Magnetic Molecularly Imprinted Chitosan Combined with a Paper-Based Analytical Device for the Smartphone Discrimination of Tryptophan Enantiomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Apparatus
2.2. Synthesis of the Magnetite Nanoparticles
2.3. Synthesis of Molecularly Imprinted Chitosan
2.4. Synthesis of Gold Nanoparticles
2.5. Synthesis of D-TRP-Grafted Polymethacrylic Acid (PMA_D-TRP)
2.6. Preparation of the Paper-Based Analytical Device (PAD)
2.7. Adsorption Study
2.8. Solid-Phase Extraction Combined with Smartphone Detection
3. Results and Discussion
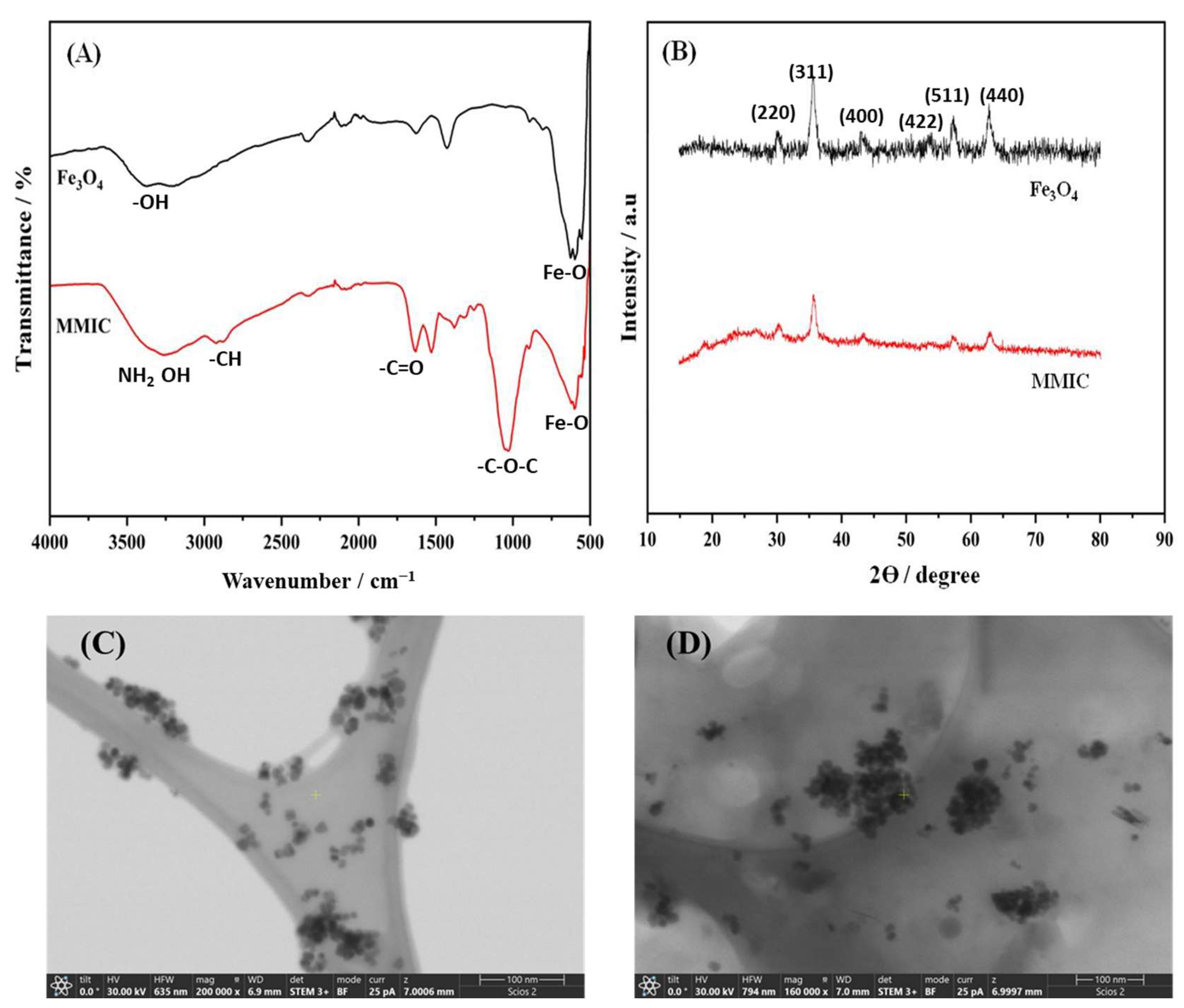
3.1. Characterization Studies of Fe3O4 and MMIC
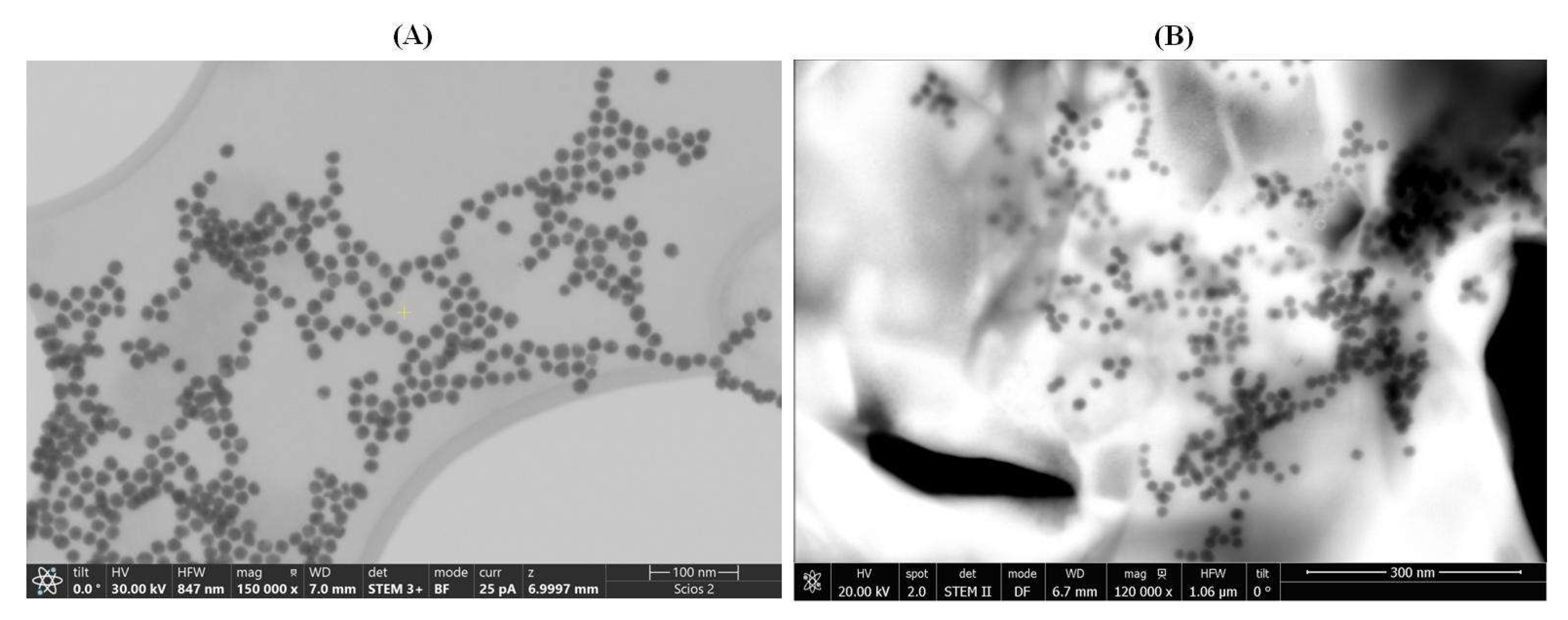
3.2. Transmission Electron Microscopy for AuNPs
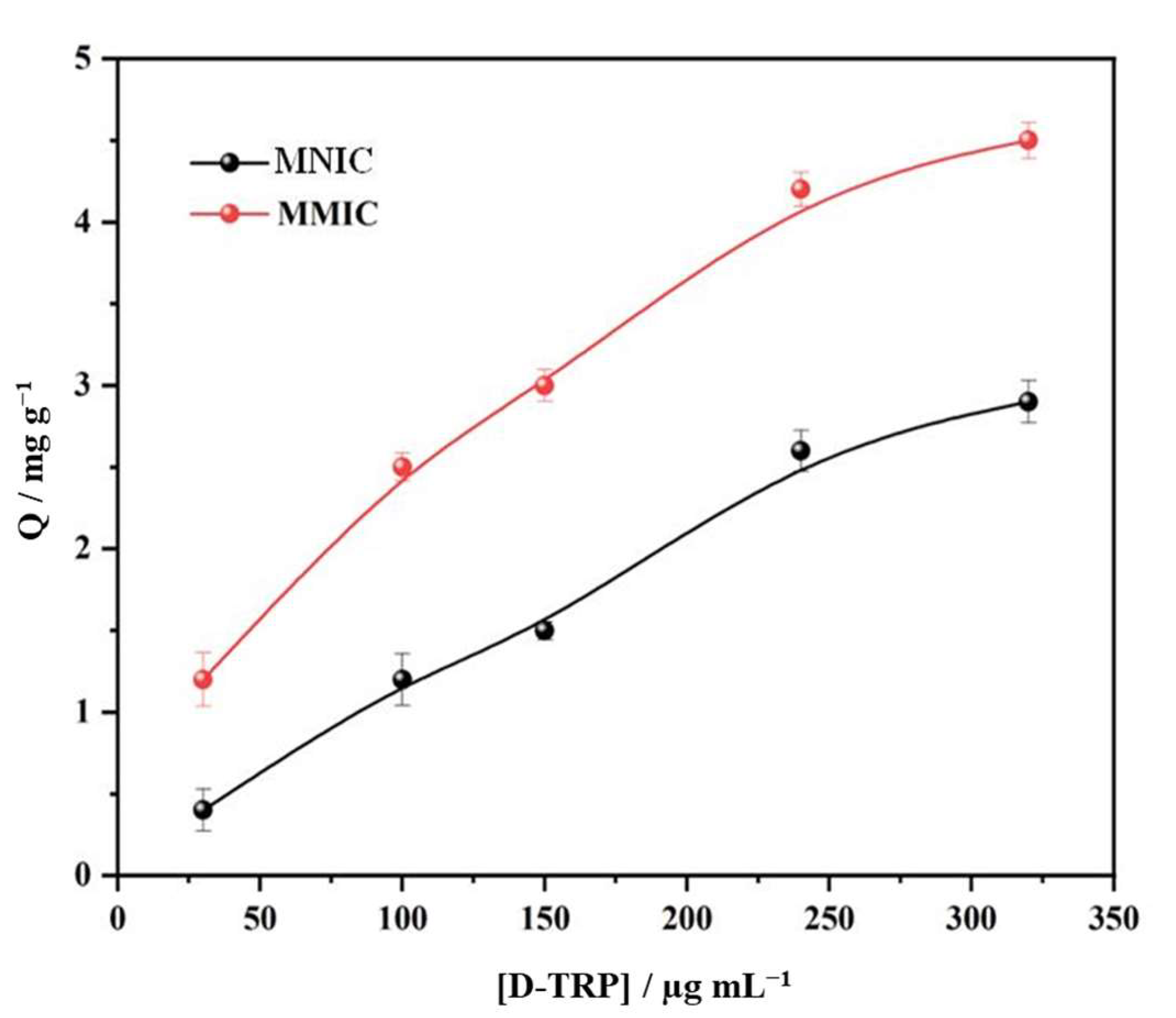
3.3. Adsorption Study
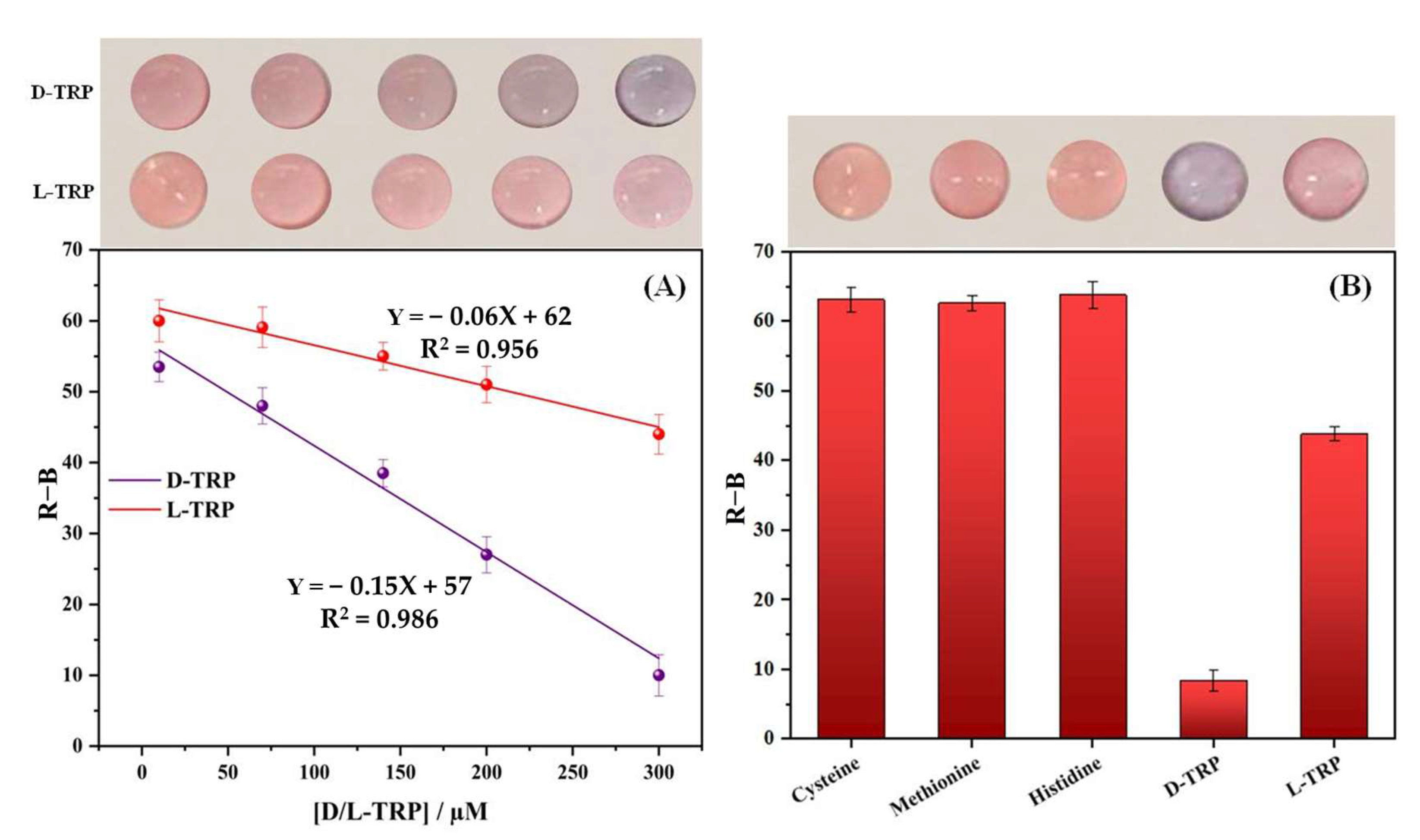
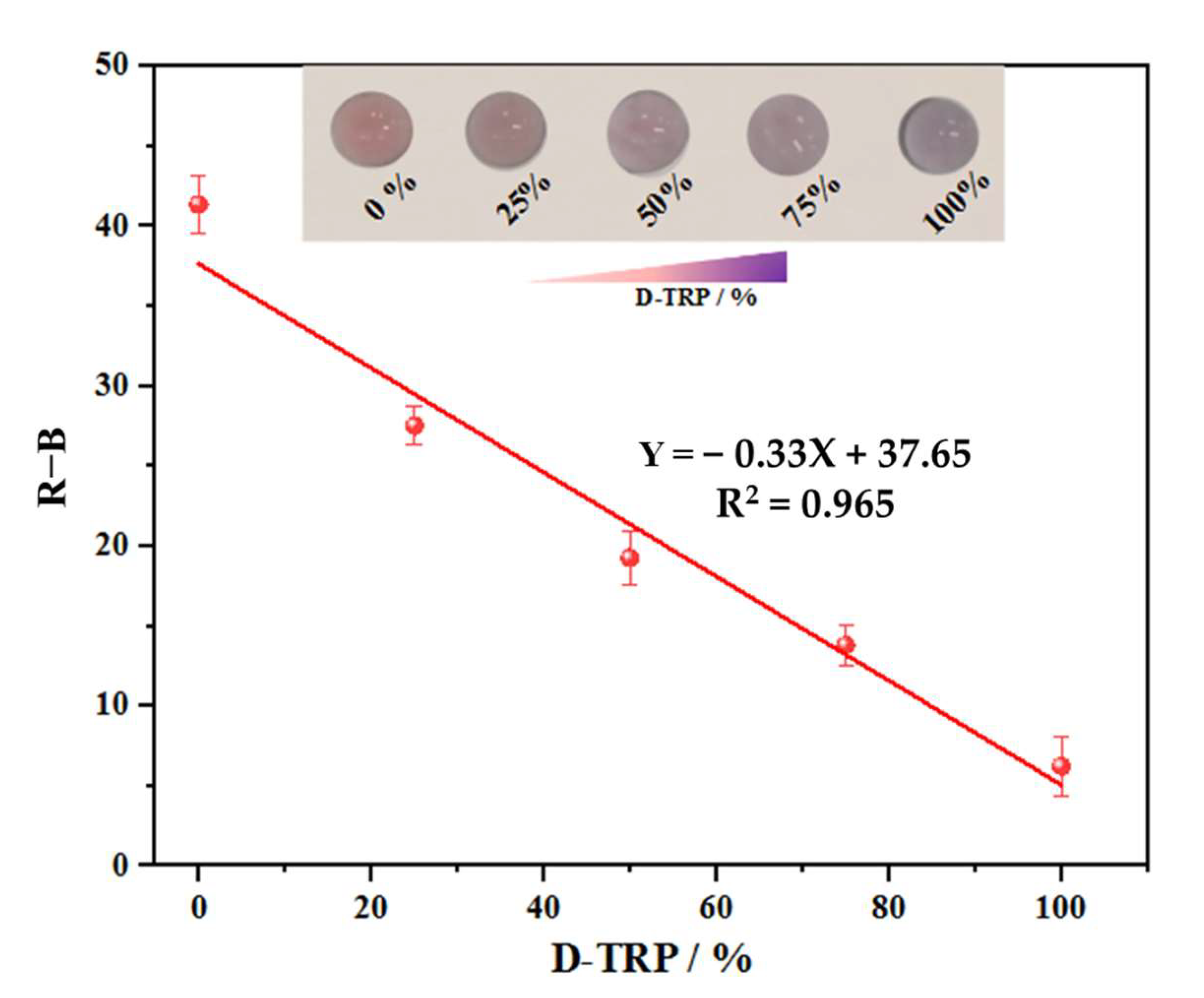
3.4. Discriminating between TRP Enantiomers
3.5. Smartphone Detection
3.6. Optimization of the Detection Procedure
3.7. Calibration Curve
3.8. Selectivity Study
3.9. Enantioselective Measurement of L/D Tryptophan Mixtures
3.10. Real Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fanali, C. Enantiomers Separation by Capillary Electrochromatography. TrAC Trends Anal. Chem. 2019, 120, 115640. [Google Scholar] [CrossRef]
- Makowski, K.; Mera, P.; Paredes, D.; Herrero, L.; Ariza, X.; Asins, G.; Hegardt, F.G.; García, J.; Serra, D. Differential Pharmacologic Properties of the Two C75 Enantiomers: (+)-C75 Is a Strong Anorectic Drug; (−)-C75 Has Antitumor Activity: Stereoselectivity of c75 enantiomers. Chirality 2013, 25, 281–287. [Google Scholar] [CrossRef]
- Mori, T.; Ito, T.; Liu, S.; Ando, H.; Sakamoto, S.; Yamaguchi, Y.; Tokunaga, E.; Shibata, N.; Handa, H.; Hakoshima, T. Structural Basis of Thalidomide Enantiomer Binding to Cereblon. Sci. Rep. 2018, 8, 1294. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Yan, S.; Chen, J.; Zhang, M.; Zhao, R.; Niu, X.; Wang, K. Fusiform-like Metal-Organic Framework for Enantioselective Discrimination of Tryptophan Enantiomers. Electrochim. Acta 2022, 419, 140409. [Google Scholar] [CrossRef]
- Jafari, M.; Tashkhourian, J.; Absalan, G. Chiral Recognition of Tryptophan Enantiomers Using Chitosan-Capped Silver Nanoparticles: Scanometry and Spectrophotometry Approaches. Talanta 2018, 178, 870–878. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Jia, L. Synthesis of Molecularly Imprinted Polymer Modified Magnetic Particles for Chiral Separation of Tryptophan Enantiomers in Aqueous Medium. J. Chromatogr. A 2020, 1622, 461147. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan Metabolism, from Nutrition to Potential Therapeutic Applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan Metabolism as a Common Therapeutic Target in Cancer, Neurodegeneration and Beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Maier, N.M.; Lindner, W. Chiral Recognition Applications of Molecularly Imprinted Polymers: A Critical Review. Anal. Bioanal. Chem. 2007, 389, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Karrat, A.; García-Guzmán, J.J.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. New Strategies for the Removal of Template from the Ion and Molecularly Imprinted Polymers: Application to the Fast and on-Site Cr(VI) Detection with a Smartphone. Sens. Actuators B Chem. 2023, 386, 133751. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Martín-Esteban, A. Molecularly Imprinted Polymer-Quantum Dot Materials in Optical Sensors: An Overview of Their Synthesis and Applications. Biosensors 2021, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Płotka-Wasylka, J.; Morrison, C.; Wieczorek, P.P.; Namieśnik, J.; Marć, M. Application of Molecularly Imprinted Polymers in Analytical Chiral Separations and Analysis. TrAC Trends Anal. Chem. 2018, 102, 91–102. [Google Scholar] [CrossRef]
- El Hani, O.; Karrat, A.; Digua, K.; Amine, A. Advanced Molecularly Imprinted Polymer-Based Paper Analytical Device for Selective and Sensitive Detection of Bisphenol-A in Water Samples. Microchem. J. 2023, 184, 108157. [Google Scholar] [CrossRef]
- Villa, C.C.; Sánchez, L.T.; Valencia, G.A.; Ahmed, S.; Gutiérrez, T.J. Molecularly Imprinted Polymers for Food Applications: A Review. Trends Food Sci. Technol. 2021, 111, 642–669. [Google Scholar] [CrossRef]
- Li, R.; Feng, Y.; Pan, G.; Liu, L. Advances in Molecularly Imprinting Technology for Bioanalytical Applications. Sensors 2019, 19, 177. [Google Scholar] [CrossRef]
- Kadhem, A.J.; Gentile, G.J.; Fidalgo de Cortalezzi, M.M. Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review. Molecules 2021, 26, 6233. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Tavengwa, N.T.; Tutu, H.; Chimuka, L. Green Aspects in Molecular Imprinting Technology: From Design to Environmental Applications. Trends Environ. Anal. Chem. 2018, 17, 14–22. [Google Scholar] [CrossRef]
- Ekmen, E.; Bilici, M.; Turan, E.; Tamer, U.; Zengin, A. Surface Molecularly-Imprinted Magnetic Nanoparticles Coupled with SERS Sensing Platform for Selective Detection of Malachite Green. Sens. Actuators B Chem. 2020, 325, 128787. [Google Scholar] [CrossRef]
- Moya Betancourt, S.N.; Cámara, C.I.; Riva, J.S. Interaction between Pharmaceutical Drugs and Polymer-Coated Fe3O4 Magnetic Nanoparticles with Langmuir Monolayers as Cellular Membrane Models. Pharmaceutics 2023, 15, 311. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Yang, H.; Zhang, S.; Song, H.; Zhu, X. Preparation and Evaluation of Magnetic Graphene Oxide Molecularly Imprinted Polymers (MIPs-GO-Fe3O4@SiO2) for the Analysis and Separation of Tripterine. React. Funct. Polym. 2021, 169, 105055. [Google Scholar] [CrossRef]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template—“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef]
- Krupadam, R.J.; Patel, G.P.; Balasubramanian, R. Removal of Cyanotoxins from Surface Water Resources Using Reusable Molecularly Imprinted Polymer Adsorbents. Environ. Sci. Pollut. Res. 2012, 19, 1841–1851. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Jaffrezic-Renault, N.; Zine, N.; Errachid, A. Electrochemical Sensors Based on Molecularly Imprinted Chitosan: A Review. TrAC Trends Anal. Chem. 2020, 130, 115982. [Google Scholar] [CrossRef]
- Mulyasuryani, A.; Prananto, Y.P.; Fardiyah, Q.; Widwiastuti, H.; Darjito, D. Application of Chitosan-Based Molecularly Imprinted Polymer in Development of Electrochemical Sensor for p-Aminophenol Determination. Polymers 2023, 15, 1818. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, M.; Bellali, F.; Karrat, A.; Bouchdoug, M.; Jaouad, A. Preparation of Teucrium polium extract-loaded chitosan-sodium lauryl sulfate beads and chitosan-alginate films for wound dressing application. AIMS Public Health 2021, 8, 754–775. [Google Scholar] [CrossRef]
- He, S.; Liu, D.; Wang, Z.; Cai, K.; Jiang, X. Utilization of Unmodified Gold Nanoparticles in Colorimetric Detection. Sci. China Phys. Mech. Astron. 2011, 54, 1757–1765. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef]
- Chahri, I.; Karrat, A.; Mohammadi, H.; Amine, A. Development of a New Route for the Immobilization of Unmodified Single-Stranded DNA on Chitosan Beads and Detection of Released Guanine after Hydrolysis. Molecules 2023, 28, 2088. [Google Scholar] [CrossRef]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef]
- Fu, L.-M.; Wang, Y.-N. Detection Methods and Applications of Microfluidic Paper-Based Analytical Devices. TrAC Trends Anal. Chem. 2018, 107, 196–211. [Google Scholar] [CrossRef]
- Afzali, M.; Mostafavi, A.; Shamspur, T. A Novel Electrochemical Sensor Based on Magnetic Core@shell Molecularly Imprinted Nanocomposite (Fe3O4@graphene Oxide@MIP) for Sensitive and Selective Determination of Anticancer Drug Capecitabine. Arab. J. Chem. 2020, 13, 6626–6638. [Google Scholar] [CrossRef]
- Cubillana-Aguilera, L.M.; Franco-Romano, M.; Gil, M.L.A.; Naranjo-Rodríguez, I.; Hidalgo-Hidalgo de Cisneros, J.L.; Palacios-Santander, J.M. New, Fast and Green Procedure for the Synthesis of Gold Nanoparticles Based on Sonocatalysis. Ultrason. Sonochemistry 2011, 18, 789–794. [Google Scholar] [CrossRef]
- Li, G.; Jiang, Y.; Huang, K.; Ding, P.; Chen, J. Preparation and Properties of Magnetic Fe3O4–Chitosan Nanoparticles. J. Alloy. Compd. 2008, 466, 451–456. [Google Scholar] [CrossRef]
- Upadhyay, Y.; Bothra, S.; Kumar, R.; Sahoo, S.K. Smartphone-Assisted Colorimetric Detection of Cr3+ Using Vitamin B6 Cofactor Functionalized Gold Nanoparticles and Its Applications in Real Sample Analyses. ChemistrySelect 2018, 3, 6892–6896. [Google Scholar] [CrossRef]
- Karrat, A.; Amine, A. Paper-Based Analytical Device for One-Step Detection of Bisphenol-A Using Functionalized Chitosan. Chemosensors 2022, 10, 450. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Seidi, S.; Lid, M.; Pedersen-Bjergaard, S.; Yamini, Y. The Modern Role of Smartphones in Analytical Chemistry. TrAC Trends Anal. Chem. 2019, 118, 548–555. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Li, Y.; Zhang, L.; Bi, N.; Gou, J.; Zhao, T.; Jia, L. A Wearable Gloved Sensor Based on Fluorescent Ag Nanoparticles and Europium Complexes for Visualized Assessment of Tetracycline in Food Samples. Food Chem. 2023, 424, 136376. [Google Scholar] [CrossRef]
- Xu, J.; Guo, S.; Jia, L.; Zhu, T.; Chen, X.; Zhao, T. A Smartphone-Integrated Method for Visual Detection of Tetracycline. Chem. Eng. J. 2021, 416, 127741. [Google Scholar] [CrossRef]



| Added (µM) | Found (µM) | Recovery (%) | RSD (%) * |
|---|---|---|---|
| 30 | 26.5 | 88.6 | 3.4 |
| 100 | 106.4 | 106.4 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karrat, A.; García-Guzmán, J.J.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. Magnetic Molecularly Imprinted Chitosan Combined with a Paper-Based Analytical Device for the Smartphone Discrimination of Tryptophan Enantiomers. Biosensors 2023, 13, 830. https://doi.org/10.3390/bios13080830
Karrat A, García-Guzmán JJ, Palacios-Santander JM, Amine A, Cubillana-Aguilera L. Magnetic Molecularly Imprinted Chitosan Combined with a Paper-Based Analytical Device for the Smartphone Discrimination of Tryptophan Enantiomers. Biosensors. 2023; 13(8):830. https://doi.org/10.3390/bios13080830
Chicago/Turabian StyleKarrat, Abdelhafid, Juan José García-Guzmán, José María Palacios-Santander, Aziz Amine, and Laura Cubillana-Aguilera. 2023. "Magnetic Molecularly Imprinted Chitosan Combined with a Paper-Based Analytical Device for the Smartphone Discrimination of Tryptophan Enantiomers" Biosensors 13, no. 8: 830. https://doi.org/10.3390/bios13080830
APA StyleKarrat, A., García-Guzmán, J. J., Palacios-Santander, J. M., Amine, A., & Cubillana-Aguilera, L. (2023). Magnetic Molecularly Imprinted Chitosan Combined with a Paper-Based Analytical Device for the Smartphone Discrimination of Tryptophan Enantiomers. Biosensors, 13(8), 830. https://doi.org/10.3390/bios13080830







