Point-of-Care Diagnostic Devices for Detection of Escherichia coli O157:H7 Using Microfluidic Systems: A Focused Review
Abstract
1. Introduction
2. SPR-Based E. coli O157:H7 Detection

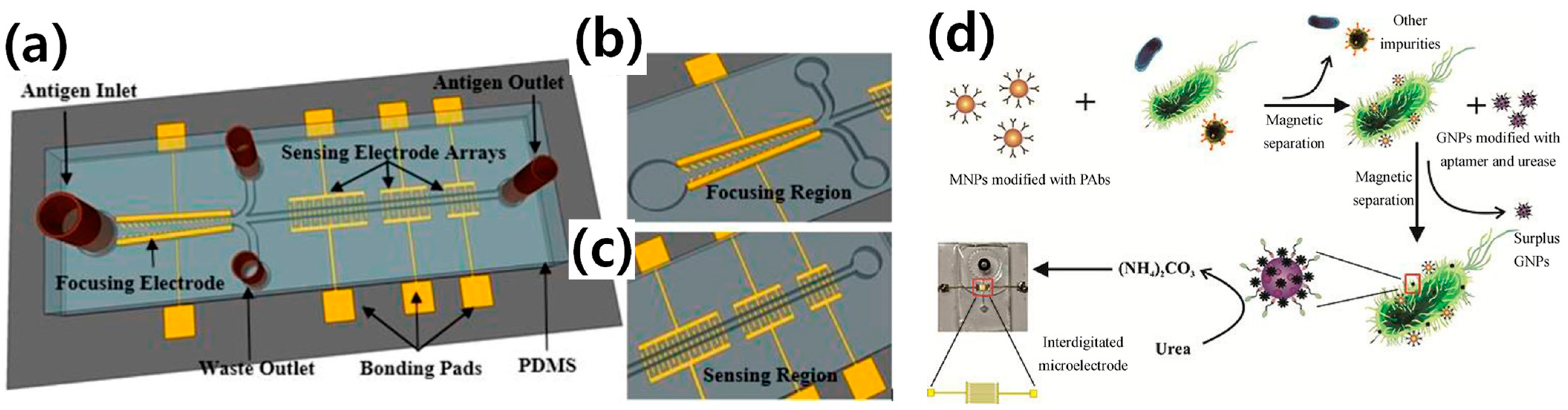
3. Electrochemical-Based E. coli O157:H7 Detection
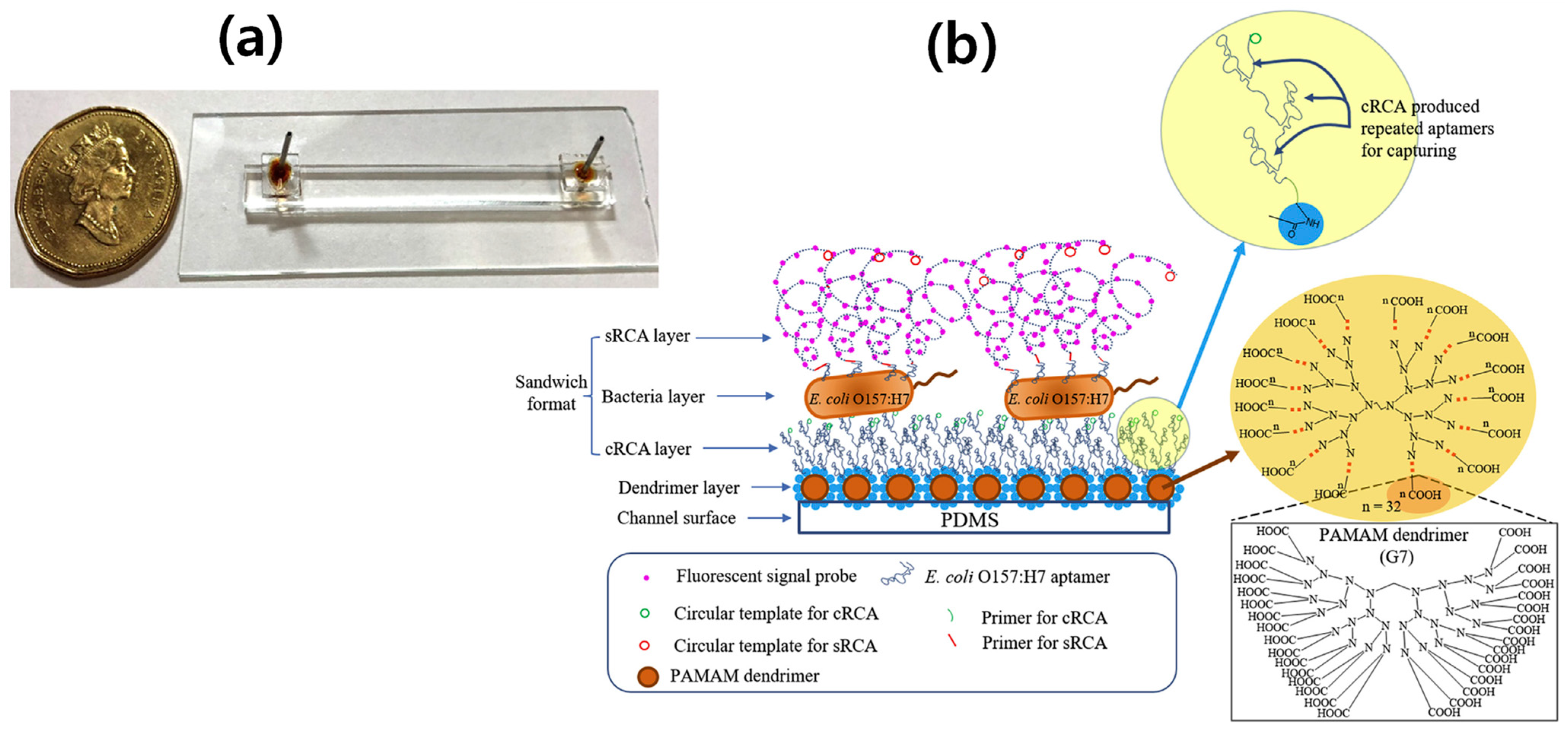
4. RCA-Based E. coli O157:H7 Detection
- ▪
- Amplification Mechanism: The fundamental amplification mechanisms of RCA and PCR differ. PCR utilizes a thermal cycling process to amplify a specific DNA segment, employing heat-stable DNA polymerases. On the other hand, RCA employs a rolling circle mechanism, where a circular template is exponentially amplified by a DNA polymerase with strand-displacement activity. This mechanism enables RCA to generate long, single-stranded DNA products, which can be advantageous for various downstream applications, such as DNA sequencing or in situ hybridization techniques.
- ▪
- Sensitivity: In certain scenarios, RCA has demonstrated higher sensitivity compared to PCR. Due to its isothermal nature, RCA can produce a larger number of amplification products, resulting in increased sensitivity for detecting low-abundance targets. This sensitivity advantage has proven particularly useful in applications such as detecting rare genetic mutations, single-cell analysis, or amplifying targets with low copy numbers.
- ▪
- Simplified Workflow: RCA offers a simplified workflow compared to PCR. RCA reactions can be performed under isothermal conditions, eliminating the need for sophisticated thermal cycling equipment. This simplification can reduce overall costs and technical complexity associated with amplification procedures, making RCA an appealing option for resource-limited settings or POC applications.
- ▪
- Product Length: As mentioned earlier, RCA can generate long, single-stranded DNA products. This feature proves advantageous in applications where longer DNA fragments are desired, such as generating templates for DNA sequencing or studying DNA–protein interactions. In contrast, PCR typically produces shorter amplicons due to limitations inherent in the polymerase enzyme and primer design considerations.
- ▪
- Enzyme Selection: RCA can be conducted using various DNA polymerases, including both strand-displacing and nick-translating enzymes. This flexibility allows researchers to choose an appropriate enzyme based on their specific requirements, such as amplification efficiency, fidelity, or compatibility with specific detection methods. In contrast, PCR primarily relies on thermostable DNA polymerases, which may have limitations in certain applications, such as amplifying challenging templates or incorporating modified nucleotides.
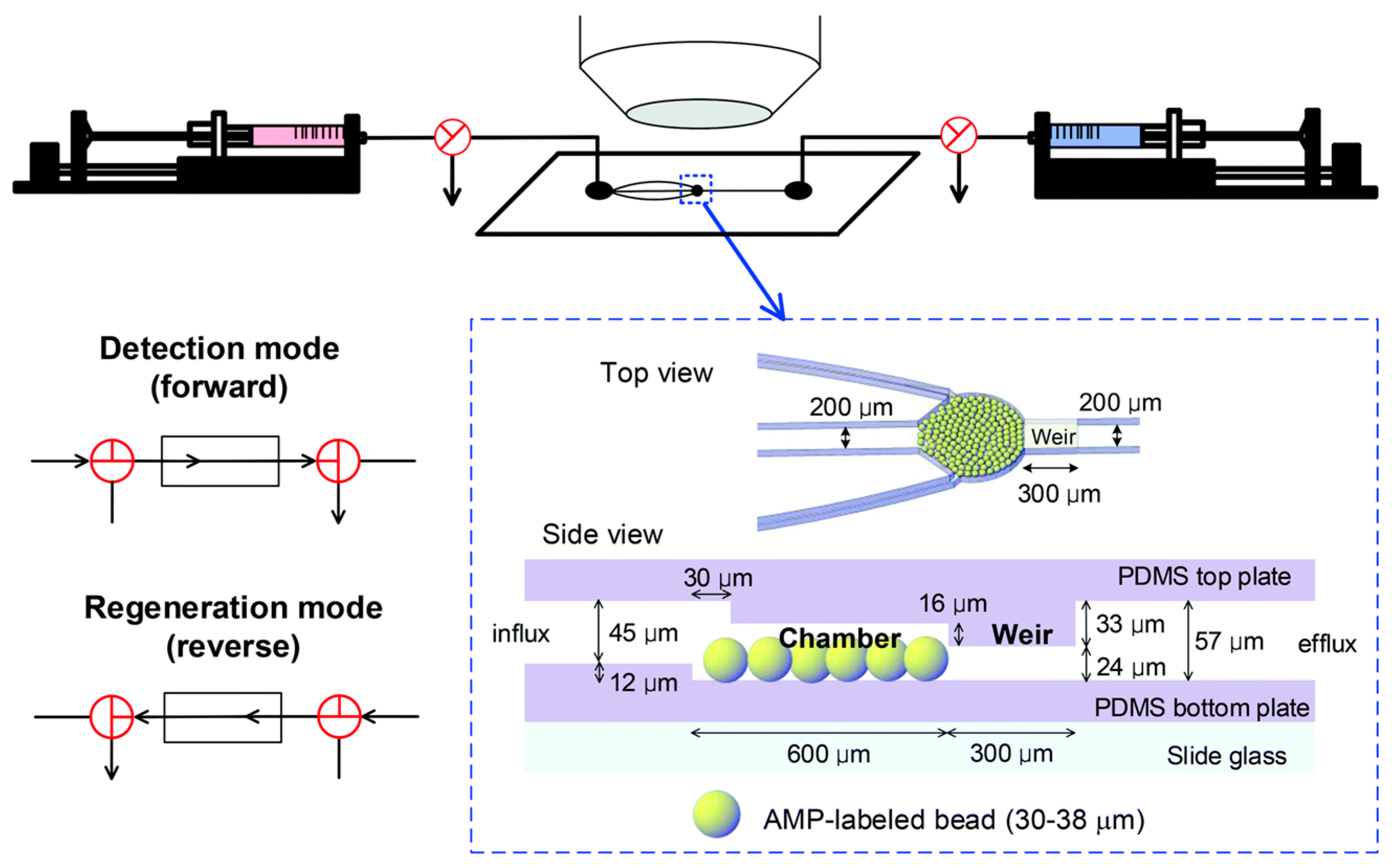
5. Attraction of Antimicrobial Peptide (AMP) for E. coli O157:H7 Detection
6. Conclusions and Future Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, V.K.; Akavaram, S.; Schaut, R.G.; Bayles, D.O. Comparative genomics reveals structural and functional features specific to the genome of a foodborne Escherichia coli O157: H7. BMC Genom. 2019, 20, 196. [Google Scholar] [CrossRef]
- Zou, Y.; Duan, N.; Wu, S.; Shen, M.; Wang, Z. Selection, identification, and binding mechanism studies of an ssDNA aptamer targeted to different stages of E. coli O157: H7. J. Agric. Food Chem. 2018, 66, 5677–5682. [Google Scholar] [CrossRef] [PubMed]
- Florentin, A.; Lizon, J.; Asensio, E.; Forin, J.; Rivier, A. Water and surface microbiologic quality of point-of-use water filters: A comparative study. Am. J. Infect. Control 2016, 44, 1061–1062. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-I.; Kim, S.-S.; Kang, D.-H. Susceptibility of Escherichia coli O157: H7 grown at low temperatures to the krypton-chlorine excilamp. Sci. Rep. 2019, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Oloketuyi, S.F.; Khan, F. Strategies for biofilm inhibition and virulence attenuation of foodborne pathogen-Escherichia coli O157: H7. Curr. Microbiol. 2017, 74, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.-Y.; Xiao, L.-M.; Liu, Y.-N.; Li, Y.-M. Prevalence of depression among Chinese University students: A meta-analysis. PLoS ONE 2016, 11, e0153454. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Scallan, E.; Jones-Bitton, A.; Sargeant, J.M.; Stapleton, J.; Angulo, F.J.; Yeung, D.H.; Kirk, M.D. Global incidence of human Shiga toxin–producing Escherichia coli infections and deaths: A systematic review and knowledge synthesis. Foodborne Pathog. Dis. 2014, 11, 447–455. [Google Scholar] [CrossRef]
- Quintela, I.A.; Vasse, T.; Lin, C.-S.; Wu, V.C.H. Advances, applications, and limitations of portable and rapid detection technologies for routinely encountered foodborne pathogens. Front. Microbiol. 2022, 13, 1054782. [Google Scholar] [CrossRef]
- Kim, J.-H.; Oh, S.-W. Rapid detection of E. coli O157: H7 by a novel access with combination of improved sample preparation and real-time PCR. Food Sci. Biotechnol. 2020, 29, 1149–1157. [Google Scholar] [CrossRef]
- Du, J.; Wu, S.; Niu, L.; Li, J.; Zhao, D.; Bai, Y. A gold nanoparticles-assisted multiplex PCR assay for simultaneous detection of Salmonella typhimurium, Listeria monocytogenes and Escherichia coli O157: H7. Anal. Methods 2020, 12, 212–217. [Google Scholar] [CrossRef]
- Kim, J.-H.; Oh, S.-W. Rapid and sensitive detection of E. coli O157: H7 and S. Typhimurium in iceberg lettuce and cabbage using filtration, DNA concentration, and qPCR without enrichment. Food Chem. 2020, 327, 127036. [Google Scholar] [CrossRef]
- Wang, C.; Xing, K.; Zhang, G.; Yuan, M.; Xu, S.; Liu, D.; Chen, W.; Peng, J.; Hu, S.; Lai, W.-H. Novel ELISA based on fluorescent quenching of DNA-stabilized silver nanoclusters for detecting E. coli O157: H7. Food Chem. 2019, 281, 91–96. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, C.; Li, L.; Song, X.; Xu, K.; Wang, J.; Liu, Y.; Fu, K.; Bao, H.; Song, D.J.; et al. Development of a low-cost paper-based ELISA method for rapid Escherichia coli O157: H7 detection. Anal. Biochem. 2018, 542, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Safavieh, M.; Ahmed, M.U.; Sokullu, E.; Ng, A.; Braescu, L.; Zourob, M. A simple cassette as point-of-care diagnostic device for naked-eye colorimetric bacteria detection. Analyst 2014, 139, 482–487. [Google Scholar] [CrossRef]
- Amin, N.; Torralba, A.S.; Álvarez-Diduk, R.; Afkhami, A.; Merkoçi, A. Lab in a tube: Point-of-care detection of Escherichia coli. Anal. Chem. 2020, 92, 4209–4216. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Chidambara, V.A.; Andreasen, S.Z.; Golabi, M.; Linh, Q.T.; Bang, D.D.; Wolff, A. Point-of-care devices for pathogen detections: The three most important factors to realize towards commercialization. TrAC-Trends Anal. Chem. 2020, 131, 116004. [Google Scholar] [CrossRef]
- Badshah, M.A.; Kim, J.; Yeom, J.; Abbas, N.; Haq, M.R.; Kim, Y.; Lu, X.; Kim, S.-M. Glass nanoimprinted plasmonic nanostructure for high power laser stable surface-enhanced Raman spectroscopy substrate. Appl. Surf. Sci. 2021, 542, 148587. [Google Scholar] [CrossRef]
- Badshah, M.A.; Michel, D.; Alam, N.E.; Madni, I.; Abbas, N.; Alameh, K.; Kim, S.-M. Enhancing the sensitivity of a surface plasmon resonance sensor with glancing angle deposited nanostructures. Plasmonics 2020, 15, 2161–2168. [Google Scholar] [CrossRef]
- Badshah, M.A.; Koh, N.Y.; Zia, A.W.; Abbas, N.; Zahra, Z.; Saleem, M.W. Recent developments in plasmonic nanostructures for metal enhanced fluorescence-based biosensing. Nanomaterials 2020, 10, 1749. [Google Scholar] [CrossRef]
- Abbas, N.; Lu, X.; Badshah, M.A.; In, J.B.; Heo, W.I.; Park, K.Y.; Lee, M.-K.; Kim, C.H.; Kang, P.; Chang, W.-J.; et al. Development of a protein microarray chip with enhanced fluorescence for identification of semen and vaginal fluid. Sensors 2018, 18, 3874. [Google Scholar] [CrossRef]
- Lu, X.; Lee, S.; Kim, J.; Abbas, N.; Badshah, M.A.; Kim, S.-M. Fabrication of Ag nanorods on micropost array for a metal-enhanced fluorescence substrate with a high signal-to-background ratio. Biosens. Bioelectron. 2021, 175, 112881. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Abbas, N.; Lee, S.; Yeom, J.; Asgar, M.A.; Badshah, M.A.; Lu, X.; Kim, Y.K.; Kim, S.-M. Fabrication of a Plasmonic Nanoantenna Array Using Metal Deposition on Polymer Nanoimprinted Nanodots for an Enhanced Fluorescence Substrate. Polymers 2021, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Fratamico, P.M.; Strobaugh, T.P.; Medina, M.B.; Gehring, A.G. Detection of Escherichia coli 0157: H7 using a surface plasmon resonance biosensor. Biotechnol. Tech. 1998, 12, 571–576. [Google Scholar] [CrossRef]
- Tokel, O.; Yildiz, U.H.; Inci, F.; Durmus, N.G.; Ekiz, O.O.; Turker, B.; Cetin, C.; Rao, S.; Sridhar, K.; Natarajan, N.; et al. Portable microfluidic integrated plasmonic platform for pathogen detection. Sci. Rep. 2015, 5, 9152. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Tiwari, U.K.; Pal, S.S.; Sinha, R.K. Rapid detection of Escherichia coli using fiber optic surface plasmon resonance immunosensor based on biofunctionalized Molybdenum disulfide (MoS2) nanosheets. Biosens. Bioelectron. 2019, 126, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Park, B. Immunoassay biosensing of foodborne pathogens with surface plasmon resonance imaging: A review. J. Agric. Food Chem. 2020, 68, 12927–12939. [Google Scholar] [CrossRef]
- Li, Y.; Gao, F.; Lu, C.; Fauconnier, M.-L.; Zheng, J. Bio-specific Au/Fe3+ porous spongy nanoclusters for sensitive SERS detection of Escherichia coli O157: H7. Biosensors 2021, 11, 354. [Google Scholar] [CrossRef]
- Yaghubi, F.; Zeinoddini, M.; Saeedinia, A.R.; Azizi, A.; Nemati, A.S. Design of localized surface plasmon resonance (LSPR) biosensor for immunodiagnostic of E. coli O157: H7 using gold nanoparticles conjugated to the chicken antibody. Plasmonics 2020, 15, 1481–1487. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157: H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 2019, 124–125, 143–149. [Google Scholar] [CrossRef]
- Lee, N.; Choi, S.-W.; Chang, H.-J.; Chun, H.S. Rapid Detection of Escherichia coli O157: H7 in fresh lettuce based on localized surface plasmon resonance combined with immunomagnetic separation. J. Food Prot. 2018, 81, 713–718. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, H.; Li, M.; Sun, C.; Ren, D.; Li, Y. Fiber optic surface plasmon resonance sensor for detection of E. coli O157: H7 based on antimicrobial peptides and AgNPs-rGO. Biosens. Bioelectron. 2018, 117, 347–353. [Google Scholar] [CrossRef]
- Song, L.; Zhang, L.; Huang, Y.; Chen, L.; Zhang, G.; Shen, Z.; Zhang, J.; Xiao, Z.; Chen, T. Amplifying the signal of localized surface plasmon resonance sensing for the sensitive detection of Escherichia coli O157: H7. Sci. Rep. 2017, 7, 3288. [Google Scholar] [CrossRef]
- Vaisocherová-Lísalová, H.; Víšová, I.; Ermini, M.L.; Špringer, T.; Song, X.C.; Mrázek, J.; Lamačová, J.; Lynn, N.S., Jr.; Šedivák, P.; Homola, J. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens. Bioelectron. 2016, 80, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tawil, N.; Sacher, E.; Mandeville, R.; Meunier, M. Surface plasmon resonance detection of E. coli and methicillin-resistant S. aureus using bacteriophages. Biosens. Bioelectron. 2012, 37, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, Z.; Si, C.; Ying, Y. Subtractive inhibition assay for the detection of E. coli O157: H7 using surface plasmon resonance. Sensors 2011, 11, 2728–2739. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Dastider, S.G.; Abdullah, A.; Jasim, I.; Yuksek, N.S.; Dweik, M.; Almasri, M. Low concentration E. coli O157: H7 bacteria sensing using microfluidic MEMS biosensor. Rev. Sci. Instrum. 2018, 89, 125009. [Google Scholar] [CrossRef]
- Yao, L.; Wang, L.; Huang, F.; Cai, G.; Xi, X.; Lin, J. A microfluidic impedance biosensor based on immunomagnetic separation and urease catalysis for continuous-flow detection of E. coli O157: H7. Sens. Actuators B 2018, 259, 1013–1021. [Google Scholar] [CrossRef]
- Qaanei, M.; Taheri, R.A.; Eskandari, K. Electrochemical aptasensor for Escherichia coli O157: H7 bacteria detection using a nanocomposite of reduced graphene oxide, gold nanoparticles and polyvinyl alcohol. Anal. Methods 2021, 13, 3101–3109. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, X.; Li, N.; Jiang, X.; Liu, C.; Xu, J.-J.; Wu, P. A portable, battery-powered photoelectrochemical aptasesor for field environment monitoring of E. coli O157: H7. Sens. Actuators B 2021, 346, 130520. [Google Scholar] [CrossRef]
- Ropero-Vega, J.L.; Redondo-Ortega, J.F.; Galvis-Curubo, Y.J.; Rondón-Villarreal, P.; Flórez-Castillo, J.M. A Bioinspired Peptide in TIR Protein as Recognition Molecule on Electrochemical Biosensors for the Detection of E. coli O157: H7 in an Aqueous Matrix. Molecules 2021, 26, 2559. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ou, G.; Chen, X.; Li, Z.; Hu, R.; Li, Y.; Yang, Y.; Liu, M. Naked-eye based point-of-care detection of E. coli O157: H7 by a signal-amplified microfluidic aptasensor. Anal. Chim. Acta 2020, 1130, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Park, J.; Lim, S.Y.; Kwon, Y.; Bae, N.H.; Park, J.-K.; Lee, S.J. Integrated pumpless microfluidic chip for the detection of foodborne pathogens by polymerase chain reaction and electrochemical analysis. Sens. Actuators B 2021, 329, 129130. [Google Scholar] [CrossRef]
- Dhull, N.; Kaur, G.; Jain, P.; Mishra, P.; Singh, D.; Ganju, L.; Gupta, V.; Tomar, M. Label-free amperometric biosensor for Escherichia coli O157: H7 detection. Appl. Surf. Sci. 2019, 495, 143548. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Cai, G.; Xiong, Y.; Li, Y.; Wang, M.; Huo, H.; Lin, J. Fast and sensitive detection of foodborne pathogen using electrochemical impedance analysis, urease catalysis and microfluidics. Biosens. Bioelectron. 2016, 86, 770–776. [Google Scholar] [CrossRef]
- Wang, Y.; Ping, J.; Ye, Z.; Wu, J.; Ying, Y. Impedimetric immunosensor based on gold nanoparticles modified graphene paper for label-free detection of Escherichia coli O157: H7. Biosens. Bioelectron. 2013, 49, 492–498. [Google Scholar] [CrossRef]
- Altintas, Z.; Akgun, M.; Kokturk, G.; Uludag, Y. A fully automated microfluidic-based electrochemical sensor for real-time bacteria detection. Biosens. Bioelectron. 2018, 100, 541–548. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Lei, C.; Sun, X.-C.; Zhou, Y. Ultrasensitive detection and quantification of E. coli O157: H7 using a giant magnetoimpedance sensor in an open-surface microfluidic cavity covered with an antibody-modified gold surface. Microchim. Acta 2016, 183, 1831–1837. [Google Scholar] [CrossRef]
- Bai, H.; Bu, S.; Liu, W.; Wang, C.; Li, Z.; Hao, Z.; Wan, J.; Han, Y. An electrochemical aptasensor based on cocoon-like DNA nanostructure signal amplification for the detection of Escherichia coli O157: H7. Analyst 2020, 145, 7340–7348. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Huang, H.; Deng, J.; Fang, L.; Luo, J.; Zhang, S.; Huang, J.; Liang, W.; Zheng, J. A sensitive electrochemical strategy via multiple amplification reactions for the detection of E. coli O157: H7. Biosens. Bioelectron. 2020, 147, 111752. [Google Scholar] [CrossRef]
- Li, T.; Zhu, F.; Guo, W.; Gu, H.; Zhao, J.; Yan, M.; Liu, S. Selective capture and rapid identification of E. coli O157: H7 by carbon nanotube multilayer biosensors and microfluidic chip-based LAMP. RSC Adv. 2017, 7, 30446–30452. [Google Scholar] [CrossRef]
- Xu, L.; Duan, J.; Chen, J.; Ding, S.; Cheng, W. Recent advances in rolling circle amplification-based biosensing strategies: A review. Anal. Chim. Acta 2021, 1148, 238187. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiu, Z.; Le, T.; Zou, S.; Cao, X. Developing a dual-RCA microfluidic platform for sensitive E. coli O157: H7 whole-cell detections. Anal. Chim. Acta 2020, 1127, 79–88. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Zhang, L.; Liu, S.; Zhang, M.; Wang, J.; Ning, B.; Peng, Y.; He, J.; Hu, Y.; et al. CRISPR-Cas9 triggered two-step isothermal amplification method for E. coli O157: H7 detection based on a metal–organic framework platform. Anal. Chem. 2020, 92, 3032–3041. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Y.; Yang, X.; Lin, M.; Dan, H.; Zou, S.; Cao, X. In situ rolling circle amplification surface modifications to improve E. coli O157: H7 capturing performances for rapid and sensitive microfluidic detection applications. Anal. Chim. Acta 2021, 1150, 338229. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zou, S.; Cao, X. A simple dendrimer-aptamer based microfluidic platform for E. coli O157: H7 detection and signal intensification by rolling circle amplification. Sens. Actuators B 2017, 251, 976–984. [Google Scholar] [CrossRef]
- Luo, F.; Li, Z.; Dai, G.; Lu, Y.; He, P.; Wang, Q. Ultrasensitive biosensing pathogenic bacteria by combining aptamer-induced catalysed hairpin assembly circle amplification with microchip electrophoresis. Sens. Actuators B 2020, 306, 127577. [Google Scholar] [CrossRef]
- Zhang, T.; Tao, Q.; Bian, X.-J.; Chen, Q.; Yan, J. Rapid Visualized Detection of Escherichia coli O157: H7 by DNA Hydrogel Based on Rolling Circle Amplification. Chin. J. Anal. Chem. 2021, 49, 377–386. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Liu, S.; Yu, J.; Pei, Q.; Leng, X.; Huang, J. A functional oligonucleotide probe from an encapsulated silver nanocluster assembled by rolling circle amplification and its application in label-free sensors. RSC Adv. 2016, 6, 88967–88973. [Google Scholar] [CrossRef]
- Makhlynets, O.V.; Caputo, G.A. Characteristics and therapeutic applications of antimicrobial peptides. Biophys. Rev. 2021, 2, 011301. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, L.; Zhu, Y.; Gao, H.; Chen, Y.; Chen, J.; Jia, Y.; He, J.; Fang, Z.; Zhu, Y.; et al. Temperature-controlled reversible exposure and hiding of antimicrobial peptides on an implant for killing bacteria at room temperature and improving biocompatibility in vivo. ACS Appl. Mater. Interfaces 2018, 10, 35830–35837. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Tan, P.; Zhu, Y.; Shao, C.; Shan, A.; Li, L. Highly stabilized α-helical coiled coils kill gram-negative bacteria by multicomplementary mechanisms under acidic condition. ACS Appl. Mater. Interfaces 2019, 11, 22113–22128. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 1995, 13, 61–92. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Duclohier, H.; Molle, G.; Spach, G. Antimicrobial peptide magainin I from Xenopus skin forms anion-permeable channels in planar lipid bilayers. Biophys. J. 1989, 56, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Sugishita, K.-i.; Harada, M.; Fujii, N.; Miyajima, K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim. Biophys. Acta 1997, 1327, 119–130. [Google Scholar] [CrossRef]
- Kulagina, N.V.; Shaffer, K.M.; Anderson, G.P.; Ligler, F.S.; Taitt, C.R. Antimicrobial peptide-based array for Escherichia coli and Salmonella screening. Anal. Chim. Acta 2006, 575, 9–15. [Google Scholar] [CrossRef]
- Chang, M.-S.; Yoo, J.H.; Woo, D.H.; Chun, M.-S. Efficient detection of Escherichia coli O157: H7 using a reusable microfluidic chip embedded with antimicrobial peptide-labeled beads. Analyst 2015, 140, 7997–8006. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Zhang, S.; Link, A.J.; McAlpine, M.C. Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2010, 107, 19207–19212. [Google Scholar] [CrossRef]
- Dong, Z.-M.; Zhao, G.-C. Label-free detection of pathogenic bacteria via immobilized antimicrobial peptides. Talanta 2015, 137, 55–61. [Google Scholar] [CrossRef]
- Bai, H.; Bu, S.; Wang, C.; Ma, C.; Li, Z.; Hao, Z.; Wan, J.; Han, Y. Sandwich immunoassay based on antimicrobial peptide-mediated nanocomposite pair for determination of Escherichia coli O157: H7 using personal glucose meter as readout. Microchim. Acta 2020, 187, 220. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Faraj, Y.; Wei, J.; Wang, W.; Xie, R.; Liu, Z.; Ju, X.-J.; Chu, L.-Y. Antimicrobial peptide-functionalized magnetic nanoparticles for rapid capture and removal of pathogenic bacteria. Microchem. J. 2020, 159, 105493. [Google Scholar] [CrossRef]
- Yang, G.; Wang, H.; Dong, Y.; Li, Z.; Wang, G.-L. High-throughput photoelectrochemical determination of E. coli O157: H7 by modulation of the anodic photoelectrochemistry of CdS quantum dots via reversible deposition of MnO2. Microchim. Acta 2020, 187, 16. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Lei, C.; Fu, Y.; Li, Y. An antimicrobial peptide-based colorimetric bioassay for rapid and sensitive detection of E. coli O157: H7. RSC Adv. 2017, 7, 15769–15775. [Google Scholar] [CrossRef]
- Qiao, Z.; Lei, C.; Fu, Y.; Li, Y. Rapid and sensitive detection of E. coli O157: H7 based on antimicrobial peptide functionalized magnetic nanoparticles and urease-catalyzed signal amplification. Anal. Methods 2017, 9, 5204–5210. [Google Scholar] [CrossRef]
- Jiang, K.; Etayash, H.; Azmi, S.; Naicker, S.; Hassanpourfard, M.; Shaibani, P.M.; Thakur, G.; Kaur, K.; Thundat, T. Rapid label-free detection of E. coli using antimicrobial peptide assisted impedance spectroscopy. Anal. Methods 2015, 7, 9744–9748. [Google Scholar] [CrossRef]
- Yoo, J.H.; Woo, D.H.; Chang, M.-S.; Chun, M.-S. Microfluidic based biosensing for Escherichia coli detection by embedding antimicrobial peptide-labeled beads. Sens. Actuators B 2014, 191, 211–218. [Google Scholar] [CrossRef]
- Li, Y.; Afrasiabi, R.; Fathi, F.; Wang, N.; Xiang, C.; Love, R.; She, Z.; Kraatz, H.-B. Impedance based detection of pathogenic E. coli O157: H7 using a ferrocene-antimicrobial peptide modified biosensor. Biosens. Bioelectron. 2014, 58, 193–199. [Google Scholar] [CrossRef]
- Schwartz, O.; Bercovici, M. Microfluidic assay for continuous bacteria detection using antimicrobial peptides and isotachophoresis. Anal. Chem. 2014, 86, 10106–10113. [Google Scholar] [CrossRef]
- Qi, W.; Zheng, L.; Hou, Y.; Duan, H.; Wang, L.; Wang, S.; Liu, Y.; Li, Y.; Liao, M.; Lin, J. A finger-actuated microfluidic biosensor for colorimetric detection of foodborne pathogens. Food Chem. 2022, 381, 131801. [Google Scholar] [CrossRef]
- Gan, Z.; Hu, H.; Deng, S.; Xiao, X.; Xiong, Y.; Peng, J.; Lai, W. An integrated colorimetric and photothermal lateral flow immunoassay based on bimetallic Ag–Au urchin-like hollow structures for the sensitive detection of E. coli O157: H7. Biosens. Bioelectron. 2023, 225, 115090. [Google Scholar]


| Authors, Year | Materials | LOD/Detection Range (CFU/mL) | Detection Time (min) | Antibody (Ab) | Sample Type | Sensors Response |
|---|---|---|---|---|---|---|
| Li et al., 2021 [27] | SERS based organic and inorganic hybrid Au/Fe3+ nanoclusters | ~2/1–106 | ~30 | monoclonal rabbit Ab | Spiked food | - |
| Yaghubi et al., 2020 [28] | LSPR based Au nanoparticle conjugated (non-covalent bond) with specific chicken anti-E. coli O157:H7 antibody | ~10/10–105 | ~120 | chicken Ab | Chicken sample | 530–543 nm |
| Kaushik et al., 2019 [25] | Functionalized 2D nanomaterial (MoS2 nanosheets) | ~94/10–8 × 103 | ~15 | E. coli monoclonal Ab | Water and orange juice | 637–644 nm |
| Zheng et al., 2019 [29] | Capture antibodies modified with magnetic nanoparticles (MNPs) and aggregation of Au nanoparticles; calorimetric biosensor | ~50/50–5 × 108 | ~30 | Ab | Chicken sample | - |
| Lee et al., 2018 [30] | LSPR based magnetic nanoparticle coated with Au shell | ~10/0–106 | ~60 | anti–E. coli O157:H7 | Fresh lettuce | 535–547 nm |
| Zhou et al., 2018 [31] | Silver nanoparticles-reduced graphene oxide (AgNPs-rGO) | ~5 × 102/103–5 × 107 | ~40 | AMP with magainin 1-C | Water and juice | 645–680 nm |
| Song et al., 2017 [32] | Immobilization of antibodies on Au NRs and Au NRs@SiO2 | 10/0.5–5 × 107 | ~70 (+10 shaking) | murine anti-E. coli O157:H7 monoclonal Ab | PBS | 718–775 nm |
| Lísalová et al., 2016 [33] | Ultra-low fouling and functionalizable poly(carboxybetaine acrylamide) brushes | 17 & 57/1–108 | ~80 | Ab | Hamburger and Cucumber | 0.0977 ± 0.03 nm |
| Tokel et al., 2015 [24] | Disposable microfluidic chips with Au coated surfaces functionalized with antibody | 105/105–3.2 × 107 | ~20 | Ab | PBS and peritoneal dialysis fluid | Angle shift of 0.01 |
| Tawil et al., 2012 [34] | T4 bacteriophage | 103/102–105 | ~20 | T4 bacteriophage | PBS | 0–35 pixels |
| Wang et al., 2011 [35] | MNPs modified with capture antibodies and aggregation of Au nanoparticles | 3 × 104/3 × 104–3 × 108 | ~10 | rabbit anti-goat IgG polyclonal Ab | - | - |
| Authors and Year | Accurate Detection Results |
|---|---|
| Li et al., 2021 [27] | E. coli O157:H7 > E. coli > S. typhimurium > S. aureus > V. parahemolyticus |
| Zheng et al., 2019 [29] | E. coli O157:H7 > Salmonella typhimurium > Listeria monocytogenes > non-target bacteria |
| Zhou et al., 2018 [31] | E. coli O157:H7 > non-pathogenic E. coli K12 > Staphylococcus aureus > hemolytic streptococcus |
| Tokel et al., 2015 [24] | E. coli O157:H7 > Staphylococcus aureus and E. coli> Staphylococcus aureus |
| Wang et al., 2011 [35] | E. coli O157:H7 > non-pathogenic E. coli DH5α |
| Authors, Year | Materials | Assay Approach | Methodology | LOD/Detection Range (CFU/mL) | Detection Time (min) | Sample Type |
|---|---|---|---|---|---|---|
| Qaanei et al., 2021 [39] | Nanocomposite of reduced graphene oxide, Au nanoparticles and polyvinyl alcohol | Aptasensor | Differential pulse voltammetry (DPV) | 17 (cucumber), 57 (hamburger)/9.2–9.2 × 108 | ~100 | Tap water, milk, meat |
| Zheng et al., 2021 [40] | Gold nanoparticles | Photoelectro chemical aptasensor | Electrochemical impedance spectroscopy (EIS) | 200/0–4 × 107 | ~40 | Water |
| Ropero-Vega et al., 2021 [41] | Au nanoparticles-modified screen-printed electrodes | Bioinspired peptide in TIR protein as recognition molecule | Cyclic voltammetry (CV), EIS, Square wave voltammetry (SWV) | 2/0–103 | ~30 | PBS |
| Li et al., 2020 [42] | Platinum nanoparticles | Aptasensor | Hybridized chemical reaction amplification | 400/102–107 | ~15 | Real milk |
| Park et al., 2021 [43] | PDMS-based finger-actuated microfluidic modules with Au electrodes | Geno-sensor | SWV | 100/0–106 | ~40 | DI water, milk |
| Dhull et al., 2019 [44] | NiO/ITO electrode based immunosensor | Immunosensor | Amperometric biosensor, CV | 1/10–107 | ~60 | Real milk |
| Chen et al., 2016 [45] | Magnetic nanoparticles and Au nanoparticles | Immunosensor | EIS | 1.6 × 102/102–105 | ~60 | Spiked lettuce |
| Wang et al., 2013 [46] | Au nanoparticles modified graphene paper | Impedimetric immunosensor | EIS | 1.5 × 102/1.5 × 102–1.5 × 107 | ~30 | Ground beef, cucumber |
| Altintas et al., 2018 [47] | Au nanoparticles amplified immunoassays | Impedimetric immunosensor | CV | 50/10–3.97 × 107 | ~35 | Water |
| Yao et al., 2018 [38] | Magnetic nanoparticles, Au nanoparticles | Impedimetric immunosensor | EIS | 12/12–1.2 × 105 | ~15 | Water |
| Yang et al., 2016 [48] | Au nano-films | Magneto-impedance sensor | Giant-magneto impedance effect | 50/0–103 | ~20 | Water |
| Bai et al., 2020 [49] | Au nanoparticles | Aptasensor | CV, EIS, DPV | 10/10–106 | ~60 | Spiked milk |
| Li et al., 2020 [50] | Hairpin primes and signal probes | Geno-sensor | CV, EIS, DPV | 7/0–104 | ~40 | Apple juice, milk |
| Li et al., 2017 [51] | Carbon nanotube | Geno-sensor | CV, EIS | 1/0–104 | ~45 | Apple juice, milk |
| Authors and Year | Accurate Detection Results |
|---|---|
| Qaanei et al., 2021 [39] | E. coli O157:H7 > non-pathogenic E. coli K12 > E. coli > Pseudomonas aeruginosa > Staphylococcus aureus > Salmonella typhimurium |
| Zheng et al., 2021 [40] | E. coli O157:H7 > Salmonella > Staphylococcus aureus > E. coli |
| Ropero-Vega et al., 2021 [41] | E. coli O157:H7 > P. aeruginosa > PBS > Staphylococcus aureus |
| Li et al., 2020 [42] | E. coli O157:H7 > E. coli K12 > Staphylococcus aureus> Buffer |
| Park et al., 2021 [43] | E. coli O157:H7 > B. cereus > S. enteritidis |
| Dhull et al., 2019 [44] | E. coli O157:H7 > Staphylococcus aureus > non-pathogenic strain of E. coli |
| Chen et al., 2016 [45] | E. coli O157:H7 > L. monocytogenes > Mixture |
| Wang et al., 2013 [46] | E. coli O157:H7 > E. coli DH 5α > S. aureus > L. monocytogenes |
| Altintas et al., 2018 [47] | E. coli O157:H7 > Salmonella > Shigella > S. aureus |
| Yao et al., 2018 [38] | E. coli O157:H7 > Salmonella typhimurium > Listeria monocytogenes |
| Bai et al., 2020 [49] | E. coli O157:H7 > S. typhimurium > S. aureus > L. monocytogenes > P. aeruginosa |
| Li et al., 2020 [50] | E. coli O157:H7 > Vibrio cholera O1 > Salmonella spp. > S. aureus > Listeria innocua |
| Authors and Year | Materials | Assay Approach | LOD/Detection Range (CFU/mL) | Detection Time (min) | Sample Type |
|---|---|---|---|---|---|
| Li et al., 2021 [55] | PDMS surface, PAMAM dendrimers, aptamer, padlock probe, primers | Aptasensor | 103–104/102–105 | ~60 | Orange juice, milk, PBS, iced tea, bottled water |
| Jiang et al., 2020 [53] | PAMAM dendrimers, signaling RCA, primers/probes | Aptasensor | 80/102–105 | ~90 | Orange juice, PBS, milk |
| Jiang et al., 2017 [56] | PDMS surface, PAMAM dendrimers, primers/probes | Aptasensor | 102/102–105 | ~60 | Orange juice, PBS, milk |
| Sun et al., 2020 [54] | UiO66 consisting of cubic framework of cationic nodes (formed in-situ via hydrolysis of ZrCl4) and 1,4-benzenedicarboxylate linkers | CRISPR (clustered regularly interspaced short palindromic repeats) based biosensor | 4 × 102/1.3 × 102–6.5 × 104 | ~120 | Spring water, skim milk, orange juice |
| Luo et al., 2020 [57] | Hairpin probes | Aptasensor | 75/2 × 102–2 × 105 | ~90 | Defatted milk |
| Zhang et al., 2021 [58] | Aptamer, padlock probe, T4 DNA ligase, phi29 DNA polymerase, primers | Aptasensor based on DNA hydrogel | 4 × 103/4 × 103–4 × 105 | ~30 | Spiked milk |
| Guo et al., 2016 [59] | Encapsulated silver nanocluster assembled by RCA | Electrochemical sensor | 31/37–3.7 × 106 | ~80 | Milk |
| Authors and Year | Accurate Detection Results |
|---|---|
| Li et al., 2021 [55] | E. coli O157:H7 > non-target E. coli ATCC25922 |
| Jiang et al., 2020 [53] | E. coli O157:H7 > E. coli ER2420 > E. coli K12 > Listeria innocua |
| Jiang et al., 2017 [56] | E. coli O157:H7 > E. coli ER2420 > E. coli K12 > Listeria innocua |
| Sun et al., 2020 [54] | E. coli O157:H7 > S. typhimurium > L. monocytogenes > V. parahemolyticus > S. Aureus > S. flexneri |
| Luo et al., 2020 [57] | E. coli O157:H7 > S. aureus > S. Typhimurium > L. monocytogenes > S. flexneri > E. coli ATCC25922 > E. coli CMCC44102> E. coli ATCC35218 |
| Zhang et al., 2021 [58] | E. coli O157:H7 > E. coli O6 > S. typhimurium > S. aureus > L. monocytogenes |
| Guo et al., 2016 [59] | E. coli O157:H7 > Salmonella > Bacillus Subtilis > Listeria |
| Authors and Year | Materials and Recognition Elements | Sensor Type | Technique | LOD/Detection Range (CFU/mL) | Detection Time (min) | Sample Type |
|---|---|---|---|---|---|---|
| Bai et al., 2020 [71] | Cu phosphate nanocomposites embedded by AMP magainin I and cecropin P1 | Immunosensor | Glucose meter readout | 10/10–107 | ~90 | Spiked milk |
| Ding et al., 2020 [72] | AMP magainin I (C-terminal) functionalized magnetic nanoparticles, consists of Fe3O4 core | Electrochemical sensor | Dynamic light scattering (DLS) | 5/5–5 × 106 | - | DI water, tap water |
| Yang et al., 2019 [73] | MnO2 on photo electrode surface, AMP magainin I as recognition element | Electrochemical sensor | Photo electrochemical | 3/10–5 × 106 | ~30 | Tap water, tomato juice |
| Qiao et al., 2017 [74] | AMP conjugated with horseradish peroxidase (AMP–HRP) | Immunosensor | UV–VIS spectroscopy | 13/102–105 | ~45 | Apple, ground beef |
| Qiao et al., 2017 [75] | AMP functionalized magnetic nanoparticles | Immunosensor | PCR and fluorescence spectroscopy | 84 (apple juice), 233 (beef)/10–106 | ~30 | Spiked apple juice, beef |
| Jiang et al., 2015 [76] | Au interdigitated electrode arrays immobilized with AMP colicin V (ColV) | Impedimetric sensor | Impedance spectroscopy | 100/102–106 | ~10 | Water |
| Dong and Jhao, 2015 [70] | AMP tagging with C-terminal cysteine for immobilization on Au electrode | Electrochemical sensor | QCM, EIS | 400/0–1.8 × 106 | ~10 | Water |
| Kulagina et al., 2006 [67] | Immobilization of AMP magainin I, cecropin P1, and parasin on microscope slide glass | Immunosensor | Fluorescent microscopy | 5 × 104/0–107 | ~90 | Chicken |
| Chang et al., 2015 [68] | Microchannel embedded with AMP magainin I labeled glass beads | Diamidino-2-phenylindole (DAPI) stained sensor | Fluorescence spectroscopy | 10/10–106 | ~20 | PBS |
| Yoo et al., 2014 [77] | Microchannel embedded with AMP magainin I labeled glass beads | DAPI-stained sensor | Fluorescence spectroscopy | 103/103–106 | ~30 | PBS |
| Li et al., 2014 [78] | Immobilization of AMP magainin I on Au surface via C-terminal cysteine | Impedimetric sensor | EIS | 103/103–107 | ~90 | Water |
| Schwartz and Bercovici, 2014 [79] | Conjugation of AMP with horseradish peroxidase (AMP–HRP) | Electrophoretic sensor | Fluorescent microscopy | 100–104/1–108 | ~60 | Water |
| Mannor et al., 2010 [69] | Immobilization of AMP magainin I on Au microelectrodes via C-terminal cysteine | Impedance-based sensor | Impedance spectroscopy | 103/10–105 | ~30 | Water |
| Authors and Year | Accurate Detection Results |
|---|---|
| Bai et al., 2020 [71] | E. coli O157:H7 > Non-pathogenic E. coli > Staphylococcus aureus > Listeria monosytogenes > invertase nanocomposites and Fe3O4 nanocomposites |
| Ding et al., 2020 [72] | E. coli O157:H7 > Staphylococcus aureus > E. coli |
| Yang et al., 2019 [73] | E. coli O157:H7 > Salmonella > Staphylococcus aureus > S. epidermidis > Listeria monosytogenes > P. aeruginosa and E. coli DH5α |
| Qiao et al., 2017 [75] | E. coli O157:H7 > S. Typhimurium > E. coli DH5α > E. coli BL21 and L. monosytogenes |
| Qiao et al., 2017 [74] | E. coli O157:H7 > S. Typhimurium > E. coli DH5α > E. coli BL21 > Listeria monosytogenes and V. parahemolyticus |
| Chang et al., 2015 [68] | E. coli O157:H7 > E. coli DH5α |
| Li et al., 2014 [78] | E. coli O157:H7 > E. coli K12 > S. epidermidis and B. subtilis |
| Mannor et al., 2010 [69] | E. coli O157:H7 > S. Typhimurium > non-pathogenic E. coli > Listeria |
| Sensing Methods | Advantages | Disadvantages |
|---|---|---|
| SPR-based sensor | Label free detection Real-time monitoring Reduces assay development time Low amount of sample volume Continuous measurement | Higher non-specific binding Expense of sensor chips Expensive instrumentation Low adoptability Poor LOD for E. coli O157:H7 |
| Electrochemical-based sensor | Small size Low cost Easy to handle High sensitivity Rapid detection Real-time monitoring Nontoxic materials | Cannot be recycled Short shelf life Limited temperature range Unstable voltage Unstable current |
| RCA-based sensor | Whole bacteria detection Good sensitivity (e.g., LOD ~ 30 CFU/mL) Better detection time High specificity | Expensive method Short shelf life Difficult to handle Large amount of sample volume Regeneration of chip is difficult |
| Effect of AMP magainin I | Cost effective Rapid detection Accurate detection High sensitivity (e.g., LOD ~10 CFU/mL) Whole bacteria detection More durable and stable results Ability to bind a variety of pathogens Long shelf-life | Ability of natural AMPs Toxicity for oral application |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, N.; Song, S.; Chang, M.-S.; Chun, M.-S. Point-of-Care Diagnostic Devices for Detection of Escherichia coli O157:H7 Using Microfluidic Systems: A Focused Review. Biosensors 2023, 13, 741. https://doi.org/10.3390/bios13070741
Abbas N, Song S, Chang M-S, Chun M-S. Point-of-Care Diagnostic Devices for Detection of Escherichia coli O157:H7 Using Microfluidic Systems: A Focused Review. Biosensors. 2023; 13(7):741. https://doi.org/10.3390/bios13070741
Chicago/Turabian StyleAbbas, Naseem, Sehyeon Song, Mi-Sook Chang, and Myung-Suk Chun. 2023. "Point-of-Care Diagnostic Devices for Detection of Escherichia coli O157:H7 Using Microfluidic Systems: A Focused Review" Biosensors 13, no. 7: 741. https://doi.org/10.3390/bios13070741
APA StyleAbbas, N., Song, S., Chang, M.-S., & Chun, M.-S. (2023). Point-of-Care Diagnostic Devices for Detection of Escherichia coli O157:H7 Using Microfluidic Systems: A Focused Review. Biosensors, 13(7), 741. https://doi.org/10.3390/bios13070741







