Photoelectrochemical Enzyme Biosensor for Malate Using Quantum Dots on Indium Tin Oxide/Plastics as a Sensing Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
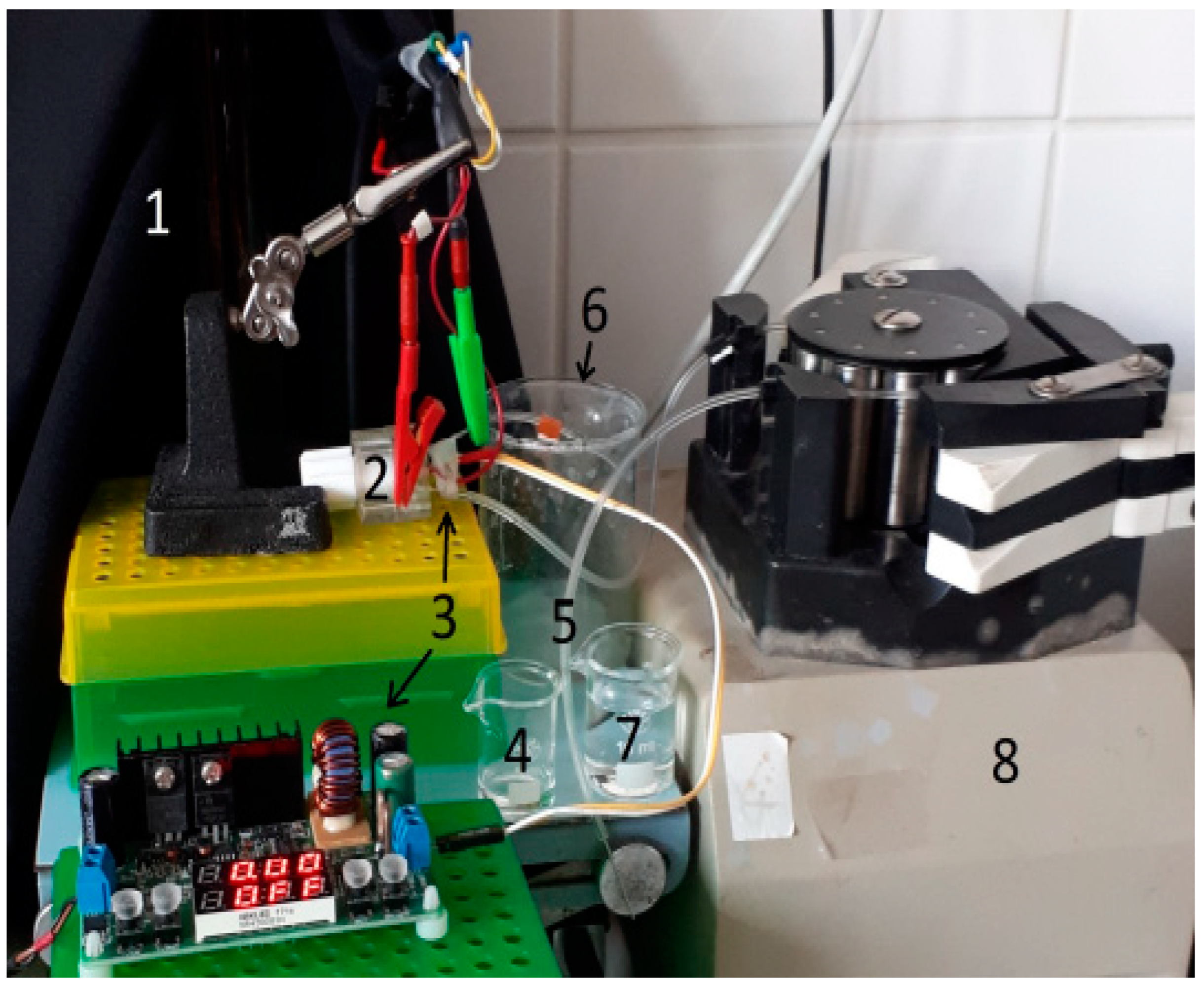
2.2. Instrumentation
2.3. ITO-PET Based Electrodes
2.4. Immobilization of MDH and QDs on ITO-PET
- Crosslinking with GA. A 40 µL mixture was prepared by combining 10 µL of MDH, 10 µL of BSA, 20 µL of PBS and 3 µL of GA. Next, 10 µL of the mixture was immediately applied on the QDs@ITO-PET.
- Gelatin solution was warmed in a water bath, and an immobilization mixture was prepared by combining 40 µL of MDH, 10 µL of BSA, 20 µL of PBS, 20 µL of gelatin and 30 µL of QDs. This mixture was quickly deposited on the ITO-PET.
- Gelatin/GA immobilization process was the same as the previous one. The immobilization mixture was composed of 40 µL of MDH, 10 µL of BSA, 20 µL of PBS, 20 µL of gelatin, 30 µL of QDs and 5 µL of GA.
- Polyethylene imine. The immobilization mixture consisted of 20 µL of PEI, 40 µL of MDH, 10 µL of BSA, 20 µL of PBS and 30 µL of QDs. 10 µL of the mixture was applied onto the ITO-PET strip and allowed to dry.
- PEI/GA was nearly the same as above, with the addition of 2.5 µL of GA (in this case, 1% solution was used).
- Nafion 1. This mixture contained 5 µL of MDH, 1.25 µL of BSA, 2.5 µL of Nafion, 4.5 µL of QDs and 10 µL of PBS. 10 µL of the mixture was applied onto the surface of the ITO-PET.
- The other three variants Nafion 2, 3 and 4 followed the same first step of immobilizing a mixture with the enzyme. The mixture used in these variants contained 20 µL of MDH, 5 µL of BSA, 13.5 µL of QDs and 10 µL of PBS. This mixture was also applied to the surface of ITO-PET and allowed to dry. The subsequent step involved coating the enzyme layer with 5 µL of Nafion at the initial concentration and diluting it with water in either a 1:1 or 1:2 ratio before allowing it to dry.
- PEI/GA/Nafion. The immobilization mixture consisted of 10 µL of MDH, 2.5 µL of BSA, 5 µL of PBS, 5 µL of PEI, 7.5 µL of QDs, and 1.25 µL of GA (1%). The mixture was applied to the ITO-PET strip and allowed to dry. Afterwards, 5 µL of Nafion diluted with water (1:1) was used to overlay the enzyme mixture.
2.5. Measurement Procedure with PEC Biosensors
3. Results and Discussion
3.1. Testing Properties of ITO-PET
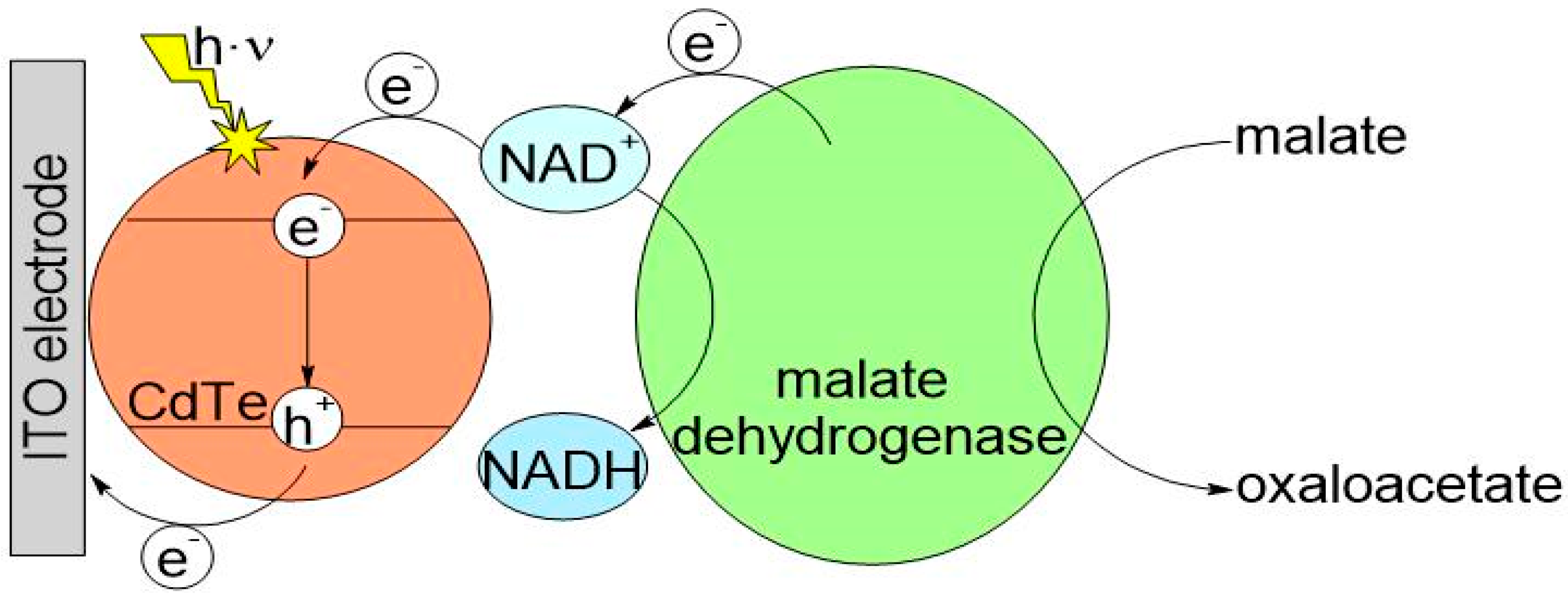
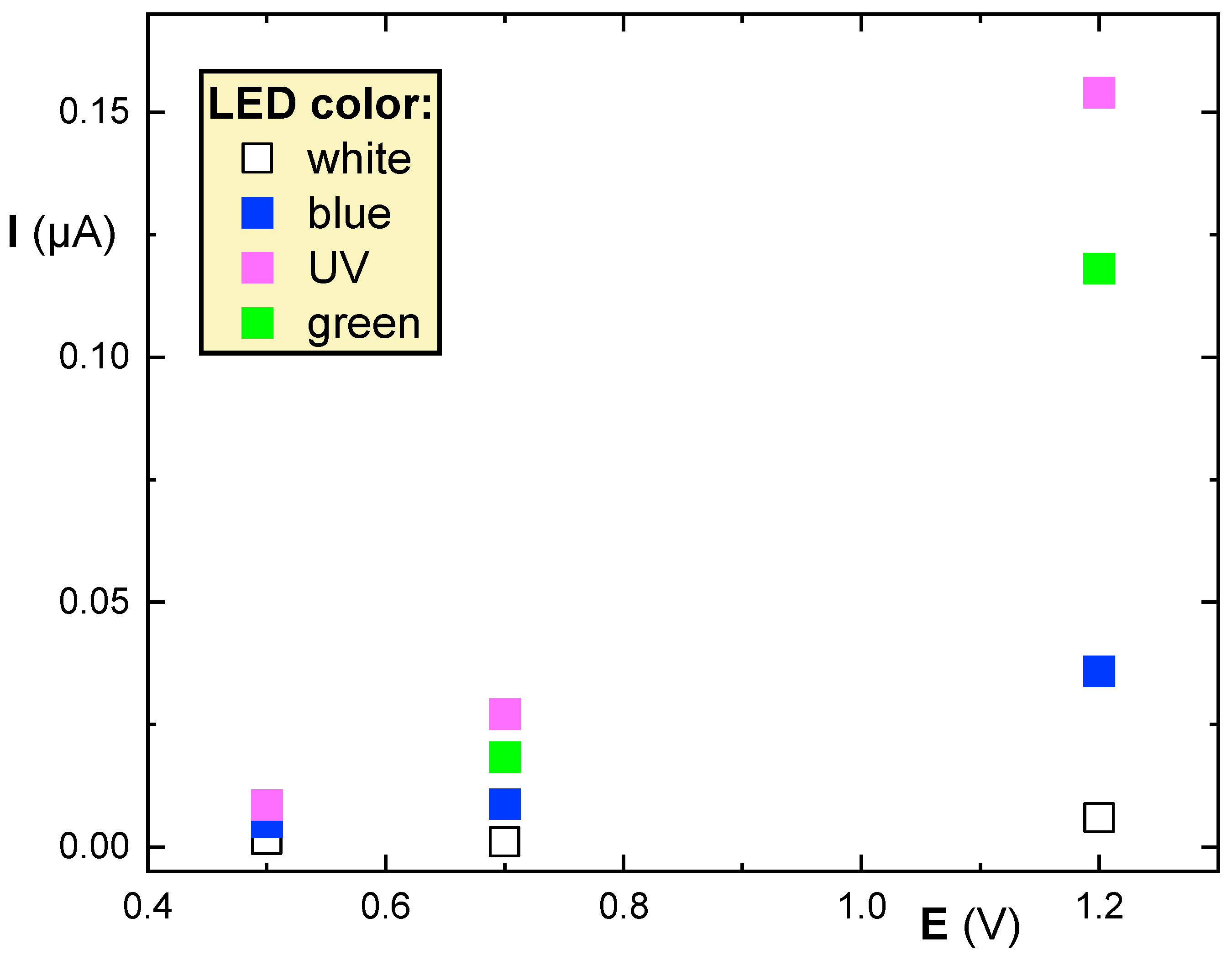
3.2. Testing the QD@ITO-PET Photoelectrochemical Properties
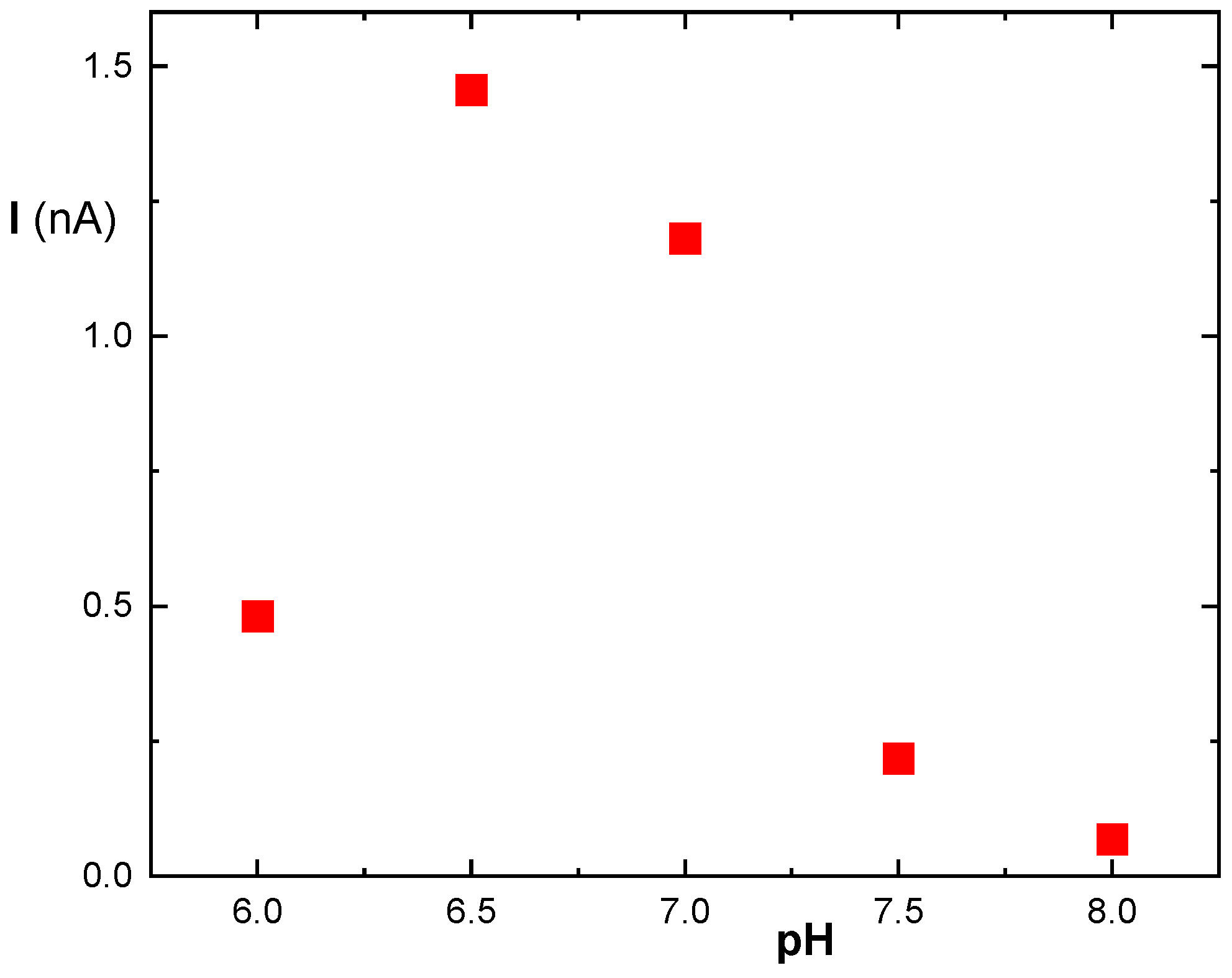
3.3. Immobilization of MDH
3.4. Determination of Malic Acid Concentration in Drinks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.-W.; Xiong, M.; Li, X.-R.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical bioanalysis: A mini review. Electrochem. Commun. 2014, 38, 40–43. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical DNA Biosensors. Chem. Rev. 2014, 114, 7421–7441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-X.; Zhao, C.-Z. Progress of Photoelectrochemical Analysis and Sensors. Chin. J. Anal. Chem. 2013, 41, 436–444. [Google Scholar] [CrossRef]
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. Photoelectrochemical biosensors: New insight into promising photoelectrodes and signal amplification strategies. J. Photochem. Photobiol. C Photochem. Reviews. 2015, 24, 43–63. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical bioanalysis: The state of the art. Chem. Soc. Rev. 2015, 44, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical enzymatic biosensors. Biosens. Bioelectron. 2017, 92, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Salimi, A.; Navaee, A. Electrochemical and Photoelectrochemical Sensing og NADH and Ethanol Based on Immobilization of Electrogenerated Chlorpromazine Sulfoxide onto Graphene-CdS Quantum Dot/Ionic Liquid Nanocomposite. Electroanalysis 2014, 26, 530–540. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Hallaj, R. Low potential detection of NADH based on Fe3O4 nanoparticles/multiwalled carbon nanotubes composite: Fabrication of integrated dehydrogenase-based lactate biosensor. Biosens. Bioelectron. 2012, 33, 60–68. [Google Scholar] [CrossRef]
- Aydın, E.B.; Sezgintürk, M.K. Indium tin oxide (ITO): A promising material in biosensing technology. Trends Anal. Chem. 2017, 97, 309–3015. [Google Scholar] [CrossRef]
- Mattox, D.M.; Mattox, V.H. Chapter: Review of transparent conductive oxides (TCO). In 50 Years of Vacuum Coating Technology and the Growth of the Society of Vacuum Coaters; Society of Vacuum Coaters: Albuquerque, NM, USA, 2007; ISBN 978-1878068279. [Google Scholar]
- Stadler, A. Transparent Conducting Oxides—An Up-To-Date Overview. Materials 2012, 5, 661–683. [Google Scholar] [CrossRef]
- Mistra, M.; Hwang, D.-K.; Kim, Y.C.; Myoung, J.-M.; Lee, T.I. Eco-friendly method of fabricating indium-tin-oxide thin films using pure aqueous sol-gel. Ceram. Int. 2018, 44, 2927–2933. [Google Scholar] [CrossRef]
- Gökceli, G.; Karatepe, N. Improving the properties of indium tin oxide thin films by the incorporation of carbon nanotubes with solution-based techniques. Thin Solid Film. 2020, 697, 137844. [Google Scholar] [CrossRef]
- Aydin, E.B.; Aydin, M.; Sezgintürk, M.K. The development of an ultra-sensitive electrochemical immunosensor using a PPyr-NHS functionalized disposable ITO sheet for the detection of interleukin 6 in real human serums. New J. Chem. 2020, 44, 14228. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Optical and electrical properties of transparent conductive ITO thin films deposited by sol-gel process. Thin Solid Film. 2000, 377, 455–459. [Google Scholar] [CrossRef]
- Demirhan, Y.; Koseoglu, H.; Turkoglu, F.; Uyanik, Z.; Ozdemir, M.; Aygun, G.; Ozyuzer, L. The controllable deposition of large area roll-to-roll sputtered ito thin films for photovoltaic applications. Renew. Energy 2020, 146, 1549–1559. [Google Scholar] [CrossRef]
- Zhang, J.; Au, K.H.; Zhu, Z.Q.; O’Shea, S. Sol-gel preparation of poly(ethylene glycol) doped indium tin oxide thin films for sensing applications. Opt. Mater. 2004, 26, 47–55. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Di, J.; Tu, Y. Disposable biosensor based on immobilization of glucose oxidase at gold nanoparticles electrodeposited on indium tin oxide electrode. Sens. Actuators B Chem. 2008, 135, 283–288. [Google Scholar] [CrossRef]
- Karaboga, M.N.S.; Simsek, C.S.; Sezgintürk, M.K. AuNPs modified, disposable, ITO based biosensor: Early diagnosis of heat shock protein 70. Biosens. Bioelectron. 2016, 81, 22–29. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Ruan, Y.-F.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Quantum-dots-based photoelectrochemical bioanalysis highlighted with recent examples. Biosens. Bioelectron. 2017, 94, 207–2018. [Google Scholar] [CrossRef]
- Riedel, M.; Sabir, N.; Scheller, F.W.; Parak, W.J.; Lisdat, F. Connecting quantum dots with enzymes: Mediator-based approaches for the light-directed read-out of glucose and fructose oxidation. Nanoscale 2017, 9, 2814–2823. [Google Scholar] [CrossRef]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum dots in bioanalysis: A review of applications across various platforms for fluorescence spectroscopy and imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [PubMed]
- Tanne, J.; Schäfer, D.; Khalid, W.; Parak, W.J.; Lisdat, F. Light-Controlled Bioelectrochemical Sensor Based on CdSe/ZnS Quantum Dots. Anal. Chem. 2011, 83, 7778–7785. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Zayats, M.; Willner, I. Semiconductor Quantum Dots for Bioanalysis. Angew. Chem. Int. Ed. Engl. 2008, 47, 7602–7625. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, T.; Kudera, S.; Liedl, T.; Javier, A.M.; Manna, L.; Parak, W.J. On the Development of Colloidal Nanoparticles towards Multifunctional Structures and their Possible Use for Biological Applications. Small 2005, 1, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zheng, Z.; Yang, J.; Chen, M.; Yao, Q.; Chen, Y.; Gao, W. The visible light-driven and self-powered photoelectrochemical biosensor for organophosphate pesticides detection based on nitrogen doped carbon quantum dots for the signal amplification. Electrochim. Acta 2019, 296, 627–636. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Yin, H.; Li, F.; Li, H.; Guo, R.; Han, Y.; Ai, S. Photoelectrochemical biosensor for histone acetyltrasferase detection based on ZnO quantum dots inhibited photoactivity of BiOI nanoflower. Sens. Actuators B Chem. 2020, 307, 127633. [Google Scholar] [CrossRef]
- McAlister-Henn, L. Evolutionary relationships among the malate dehydrogenases. Trends Biochem. Sci. 1988, 13, 178–181. [Google Scholar] [CrossRef]
- Hall, M.D.; Levitt, D.G.; Banaszak, L.J. Crystal Structure of Escherichia coli Malate Dehydrogenase. A Complex of the Apoenzyme and Citrate at 1.87 A Resolution. J. Mol. Biol. 1992, 226, 867–882. [Google Scholar] [CrossRef]
- Goward, C.R.; Nicholls, D. Malate dehydrogenase: A model for structure, evolution, and catalysis. Protein Sci. 1994, 3, 1883–1888. [Google Scholar] [CrossRef]
- Porzani, S.J.; Samaneh, J.; Lorenzi, A.S.; Eghtedari, M.; Nowruzi, B. Interaction of Dehydrogenase Enzymes with Nanoparticles in Industrial and Medical Applications, and the Associated Challenges: A Mini-review. Mini Rev. Med. Chem. 2021, 21, 1351–1366. [Google Scholar] [CrossRef]
- Ravariu, C.; Srinivasulu, A.; Mihaiescu, D.E.; Musala, S. Generalized Analytical Model for Enzymatic BioFET Transistors. Biosensors 2022, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Gur, B.; Isik, M.; Kiransan, K.D.; Alanyalioglu, M.; Beydemir, S.; Meral, K. High enzymatic activity preservation of malate dehydrogenase immobilized in a Langmuir-Blodgett film and its electrochemical biosensor application for malic acid detection. RSC Adv. 2016, 6, 79792–79797. [Google Scholar] [CrossRef]
- Matthews, C.J.; Andrews, E.S.V.; Patrick, W.M. Enzyme-based amperometric biosensors for malic acid—A review. Anal. Chim. Acta 2021, 1156, 338218. [Google Scholar] [CrossRef]
- Palleschi, G.; Volpe, G.; Compagnone, D.; Notte, E.L.; Esti, M. Bioelectrochemical determination of lactic and malic acids in wine. Talanta 1994, 41, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Arvinte, A.; Rotariu, L.; Bala, C. Amperometric Low-Potential Detection of Malic Acid Using Single-Wall Carbon Nanotubes Based Electrodes. Sensors 2008, 8, 1497–1507. [Google Scholar] [CrossRef]
- Gimenez-Gomez, P.; Gutierrez-Capitan, M.; Capdevila, F.; Puig-Pujol, A.; Fernandez-Sanchez, C.; Jimenez-Jorquera, C. Robust l-malate bienzymatic biosensor to enable the on-site monitoring of malolactic fermentation of red wines. Anal. Chim. Acta 2017, 954, 105–113. [Google Scholar] [CrossRef]
- Monošík, R.; Streďanský, M.; Greif, G.; Šturdík, E. Comparison of biosensors based on gold and nanocomposite electrodes for monitoring of malic acid in wine. Cent. Eur. J. Chem. 2012, 10, 157–164. [Google Scholar] [CrossRef]
- Vasilescu, A.; Fanjul-Bolado, P.; Titoiu, A.M.; Porum, R.; Epure, P. Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production. Chemosensors 2019, 7, 66. [Google Scholar] [CrossRef]



| Construction | Method | LOD/Upper Limit (mmol/L) | Ref. |
|---|---|---|---|
| glass electrode | pH sensor | 0.75/30 | [35] |
| gC, SWCNT, MDH in Nafion | amperometry | 0.033/1.3 | [36] |
| Au, MDH and DP in polypyrrole | amperometry | 0.000063/0.001 | [37] |
| Au, MWCNT, MDH and DP | amperometry | 0.0016/0.61 | [38] |
| ITO, Langmuir-Blodget film | voltammetry | 3/50 | [33] |
| ITO, MDH and QDs in Nafion layer | photoelectrochemical | 0.28/2 | this work |
| Samples | Malate (mmol/L) | SD (mmol/L) |
|---|---|---|
| Fruit juices | ||
| Aquila Fruttimo apple | 7.2 | 2.6 |
| Relax juice 100% apple | 14.2 | 8.5 |
| Relax Exotica mango | 10.5 | 4.2 |
| Tesco 100% apple—directly pressed | 24.1 | 3.4 |
| Tesco 100% apple—purefruit | 22.6 | 8.7 |
| Wines | ||
| Irsai Oliver | 14.0 | 3.7 |
| Zweigelt Claret | 9.3 | 2.5 |
| Sample/Spiked Level (mmol/L) | Malate ± SD (mmol/L) | Recovery (%) |
|---|---|---|
| Tesco 100% apple—directly pressed/0 | 2.41 ± 0.34 | - |
| Tesco 100% apple—directly pressed/0.5 | 2.95 ± 0.21 | 108 |
| Tesco 100% apple—directly pressed/1.0 | 3.50 ± 0.29 | 109 |
| Relax Exotica mango/0 | 1.05 ± 0.42 | - |
| Relax Exotica mango/0.5 | 2.05 ± 0.29 | 200 |
| Relax Exotica mango/1.0 | 3.26 ± 0.97 | 226 |
| Irsai oliver/0 | 1.40 ± 0.37 | - |
| Irsai oliver/0.5 | 2.06 ± 0.38 | 132 |
| Irsai oliver/1.0 | 2.59 ± 0.34 | 119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlaváčová, T.; Skládal, P. Photoelectrochemical Enzyme Biosensor for Malate Using Quantum Dots on Indium Tin Oxide/Plastics as a Sensing Surface. Biosensors 2024, 14, 11. https://doi.org/10.3390/bios14010011
Hlaváčová T, Skládal P. Photoelectrochemical Enzyme Biosensor for Malate Using Quantum Dots on Indium Tin Oxide/Plastics as a Sensing Surface. Biosensors. 2024; 14(1):11. https://doi.org/10.3390/bios14010011
Chicago/Turabian StyleHlaváčová, Tereza, and Petr Skládal. 2024. "Photoelectrochemical Enzyme Biosensor for Malate Using Quantum Dots on Indium Tin Oxide/Plastics as a Sensing Surface" Biosensors 14, no. 1: 11. https://doi.org/10.3390/bios14010011
APA StyleHlaváčová, T., & Skládal, P. (2024). Photoelectrochemical Enzyme Biosensor for Malate Using Quantum Dots on Indium Tin Oxide/Plastics as a Sensing Surface. Biosensors, 14(1), 11. https://doi.org/10.3390/bios14010011






