Methylene-Blue-Encapsulated Metal-Organic-Framework-Based Electrochemical POCT Platform for Multiple Detection of Heavy Metal Ions in Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instrumentation
2.3. Preparation of AuNPs
2.4. Preparation of Zr-MOFs
2.5. Preparation of MB@Zr-MOFs
2.6. Preparation of MB@Zr-MOFs-apt and MB-apt
2.7. Fabrication of the Portable Electrochemical Platform
3. Results and Discussion
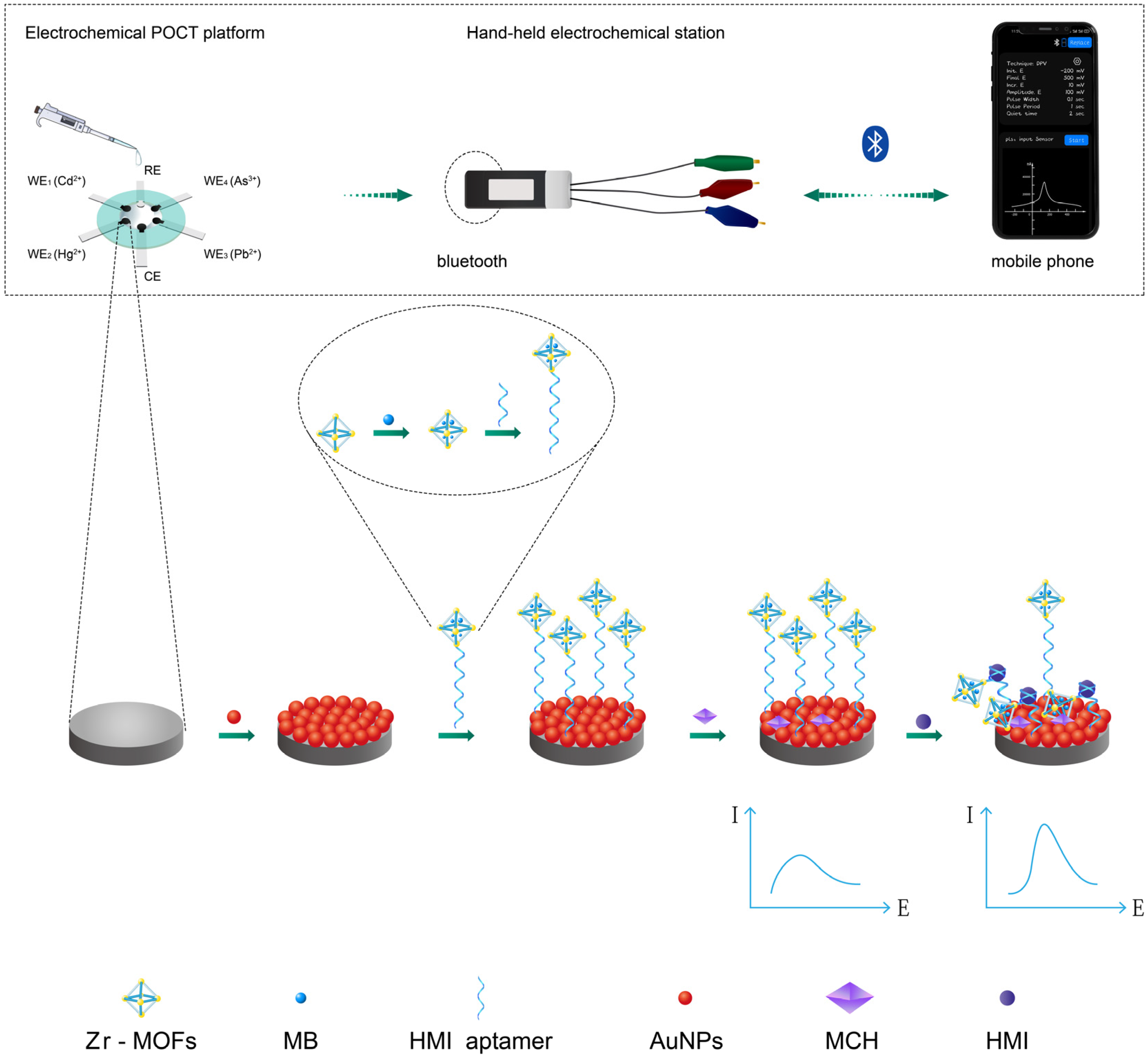
3.1. Detection Principle of the Electrochemical POCT Platform
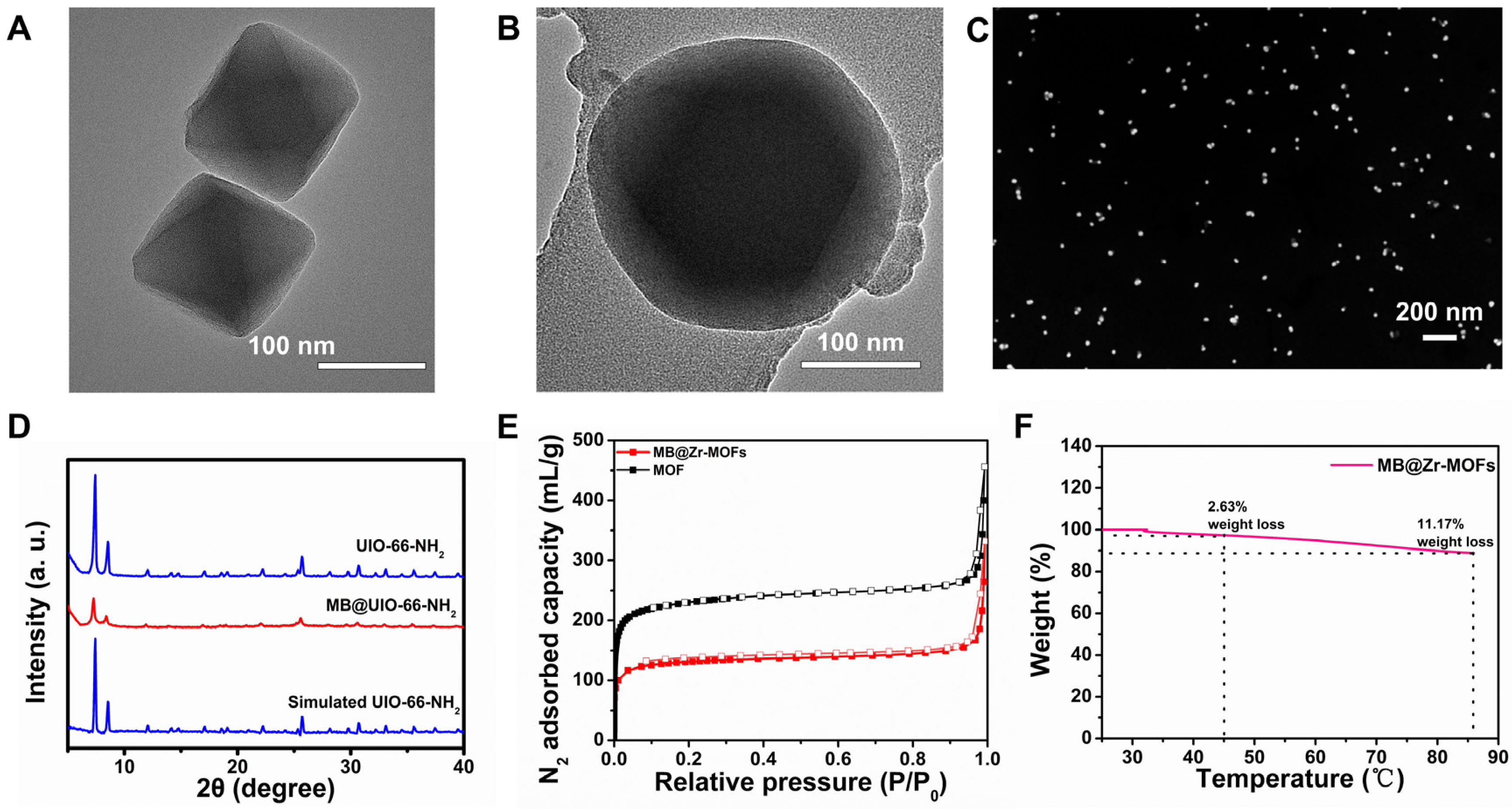
3.2. Characterization of Zr-MOFs and MB@Zr-MOFs
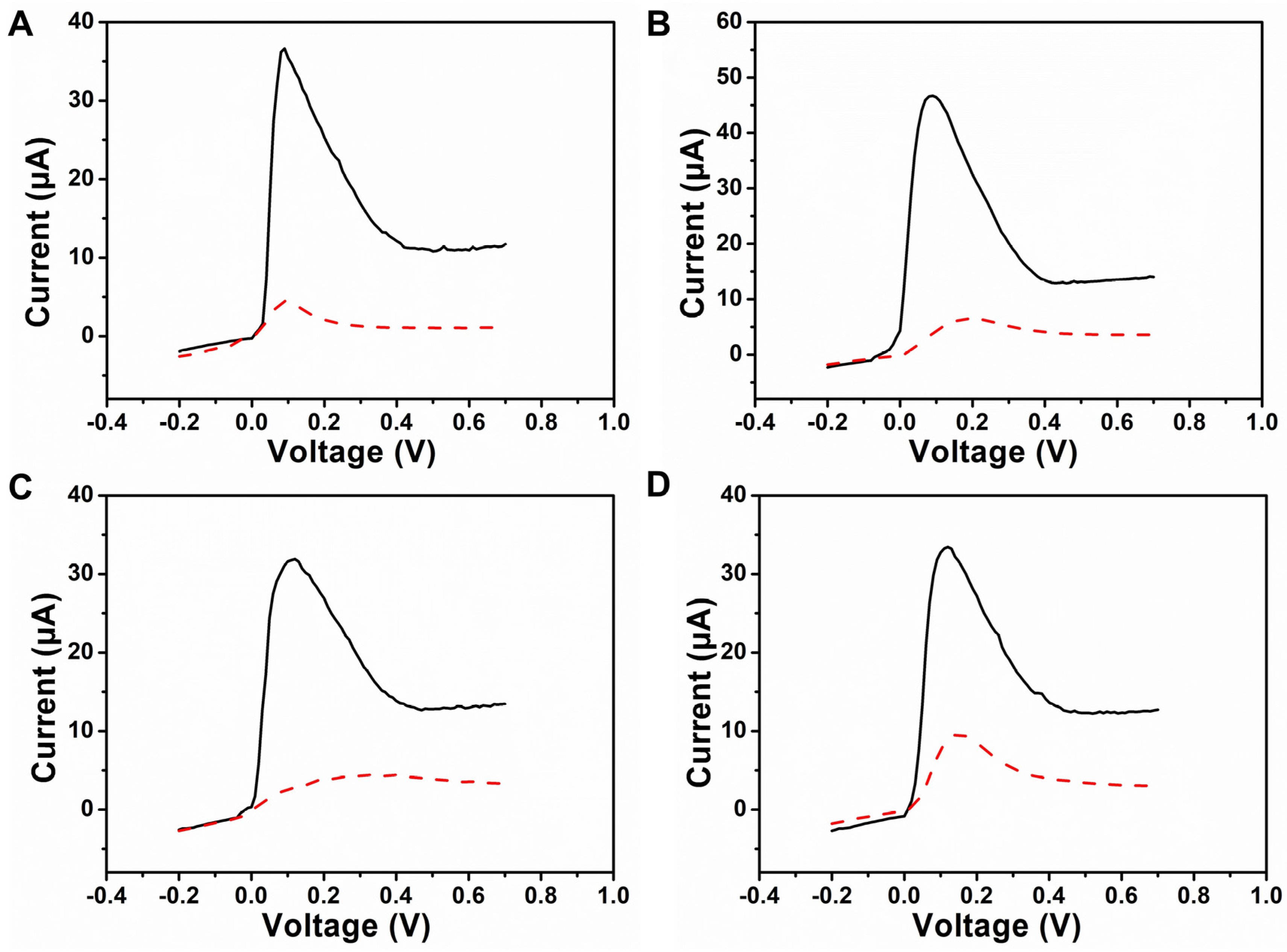
3.3. Amplification Electrochemical Behavior of the POCT Sensor
3.4. Electrochemical Behavior of the POCT Sensor
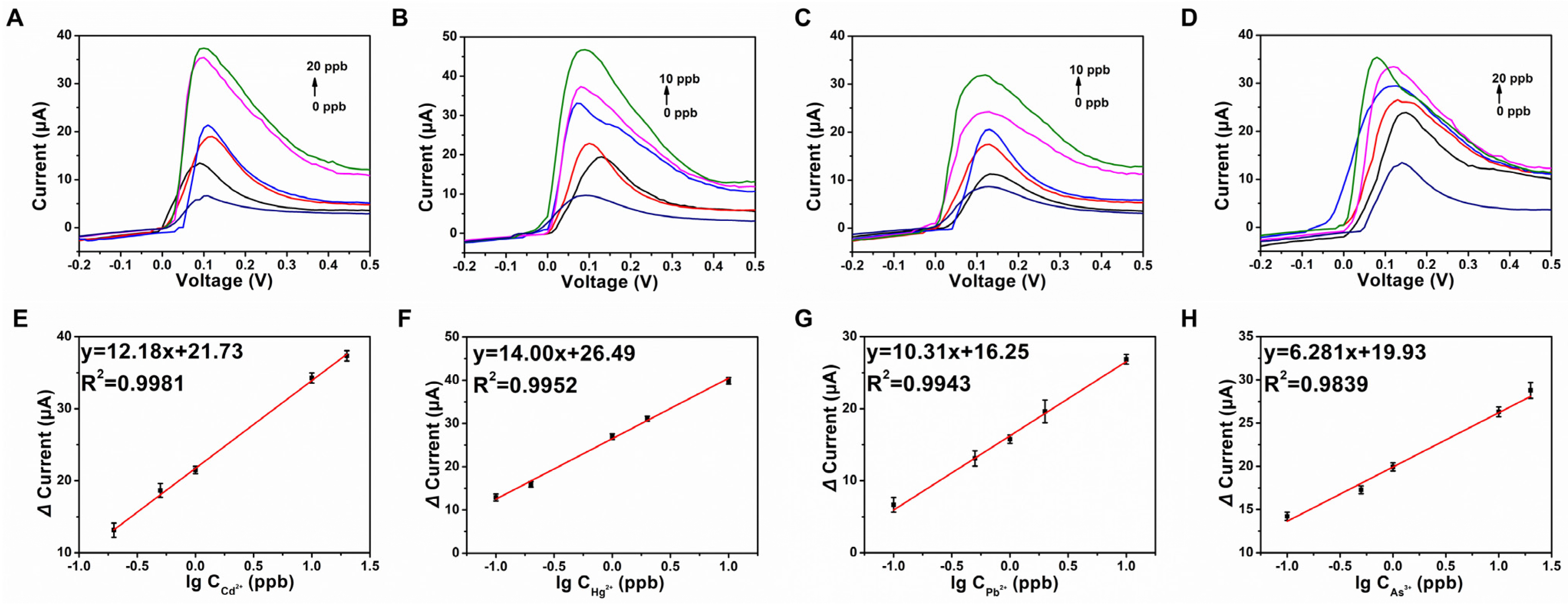
3.5. Analytical Performance of the Electrochemical POCT Sensor
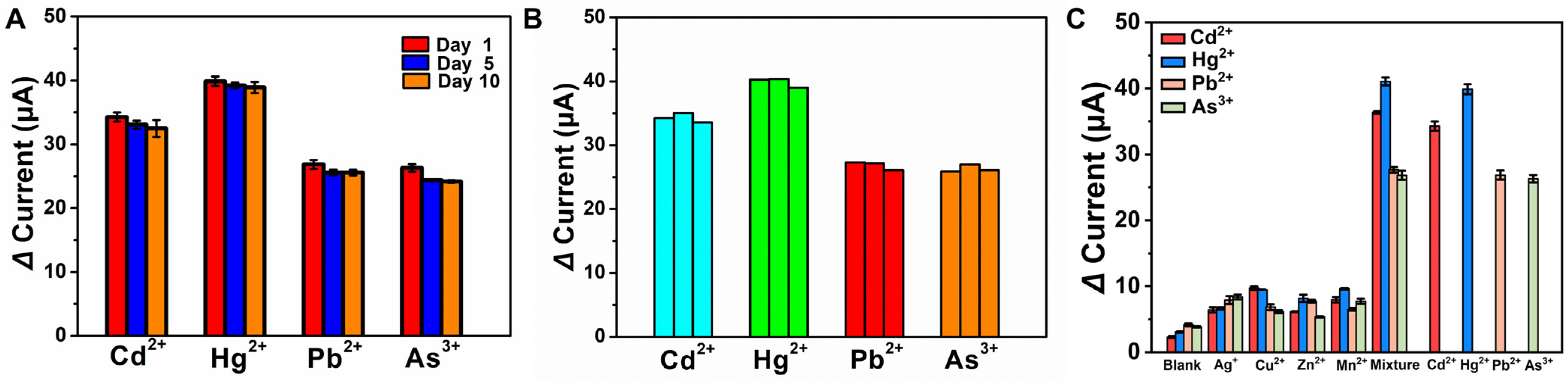
3.6. Stability, Reproducibility, and Selectivity of Electrochemical POCT Platform
3.7. Real Sample Analysis Using Milk
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, A.; Riaz, M.; Akhtar, S.; Goodwill, J.E.; Sun, J. Heavy metals in milk: Global prevalence and health risk assessment. Toxin Rev. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Devadhasan, J.P.; Kim, J. A chemically functionalized paper-based microfluidic platform for multiplex heavy metal detection. Sens. Actuators B Chem. 2018, 273, 18–24. [Google Scholar] [CrossRef]
- Ding, Q.; Li, C.; Wang, H.; Xu, C.; Kuang, H. Electrochemical detection of heavy metal ions in water. Chem. Commun. 2021, 57, 7215–7231. [Google Scholar] [CrossRef] [PubMed]
- Thongsaw, A.; Udnan, Y.; Ross, G.M.; Chaiyasith, W.C. Speciation of mercury in water and biological samples by eco-friendly ultrasound-assisted deep eutectic solvent based on liquid phase microextraction with electrothermal atomic absorption spectrometry. Talanta 2019, 197, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.-T.; Chen, J.-Y.; Luo, Y.-T.; Lin, C.-H.; Liu, Y.-H.; Su, Y.-A.; Chao, P.-C.; Sun, Y.-C. Development of a titanium dioxide-assisted preconcentration/on-site vapor-generation chip hyphenated with inductively coupled plasma-mass spectrometry for online determination of mercuric ions in urine samples. Anal. Chim. Acta 2019, 1063, 82–90. [Google Scholar] [CrossRef]
- Zheng, H.; Hong, J.; Luo, X.; Li, S.; Wang, M.; Yang, B.; Wang, M. Combination of sequential cloud point extraction and hydride generation atomic fluorescence spectrometry for preconcentration and determination of inorganic and methyl mercury in water samples. Microchem. J. 2019, 145, 806–812. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, T.; Wu, Z.; Yu, R. Novel ratiometric surface-enhanced raman spectroscopy aptasensor for sensitive and reproducible sensing of Hg2+. Biosens. Bioelectron. 2018, 99, 646–652. [Google Scholar] [CrossRef]
- Zeng, Y.; Ren, J.; Shen, A.; Hu, J. Field and Pretreatment-Free Detection of Heavy-Metal Ions in Organic Polluted Water through an Alkyne-Coded SERS Test Kit. ACS Appl. Mater. Interfaces 2016, 8, 27772–27778. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Devadhasan, J.P.; Howse, R.; Kim, J. A Chemically Patterned Microfluidic Paper-based Analytical Device (C-µPAD) for Point-of-Care Diagnostics. Sci. Rep. 2017, 7, 1188. [Google Scholar] [CrossRef]
- Pan, J.; Li, Q.; Zhou, D.; Chen, J. Ultrasensitive aptamer biosensor for arsenic (III) detection based on label-free triple-helix molecular switch and fluorescence sensing platform. Talanta 2018, 189, 370–376. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Wang, Y.; Luo, J.; Xu, H.; Xu, S.; Cai, X. A wireless point-of-care testing system for the detection of neuron-specific enolase with microfluidic paper-based analytical devices. Biosens. Bioelectron. 2017, 95, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Ye, X.; Li, Y.; Qi, J.; Fang, X.; Kong, J. Efficient Microfluidic-Based Air Sampling/Monitoring Platform for Detection of Aerosol SARS-CoV-2 On-site. Anal. Chem. 2021, 93, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Ye, X.; Li, Y.; Wang, L.; Zhang, J.; Fang, X.; Kong, J. Rapid Differential Diagnosis of Seven Human Respiratory Coronaviruses Based on Centrifugal Microfluidic Nucleic Acid Assay. Anal. Chem. 2020, 92, 14297–14302. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, B. Detection and extraction of heavy metal ions using paper-based analytical devices fabricated via atom stamp printing. Microsyst. Nanoeng. 2020, 6, 14. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, P.; Wang, D.; Jiang, J.; Chen, X.; Liu, Y.; Zhang, Z.; Tang, B.Z.; Li, P. AIEgens enabled ultrasensitive point-of-care test for multiple targets of food safety: Aflatoxin B1 and cyclopiazonic acid as an example. Biosens. Bioelectron. 2021, 182, 113188. [Google Scholar] [CrossRef] [PubMed]
- Valtonen, M.; Waris, M.; Vuorinen, T.; Eerola, E.; Hakanen, A.J.; Mjosund, K.; Grönroos, W.; Heinonen, O.J.; Ruuskanen, O. Common cold in Team Finland during 2018 Winter Olympic Games (PyeongChang): Epidemiology, diagnosis including molecular point-of-care testing (POCT) and treatment. Br. J. Sports Med. 2019, 53, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xia, A.; Wang, D.; Zhang, Y.; Deng, S.; Lu, W.; Luo, J.; Zhong, Q.; Zhang, F.; Zhou, L.; et al. An ultraportable and versatile point-of-care DNA testing platform. Sci. Adv. 2020, 6, eaaz7445. [Google Scholar] [CrossRef]
- Gao, H.; Wen, L.; Tian, J.; Wu, Y.; Liu, F.; Lin, Y.; Hua, W.; Wu, G. A portable electrochemical immunosensor for highly sensitive point-of-care testing of genetically modified crops. Biosens. Bioelectron. 2019, 142, 111504. [Google Scholar] [CrossRef]
- Kanno, Y.; Zhou, Y.; Fukuma, T.; Takahashi, Y. Alkaline Phosphatase-Based Electrochemical Analysis for Point-of-Care Testing. Electroanalysis 2021, 34, 161–167. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanomaterial-based devices for point-of-care diagnostic applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef]
- Joe, C.; Lee, B.H.; Kim, S.H.; Ko, Y.; Gu, M.B. Aptamer duo-based portable electrochemical biosensors for early diagnosis of periodontal disease. Biosens. Bioelectron. 2022, 199, 113884. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Z.; Xiao, M.; Jiang, L.; Yi, C. A smartphone-based quantitative point-of-care testing (POCT) system for simultaneous detection of multiple heavy metal ions. Chem. Eng. J. 2020, 394, 124966. [Google Scholar] [CrossRef]
- Khanwalker, M.; Fujita, R.; Lee, J.; Wilson, E.; Ito, K.; Asano, R.; Ikebukuro, K.; LaBelle, J.; Sode, K. Development of a POCT type insulin sensor employing anti-insulin single chain variable fragment based on faradaic electrochemical impedance spectroscopy under single frequency measurement. Biosens. Bioelectron. 2022, 200, 113901. [Google Scholar] [CrossRef]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in Diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Wieczerzak, M. Aptamer based tools for environmental and therapeutic monitoring: A review of developments, applications, future perspectives. Crit. Rev. Environ. Sci. Technol. 2020, 50, 816–867. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Zhu, Z.; Yang, C. Recent Progress in Aptamer-Based Functional Probes for Bioanalysis and Biomedicine. Chem. Eur. J. 2016, 22, 9886–9900. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, F.; Sang, Y.; Katouzian, I.; Jafari, S.M.; Wang, X.; Li, W.; Wang, J.; Mohammadi, Z. Screening, identification, and application of nucleic acid aptamers applied in food safety biosensing. Trends Food Sci. Technol. 2022, 123, 355–375. [Google Scholar] [CrossRef]
- Bunka, D.H.J.; Stockley, P.G. Aptamers come of age–at last. Nat. Rev. Microbiol. 2006, 4, 588–596. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, C.; Ma, T.; Liu, X.; Chen, Z.; Li, S.; Deng, Y. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J. Nanobiotechnol. 2021, 19, 166. [Google Scholar] [CrossRef]
- Abu-Ali, H.; Nabok, A.; Smith, T.J. Development of Novel and Highly Specific ssDNA-Aptamer-Based Electrochemical Biosensor for Rapid Detection of Mercury (II) and Lead (II) Ions in Water. Chemosensors 2019, 7, 27. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal-Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Li, X.; Liu, Q.; Kushwaha, A.; Prakash, O.; Sakiyama, H.; Muddassir, M.; Kumar, A.; Liu, J. Selective and Sensitive Sensing of Copper(II) by natural compound C-21 Steroid Caudatin. Polyhedron 2023, 243, 116537. [Google Scholar] [CrossRef]
- Dong, X.; Li, D.; Li, Y.; Sakiyama, H.; Muddassir, M.; Pan, Y.; Srivastava, D.; Kumar, A. A 3,8-connected Cd(ii)-based metal–organic framework as an appropriate luminescent sensor for the antibiotic sulfasalazine. Crystengcomm 2022, 24, 7157–7165. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Liu, S.; Zhang, Y.; He, Z.; Li, W.; Zhang, F.; Shi, Y.; Lü, W.; Li, Y.; et al. Ultrathin Metal-Organic Framework: An Emerging Broadband Nonlinear Optical Material for Ultrafast Photonics. Adv. Opt. Mater. 2018, 6, 1800561. [Google Scholar] [CrossRef]
- Pascanu, V.; Miera, G.G.; Inge, A.K.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef]
- Wu, M.-X.; Yang, Y.-W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Lin, M.; Lu, C.; Kumar, A.; Pan, Y.; Liu, J.-Q.; Peng, Y. Current and promising applications of Hf(iv)-based MOFs in clinical cancer therapy. J. Mater. Chem. B 2023, 11, 5693–5714. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Graphene Aerogel–Metal–Organic Framework-Based Electrochemical Method for Simultaneous Detection of Multiple Heavy-Metal Ions. Anal. Chem. 2019, 91, 888–895. [Google Scholar] [CrossRef]
- Du, D.; Shu, J.; Guo, M.; Haghighatbin, M.A.; Yang, D.; Bian, Z.; Cui, H. Potential-Resolved Differential Electrochemiluminescence Immunosensor for Cardiac Troponin I Based on MOF-5-Wrapped CdS Quantum Dot Nanoluminophores. Anal. Chem. 2020, 92, 14113–14121. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Z.; Wang, F.; Cai, J.; Guo, L.; Su, J.; Liu, Y. Highly sensitive photoelectrochemical biosensor for kinase activity detection and inhibition based on the surface defect recognition and multiple signal amplification of metal-organic frameworks. Biosens. Bioelectron. 2017, 97, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, L.; Wu, S.; Pan, Y.; Dong, Y.; Zhu, S.; Yang, J.; Yin, Y.; Li, G. An Electrochemical Biosensor Designed by Using Zr-Based Metal–Organic Frameworks for the Detection of Glioblastoma-Derived Exosomes with Practical Application. Anal. Chem. 2020, 92, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Dong, X. Hierarchically porous Zr-MOFs labelled methylene blue as signal tags for electrochemical patulin aptasensor based on ZnO nano flower. Sens. Actuators B Chem. 2019, 294, 192–198. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, T.; Li, X.; Qian, H.; Bian, X.; Li, X.; Bai, H.; Ding, S.; Yan, Y. Femtomolar and locus-specific detection of N6-methyladenine in DNA by integrating double-hindered replication and nucleic acid-functionalized MB@Zr-MOF. J. Nanobiotechnol. 2021, 19, 408. [Google Scholar] [CrossRef]
- Xu, L.; Liang, J.; Wang, Y.; Ren, S.; Wu, J.; Zhou, H.; Gao, Z. Highly Selective, Aptamer-Based, Ultrasensitive Nanogold Colorimetric Smartphone Readout for Detection of Cd(II). Molecules 2019, 24, 2745. [Google Scholar] [CrossRef]
- Li, L.; Chen, B.; Luo, L.; Liu, X.; Bi, X.; You, T. Sensitive and selective detection of Hg2+ in tap and canal water via self-enhanced ECL aptasensor based on NH2–Ru@SiO2-NGQDs. Talanta 2021, 222, 121579. [Google Scholar] [CrossRef]
- Li, D.; Yuan, X.; Li, C.; Luo, Y.; Jiang, Z. A novel fluorescence aptamer biosensor for trace Pb(II) based on gold-doped carbon dots and DNAzyme synergetic catalytic amplification. J. Lumin. 2020, 221, 117056. [Google Scholar] [CrossRef]
- Wen, S.-H.; Wang, Y.; Yuan, Y.-H.; Liang, R.-P.; Qiu, J.-D. Electrochemical sensor for arsenite detection using graphene oxide assisted generation of prussian blue nanoparticles as enhanced signal label. Anal. Chim. Acta 2018, 1002, 82–89. [Google Scholar] [CrossRef]
- Xiong, H.; Gao, J.; Wang, Y.; Chen, Z.; Chen, M.-M.; Zhang, X.; Wang, S. Construction of an ultrasensitive electrochemiluminescent aptasensor for ractopamine detection. Analyst 2019, 144, 2550–2555. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, S.; Ma, J.; Shi, H.; Wang, L.; Sheng, A.; Yin, T.; Sun, L.; Li, G. Colorimetric Sensor Array for Human Semen Identification Designed by Coupling Zirconium Metal–Organic Frameworks with DNA-Modified Gold Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 36316–36323. [Google Scholar] [CrossRef]
- Xiong, H.; Huang, Z.; Lin, Q.; Yang, B.; Yan, F.; Liu, B.; Chen, H.; Kong, J. Surface Plasmon Coupling Electrochemiluminescence Immunosensor Based on Polymer Dots and AuNPs for Ultrasensitive Detection of Pancreatic Cancer Exosomes. Anal. Chem. 2022, 94, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Fakude, C.T.; Arotiba, O.A.; Mabuba, N. Electrochemical aptasensing of cadmium (II) on a carbon black-gold nano-platform. J. Electroanal. Chem. 2020, 858, 113796. [Google Scholar] [CrossRef]
- Li, C.; Hu, X.; Lu, J.; Mao, X.; Xiang, Y.; Shu, Y.; Li, G. Design of DNA nanostructure-based interfacial probes for the electrochemical detection of nucleic acids directly in whole blood. Chem. Sci. 2018, 9, 979–984. [Google Scholar] [CrossRef]
- Ma, D.; Li, Z.; Zhu, J.; Zhou, Y.; Chen, L.; Mai, X.; Liufu, M.; Wu, Y.; Li, Y. Inverse and highly selective separation of CO2/C2H2 on a thulium–organic framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Nie, Y.; Liu, Y.; Ma, Q. Wavelength-Dependent Surface Plasmon Coupling Electrochemiluminescence Biosensor Based on Sulfur-Doped Carbon Nitride Quantum Dots for K-RAS Gene Detection. Anal. Chem. 2019, 91, 13780–13786. [Google Scholar] [CrossRef] [PubMed]
- Commission, E. Commission Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Commission Regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006, 173, 5–24. [Google Scholar]
- Tripathi, R.; Raghunath, R.; Sastry, V.; Krishnamoorthy, T. Daily intake of heavy metals by infants through milk and milk products. Sci. Total Environ. 1999, 227, 229–235. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Yang, Y.; Liang, W.-B.; Yao, L.-Y.; Yuan, R.; Xiao, D.-R. Highly Stable Covalent Organic Framework Nanosheets as a New Generation of Electrochemiluminescence Emitters for Ultrasensitive MicroRNA Detection. Anal. Chem. 2021, 93, 3258–3265. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Kim, J.; Gwak, H.; Hyun, K.-A.; Bae, N.H.; Lee, S.J.; Jung, H.-I. A microfluidic electrochemical aptasensor for enrichment and detection of bisphenol A. Biosens. Bioelectron. 2018, 117, 457–463. [Google Scholar] [CrossRef]
- Guo, H.; Li, J.; Li, Y.; Wu, D.; Ma, H.; Wei, Q.; Du, B. A turn-on fluorescent sensor for Hg2+ detection based on graphene oxide and DNA aptamers. New J. Chem. 2018, 42, 11147–11152. [Google Scholar] [CrossRef]
- Fan, G. Highly Sensitive Electrochemical Determination of Cadmium (II) in Environmental Water Based on the Electrodeposited Bismuth Nanoparticles. Int. J. Electrochem. Sci. 2016, 11, 4362–4370. [Google Scholar] [CrossRef]
- Kitte, S.A.; Li, S.; Nsabimana, A.; Gao, W.; Lai, J.; Liu, Z.; Xu, G. Stainless steel electrode for simultaneous stripping analysis of Cd(II), Pb(II), Cu(II) and Hg(II). Talanta 2019, 191, 485–490. [Google Scholar] [CrossRef] [PubMed]

| Sample | Added (ppb) | Found (ppb) | Recovery (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd2+ | Hg2+ | Pb2+ | As3+ | Cd2+ | Hg2+ | Pb2+ | As3+ | Cd2+ | Hg2+ | Pb2+ | As3+ | |
| Milk 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Milk 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Milk 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Milk 1 | 0.100 | 0.100 | 0.100 | 0.100 | 0.0920 | 0.104 | 0.096 | 0.106 | 92.0 | 104 | 96.0 | 106 |
| Milk 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.04 | 1.08 | 1.02 | 1.08 | 104 | 108 | 102 | 108 |
| Milk 3 | 10.0 | 10.0 | 10.0 | 10.0 | 10.5 | 9.78 | 9.86 | 9.53 | 105 | 97.8 | 98.6 | 95.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, H.; Li, P.; Cun, F.; Chen, H.; Kong, J. Methylene-Blue-Encapsulated Metal-Organic-Framework-Based Electrochemical POCT Platform for Multiple Detection of Heavy Metal Ions in Milk. Biosensors 2023, 13, 783. https://doi.org/10.3390/bios13080783
Xiong H, Li P, Cun F, Chen H, Kong J. Methylene-Blue-Encapsulated Metal-Organic-Framework-Based Electrochemical POCT Platform for Multiple Detection of Heavy Metal Ions in Milk. Biosensors. 2023; 13(8):783. https://doi.org/10.3390/bios13080783
Chicago/Turabian StyleXiong, Huiwen, Pintao Li, Fei Cun, Hui Chen, and Jilie Kong. 2023. "Methylene-Blue-Encapsulated Metal-Organic-Framework-Based Electrochemical POCT Platform for Multiple Detection of Heavy Metal Ions in Milk" Biosensors 13, no. 8: 783. https://doi.org/10.3390/bios13080783
APA StyleXiong, H., Li, P., Cun, F., Chen, H., & Kong, J. (2023). Methylene-Blue-Encapsulated Metal-Organic-Framework-Based Electrochemical POCT Platform for Multiple Detection of Heavy Metal Ions in Milk. Biosensors, 13(8), 783. https://doi.org/10.3390/bios13080783





