1. Introduction
In the context of biosensors for medical diagnostics, a number of trends can be observed. Firstly, during the development of novel biosensing concepts there is a continuous search for biosensors that are able to detect specific disease-related biomarkers with increased sensitivity and specificity. This way, low amounts of these biomarkers can be detected, which is crucial for the prevention and early stage detection of many diseases (e.g., cancer or Alzheimer’s disease). Another trend in the diagnostic field focuses on the ability to interrogate multiple disease-related biomarkers with a single test, known as multiplexing. The analysis of multiple biomarkers at the same time allows for more detailed and reliable disease diagnosis and thus an improved outcome for the patient. Moreover, simultaneously testing several diseases drastically improves the diagnostic efficiency with respect to sample consumption, cost and time [
1,
2]. In a broader context, these advancements in the biosensing field align with cornerstones for achieving health-related targets set by the World Health Organization.
Two of the most commonly used approaches that enable high multiplex capacity for DNA or protein detection depend on x–y coordinates for coding of planar arrays (e.g., RayBiotech microarray) or color-coded microparticles (e.g., xMAP Luminex) [
3]. Planar microarrays rely on a high spot density for the screening of high numbers of a target on a single slide. However, the flexibility of such an approach is often limited since the panel has to be defined prior to array manufacturing. Bead-based methods, on the other hand, are more flexible as the multiplex panel can be easily adjusted according to the assay application by coupling different capture molecules to different populations of beads. However, to be cost-efficient, these tests are often performed in batches (e.g., 96-well plates) requiring more sample [
4]. Notwithstanding their commercial success, these techniques still demand considerable hands-on time. To reduce the handling time and move towards more automated platforms, microfluidics has been successfully combined in literature with these types of multiplexed detection [
5,
6,
7]. In this context, an innovative and commercially oriented microfluidic Evalution™ platform was developed (MyCartis NV, Gent, Belgium) [
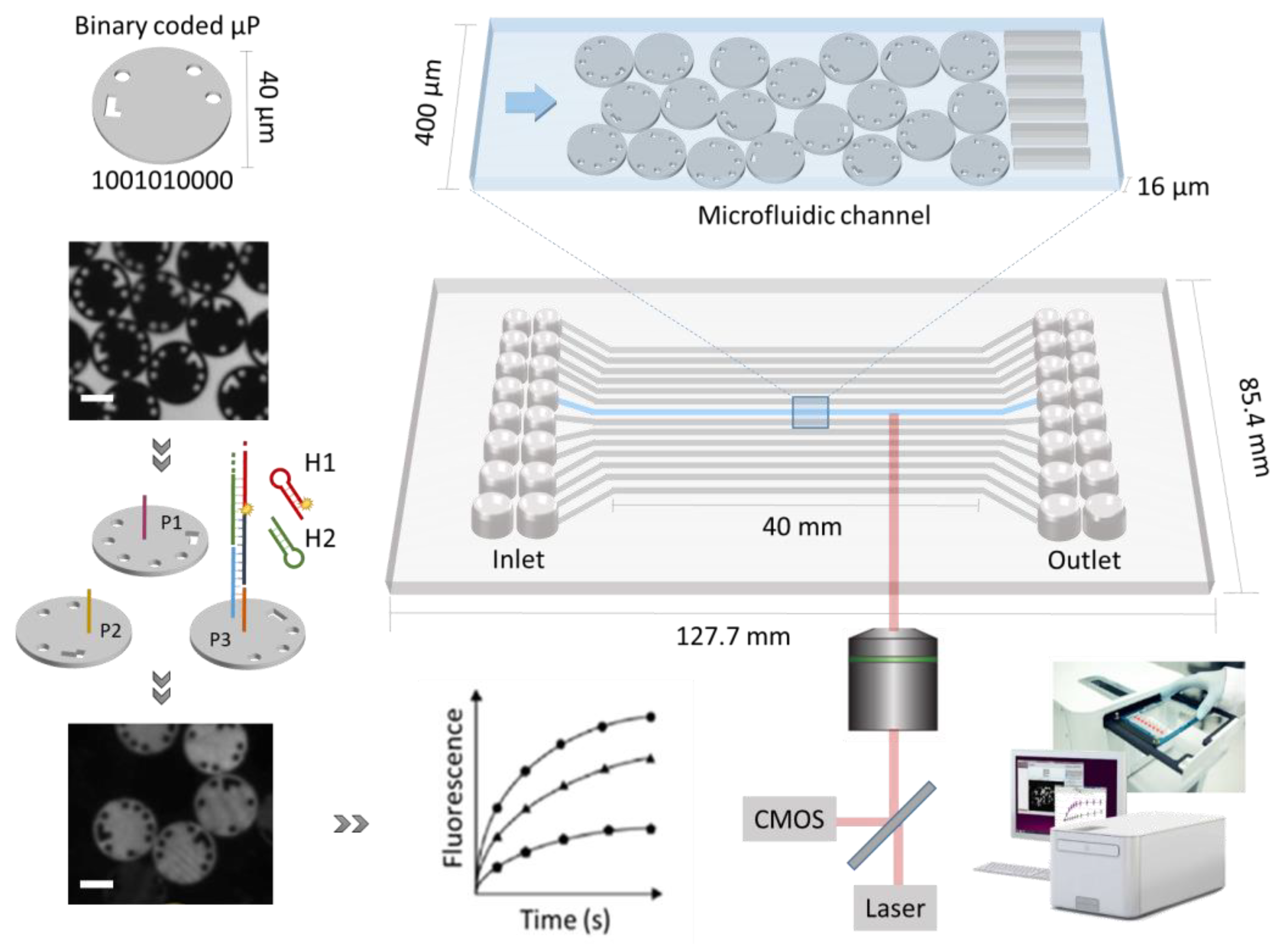
4]. This platform creates a high-throughput multiplex environment by combining uniquely encoded microparticles with channel-based microfluidics (
Figure 1) that allows for the integration of the entire assay workflow. This unique coding is represented by physical holes that go through the microparticle, which allows different codes to be created without spectral overlap or the risk of light-induced or chemical damage. In addition, the binary coded microparticles allow a high level of multiplex capacity, up to 1024 different codes and, together with the possibility to address 16 individual channels simultaneously, enable an elaborate collection of information [
8,
9]. The analysis costs related to a 150-plex analysis are estimated to be approximately EUR 10 per sample. Moreover, the short channel length, in combination with the narrow channel dimension (400 µm width and 16 µm height) results in a low sample consumption in the microliter range. Although the binary coding of the Evalution™ platform provides a higher encoding capacity compared to conventional methods that rely on spectral target discrimination [
10], its ability to detect low concentrations of biomarker currently remains unexplored.
In order to achieve the high sensitivity, necessary to detect low target concentrations, signal amplification strategies can be used. Two main strategies for signal amplification can be discerned: the signaling molecules (1) can diffuse or flow away from the signal amplification system or (2) remain tethered to it. The latter is of particular interest when the bioassay is directly exposed to the flow of liquid, as is the case for the Evalution™. To achieve this localized accumulation of signaling molecules, DNA-based nanostructures can be used. While formation of a DNA construct often is protein-enzyme mediated (e.g., rolling circle amplification [
11,
12]), isothermal amplification techniques can still suffer from the drawbacks related to the limited stability of the enzymes and their stringent buffer requirements. As an alternative, a protein-free DNA amplification strategy, hybridization chain reaction (HCR), was developed by Dirks and Pierce [
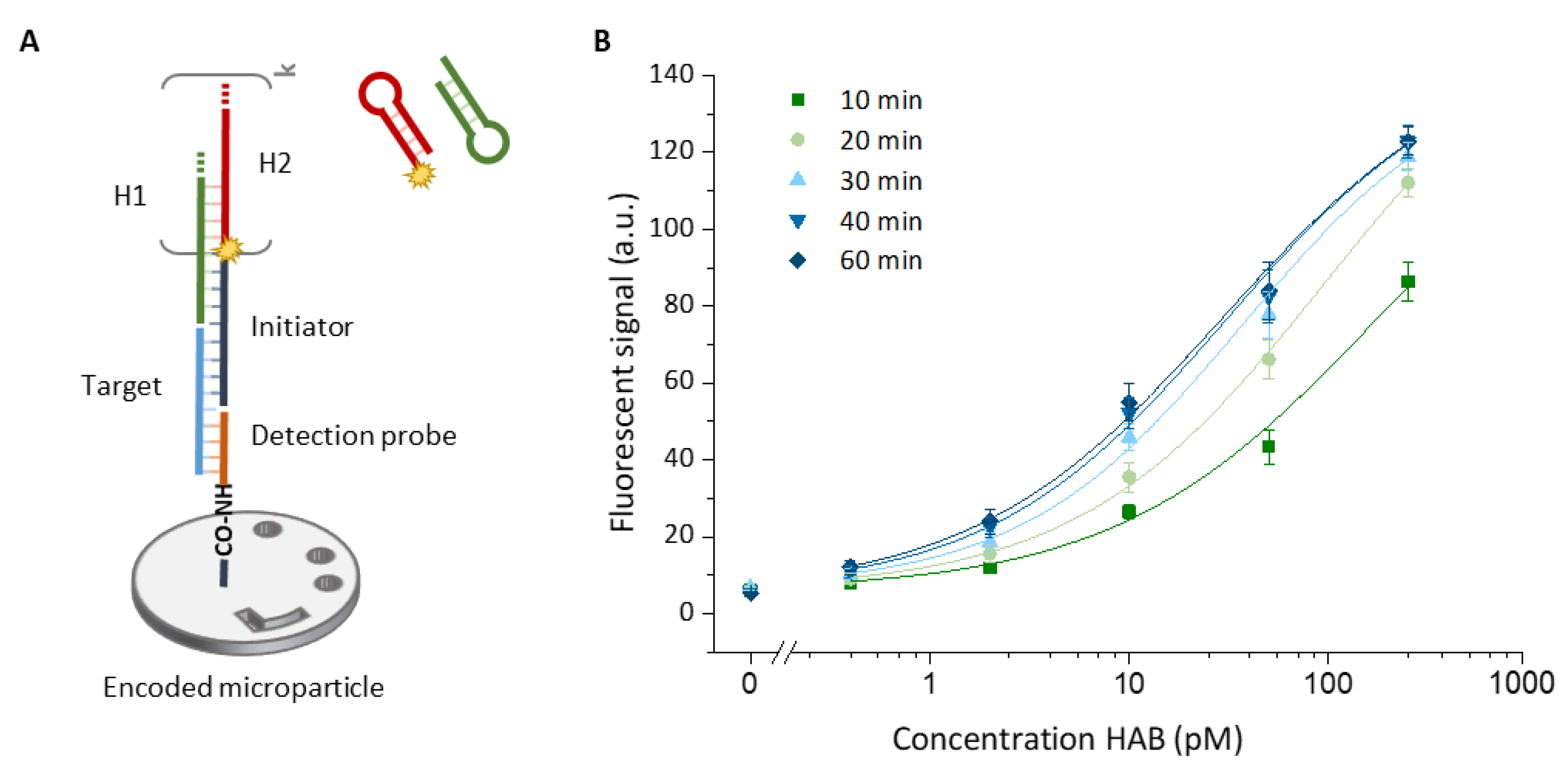
13]. HCR is an enzyme-free, isothermal and robust amplification technique of low complexity and therefore easy to use. The key driving force of HCR is a pair of DNA hairpins that propagate a chain reaction of hybridization events. In a typical HCR, the target initiates a toehold-mediated strand displacement, triggering a cascade reaction that consists of the cross-opening of two meta-stable DNA hairpins (H1 and H2, in
Figure 1 and
Figure 2A). This reaction results in the assembly of a nicked double-helix construct [
14,
15]. Detection of the target can be achieved by using hairpin structures that are modified with signaling molecules [
16]. This way HCR has been successfully employed as a signal amplification technique in several solid-phase biosensing applications (e.g., electrochemistry) for nucleic acid target detection [
14,
16,
17]. Although these applications provide already evidence that by using HCR as a signal amplification technique for solid-phase assays, the detection limit can be improved by several orders of magnitude (100-fold on average); so far, this has never been demonstrated in a microfluidic system in combination with multiplexed solid-phase assays [
14].
Benefiting from the advantages of both systems, in this paper we combine, for the first time, HCR as an amplification strategy with encoded microparticles in a microfluidic platform for the sensitive multiplex detection of six different molecular biomarkers, without the aforementioned drawbacks related to spectral encoding techniques, protein enzymes, thermal cycling or non-automated systems. To this purpose, a panel of six different nucleic acid targets, derived from the genetic material of both pathogenic bacteria and viruses causing respiratory tract infections, is used as a model system. The selected target sequences demonstrate the ability to specifically recognize and discriminate: viruses from bacteria, virus subtypes (human adenovirus type B and D) [
18], virus strains (human adenovirus and human bocavirus) [
19] and antibiotic-susceptible bacteria strains from resistant strains (
Streptococcus pneumonia) [
20]. To obtain a generic approach suitable for the multiplexed detection of a wide variety of target molecules, the HCR initiators are designed to match with a sole hairpin-ensemble. Moreover, the robustness of the system in clinically relevant matrices will be demonstrated in spiked nasopharyngeal swab samples. As such, we will evaluate the Evalution™ platform combined with HCR as a universal signal amplification tool to achieve highly sensitive and high-throughput multiplexed target detection and quantification.
2. Materials and Methods
2.1. Reagents
Reagents were of analytical grade and purchased from Sigma-Aldrich (Diegem, Belgium) unless stated otherwise. All unmodified, amino-modified and fluorescently labeled oligonucleotides were purchased from Integrated DNA Technologies (IDT, Haasrode, Belgium). Each of these sequences can be found in
Supporting Information (Table S1).
2.2. Evalution™ Instrument
The Evalution™ (MyCartis, Gent, Belgium), a microfluidic platform, relies on encoded microparticles [
4]. These disk-shaped silicon microparticles (40 µm diameter and 10 µm height) carried a 10-bit digital code that allowed identification. The microparticles were modified with carboxyl groups that allowed functionalization with bioreceptors. Different bioreceptors were immobilized on differently encoded microparticle populations (i.e., particles with identical coding belonged to the same population). A mixture of these pre-functionalized microparticles was then loaded into a microfluidic cartridge for multiplex detection. The cartridge has a size of 127.7 × 85.4 mm, similar to a standard 96-well plate. This cartridge, accommodating 16 individual microfluidic channels, was inserted into the instrument. The microfluidic flow was controlled by applying a pressure difference (set to 300 mbar) over the in- and outlet of each of the channels. This pressure difference yielded a flow rate of approximately 30 nL/s. More technical details can be found in Falconnet et al., 2015 [
4]. In addition, the temperature in the channels was controlled, ranging from 25 °C to 55 °C. At the start of each experiment, the background fluorescence signal of the microparticles was determined by scanning each of the channels from the bottom of the cartridge as depicted in
Figure 1. The imaging system that was integrated in the instrument relied on a 10× objective (NA 0.3) and a highly sensitive CMOS camera, mounted on an automated stage that moves in x, y and z directions to scan the channels. Each channel is scanned sequentially and in multiple fields of view (each containing approximately 240 microparticles). Each microparticle generated over 1800 results corresponding to each of the pixels. After the first scan, the required assay steps were performed, which include target incubation, washing and fluorescent signal generation. Visualization of the fluorophores (ATTO 550) was obtained through exposure to a green laser (532 nm, 20 mW and 1000 ms), also incorporated in the instrument. The recorded signals were linked to the corresponding particle codes that were precedingly determined in brightfield. In summary, a typical assay was performed in the following steps: (1) microparticle functionalization (
Section 2.3), (2) loading of the microparticle mixture into the microfluidic cartridge (
Section 2.4), (3) insertion of the microfluidic cartridge in the integrated instrument to perform a predefined protocol (
Section 2.5,
Section 2.6 and
Section 2.7).
2.3. Microparticle Functionalization
Each batch of uniquely coded microparticles (µP) (MyCartis, Gent, Belgium) was resuspended in 200 µL of nuclease free water and washed three times in 500 µL of activation buffer (100 mM MES, 0.3% Tween 20, pH 5). Subsequently, the carboxyl groups on the microparticle surface were activated by adding a mixture of 500 µL of 10 mg/mL sulfo-NHS solution and 100 µL of 50 mg/mL EDC solution, both prepared in activation buffer. The homogenized microparticle suspension was incubated for 40 min at 1100 rpm and 22 °C. Following the activation, the microparticles were washed three times with coupling buffer (100 mM MES, 0.3% Tween 20, pH 5.8). Afterwards, the activated microparticles were incubated with 3 µM of amino-modified DNA probes (detection probes) in 600 µL coupling buffer for 40 min at 1100 rpm and 22 °C. The detection probe sequences can be found in
Supporting Information (Table S1). In a final step, the microparticles, functionalized with detection probes, were washed three times in PBST buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na
2HPO
4, 1.8 mM KH2PO4, 0.3% Tween 20, pH 7.4), aliquoted and stored at −20 °C until further use.
2.4. Microfluidic Cartridge Loading
The functionalized microparticles were loaded into the channels of the microfluidic cartridge in a semi-automated fashion. To this purpose, a loading station (MyCartis, Gent, Belgium), optimally exploiting gravitational forces, was used. In combination with the filter structure and the end of the channels, the microparticles form a static monolayer in the detection zone as schematically represented in
Figure 1. Moreover, the microparticles are equipped with supporting units on the top to allow sufficient flow between the channels surface and the microparticle surface. First, a microparticles mixture was prepared in PBST buffer with a concentration of 5000 µP/mL. This mixture comprised multiple uniquely coded microparticle populations, depending on the required multiplex factor of the test. Prior to loading, the channels and inlet wells were prewetted with ethanol (20 µL), followed by PBST buffer (110 µL). The channels were loaded using 100 µL of the microparticle mix per channel. Depending on the multiplex factor, the channels were filled with the microparticle mixture, ensuring that a minimum of 30 microparticles represented each microparticle population per channel. Finally, the solution in the inlet wells was replaced with 100 µL of storage buffer, after which the microfluidic cartridge was inserted into the Evalution™ instrument.
2.5. Singleplex Assay Characterization
The effect of the HCR amplification on the recorded signals was investigated based on the human adenovirus type B (HAB) target. Target detection was achieved through following steps: (1) 20 min incubation with different target concentrations (250, 50, 10, 2, 0.4 pM and blank), (2) 20 min incubation with 1 µM excess of HAB initiator and (3) 60 min incubation with a 1 µM mixture of hairpin 1 (H1) and fluorescently labeled hairpin 2 (H2). During this last incubation, the fluorescent signal was recorded with 5 min intervals. All incubation steps were performed in SPSC buffer (1 M NaCl, 50 mM Na2HPO4, 0.05% Tween 20, pH 7.5) at 25 °C. The assay steps were separated by a 2 min wash with SPSC buffer (0.3% Tween 20). In a similar way a reference signal (i.e., non-amplified signal) was established. Here, to ensure only one HCR cycle to occur, resulting in only one fluorescent label per target molecule, H1 and H2 were flowed sequentially for 20 min and separated by a washing step.
2.6. Multiplex Detection in Buffer
The detection of a panel of 6 different targets was performed in multiplex format. The investigated targets included human adenovirus type B (HAB), human adenovirus type D (HAD), human bocavirus (HB), Streptococcus pneumonia (SP) and two antibiotic-resistant strains of Streptococcus pneumonia (SPR1 and SPR2). First, the occurrence of cross-reactivity was studied and challenged by applying elevated temperatures during target incubation, ranging from 35 to 55 °C with 5 °C intervals for a target concentration of 250 pM. Next, multiplex target detection (250, 50, 10, 2, 0.4 pM and blank) was performed at 45 °C using the same assay configuration as described in the previous paragraph for the singleplex detection using HCR. To address all 6 targets at once, an initiator mixture of 1 µM, containing all initiator probes, was used.
2.7. Detection in Nasopharyngeal Swabs
Following the protocol described in the previous paragraphs, the detection of human adenovirus type B (HAB) was evaluated in nasopharyngeal samples, thereby mimicking a respiratory tract infection to be identified using the developed multiplex model case presented in this work. The used swab samples were obtained from healthy donors and eluted in 3 mL of universal transport medium (Copan Flock technologies S.R.L., Brescia, Italy). For further analysis, the samples were diluted 10-fold in SPSC buffer and subsequently spiked with HAB target at the same concentrations as in buffer. In addition, 0.5% (SDS) was added to the sample for lysis purposes. This procedure was based on the procedure previously described in Leirs et al., 2016 [
21].
2.8. Data Analysis
The average fluorescence intensity for each microparticle population was recorded using the supplied software (Evalution Control 4.2, MyCartis, Gent, Belgium). Both end-point measurements as well as measurements over time were performed. In the latter situation, fluorescent label was present in the channels, causing a background signal. This constant channel background was subtracted from the average fluorescent signal obtained after HCR. Data were further processed using Origin 7 (OriginLab, Northampton, MA, USA). Calibration curves were fitted with a four-parameter logistic fit. LOD values were calculated based on the statistical method described by Holstein et al. [
22]. The LOD is estimated using information of both blank and test samples. A more detailed description of the formulas that were used can be found in
Supporting Information. This method generates a 95% confidence interval for the calculated LOD values that allows a statistical comparison of the obtained values [
22].
4. Conclusions
The Evalution™ platform was evaluated as a technology for highly sensitive and high-throughput multiplex nucleic acid analysis enabled by the unprecedented microparticle encoding capacity. To achieve the highest possible sensitivity, HCR was introduced as a universal signal amplification tool, benefiting from an isothermal and protein enzyme-free amplification. Singleplex detection of a nucleic acid target (HAB) indicated that different reaction times led to different levels of sensitivity, improving the limit of detection from 103-fold after 10 min to 104-fold after 60 min, compared to a non-amplified reference system. Varying the HCR reaction time offers the possibility to tune the assay for assay speed and/or sensitivity, as desired. The potential of sensitive and specific multiplex detection was further demonstrated for a multiplex panel of six different nucleic acid target molecules. This model system included both viruses (HAB, HAD and HB) and bacteria (SP, SPR1 and SPR2). The ability to distinguish between these two categories, as well as different virus strains (HA and HB), virus subtypes (HAB and HAD) and antibiotic susceptible bacterial strains (SP) from resistant strains (SPR1 and SPR2), is diagnostically relevant as specific species and their quantity are often associated with a unique disease management and treatment. A difference in assay performance was noticed for different target sequences. This difference can be attributed to the difference in the target and detection sequences, affecting hybridization efficiency and in some cases imposing undesired secondary structures.
In buffer conditions, detection limits as low as 33 ± 4, 51 ± 5; 106 ± 9, 88 ± 6, 151 ± 12 and 136 ± 11 fM were calculated based on the detection of HAB; HAD, HB, SP, SPR1 and SPR2 targets, respectively. Lastly, the direct detection of the HAB target in clinically relevant nasopharyngeal swab samples was demonstrated without the need for elaborate sample preparation steps. Using the highly simplified and protein-enzyme-free detection method, a calculated detection limit of 309 ± 80 fM was reached in a 10-fold diluted complex sample matrix, approximating the low fM levels detectable with the current gold standard analysis method, being PCR [
18]. It should be noted that the use of branched HCR could potentially lead to even more efficient signal amplification resulting in even lower limits of detection [
25]. If we compare the results obtained for the developed assay with other commonly used multiplex technologies described in literature, we see that the obtained detection limit of 1.1 pg for the detection of the HAB target in the sample matrix exceeds the detection limit of a recent study using the xMAP approach in combination with PCR amplification that reports a detection limit between 0.05 and 0.01 ng of DNA [
26]. The microarray approach commercialized by Raybiotech reaches a detection limit between 0.005 and 0.05 pg for protein targets. In addition, a recent study using a similar microarray system in combination with PCR amplification to detect DNA targets reports a detection limit of 0.4 fg [
27], indicating that the sensitivity or a reported approach strongly varies for different assays. Although more sensitive, a drawback to the reported microarray approach is the limited multiplex capacity of up to 40 different capture molecules, which is required to be able to link the spot position to one particular target on the area of each subarray [
28]. If we look at the multiplexing capacity of the xMAP system, involving microspheres internally dyed with a fluorophore allows as many as 100 unique codes to detect 100 different targets simultaneously [
29]. Since the Evalution does not rely on spectral encoding but rather on a 10-digit physical code, the encoding capacity goes up to 1024 different codes. Additionally, compared to spectral encoding strategies, the digital code intrinsic to the Evalution system does not suffer from spectral overlap and cannot be altered by light-induced damage or chemical degradation. Moreover, the reported detection strategy can be applied for a wide range of target molecules in a multiplex format, being not only nucleic acid targets but also proteins, by linking the HCR initiator sequence to target-specific bioreceptor molecules such as antibodies or aptamers [
14]. As such, this approach has the potential to improve early stage disease diagnosis through the combined detection of both protein and nucleic-acid-based biomarkers in a high-throughput fashion.