Characterization of Benchtop-Fabricated Arrays of Nanowrinkled Surface Electrodes as a Nitric Oxide Electrochemical Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
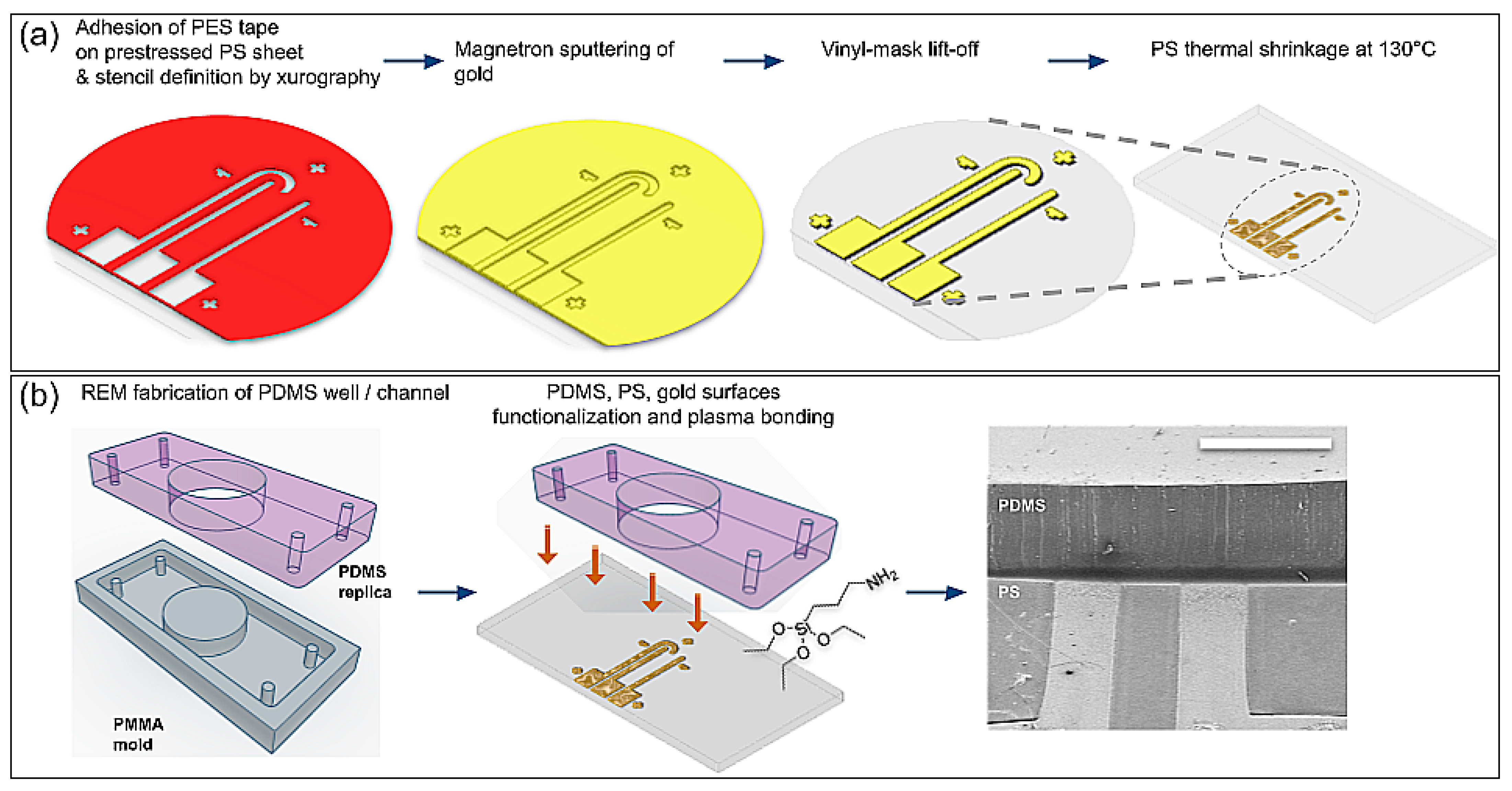
2.2. Fabrication of Arrays of Nanowrinkled-Surface Electrodes
2.3. Geometric and Topographical Characterizations
2.4. Electrochemical Characterizations
2.4.1. Characterization of Electrochemically Active Surface Area
2.4.2. Evaluation of ANSE’s Electrochemical Performance
2.5. Electrochemical Detection of Dissolved Nitric Oxide with an ANSE
2.5.1. Preparation of Nitric Oxide Aqueous Solutions for Calibration
2.5.2. NO Electrooxidation Calibration Curve
2.6. Detection of Nitric Oxide Released from Stimulated Endothelial Cells in Culture
2.6.1. Cell Culture Conditions
2.6.2. Nitric Oxide Colorimetric Detection
2.6.3. Detection of Nitric Oxide with Fluorescent DAF-FM Diacetate
2.6.4. Detection of Nitric Oxide with the ANSE-Based Electrochemical Sensor
3. Results
3.1. Fabrication and Characterization of Nanowrinkled-Surface Electrodes
3.2. ANSE Characterization as Electrochemical Cells in the Diffusion-Controlled Mass Transport Regime
3.3. Detection of Nitric Oxide Released by Stimulated Endothelial Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Xu, H.; Zhang, J.; Li, G. Electrochemical Sensors for Clinic Analysis. Sensors 2008, 8, 2043–2081. [Google Scholar] [CrossRef]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical sensors for environmental monitoring: Design, development and applications. J. Environ. Monit. 2004, 6, 657–664. [Google Scholar] [CrossRef]
- Sharifi, K.; Pirsa, S. Electrochemical sensors; Types and applications in the food industry. Chem. Rev. Lett. 2020, 3, 192–201. [Google Scholar]
- Wu, J.; He, Z.; Chen, Q.; Lin, J. Biochemical analysis on microfluidic chips. Trends Anal. Chem. 2016, 80, 213–231. [Google Scholar] [CrossRef]
- Ferrari, E.; Palma, C.; Vesentini, S.; Occhetta, P.; Rasponi, M. Integrating Biosensors in Organs-on-Chip Devices: A Perspective on Current Strategies to Monitor Microphysiological Systems. Biosensors 2020, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Sticker, D.; Charwat, V.; Kasper, C.; Lepperdinger, G. Lab-on-a-chip technologies for stem cell analysis. Trends Biotechnol. 2014, 32, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, L. Enzymatic Strips for Detection of Serum Total Cholesterol with Point-of-Care Testing (POCT) Devices: Current Status and Future Prospect. J. Electrochem. Soc. 2020, 167, 037535. [Google Scholar] [CrossRef]
- Nemčeková, K.; Labuda, J. Advanced materials-integrated electrochemical sensors as promising medical diagnostics tools: A review. Mater. Sci. Eng. C 2021, 120, 111751. [Google Scholar] [CrossRef] [PubMed]
- Mohan, J.; Amreen, K.; Javed, A.; Dubey, K.; Goel, S. Emerging trends in miniaturized and microfluidic electrochemical sensing platforms. Curr. Opin. Electrochem. 2022, 33, 100930. [Google Scholar] [CrossRef]
- Sonney, S.; Shek, N.; Moran-Mirabal, J.M. Rapid bench-top fabrication of poly(dimethylsiloxane)/polystyrene microfluidic devices incorporating high-surface-area sensing electrodes. Biomicrofluidics 2015, 9, 026501. [Google Scholar] [CrossRef]
- Moya, A.; Ortega-Ribera, M.; Guimerà, X.; Sowade, E.; Zea, M.; Illa, X.; Ramon, E.; Villa, R.; Gracia-Sancho, J.; Gabriel, G. Online oxygen monitoring using integrated inkjet-printed sensors in a liver-on-a-chip system. Lab Chip 2018, 18, 2023–2035. [Google Scholar] [CrossRef] [PubMed]
- Batista Deroco, P.; Giarola, J.; Wachholz Júnior, D.; Arantes Lorga, G.; Tatsuo Kubota, L. Comprehensive Analytical Chemistry. In Paper-Based Electrochemical Sensing Devices; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–137. [Google Scholar]
- Elliott, J.; Duay, J.; Simoska, O.; Shear, J.B.; Stevenson, K.J. Gold Nanoparticle Modified Transparent Carbon Ultramicroelectrode Arrays for the Selective and Sensitive Electroanalytical Detection of Nitric Oxide. Anal. Chem. 2017, 89, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lang, X.; Hirata, A.; Chen, M. Wrinkled Nanoporous Gold Films with Ultrahigh Surface-Enhanced Raman Scattering Enhancement. ACS Nano 2011, 5, 4407–4413. [Google Scholar] [CrossRef] [PubMed]
- Pegan, J.D.; Ho, A.Y.; Bachman, M.; Khine, M. Flexible shrink-induced high surface area electrodes for electrochemiluminescent sensing. Lab Chip 2013, 13, 4205–4209. [Google Scholar] [CrossRef]
- Gabardo, C.M.; Zhu, Y.; Soleymani, L.; Moran-Mirabal, J.M. Bench-Top Fabrication of Hierarchically Structured High-Surface-Area Electrodes. Adv. Funct. Mater. 2013, 23, 3030–3039. [Google Scholar] [CrossRef]
- Zhu, Y.; Moran-Mirabal, J. Highly Bendable and Stretchable Electrodes Based on Micro/Nanostructured Gold Films for Flexible Sensors and Electronics. Adv. Electron. Mater. 2016, 2, 1500345. [Google Scholar] [CrossRef]
- Ding, X.; Moran-Mirabal, J.M. Efficient Multi-Material Structured Thin Film Transfer to Elastomers for Stretchable Electronic Devices. Micromachines 2022, 13, 334. [Google Scholar] [CrossRef]
- Tremiliosi-Filho, G.; Dall’antonia, L.; Jerkiewicz, G. Growth of surface oxides on gold electrodes under well-defined potential, time and temperature conditions. J. Electroanal. Chem. 2005, 578, 1–8. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2017, 95, 197–206. [Google Scholar] [CrossRef]
- Mattson, B.; Anderson, M.P.; Mattson, S. Microscale Gas Chemistry. Sch. Sci. Rev. 2006, 92, 43–48. [Google Scholar] [CrossRef]
- Hood, J.D.; Meininger, C.J.; Ziche, M.; Granger, H.J. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am. J. Physiol. 1998, 274, H1054–H1058. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.C.; Grimes, A.; Long, M.; Ferri, C.G.; Rich, B.D.; Ghosh, S.; Ghosh, S.; Lee, L.P.; Gopinathan, A.; Khine, M. Tunable Nanowrinkles on Shape Memory Polymer Sheets. Adv. Mater. 2009, 21, 4472–4476. [Google Scholar] [CrossRef]
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, biosensors and microfluidics: A convergence of fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef]
- Brown, M.D.; Schoenfisch, M.H. Electrochemical Nitric Oxide Sensors: Principles of Design and Characterization. Chem. Rev. 2019, 119, 11551–11575. [Google Scholar] [CrossRef]
- Koprowski, H.; Maeda, H. The Role of Nitric Oxide in Physiology and Pathophysiology; Springer Science & Business Media: Boston, NY, USA, 2012. [Google Scholar]
- Zhang, F.; Liu, J.; Ivanov, I.; Hatzell, M.C.; Yang, W.; Ahn, Y.; Logan, B.E. Reference and counter electrode positions affect electrochemical characterization of bioanodes in different bioelectrochemical systems. Biotechnol. Bioeng. 2014, 111, 1931–1939. [Google Scholar] [CrossRef]
- Bouloumié, A.; Schini-Kerth, V.B.; Busse, R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc. Res. 1999, 41, 773–780. [Google Scholar] [CrossRef]
- Cai, G.; Satoh, T.; Hoshi, H. Purification and characterization of an endothelial cell-viability maintaining factor from fetal bovine serum. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1995, 1269, 13–18. [Google Scholar] [CrossRef][Green Version]
- Matsumura, T.; Takesue, M.; Westerman, K.A.; Okitsu, T.; Sakaguchi, M.; Fukazawa, T.; Totsugawa, T.; Noguchi, H.; Yamamoto, S.; Stolz, D.B.; et al. Establishment of an immortalized human-liver endothelial cell line with SV40T and hTERT. Transplantation 2004, 77, 1357–1365. [Google Scholar] [CrossRef]
- Chan, Y.; Skreta, M.; McPhee, H.; Saha, S.; Deus, R.; Soleymani, L. Solution-processed wrinkled electrodes enable the development of stretchable electrochemical biosensors. Analyst 2018, 144, 172–179. [Google Scholar] [CrossRef]
- Saem, S.; Zhu, Y.; Luu, H.; Moran-Mirabal, J. Bench-Top Fabrication of an All-PDMS Microfluidic Electrochemical Cell Sensor Integrating Micro/Nanostructured Electrodes. Sensors 2017, 17, 732. [Google Scholar] [CrossRef] [PubMed]
- Oni, J.; Pailleret, A.; Isik, S.; Diab, N.; Radtke, I.; Jackson, M.; Bedioui, F.; Schuhmann, W.; Blöchl, A. Functionalised electrode array for the detection of nitric oxide released by endothelial cells using different NO-sensing chemistries. Anal. Bioanal. Chem. 2004, 378, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Pereira-Rodrigues, N.; Henderson, J.R.; Cole, A.; Bedioui, F.; McNeil, C.J. An electrochemical sensor array system for the direct, simultaneous in vitro monitoring of nitric oxide and superoxide production by cultured cells. Biosens. Bioelectron. 2005, 21, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Forsyth, H.; Khaper, N.; Chen, A. Sensitive electrochemical detection of nitric oxide based on AuPt and reduced graphene oxide nanocomposites. Analyst 2016, 141, 4074–4083. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, S.Z.; Kwasny, D.; Amato, L.; Brøgger, A.L.; Bosco, F.G.; Andersen, K.B.; Svendsen, W.E.; Boisen, A. Integrating electrochemical detection with centrifugal microfluidics for real-time and fully automated sample testing. RSC Adv. 2015, 5, 17187–17193. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, J.; Cardoso, L.; Broderick, M.; Darley-Usmar, V. A Novel Microchip Nitric Oxide Sensor with sub-nM Detection Limit. Electroanalysis 2002, 14, 697–703. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 2022. [Google Scholar]
- Finnerty, N.J.; O’Riordan, S.L.; Brown, F.O.; Serra, P.A.; O’Neill, R.D.; Lowry, J.P. In vivo characterisation of a Nafion®-modified Pt electrode for real-time nitric oxide monitoring in brain extracellular fluid. Anal. Methods 2012, 4, 550–557. [Google Scholar] [CrossRef]
- Bedioui, F.; Villeneuve, N. Electrochemical Nitric Oxide Sensors for Biological Samples—Principle, Selected Examples and Applications. Electroanalysis 2003, 15, 5–18. [Google Scholar] [CrossRef]
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Mikhaylov, V.; Demchenko, A.V.; Stine, K.J. Preparation, Modification, Characterization, and Biosensing Application of Nanoporous Gold Using Electrochemical Techniques. Nanomaterials 2018, 8, 171. [Google Scholar] [CrossRef]
- Liu, Z.; Manikandan, V.S.; Chen, A. Recent advances in nanomaterial-based electrochemical sensing of nitric oxide and nitrite for biomedical and food research. Curr. Opin. Electrochem. 2019, 16, 127–133. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peto-Gutiérrez, C.; Vázquez-Victorio, G.; Hautefeuille, M. Characterization of Benchtop-Fabricated Arrays of Nanowrinkled Surface Electrodes as a Nitric Oxide Electrochemical Sensor. Biosensors 2023, 13, 794. https://doi.org/10.3390/bios13080794
Peto-Gutiérrez C, Vázquez-Victorio G, Hautefeuille M. Characterization of Benchtop-Fabricated Arrays of Nanowrinkled Surface Electrodes as a Nitric Oxide Electrochemical Sensor. Biosensors. 2023; 13(8):794. https://doi.org/10.3390/bios13080794
Chicago/Turabian StylePeto-Gutiérrez, Cindy, Genaro Vázquez-Victorio, and Mathieu Hautefeuille. 2023. "Characterization of Benchtop-Fabricated Arrays of Nanowrinkled Surface Electrodes as a Nitric Oxide Electrochemical Sensor" Biosensors 13, no. 8: 794. https://doi.org/10.3390/bios13080794
APA StylePeto-Gutiérrez, C., Vázquez-Victorio, G., & Hautefeuille, M. (2023). Characterization of Benchtop-Fabricated Arrays of Nanowrinkled Surface Electrodes as a Nitric Oxide Electrochemical Sensor. Biosensors, 13(8), 794. https://doi.org/10.3390/bios13080794




