Wearable Biosensor with Molecularly Imprinted Conductive Polymer Structure to Detect Lentivirus in Aerosol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Virus-Imprinted Polyaniline Structure
2.3. Fabrication of Virus MIP Paper Sensor
2.4. Lentivirus Detection and Resistance Measurement
3. Results and Discussion
3.1. Calibration Curve: Lentivirus Concentration from Liquid on Virus MIP Sensors
3.2. Sensor Selectivity
3.3. Lentivirus Detection from Aerosol on Virus MIP Sensors
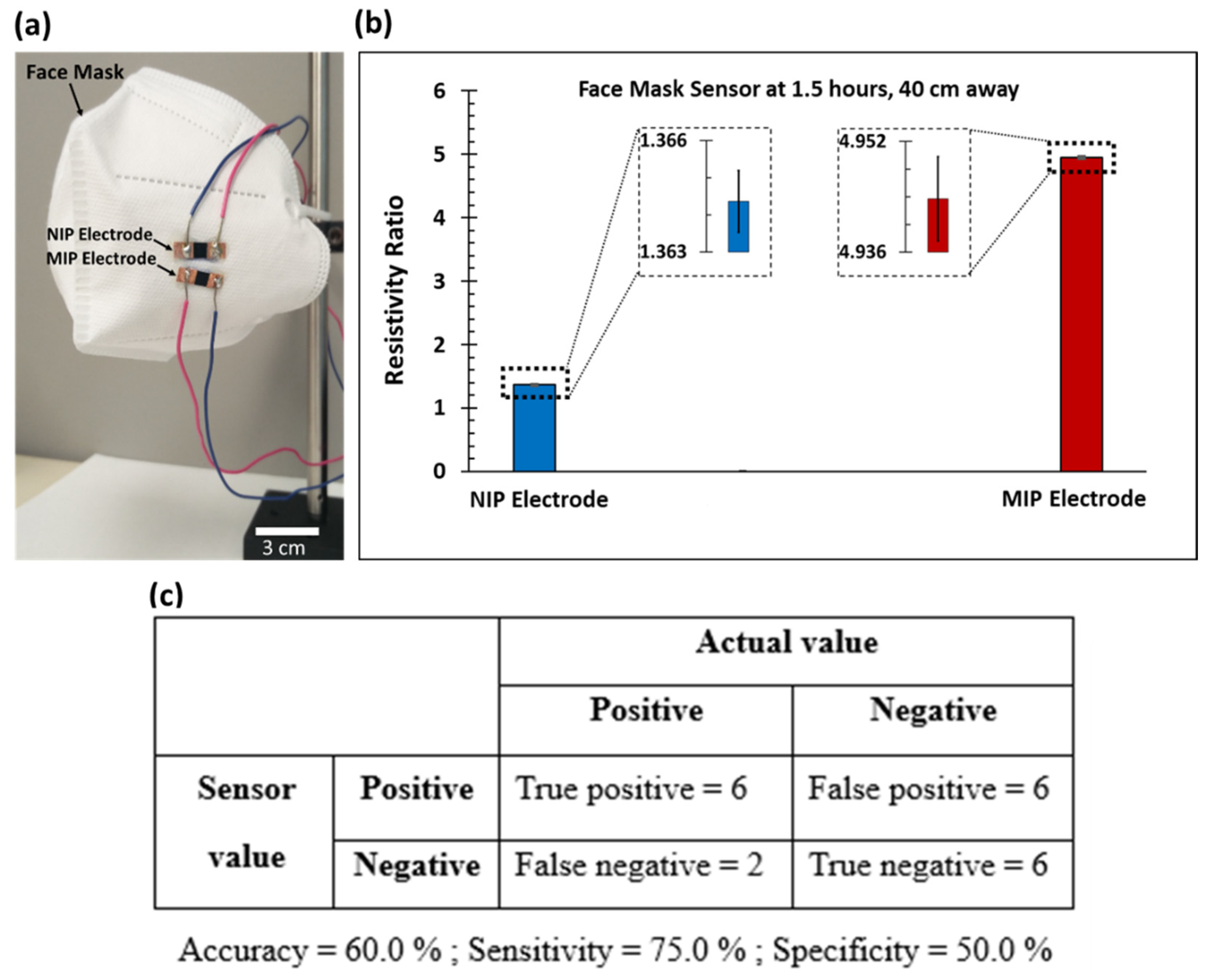
3.4. Face Mask Application and Sensor Accuracy, Sensitivity, and Specificity
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greer, S.; Alexander, G.J. Viral serology and detection. Baillière’s Clin. Gastroenterol. 1995, 9, 689–721. [Google Scholar] [CrossRef]
- Gullett, J.C.; Nolte, F.S. Quantitative nucleic acid amplification methods for viral infections. Clin. Chem. 2015, 61, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, W.; Xu, T.; Chen, H.; Jin, Z.; Zhang, Z.; Song, Q.; Tang, Y. Miniaturized paper-based smartphone biosensor for differential diagnosis of wild-type pseudorabies virus infection versus vaccination immunization. Sens. Actuators B Chem. 2021, 327, 128893. [Google Scholar]
- Mahendra, C.; Kaisar, M.M.M.; Vasandani, S.R.; Surja, S.S.; Tjoa, E.; Chriestya, F.; Junusmin, K.I.; Widowati, T.A.; Irwanto, A.; Ali, S. Wide Application of Minimally Processed Saliva on Multiple RT-qPCR Kits for SARS-CoV-2 Detection in Indonesia. Front. Cell. Infect. Microbiol. 2021, 11, 691538. [Google Scholar] [CrossRef] [PubMed]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real time quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shen, X.-L.; Zeng, Q.; Wang, H.-S.; Wang, L.-S. A multi-walled carbon nanotubes based molecularly imprinted polymers electrochemical sensor for the sensitive determination of HIV-p24. Talanta 2017, 164, 121–127. [Google Scholar] [CrossRef]
- Mojsoska, B.; Larsen, S.; Olsen, D.A.; Madsen, J.S.; Brandslund, I.; Alatraktchi, F.A. Rapid SARS-CoV-2 detection using electrochemical immunosensor. Sensors 2021, 21, 390. [Google Scholar] [CrossRef]
- Li, J.; Lin, R.; Yang, Y.; Zhao, R.; Song, S.; Zhou, Y.; Shi, J.; Wang, L.; Song, H.; Hao, R. Multichannel Immunosensor Platform for the Rapid Detection of SARS-CoV-2 and Influenza A (H1N1) Virus. ACS Appl. Mater. Interfaces 2021, 13, 22262–22270. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Sharma, A.; Jang, J. Vertical flow-based paper immunosensor for rapid electrochemical and colorimetric detection of influenza virus using a different pore size sample pad. Biosens. Bioelectron. 2019, 126, 36–43. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Buchsbaum, S.F.; Wu, T.-T.; Hsieh, K.; Xiao, Y.; Sun, R.; Soh, H.T. Genetic analysis of H1N1 influenza virus from throat swab samples in a microfluidic system for point-of-care diagnostics. J. Am. Chem. Soc. 2011, 133, 9129–9135. [Google Scholar] [CrossRef]
- Han, J.-H.; Lee, D.; Chew, C.H.C.; Kim, T.; Pak, J.J. A multi-virus detectable microfluidic electrochemical immunosensor for simultaneous detection of H1N1, H5N1, and H7N9 virus using ZnO nanorods for sensitivity enhancement. Sens. Actuators B Chem. 2016, 228, 36–42. [Google Scholar] [CrossRef]
- Eissa, S.; Alhadrami, H.A.; Al-Mozaini, M.; Hassan, A.M.; Zourob, M. Voltammetric-based immunosensor for the detection of SARS-CoV-2 nucleocapsid antigen. Microchim. Acta 2021, 188, 199. [Google Scholar]
- Zhou, C.H.; Wu, Z.; Chen, J.J.; Xiong, C.; Chen, Z.; Pang, D.W.; Zhang, Z.L. Biometallization-Based Electrochemical Magnetoimmunosensing Strategy for Avian Influenza A (H7N9) Virus Particle Detection. Chem. –Asian J. 2015, 10, 1387–1393. [Google Scholar]
- Dong, S.; Zhao, R.; Zhu, J.; Lu, X.; Li, Y.; Qiu, S.; Jia, L.; Jiao, X.; Song, S.; Fan, C.; et al. Electrochemical DNA biosensor based on a tetrahedral nanostructure probe for the detection of avian influenza A (H7N9) virus. ACS Appl. Mater. Interfaces 2015, 7, 8834–8842. [Google Scholar] [CrossRef]
- Tancharoen, C.; Sukjee, W.; Thepparit, C.; Jaimipuk, T.; Auewarakul, P.; Thitithanyanont, A.; Sangma, C. Electrochemical biosensor based on surface imprinting for zika virus detection in serum. ACS Sens. 2018, 4, 69–75. [Google Scholar]
- Afsahi, S.; Lerner, M.B.; Goldstein, J.M.; Lee, J.; Tang, X.; Bagarozzi, D.A., Jr.; Pan, D.; Locascio, L.; Walker, A.; Barron, F.; et al. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens. Bioelectron. 2018, 100, 85–88. [Google Scholar] [CrossRef]
- Altintas, Z.; Gittens, M.; Guerreiro, A.; Thompson, K.-A.; Walker, J.; Piletsky, S.; Tothill, I.E. Detection of waterborne viruses using high affinity molecularly imprinted polymers. Anal. Chem. 2015, 87, 6801–6807. [Google Scholar] [CrossRef]
- Wangchareansak, T.; Thitithanyanont, A.; Chuakheaw, D.; Gleeson, M.P.; Lieberzeit, P.A.; Sangma, C. Influenza A virus molecularly imprinted polymers and their application in virus sub-type classification. J. Mater. Chem. B 2013, 1, 2190–2197. [Google Scholar]
- Sukjee, W.; Thitithanyanont, A.; Wiboon-Ut, S.; Lieberzeit, P.A.; Gleeson, M.P.; Navakul, K.; Sangma, C. An influenza A virus agglutination test using antibody-like polymers. J. Biomater. Sci. Polym. Ed. 2017, 28, 1786–1795. [Google Scholar] [CrossRef]
- Liang, C.; Wang, H.; He, K.; Chen, C.; Chen, X.; Gong, H.; Cai, C. A virus-MIPs fluorescent sensor based on FRET for highly sensitive detection of JEV. Talanta 2016, 160, 360–366. [Google Scholar] [CrossRef]
- Navakul, K.; Warakulwit, C.; Yenchitsomanus, P.-t.; Panya, A.; Lieberzeit, P.A.; Sangma, C. A novel method for dengue virus detection and antibody screening using a graphene-polymer based electrochemical biosensor. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, K.; Roushani, M. A nanohybrid probe based on double recognition of an aptamer MIP grafted onto a MWCNTs-Chit nanocomposite for sensing hepatitis C virus core antigen. Sens. Actuators B Chem. 2018, 258, 1066–1071. [Google Scholar] [CrossRef]
- Chen, Z.; Wright, C.; Dincel, O.; Chi, T.-Y.; Kameoka, J. A Low-Cost Paper Glucose Sensor with Molecularly Imprinted Polyaniline Electrode. Sensors 2020, 20, 1098. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.-Y.; Chen, Z.; Kameoka, J. Perfluorooctanesulfonic Acid Detection Using Molecularly Imprinted Polyaniline on a Paper Substrate. Sensors 2020, 20, 7301. [Google Scholar] [CrossRef]
- Chen, Z.; Chi, T.-Y.; Dincel, O.; Tong, L.; Kameoka, J. A Low-cost and Enzyme-free Glucose Paper Sensor. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 4097–4100. [Google Scholar]
- Lee, D.-F.; Su, J.; Ang, Y.-S.; Carvajal-Vergara, X.; Mulero-Navarro, S.; Pereira, C.F.; Gingold, J.; Wang, H.-L.; Zhao, R.; Sevilla, A.; et al. Regulation of embryonic and induced pluripotency by aurora kinase-p53 signaling. Cell Stem Cell 2012, 11, 179–194. [Google Scholar] [CrossRef]
- Panggabean, A.S.; Silaban, H.S.; Pasaribu, S.P.; Alimuddin. Method validation of Cd (II) determination in lubrication oil by direct dilution method using atomic absorption spectrophotometer. J. Phys. Conf. Ser. 2019, 1277, 012004. [Google Scholar] [CrossRef]
- Arkhipenko, M.V.; Nikitin, N.A.; Baranov, O.A.; Evtushenko, E.A.; Atabekov, J.G.; Karpova, O.V. Surface charge mapping on virions and virus-like particles of helical plant viruses. Acta Naturae 2019, 11, 73–78. [Google Scholar] [CrossRef]
- Tsuda, A.; Henry, F.S.; Butler, J.P. Particle Transport and Deposition: Basic Physics of Particle Kinetics. Compr. Physiol. 2013, 3, 1437–1471. [Google Scholar]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef]
- Breshears, L.E.; Nguyen, B.T.; Robles, S.M.; Wu, L.; Yoon, J.-Y. Biosensor detection of airborne respiratory viruses such as SARS-CoV-2. SLAS Technol. 2022, 27, 4–17. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; He, Q.; Yan, J.; Xiong, H.; Wen, N.; Cai, S.; Peng, D.; Liu, Y.; Liu, Z. Metal-organic frameworks for virus detection. Biosens. Bioelectron. 2020, 169, 112604. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Zion, P.L.; Batra, J.; Pursell, C.J. Correlation for the convective and diffusive evaporation of a sessile drop. Int. J. Heat Mass Transf. 2013, 64, 278–285. [Google Scholar] [CrossRef]
- Kelly-Zion, P.L.; Pursell, C.J.; Vaidya, S.; Batra, J. Evaporation of sessile drops under combined diffusion and natural convection. Colloids Surf. A Physicochem. Eng. Asp. 2011, 381, 31–36. [Google Scholar] [CrossRef]
- Olad, A.; Khatamian, M.; Naseri, B. Removal of toxic hexavalent chromium by polyaniline modified clinoptilolite nanoparticles. J. Iran. Chem. Soc. 2011, 8, S141–S151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batra, J.S.; Chi, T.-Y.; Huang, M.-F.; Zhu, D.; Chen, Z.; Lee, D.-F.; Kameoka, J. Wearable Biosensor with Molecularly Imprinted Conductive Polymer Structure to Detect Lentivirus in Aerosol. Biosensors 2023, 13, 861. https://doi.org/10.3390/bios13090861
Batra JS, Chi T-Y, Huang M-F, Zhu D, Chen Z, Lee D-F, Kameoka J. Wearable Biosensor with Molecularly Imprinted Conductive Polymer Structure to Detect Lentivirus in Aerosol. Biosensors. 2023; 13(9):861. https://doi.org/10.3390/bios13090861
Chicago/Turabian StyleBatra, Jaskirat Singh, Ting-Yen Chi, Mo-Fan Huang, Dandan Zhu, Zheyuan Chen, Dung-Fang Lee, and Jun Kameoka. 2023. "Wearable Biosensor with Molecularly Imprinted Conductive Polymer Structure to Detect Lentivirus in Aerosol" Biosensors 13, no. 9: 861. https://doi.org/10.3390/bios13090861
APA StyleBatra, J. S., Chi, T.-Y., Huang, M.-F., Zhu, D., Chen, Z., Lee, D.-F., & Kameoka, J. (2023). Wearable Biosensor with Molecularly Imprinted Conductive Polymer Structure to Detect Lentivirus in Aerosol. Biosensors, 13(9), 861. https://doi.org/10.3390/bios13090861