Interfaces 2020, 12, 40629–40634. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.T.; Jun, S.W.; Cha, S.H.; Park, J.Y.; Lee, S.; Shin, G.A.; Ahn, Y.H. Enhanced sensitivity in THz plasmonic sensors with silver nanowires. Sci. Rep. 2018, 8, 15536. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, J.; de la Chapelle, M.L.; Huang, G.; Wang, Y.; Zhang, J.; Xu, D.; Yao, J.; Yang, X.; Fu, W. A terahertz metamaterial biosensor for sensitive detection of microRNAs based on gold-nanoparticles and strand displacement amplification. Biosens. Bioelectron. 2021, 175, 112874. [Google Scholar] [CrossRef]
- Amini, B.; Atlasbaf, Z. Design and analysis of high-sensitivity tunable graphene sensors for cancer detection. Opt. Quantum Electron. 2023, 55, 446. [Google Scholar] [CrossRef]
- Zamzam, P.; Rezaei, P.; Abdulkarim, Y.I.; Daraei, O.M. Graphene-based polarization-insensitive metamaterials with perfect absorption for terahertz biosensing applications: Analytical approach. Opt. Laser Technol. 2023, 163, 109444. [Google Scholar] [CrossRef]
- Xu, W.; Xie, L.; Zhu, J.; Tang, L.; Singh, R.; Wang, C.; Ma, Y.; Chen, H.-T.; Ying, Y. Terahertz biosensing with a graphene-metamaterial heterostructure platform. Carbon 2019, 141, 247–252. [Google Scholar] [CrossRef]
- Cen, C.; Zhang, Y.; Chen, X.; Yang, H.; Yi, Z.; Yao, W.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. A dual-band metamaterial absorber for graphene surface plasmon resonance at terahertz frequency. Phys. E Low Dimens. Syst. Nanostruct. 2020, 117, 113840. [Google Scholar] [CrossRef]
- The different dimensions of nanotechnology. Nat. Nanotechnol. 2009, 4, 135. [CrossRef] [PubMed]
- Zhao, J.; Zhang, W.; Sherrell, P.; Razal, J.M.; Huang, X.-F.; Minett, A.I.; Chen, J. Carbon Nanotube Nanoweb–Bioelectrode for Highly Selective Dopamine Sensing. ACS Appl. Mater. Interfaces 2012, 4, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Ji, W.; Fan, H.; Zhang, L.; Wan, X.; Fan, Z.; Liu, G.L.; Peng, Q.; Huang, L. A Metasurface Plasmonic Analysis Platform Combined with Gold Nanoparticles for Ultrasensitive Quantitative Detection of Small Molecules. Biosensors 2023, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xie, L.; Zhu, J.; Xu, X.; Ye, Z.; Wang, C.; Ma, Y.; Ying, Y. Gold Nanoparticle-Based Terahertz Metamaterial Sensors: Mechanisms and Applications. ACS Photonics 2016, 3, 2308–2314. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, R.; Liu, Y.; Chen, X.; Li, K.; Pickwell-Macpherson, E. Gold nanoparticle enhanced detection of EGFR with a terahertz metamaterial biosensor. Biomed. Opt. Express 2021, 12, 1559–1567. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Q.; Liu, K.; Chen, Z.; Li, K.; Zhang, X.; Xu, J.; Pickwell-MacPherson, E. Terahertz Microfluidic Metamaterial Biosensor for Sensitive Detection of Small-Volume Liquid Samples. IEEE Trans. Terahertz Sci. Technol. 2019, 9, 209–214. [Google Scholar] [CrossRef]
- Miyamaru, F.; Hattori, K.; Shiraga, K.; Kawashima, S.; Suga, S.; Nishida, T.; Takeda, M.W.; Ogawa, Y. Highly Sensitive Terahertz Sensing of Glycerol-Water Mixtures with Metamaterials. J. Infrared Millim. Terahertz Waves 2013, 35, 198–207. [Google Scholar] [CrossRef]
- Yang, J.; Qi, L.; Li, B.; Wu, L.; Shi, D.; Uqaili, J.A.; Tao, X. A terahertz metamaterial sensor used for distinguishing glucose concentration. Results Phys. 2021, 26, 104332. [Google Scholar] [CrossRef]
- Han, B.; Han, Z.; Qin, J.; Wang, Y.; Zhao, Z. A sensitive and selective terahertz sensor for the fingerprint detection of lactose. Talanta 2019, 192, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Kang, J.H.; Lee, J.S.; Kim, H.S.; Kim, C.; Kim, J.H.; Lee, T.; Son, J.H.; Park, Q.H.; Seo, M. Highly sensitive and selective sugar detection by terahertz nano-antennas. Sci. Rep. 2015, 5, 15459. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xia, L.; Wei, D.; Yan, S.; Zhang, M.; Yang, Z.; Wang, H.; Du, C.; Cui, H.-L. Rapid and label-free metamaterial-based biosensor for fatty acid detection with terahertz time-domain spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117736. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xie, L.; Zhu, J.; Wang, W.; Ye, Z.; Ma, Y.; Tsai, C.-Y.; Chen, S.; Ying, Y. Terahertz sensing of chlorpyrifos-methyl using metamaterials. Food Chem. 2017, 218, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Li, Z.; Hu, F.; Hu, C.; Chen, T.; Zhang, H.; Zhao, Y. Highly Sensitive Detection of Carbendazim by Using Terahertz Time-Domain Spectroscopy Combined with Metamaterial. IEEE Trans. Terahertz Sci. Technol. 2018, 8, 149–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Y.; Song, X.; Yang, M.; Ren, Y.; Ren, X.; Liang, L.; Yao, J. High-sensitivity detection of chlorothalonil via terahertz metasensor. Mater. Res. Express 2020, 7, 095801. [Google Scholar] [CrossRef]
- Bui, T.S.; Dao, T.D.; Dang, L.H.; Vu, L.D.; Ohi, A.; Nabatame, T.; Lee, Y.; Nagao, T.; Hoang, C.V. Metamaterial-enhanced vibrational absorption spectroscopy for the detection of protein molecules. Sci. Rep. 2016, 6, 32123. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhu, F.; Lang, T.; Liu, J.; Hong, Z.; Qin, J. All-metal terahertz metamaterial biosensor for protein detection. Nanoscale Res. Lett. 2021, 16, 109. [Google Scholar] [CrossRef]
- Li, D.; Lin, S.; Hu, F.; Chen, Z.; Zhang, W.; Han, J. Metamaterial Terahertz Sensor for Measuring Thermal-Induced Denaturation Temperature of Insulin. IEEE Sens. J. 2020, 20, 1821–1828. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Liu, X.; Lin, S.; Deng, B.; Shen, J.; Li, F.; Zhu, J. Plasmonic metasurface enhanced by nanobumps for label-free biosensing of lung tumor markers in serum. Talanta 2023, 264, 124731. [Google Scholar] [CrossRef]
- Li, R.; Fan, H.; Zhou, H.; Chen, Y.; Yu, Q.; Hu, W.; Liu, G.L.; Huang, L. Nanozyme-Catalyzed Metasurface Plasmon Sensor-Based Portable Ultrasensitive Optical Quantification Platform for Cancer Biomarker Screening. Adv. Sci. 2023, 10, 2301658. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Liu, W.; Lin, S.; Chen, Z.; Zeng, L.; Hu, F. Aptamer HB5 modified terahertz metasurface biosensor used for specific detection of HER2. Sens. Actuators B Chem. 2021, 355, 131337. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, X.; Fan, Z.; Lv, X.; Chen, H. A Route to Terahertz Metamaterial Biosensor Integrated with Microfluidics for Liver Cancer Biomarker Testing in Early Stage. Sci. Rep. 2017, 7, 16378. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Guan, M.; Xu, M.; Fang, W.; Zhang, Y.; Zhao, C.; Zeng, Y. Design and application of terahertz metamaterial sensor based on DSRRs in clinical quantitative detection of carcinoembryonic antigen. Opt. Express 2020, 28, 16834–16844. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Yang, S.; Huang, G.; Yang, L.; Zhang, Y.; Tian, H.; Xie, F.; de la Chapelle, M.L.; Yang, X.; Fu, W. Streptavidin-functionalized terahertz metamaterials for attomolar exosomal microRNA assay in pancreatic cancer based on duplex-specific nuclease-triggered rolling circle amplification. Biosens. Bioelectron. 2021, 188, 113314. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, D.; Zhang, W. High-sensitivity and label-free identification of a transgenic genome using a terahertz meta-biosensor. Opt. Express 2018, 26, 31589–31598. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Choe, J.-H.; Kim, C.; Bae, S.; Kim, J.-S.; Park, Q.H.; Seo, M. Graphene assisted terahertz metamaterials for sensitive bio-sensing. Sens. Actuators B Chem. 2020, 310, 127841. [Google Scholar] [CrossRef]
- Park, S.J.; Hong, J.T.; Choi, S.J.; Kim, H.S.; Park, W.K.; Han, S.T.; Park, J.Y.; Lee, S.; Kim, D.S.; Ahn, Y.H. Detection of microorganisms using terahertz metamaterials. Sci. Rep. 2014, 4, 4988. [Google Scholar] [CrossRef]
- Park, S.J.; Son, B.H.; Choi, S.J.; Kim, H.S.; Ahn, Y.H. Sensitive detection of yeast using terahertz slot antennas. Opt. Express 2014, 22, 30467–30472. [Google Scholar] [CrossRef]
- Park, S.J.; Cha, S.H.; Shin, G.A.; Ahn, Y.H. Sensing viruses using terahertz nano-gap metamaterials. Biomed. Opt. Express 2017, 8, 3551–3558. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, H.; Yan, X.; Liang, L.; Wei, D.; Wang, M.; Yang, Q.; Yao, J. Sensitive detection of cancer cell apoptosis based on the non-bianisotropic metamaterials biosensors in terahertz frequency. Opt. Mater. Express 2018, 8, 659–667. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, L.; Ding, L.; Jin, B.; Hou, Y.; Li, C.; Jiang, L.; Liu, W.; Hu, W.; Lu, Y.; et al. Label-free measurements on cell apoptosis using a terahertz metamaterial-based biosensor. Appl. Phys. Lett. 2016, 108, 241105. [Google Scholar] [CrossRef]
- Huang, S.T.; Hsu, S.F.; Tang, K.Y.; Yen, T.J.; Yao, D.J. Application of a Terahertz System Combined with an X-Shaped Metamaterial Microfluidic Cartridge. Micromachines 2020, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, M.; Xia, L.; Yu, W.; Peng, J.; Zhang, Y.; de la Chapelle, M.L.; Yang, K.; Cui, H.-L.; Fu, W. Cell viability and hydration assay based on metamaterial-enhanced terahertz spectroscopy. RSC Adv. 2017, 7, 53963–53969. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, L.; Ma, S.; Wang, J.; Su, J.; Liu, A. Terahertz metamaterial designs for capturing and detecting circulating tumor cells. Mater. Res. Express 2018, 6, 045805. [Google Scholar] [CrossRef]

| Detection Basis | Metamaterial Structure | Analytes | Sensitivity | Resolution | Linearity | Ref | |
|---|---|---|---|---|---|---|---|
| Sensing principle | Transmission dip shift | Split-ring resonator | BSA | 95 GHz/(mmol/L) | 17.7 µmol/L | - | [48] |
| Mirror-asymmetric SRRs | A549 cells | 213 GHz for 5 × 105 cells/mL | - | - | [53] | ||
| Absorption peak shift | Loop shape | Organochlorine pesticide | - | 0.213 mg/L | 0.9863 | [55] | |
| Bi-layer structure | Biotin | 153 GHz/µM | - | - | [56] | ||
| Microchannel assisted | Ar/ethanol/glucose | 0.2 THz/RIU | - | - | [57] | ||
| Square and split-ring hybrid structure | 2,4-dichlorophenoxyacetic acid | - | 0.1 ppm | - | [58] | ||
| Polarization rotation | Hexagon structure | Tumor cells | - | 3 × 103 cells/mL | - | [60] | |
| EIT-like resonance | Cut-wire structure and a split-ring resonator | lung cancer cells | - | 5 × 105 cells/mL | - | [70] | |
| Fano resonance | Asymmetric double-cut wire | water-methanol mixture | 160 GHz/RIU | - | - | [72] | |
| Asymmetric gap | ethanol solution | 112 GHz/RIU | - | - | [73] | ||
| Ultra-thin substrate | protein A/G | 240 GHz/RIU | - | - | [74] | ||
| Free of substrate | galactose | 91.7 GHz/RIU | - | - | [75] | ||
| Stacked nanorods | IPA/metanol/ethanol | 1 THz/RIU | - | - | [76] | ||
| Toroidal resonance | Symmetrically arranged split-ring pair | Lung cancer cells | 485.3 GHz/RIU | - | - | [79] | |
| SPP resonance | Metal holes | 12 CH3OH and 12 CD3OD | 0.4 THz/RIU | - | - | [86] | |
| Quasi-BIC | Triple micro-rods | Interleukin-6 | - | 1 nM | - | [96] | |
| Split-ring resonator | C-reactive protein | 674 GHz/RIU | 1 pM | - | [100] | ||
| Special materials | All-dielectric materials | Ring and a cylinder | Chlorpyrifos | - | 0.1 mg/L | 0.9943 | [103] |
| Cylinder dimers | Lactose | - | 1.53 mg /cm2 | - | [106] | ||
| Glucose | - | 1.54 mg /cm2 | - | [106] | |||
| Zigzag array of tilted silicon bars | Tyrosine | - | 6.7 mg/cm2 | - | [107] | ||
| Santonin | - | 59.35 mg/cm2 | - | [107] | |||
| Carbon assisted | CNT coated | Lactose | - | 40 ng/mL | - | [108] | |
| Glucose | - | 30 ng/ml | - | [108] | |||
| NW and NP | AuNPs conjugated | Avidin | - | 7.8 fmol | 0.9371 | [118] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Lin, J.; Yuan, Z.; Lin, Y.; Shang, W.; Chin, L.K.; Zhang, M. Terahertz Metamaterials for Biosensing Applications: A Review. Biosensors 2024, 14, 3. https://doi.org/10.3390/bios14010003
Zhang W, Lin J, Yuan Z, Lin Y, Shang W, Chin LK, Zhang M. Terahertz Metamaterials for Biosensing Applications: A Review. Biosensors. 2024; 14(1):3. https://doi.org/10.3390/bios14010003
Chicago/Turabian StyleZhang, Wu, Jiahan Lin, Zhengxin Yuan, Yanxiao Lin, Wenli Shang, Lip Ket Chin, and Meng Zhang. 2024. "Terahertz Metamaterials for Biosensing Applications: A Review" Biosensors 14, no. 1: 3. https://doi.org/10.3390/bios14010003
APA StyleZhang, W., Lin, J., Yuan, Z., Lin, Y., Shang, W., Chin, L. K., & Zhang, M. (2024). Terahertz Metamaterials for Biosensing Applications: A Review. Biosensors, 14(1), 3. https://doi.org/10.3390/bios14010003