Toward Continuous Molecular Testing Using Gold-Coated Threads as Multi-Target Electrochemical Biosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
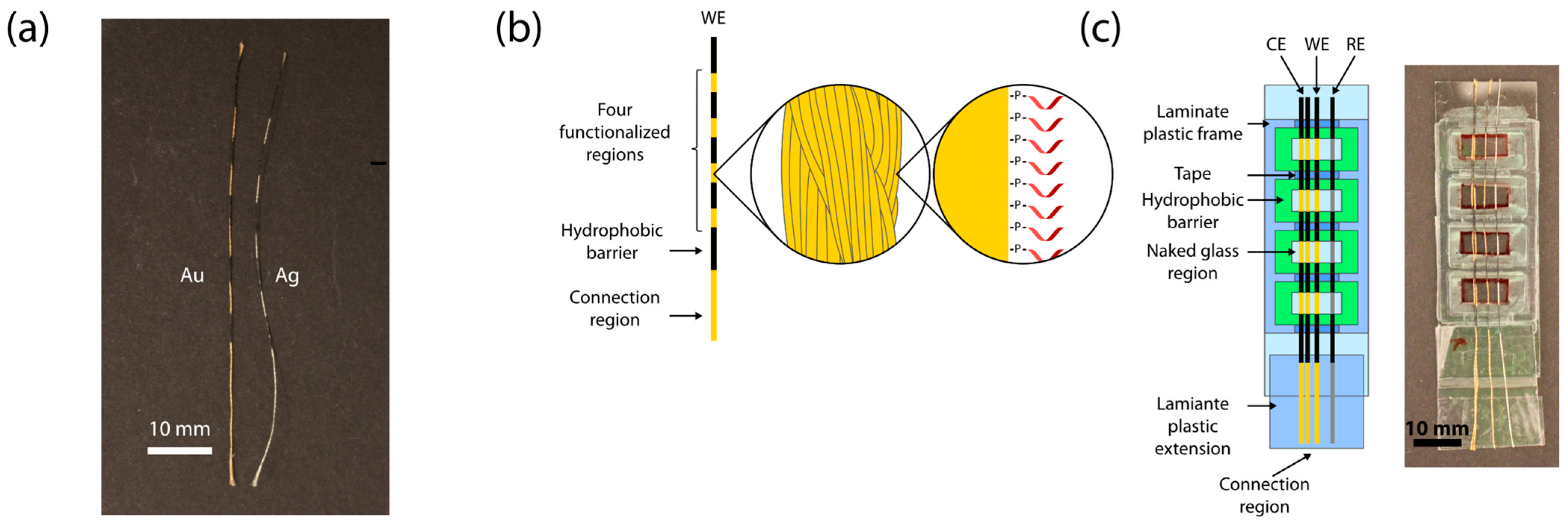
2.2. Device Assembly
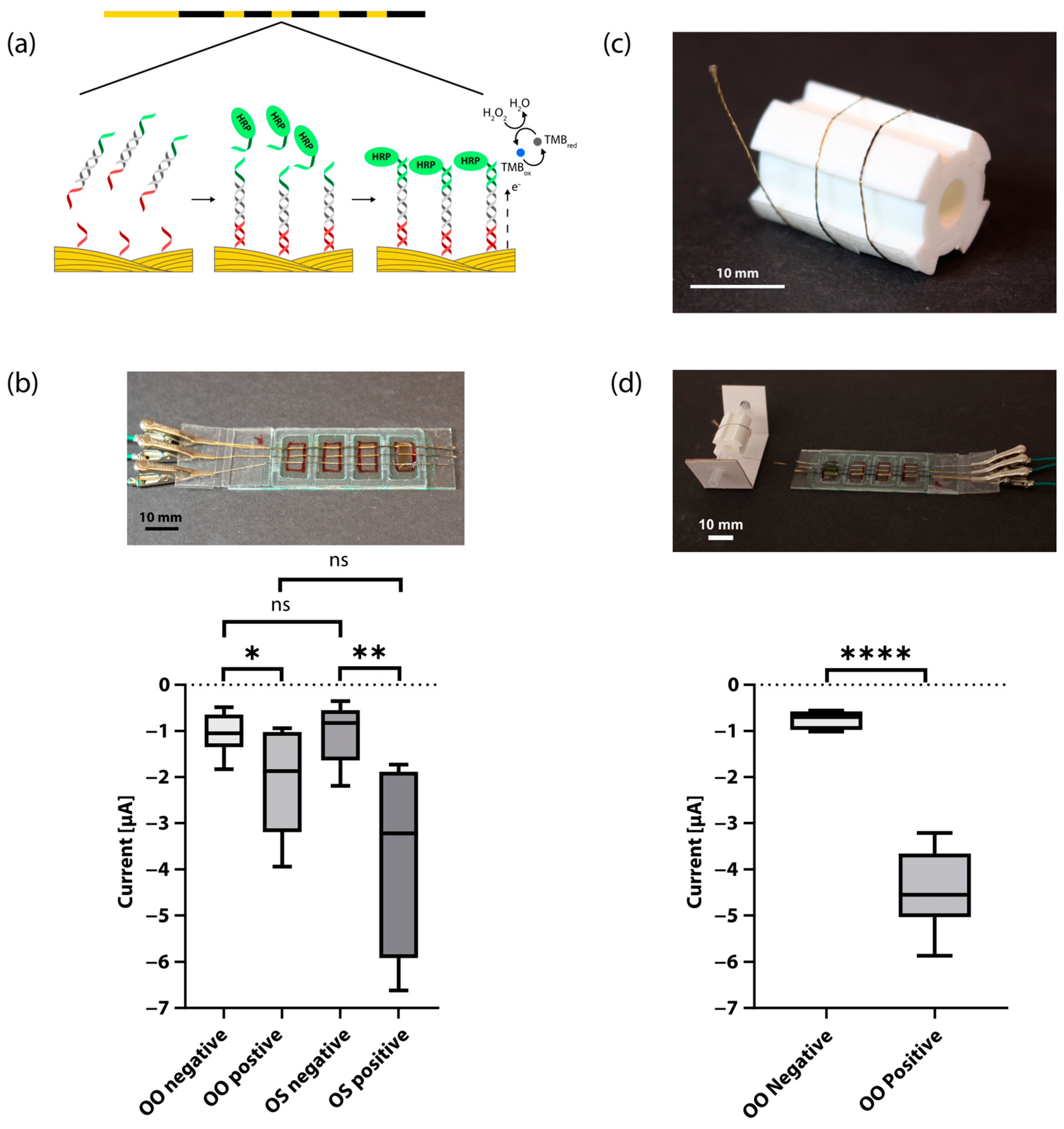
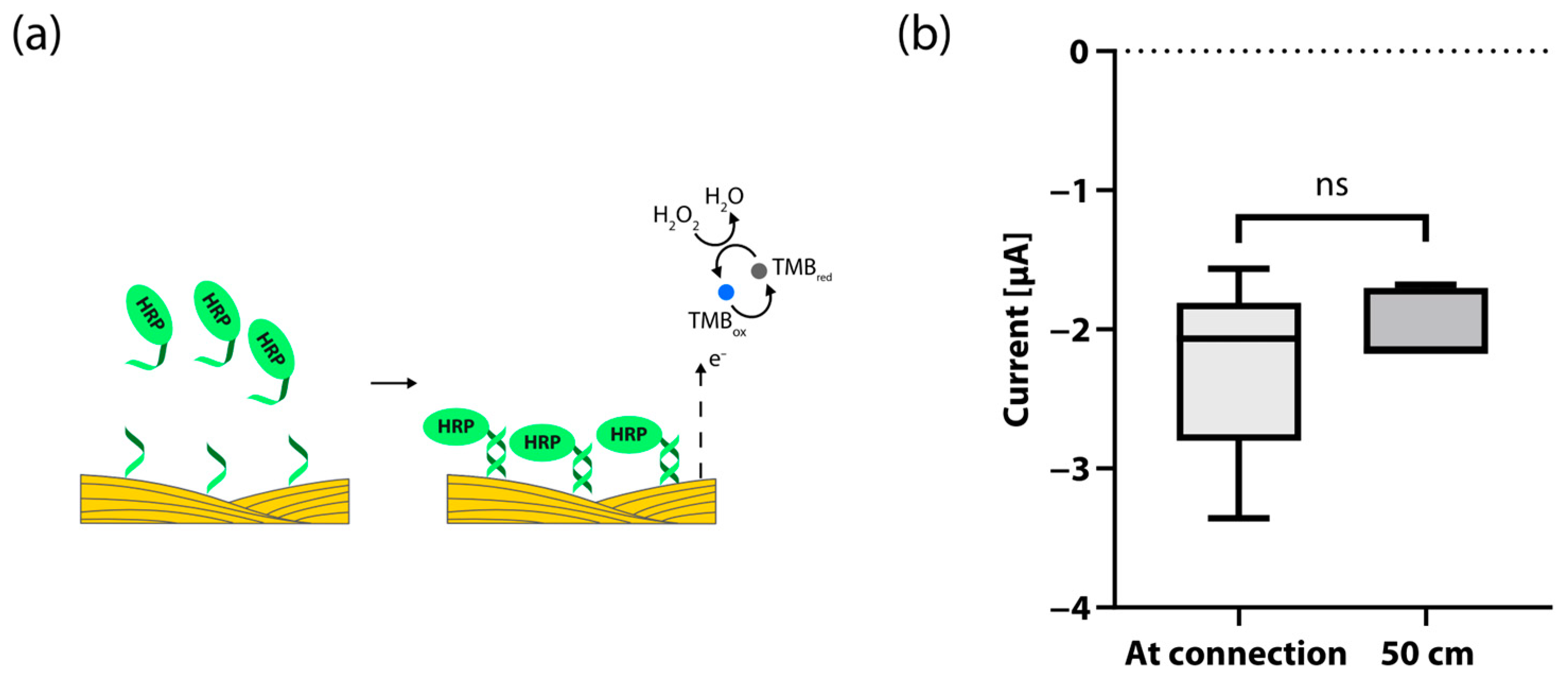
2.3. Functionalization of Au Threads
2.4. Rolling Threads on Spool
2.5. DNA Amplification
2.6. Gel Electrophoresis
2.7. Sandwich Hybridization Assay and Electrochemical Detection
2.8. Electrical Signal Comparison
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaliliazar, S.; Ouyang1, L.; Piper, A.; Chondrogiannis, G.; Hanze, M.; Herland, A.; Hamedi, M.M. Electrochemical Detection of Genomic DNA Utilizing Recombinase Polymerase Amplification (RPA) and Stem-Loop Probe. ACS Omega 2020, 5, 12103–12109. [Google Scholar] [CrossRef] [PubMed]
- Khaliliazar, S.; Öberg Månsson, I.; Piper, A.; Ouyang, L.; Réu, P.; Hamedi, M.M. Woven Electroanalytical Biosensor for Nucleic Acid Amplification Tests. Adv. Healthc. Mater. 2021, 10, 2100034. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, S.; Caraballo, M.; Guiducci, C.; Martini, P.; Ross, M.; Santaana, M.; Danley, D.; West, T.; Lanfranchi, G. Overview of Electrochemical DNA Biosensors: New Approaches to Detect the Expression of Life. Sensors 2009, 9, 3122–3148. [Google Scholar] [CrossRef]
- Oliveira, B.B.; Veigas, B.; Baptista, P.V. Isothermal Amplification of Nucleic Acids: The Race for the Next “Gold Standard”. Front. Sens. 2021, 2, 752600. [Google Scholar] [CrossRef]
- Mayboroda, O.; Katakis, I.; O’Sullivan, C.K. Multiplexed Isothermal Nucleic Acid Amplification. Anal. Biochem. 2018, 545, 20–30. [Google Scholar] [CrossRef]
- Hønsvall, B.K.; Robertson, L.J. From Research Lab to Standard Environmental Analysis Tool: Will NASBA Make the Leap? Water Res. 2017, 109, 389–397. [Google Scholar] [CrossRef]
- Ju, Y.; Kim, H.Y.; Ahn, J.K.; Park, H.G. Ultrasensitive Version of Nucleic Acid Sequence-Based Amplification (NASBA) Utilizing a Nicking and Extension Chain Reaction System. Nanoscale 2021, 13, 10785–10791. [Google Scholar] [CrossRef]
- Li, Y.; Fan, P.; Zhou, S.; Zhang, L. Loop-Mediated Isothermal Amplification (LAMP): A Novel Rapid Detection Platform for Pathogens. Microb. Pathog. 2017, 107, 54–61. [Google Scholar] [CrossRef]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a Loop-Mediated Isothermal Amplification Reaction for Diagnostic Applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef]
- Mori, Y.; Kanda, H.; Notomi, T. Loop-Mediated Isothermal Amplification (LAMP): Recent Progress in Research and Development. J. Infect. Chemother. 2013, 19, 404–411. [Google Scholar] [CrossRef]
- Hellyer, T.J.; Nadeau, J.G. Strand Displacement Amplification: A Versatile Tool for Molecular Diagnostics. Expert Rev. Mol. Diagn. 2014, 4, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yin, F.; Chen, H.; Tian, Y.; Zhou, N. A Fluorescent Aptasensor for Staphylococcus Aureus Based on Strand Displacement Amplification and Self-Assembled DNA Hexagonal Structure. Microchim. Acta 2020, 187, 304. [Google Scholar] [CrossRef] [PubMed]
- Barreda-García, S.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Helicase-Dependent Isothermal Amplification: A Novel Tool in the Development of Molecular-Based Analytical Systems for Rapid Pathogen Detection. Anal. Bioanal. Chem. 2018, 410, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ali, M.M.; Brook, M.A.; Li, Y. Rolling Circle Amplification: Applications in Nanotechnology and Biodetection with Functional Nucleic Acids. Angew. Chem. Int. Ed. 2008, 47, 6330–6337. [Google Scholar] [CrossRef] [PubMed]
- Demidov, V.V. Rolling-Circle Amplification in DNA Diagnostics: The Power of Simplicity. Expert Rev. Mol. Diagn. 2014, 2, 542–548. [Google Scholar] [CrossRef]
- Tan, M.; Liao, C.; Liang, L.; Yi, X.; Zhou, Z.; Wei, G. Recent Advances in Recombinase Polymerase Amplification: Principle, Advantages, Disadvantages and Applications. Front. Cell Infect. Microbiol. 2022, 12, 1019071. [Google Scholar] [CrossRef]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase Polymerase Amplification: Basics, Applications and Recent Advances. TrAC Trends Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Li, J.; Macdonald, J.; Von Stetten, F. Review: A Comprehensive Summary of a Decade Development of the Recombinase Polymerase Amplification. Analyst 2018, 144, 31–67. [Google Scholar] [CrossRef]
- Daum, L.T.; Barnes, W.J.; McAvin, J.C.; Neidert, M.S.; Cooper, L.A.; Huff, W.B.; Gaul, L.; Riggins, W.S.; Morris, S.; Salmen, A.; et al. Real-Time PCR Detection of Salmonella in Suspect Foods from a Gastroenteritis Outbreak in Kerr County, Texas. J. Clin. Microbiol. 2002, 40, 3050. [Google Scholar] [CrossRef]
- Nybond, S.; Réu, P.; Rhedin, S.; Svedberg, G.; Alfvén, T.; Gantelius, J.; Svahn, H.A. Adenoviral Detection by Recombinase Polymerase Amplification and Vertical Flow Paper Microarray. Anal. Bioanal. Chem. 2019, 411, 813. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, N.; Comini, E. The Role of Self-Assembled Monolayers in Electronic Devices. J. Mater. Chem. C 2020, 8, 3938–3955. [Google Scholar] [CrossRef]
- Kim, S.; Yoo, H. Self-Assembled Monolayers: Versatile Uses in Electronic Devices from Gate Dielectrics, Dopants, and Biosensing Linkers. Micromachines 2021, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Carr, O.; Raymundo-Pereira, P.A.; Shimizu, F.M.; Sorroche, B.P.; Melendez, M.E.; de Oliveira Pedro, R.; Miranda, P.B.; Carvalho, A.L.; Reis, R.M.; Arantes, L.M.R.B.; et al. Genosensor Made with a Self-Assembled Monolayer Matrix to Detect MGMT Gene Methylation in Head and Neck Cancer Cell Lines. Talanta 2020, 210, 120609. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, Y.; Tilley, R.D.; Gooding, J.J. Rapid and Ultrasensitive Electrochemical Detection of DNA Methylation for Ovarian Cancer Diagnosis. Biosens. Bioelectron. 2022, 206, 114126. [Google Scholar] [CrossRef]
- Khaliliazar, S.; Toldrà, A.; Chondrogiannis, G.; Hamedi, M.M. Electroanalytical Paper-Based Nucleic Acid Amplification Biosensors with Integrated Thread Electrodes. Anal. Chem. 2021, 93, 14187–14195. [Google Scholar] [CrossRef]
- Toldrà, A.; Ainla, A.; Khaliliazar, S.; Landin, R.; Chondrogiannis, G.; Hanze, M.; Réu, P.; Hamedi, M.M. Portable Electroanalytical Nucleic Acid Amplification Tests Using Printed Circuit Boards and Open-Source Electronics. Analyst 2022, 147, 4249–4256. [Google Scholar] [CrossRef]
- Travaglini, L.; Micolich, A.P.; Cazorla, C.; Zeglio, E.; Lauto, A.; Mawad, D. Single-Material OECT-Based Flexible Complementary Circuits Featuring Polyaniline in Both Conducting Channels. Adv. Funct. Mater. 2021, 31, 2007205. [Google Scholar] [CrossRef]
- Huang, Y.; Mason, A.J. Lab-on-CMOS: Integrating Microfluidics and Electrochemical Sensor on CMOS. In Proceedings of the NEMS 2011—6th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Kaohsiung, Taiwan, 20–23 February 2011; pp. 690–693. [Google Scholar] [CrossRef]
- Chittuam, K.; Jampasa, S.; Vilaivan, T.; Tangkijvanich, P.; Chuaypen, N.; Avihingsanon, A.; Sain, M.; Panraksa, Y.; Chailapakul, O. Electrochemical Capillary-Driven Microfluidic DNA Sensor for HIV-1 and HCV Coinfection Analysis. Anal. Chim. Acta 2023, 1265, 341257. [Google Scholar] [CrossRef]
- Imani, S.M.; Osman, E.; Bakhshandeh, F.; Qian, S.; Sakib, S.; MacDonald, M.; Gaskin, M.; Zhitomirsky, I.; Yamamura, D.; Li, Y.; et al. Liquid NanoBiosensors Enable One-Pot Electrochemical Detection of Bacteria in Complex Matrices. Adv. Sci. 2023, 10, 2207223. [Google Scholar] [CrossRef]
- Choudhary, S.; Soni, M.; Sharma, S.K.; Das, P.K.; Adil, O.; Shamsi, M.H. First Multiplexed Electrochemical Wax-on-Plastic Chip: PNA/GO Interface Integration for DNA Detection. J. Micromech. Microeng. 2023, 33, 097001. [Google Scholar] [CrossRef]
- Izuan, J.; Rashid, A.; Yusof, A.; Abdullah, J.; Hanim, R.; Shueb, S. Strategies in the Optimization of DNA Hybridization Conditions and Its Role in Electrochemical Detection of Dengue Virus (DENV) Using Response Surface Methodology (RSM). RSC Adv. 2023, 13, 18748–18759. [Google Scholar] [CrossRef]
- Thoeny, V.; Melnik, E.; Mehrabi, P.; Huetter, M.; Schalkhammer, T.; Maier, T.; Mutinati, G.C.; Lieberzeit, P.; Hainberger, R. Redox Indicator-Based Electrochemical DNA Detection. IEEE Sens. Lett. 2023, 7, 4501804. [Google Scholar] [CrossRef]
- Safavieh, M.; Ahmed, M.U.; Ng, A.; Zourob, M. High-Throughput Real-Time Electrochemical Monitoring of LAMP for Pathogenic Bacteria Detection. Biosens. Bioelectron. 2014, 58, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Carnicer, O.; García-Altares, M.; Andree, K.B.; Tartaglione, L.; Dell’Aversano, C.; Ciminiello, P.; de la Iglesia, P.; Diogène, J.; Fernández-Tejedor, M. Ostreopsis Cf. Ovata from Western Mediterranean Sea: Physiological Responses under Different Temperature and Salinity Conditions. Harmful Algae 2016, 57, 98–108. [Google Scholar] [CrossRef]
- Berdalet, E.; Tester, P.A.; Chinain, M.; Fraga, S.; Lemée, R.; Litaker, W.; Penna, A.; Usup, G.; Vila, M.; Zingone, A. Harmful Algal Blooms in Benthic Systems: Recent Progress and Future Research. Oceanography 2017, 30, 36–45. [Google Scholar] [CrossRef]
- Toldrà, A.; Alcaraz, C.; Andree, K.B.; Fernández-Tejedor, M.; Diogène, J.; Katakis, I.; O’Sullivan, C.K.; Campàs, M. Colorimetric DNA-Based Assay for the Specific Detection and Quantification of Ostreopsis Cf. Ovata and Ostreopsis Cf. Siamensis in the Marine Environment. Harmful Algae 2019, 84, 27–35. [Google Scholar] [CrossRef]
- Öberg Månsson, I.; Piper, A.; Max Hamedi, M.M. Weaving Off-The-Shelf Yarns into Textile Micro Total Analysis Systems (ΜTAS). Macromol. Biosci. 2020, 20, 2000150. [Google Scholar] [CrossRef]
- Piper, A.; Öberg Månsson, I.; Khaliliazar, S.; Landin, R.; Hamedi, M.M. A Disposable, Wearable, Flexible, Stitched Textile Electrochemical Biosensing Platform. Biosens. Bioelectron. 2021, 194, 113604. [Google Scholar] [CrossRef]
- Pensa, E.; Cortés, E.; Corthey, G.; Carro, P.; Vericat, C.; Fonticelli, M.H.; Benítez, G.; Rubert, A.A.; Salvarezza, R.C. The Chemistry of the Sulfur-Gold Interface: In Search of a Unified Model. Acc. Chem. Res. 2012, 45, 1183–1192. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Ranjbari, M.; Shayan, M.; Mehrgardi, M.A.; Kiani, A. Wireless Electrochemiluminescence Bipolar Electrode Array for Visualized Genotyping of Single Nucleotide Polymorphism. Anal. Chem. 2015, 87, 8123–8131. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Mehrgardi, M.A. Amplified Electrochemical Genotyping of Single-Nucleotide Polymorphisms Using a Graphene–Gold Nanoparticles Modified Glassy Carbon Platform. RSC Adv. 2015, 5, 29285–29293. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Mehrgardi, M.A. Dual Amplification of Single Nucleotide Polymorphism Detection Using Graphene Oxide and Nanoporous Gold Electrode Platform. Analyst 2014, 139, 5192–5199. [Google Scholar] [CrossRef]
- Casabianca, S.; Casabianca, A.; Riobó, P.; Franco, J.M.; Vila, M.; Penna, A. Quantification of the Toxic Dinoflagellate Ostreopsis Spp. by QPCR Assay in Marine Aerosol. Environ. Sci. Technol. 2013, 47, 3788–3795. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, M.; Penna, A.; Sbrana, F.; Casabianca, S.; Gjeci, N.; Capellacci, S.; Asnaghi, V.; Ottaviani, E.; Giussani, V.; Pugliese, L.; et al. Intercalibration of Counting Methods for Ostreopsis Spp. Blooms in the Mediterranean Sea. Ecol. Indic. 2018, 85, 1092–1100. [Google Scholar] [CrossRef]
- Nicholls, P.J.; Malcolm, A.D.B. Nucleic Acid Analysis by Sandwich Hybridization. J. Clin. Lab. Anal. 1989, 3, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Castro, R.; de los Santos-Álvarez, N.; Lobo-Castañón, M.J. Understanding the Factors Affecting the Analytical Performance of Sandwich-Hybridization Genosensors on Gold Electrodes. Electroanalysis 2018, 30, 1229–1240. [Google Scholar] [CrossRef]
- Khuda, N.; Somasundaram, S.; Urgunde, A.B.; Easley, C.J. Ionic Strength and Hybridization Position near Gold Electrodes Can Significantly Improve Kinetics in DNA-Based Electrochemical Sensors. ACS Appl. Mater. Interfaces 2023, 15, 5019–5027. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanze, M.; Khaliliazar, S.; Réu, P.; Toldrà, A.; Hamedi, M.M. Toward Continuous Molecular Testing Using Gold-Coated Threads as Multi-Target Electrochemical Biosensors. Biosensors 2023, 13, 844. https://doi.org/10.3390/bios13090844
Hanze M, Khaliliazar S, Réu P, Toldrà A, Hamedi MM. Toward Continuous Molecular Testing Using Gold-Coated Threads as Multi-Target Electrochemical Biosensors. Biosensors. 2023; 13(9):844. https://doi.org/10.3390/bios13090844
Chicago/Turabian StyleHanze, Martin, Shirin Khaliliazar, Pedro Réu, Anna Toldrà, and Mahiar M. Hamedi. 2023. "Toward Continuous Molecular Testing Using Gold-Coated Threads as Multi-Target Electrochemical Biosensors" Biosensors 13, no. 9: 844. https://doi.org/10.3390/bios13090844
APA StyleHanze, M., Khaliliazar, S., Réu, P., Toldrà, A., & Hamedi, M. M. (2023). Toward Continuous Molecular Testing Using Gold-Coated Threads as Multi-Target Electrochemical Biosensors. Biosensors, 13(9), 844. https://doi.org/10.3390/bios13090844




