Electrochemical Immunosensors Developed for Amyloid-Beta and Tau Proteins, Leading Biomarkers of Alzheimer’s Disease
Abstract
1. Introduction
2. Electrochemical Immunosensing of Aβ
3. Electrochemical Sensing of Tau Protein
4. The Fundamental Elements Involved in the Design and Development of Electrochemical Immunosensors for the Detection of AD Biomarkers
5. Challenges, Limitations, and Aspects for the Development of Electrochemical Immunosensors for AD
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atri, A. The Alzheimer’s disease clinical spectrum: Diagnosis and management. Med. Clin. 2019, 103, 263–293. [Google Scholar]
- Gallaway, P.J.; Miyake, H.; Buchowski, M.S.; Shimada, M.; Yoshitake, Y.; Kim, A.S.; Hongu, N. Physical activity: A viable way to reduce the risks of mild cognitive impairment, Alzheimer’s disease, and vascular dementia in older adults. Brain Sci. 2017, 7, 22. [Google Scholar] [CrossRef]
- Zvěřová, M. Clinical aspects of Alzheimer’s disease. Clin. Biochem. 2019, 72, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Durongbhan, P.; Zhao, Y.; Chen, L.; Zis, P.; De Marco, M.; Unwin, Z.C.; Venneri, A.; He, X.; Li, S.; Zhao, Y. A dementia classification framework using frequency and time-frequency features based on EEG signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 826–835. [Google Scholar] [CrossRef]
- Ancelin, M.L.; Artero, S.; Portet, F.; Dupuy, A.-M.; Touchon, J.; Ritchie, K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: Longitudinal cohort study. BMJ 2006, 332, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2022 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2014, 10, e47–e92. [Google Scholar]
- Sheladia, S.; Reddy, P.H. Age-related chronic diseases and Alzheimer’s disease in Texas: A Hispanic focused study. J. Alzheimer’s Dis. Rep. 2021, 5, 121–133. [Google Scholar] [CrossRef]
- Joe, E.; Ringman, J.M. Cognitive symptoms of Alzheimer’s disease: Clinical management and prevention. BMJ 2019, 367, 16217. [Google Scholar] [CrossRef]
- Grossberg, G.T.; Tong, G.; Burke, A.D.; Tariot, P.N. Present algorithms and future treatments for Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 67, 1157–1171. [Google Scholar] [CrossRef]
- Rossini, P.M.; Di Iorio, R.; Vecchio, F.; Anfossi, M.; Babiloni, C.; Bozzali, M.; Bruni, A.C.; Cappa, S.F.; Escudero, J.; Fraga, F.J. Early diagnosis of Alzheimer’s disease: The role of biomarkers including advanced EEG signal analysis. Report from the IFCN-sponsored panel of experts. Clin. Neurophysiol. 2020, 131, 1287–1310. [Google Scholar] [CrossRef] [PubMed]
- Larner, A. Diagnostic Test Accuracy Studies in Dementia: A Pragmatic Approach; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Bonakdarpour, B.; Takarabe, C. Brain Networks, Clinical Manifestations, and Neuroimaging of Cognitive Disorders: The Role of Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Positron Emission Tomography (PET), and Other Advanced Neuroimaging Tests. Clin. Geriatr. Med. 2023, 39, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Gao, F. Integrated positron emission tomography/magnetic resonance imaging in clinical diagnosis of Alzheimer’s disease. Eur. J. Radiol. 2021, 145, 110017. [Google Scholar] [CrossRef]
- Chaves, R.; Ramírez, J.; Górriz, J.; Puntonet, C.G.; Initiative, A.s.D.N. Association rule-based feature selection method for Alzheimer’s disease diagnosis. Expert Syst. Appl. 2012, 39, 11766–11774. [Google Scholar] [CrossRef]
- Ferreira, L.K.; Busatto, G.F. Neuroimaging in Alzheimer’s disease: Current role in clinical practice and potential future applications. Clinics 2011, 66, 19–24. [Google Scholar] [CrossRef]
- Ruan, D.; Sun, L. Amyloid-β PET in Alzheimer’s disease: A systematic review and Bayesian meta-analysis. Brain Behav. 2023, 13, e2850. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Li, L.; Man, Z.; Xu, Z.; Cui, H.; Zhan, R.; He, Q.; Zheng, L.; Fu, H. Polarity-activated super-resolution imaging probe for the formation and morphology of amyloid fibrils. Nano Res. 2020, 13, 2556–2563. [Google Scholar] [CrossRef]
- Wei, T.-Y.; Fu, Y.; Chang, K.-H.; Lin, K.-J.; Lu, Y.-J.; Cheng, C.-M. Point-of-care devices using disease biomarkers to diagnose neurodegenerative disorders. Trends Biotechnol. 2018, 36, 290–303. [Google Scholar] [CrossRef]
- Chethana, H.; Hemachandra, G.; Sidhu, A. Biomarkers: Potential Perspectives in Detection, Diagnosis, and Prognosis of Neurodegenerative Disorders. In Functional Foods and Therapeutic Strategies for Neurodegenerative Disorders; Springer: Berlin/Heidelberg, Germany, 2022; pp. 203–222. [Google Scholar]
- Turner, P.R.; O’Connor, K.; Tate, W.P.; Abraham, W.C. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 2003, 70, 1–32. [Google Scholar] [CrossRef]
- Blaikie, L.; Kay, G.; Lin, P.K.T. Current and emerging therapeutic targets of alzheimer’s disease for the design of multi-target directed ligands. MedChemComm 2019, 10, 2052–2072. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, K.; Guo, H.; Zeng, J.; Wang, S.; Xu, H.; Ge, A.; Zeng, L.; Chen, S.; Ge, J. Mechanisms of ferroptosis in Alzheimer’s disease and therapeutic effects of natural plant products: A review. Biomed. Pharmacother. 2023, 164, 114312. [Google Scholar] [CrossRef]
- Luiten, P.; Nyakas, C.; Eisel, U.; Van der Zee, E. Aging of the Brain. In Neuroscience in the 21st Century: From Basic to Clinical; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3115–3149. [Google Scholar]
- Gallo, A.; Pillet, L.-E.; Verpillot, R. New frontiers in Alzheimer’s disease diagnostic: Monoamines and their derivatives in biological fluids. Exp. Gerontol. 2021, 152, 111452. [Google Scholar] [CrossRef]
- Michno, W.; Blennow, K.; Zetterberg, H.; Brinkmalm, G. Refining the amyloid β peptide and oligomer fingerprint ambiguities in Alzheimer’s disease: Mass spectrometric molecular characterization in brain, cerebrospinal fluid, blood, and plasma. J. Neurochem. 2021, 159, 234–257. [Google Scholar] [CrossRef]
- Delaby, C.; Hirtz, C.; Lehmann, S. Overview of the blood biomarkers in Alzheimer’s disease: Promises and challenges. Rev. Neurol. 2022, 179, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Urayama, A.; Moreno-Gonzalez, I.; Morales-Scheihing, D.; Kharat, V.; Pritzkow, S.; Soto, C. Preventive and therapeutic reduction of amyloid deposition and behavioral impairments in a model of Alzheimer’s disease by whole blood exchange. Mol. Psychiatry 2022, 27, 4285–4296. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wan, J.; Liu, A.; Sun, J. Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: A potential therapeutic molecule for Alzheimer’s disease. Biomed. Pharmacother. 2020, 132, 110887. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ali, R.; Verma, S. Aβ-oligomers: A potential therapeutic target for Alzheimer’s disease. Int. J. Biol. Macromol. 2023, 239, 124231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Hu, J.; Zhu, H.; Chen, Q.; Koh, K.; Chen, H.; Xu, X.-H. A facile and effective immunoassay for sensitive detection of phosphorylated tau: The role of flower-shaped TiO2 in specificity and signal amplification. Sens. Actuators B Chem. 2022, 366, 132015. [Google Scholar] [CrossRef]
- Hampel, H.; Goernitz, A.; Buerger, K. Advances in the development of biomarkers for Alzheimer’s disease: From CSF total tau and Aβ1–42 proteins to phosphorylated tau protein. Brain Res. Bull. 2003, 61, 243–253. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated tau in Alzheimer’s disease and other tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Penke, B.; Bogár, F.; Paragi, G.; Gera, J.; Fülöp, L. Key peptides and proteins in Alzheimer’s disease. Curr. Protein Pept. Sci. 2019, 20, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, S. Alzheimer’s Disease Pathology: A Tau Perspective. Alzheimer’s Dis. 2022, 58. [Google Scholar]
- Phan, L.M.T.; Hoang, T.X.; Vo, T.A.T.; Pham, H.L.; Le, H.T.N.; Chinnadayyala, S.R.; Kim, J.Y.; Lee, S.-M.; Cho, W.W.; Kim, Y.H. Nanomaterial-based Optical and Electrochemical Biosensors for Amyloid beta and Tau: Potential for early diagnosis of Alzheimer’s Disease. Expert Rev. Mol. Diagn. 2021, 21, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Shabaninejad, Z.; Movahedpour, A.; Sahebkar, A.; Mohammadi, S.; Hosseindoost, S.; Ebrahimi, M.S.; Savardashtaki, A.; Karimipour, M.; Mirzaei, H. Biosensors for detection of Tau protein as an Alzheimer’s disease marker. Int. J. Biol. Macromol. 2020, 162, 1100–1108. [Google Scholar] [CrossRef]
- Bouwman, F.H.; Frisoni, G.B.; Johnson, S.C.; Chen, X.; Engelborghs, S.; Ikeuchi, T.; Paquet, C.; Ritchie, C.; Bozeat, S.; Quevenco, F.C. Clinical application of CSF biomarkers for Alzheimer’s disease: From rationale to ratios. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12314. [Google Scholar] [CrossRef] [PubMed]
- Mankhong, S.; Kim, S.; Lee, S.; Kwak, H.-B.; Park, D.-H.; Joa, K.-L.; Kang, J.-H. Development of Alzheimer’s disease biomarkers: From CSF-to blood-based biomarkers. Biomedicines 2022, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Winston, C.N.; Goetzl, E.J.; Akers, J.C.; Carter, B.S.; Rockenstein, E.M.; Galasko, D.; Masliah, E.; Rissman, R.A. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2016, 3, 63–72. [Google Scholar] [CrossRef]
- Nangare, S.; Patil, P. Nanoarchitectured bioconjugates and bioreceptors mediated surface plasmon resonance biosensor for in vitro diagnosis of Alzheimer’s disease: Development and future prospects. Crit. Rev. Anal. Chem. 2022, 52, 1139–1169. [Google Scholar] [CrossRef]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine 2016, 6, 42–49. [Google Scholar] [CrossRef]
- Viola, K.L.; Klein, W.L. Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015, 129, 183–206. [Google Scholar] [CrossRef]
- Bivona, G.; Sasso, B.L.; Iacolino, G.; Gambino, C.M.; Scazzone, C.; Agnello, L.; Ciaccio, M. Standardized measurement of circulating vitamin D [25 (OH) D] and its putative role as a serum biomarker in Alzheimer’s disease and Parkinson’s disease. Clin. Chim. Acta 2019, 497, 82–87. [Google Scholar] [CrossRef]
- Kováč, A.; Majerová, P.; Nytka, M.; Cechová, M.Z.; Bednář, P.; Hájek, R.; Cooper-Shepherd, D.A.; Muck, A.; Lemr, K. Separation of Isomeric Tau Phosphopeptides from Alzheimer’s Disease Brain by Cyclic Ion Mobility Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2023, 34, 394–400. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Rahbarghazi, R.; Fathi, F. Surface plasmon resonance biosensors for detection of Alzheimer’s biomarkers; an effective step in early and accurate diagnosis. Biosens. Bioelectron. 2020, 167, 112511. [Google Scholar] [CrossRef]
- Yang, J.K.; Hwang, I.J.; Cha, M.G.; Kim, H.I.; Yim, D.; Jeong, D.H.; Lee, Y.S.; Kim, J.H. Reaction Kinetics-Mediated Control over Silver Nanogap Shells as Surface-Enhanced Raman Scattering Nanoprobes for Detection of Alzheimer’s Disease Biomarkers. Small 2019, 15, 1900613. [Google Scholar] [CrossRef]
- Korecka, M.; Shaw, L.M. Mass spectrometry-based methods for robust measurement of Alzheimer’s disease biomarkers in biological fluids. J. Neurochem. 2021, 159, 211–233. [Google Scholar] [CrossRef]
- Brazaca, L.C.; Moreto, J.R.; Martín, A.; Tehrani, F.; Wang, J.; Zucolotto, V. Colorimetric paper-based immunosensor for simultaneous determination of fetuin B and clusterin toward early Alzheimer’s diagnosis. ACS Nano 2019, 13, 13325–13332. [Google Scholar] [CrossRef]
- Agnello, L.; Piccoli, T.; Vidali, M.; Cuffaro, L.; Lo Sasso, B.; Iacolino, G.; Giglio, V.R.; Lupo, F.; Alongi, P.; Bivona, G. Diagnostic accuracy of cerebrospinal fluid biomarkers measured by chemiluminescent enzyme immunoassay for Alzheimer disease diagnosis. Scand. J. Clin. Lab. Investig. 2020, 80, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Luo, Y.; Xu, T.; Cheng, G.; Cai, H.; Zhang, X. Acoustic aggregation-induced separation for enhanced fluorescence detection of Alzheimer’s biomarker. Talanta 2021, 233, 122517. [Google Scholar] [CrossRef] [PubMed]
- Dehdari Vais, R.; Yadegari, H.; Heli, H.; Sattarahmady, N. A β-Amyloid((1-42)) Biosensor Based on Molecularly Imprinted Poly-Pyrrole for Early Diagnosis of Alzheimer’s Disease. J. Biomed. Phys. Eng. 2021, 11, 215–228. [Google Scholar] [CrossRef]
- Negahdary, M.; Heli, H. An electrochemical peptide-based biosensor for the Alzheimer biomarker amyloid-β(1-42) using a microporous gold nanostructure. Microchim. Acta 2019, 186, 766. [Google Scholar] [CrossRef] [PubMed]
- Negahdary, M.; Heli, H. An ultrasensitive electrochemical aptasensor for early diagnosis of Alzheimer’s disease, using a fern leaves-like gold nanostructure. Talanta 2019, 198, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, R.; Luo, F.; Wang, P.; Lin, Z. Miniaturized electrochemical sensors and their point-of-care applications. Chin. Chem. Lett. 2020, 31, 589–600. [Google Scholar] [CrossRef]
- Negahdary, M.; Veloso, W.B.; Bacil, R.P.; Buoro, R.M.; Gutz, I.G.R.; Paixão, T.R.L.C.; do Lago, C.L.; Sakata, S.K.; Meloni, G.N.; França, M.C.; et al. Aptasensing of beta-amyloid (Aβ(1-42)) by a 3D-printed platform integrated with leaf-shaped gold nanodendrites. Sens. Actuators B Chem. 2023, 393, 134130. [Google Scholar] [CrossRef]
- Kumar, S.; Ahlawat, W.; Kumar, R.; Dilbaghi, N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015, 70, 498–503. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef]
- Negahdary, M.; Angnes, L. Electrochemical aptamer-based nanobiosensors for diagnosing Alzheimer’s disease: A review. Biomater. Adv. 2022, 135, 112689. [Google Scholar] [CrossRef]
- Negahdary, M. Electrochemical aptasensors based on the gold nanostructures. Talanta 2020, 216, 120999. [Google Scholar] [CrossRef]
- Sharma, A.; Jang, J. Flexible electrical aptasensor using dielectrophoretic assembly of graphene oxide and its subsequent reduction for cardiac biomarker detection. Sci. Rep. 2019, 9, 5970. [Google Scholar] [CrossRef]
- Sharma, A.; Han, C.-H.; Jang, J. Rapid electrical immunoassay of the cardiac biomarker troponin I through dielectrophoretic concentration using imbedded electrodes. Biosens. Bioelectron. 2016, 82, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bhardwaj, J.; Jang, J. Label-free, highly sensitive electrochemical aptasensors using polymer-modified reduced graphene oxide for cardiac biomarker detection. ACS Omega 2020, 5, 3924–3931. [Google Scholar] [CrossRef] [PubMed]
- Vais, R.D.; Sattarahmady, N.; Heli, H. Green electrodeposition of gold nanostructures by diverse size, shape, and electrochemical activity. Gold Bull. 2016, 49, 95–102. [Google Scholar] [CrossRef]
- Abdelnour, C.; Agosta, F.; Bozzali, M.; Fougère, B.; Iwata, A.; Nilforooshan, R.; Takada, L.T.; Viñuela, F.; Traber, M. Perspectives and challenges in patient stratification in Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 112. [Google Scholar] [CrossRef]
- Hao, N.; Wang, Z.; Liu, P.; Becker, R.; Yang, S.; Yang, K.; Pei, Z.; Zhang, P.; Xia, J.; Shen, L.; et al. Acoustofluidic multimodal diagnostic system for Alzheimer’s disease. Biosens. Bioelectron. 2022, 196, 113730. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; You, X.; Jang, Y.; Nam, Y.; Kim, M.J.; Min, N.K.; Pak, J.J. ZnO nanorod matrix based electrochemical immunosensors for sensitivity enhanced detection of Legionella pneumophila. Sens. Actuators B Chem. 2014, 200, 173–180. [Google Scholar] [CrossRef]
- Abbasi, H.Y.; Tehrani, Z.; Devadoss, A.; Ali, M.M.; Moradi-Bachiller, S.; Albani, D.; Guy, O.J. Graphene based electrochemical immunosensor for the ultra-sensitive label free detection of Alzheimer’s beta amyloid peptides Aβ(1-42). Nanoscale Adv. 2021, 3, 2295–2304. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Chuang, H.-C.; Tripathi, A.; Wang, Y.-L.; Ko, M.-L.; Chuang, C.-C.; Chen, J.-C. High-Sensitivity and Trace-Amount Specimen Electrochemical Sensors for Exploring the Levels of β-Amyloid in Human Blood and Tears. Anal. Chem. 2021, 93, 8099–8106. [Google Scholar] [CrossRef]
- Posa, A.; Bräuer, L.; Schicht, M.; Garreis, F.; Beileke, S.; Paulsen, F. Schirmer strip vs. capillary tube method: Non-invasive methods of obtaining proteins from tear fluid. Ann. Anat.-Anat. Anz. 2013, 195, 137–142. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Gu, B.-C.; Wang, G.-J.; Yang, Y.-H.; Wu, C.-C. Detection of Amyloid-β(1-42) Aggregation With a Nanostructured Electrochemical Sandwich Immunoassay Biosensor. Front. Bioeng. Biotechnol. 2022, 10, 853947. [Google Scholar] [CrossRef]
- Tehrani, Z.; Abbasi, H.Y.; Devadoss, A.; Evans, J.E.; Guy, O.J. Assessing Surface Coverage of Aminophenyl Bonding Sites on Diazotised Glassy Carbon Electrodes for Optimised Electrochemical Biosensor Performance. Nanomaterials 2021, 11, 416. [Google Scholar] [CrossRef]
- Kasturi, S.; Torati, S.R.; Eom, Y.; Kim, C. Microvalve-controlled miniaturized electrochemical lab-on-a-chip based biosensor for the detection of β-amyloid biomarker. J. Ind. Eng. Chem. 2021, 97, 349–355. [Google Scholar] [CrossRef]
- Ding, M.; Shu, Q.; Zhang, N.; Yan, C.; Niu, H.; Li, X.; Guan, P.; Hu, X. Electrochemical Immunosensor for the Sensitive Detection of Alzheimer’s Biomarker Amyloid-β (1-42) Using the Heme-amyloid-β (1–42) Complex as the Signal Source. Electroanalysis 2022, 34, 263–274. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Ren, J.; Zhao, H.; Cui, M.; Li, N.; Li, M.; Zhang, C. Electrocatalysis of Copper Sulfide Nanoparticle-Engineered Covalent Organic Frameworks for Ratiometric Electrochemical Detection of Amyloid-β Oligomer. Anal. Chem. 2022, 94, 11201–11208. [Google Scholar] [CrossRef]
- Supraja, P.; Tripathy, S.; Vanjari, S.R.K.; Singh, R.; Singh, V.; Singh, S.G. Label-free detection of β-Amyloid (1-42) in plasma using electrospun SnO2 nanofiber based electro-analytical sensor. Sens. Actuators B Chem. 2021, 346, 130522. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Xu, Q.; Zhang, L.; Liu, Q.; Xu, T. Portable electrochemical micro-workstation platform for simultaneous detection of multiple Alzheimer’s disease biomarkers. Microchim. Acta 2022, 189, 91. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, H.J.; Lee, J.-H.; Park, J.H.; Kim, J.; Hwang, K.S.; Lee, B.C. Amyloid Beta Detection by Faradaic Electrochemical Impedance Spectroscopy Using Interdigitated Microelectrodes. Sensors 2018, 18, 426. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhao, G.; Dong, X.; Li, X.; Miao, J.; Wei, Q.; Cao, W. Ultrasensitive electrochemiluminescence immunosensor for the detection of amyloid-β proteins based on resonance energy transfer between g-C3N4 and Pd NPs coated NH2-MIL-53. Biosens. Bioelectron. 2019, 142, 111517. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Xu, Y.; Liu, Q.; Gu, H.; Zhu, A.; Shi, G. Interface engineering of microelectrodes toward ultrasensitive monitoring of β-amyloid peptides in cerebrospinal fluid in Alzheimer’s disease. Analyst 2020, 145, 2331–2338. [Google Scholar] [CrossRef]
- Song, Y.; Xu, T.; Zhu, Q.; Zhang, X. Integrated individually electrochemical array for simultaneously detecting multiple Alzheimer’s biomarkers. Biosens. Bioelectron. 2020, 162, 112253. [Google Scholar] [CrossRef]
- Park, Y.M.; Ahn, J.; Choi, Y.S.; Jeong, J.-M.; Lee, S.J.; Lee, J.J.; Choi, B.G.; Lee, K.G. Flexible nanopillar-based immunoelectrochemical biosensor for noninvasive detection of Amyloid beta. Nano Converg. 2020, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Ramli, M.Z.; Ramasamy, K.; Meng, L.S.; Yean, C.Y.; Banga Singh, K.K.; Zain, Z.M.; Low, K.-F. An impedimetric micro-immunosensing assay to detect Alzheimer’s disease biomarker: Aβ40. Anal. Biochem. 2018, 555, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ngoc Le, H.T.; Park, J.; Chinnadayyala, S.R.; Cho, S. Sensitive electrochemical detection of amyloid beta peptide in human serum using an interdigitated chain-shaped electrode. Biosens. Bioelectron. 2019, 144, 111694. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, N.; Li, Y.; Yang, L.; Wei, D.; Yan, T.; Ju, H.; Du, B.; Wei, Q. Cobalt-based metal-organic frameworks as co-reaction accelerator for enhancing electrochemiluminescence behavior of N-(aminobutyl)-N-(ethylisoluminol) and ultrasensitive immunosensing of amyloid-β protein. Sens. Actuators B Chem. 2019, 291, 319–328. [Google Scholar] [CrossRef]
- Sethi, J.; Van Bulck, M.; Suhail, A.; Safarzadeh, M.; Perez-Castillo, A.; Pan, G. A label-free biosensor based on graphene and reduced graphene oxide dual-layer for electrochemical determination of beta-amyloid biomarkers. Microchim. Acta 2020, 187, 288. [Google Scholar] [CrossRef]
- Le, H.T.N.; Park, J.; Cho, S. A Probeless Capacitive Biosensor for Direct Detection of Amyloid Beta 1-42 in Human Serum Based on an Interdigitated Chain-Shaped Electrode. Micromachines 2020, 11, 791. [Google Scholar] [CrossRef]
- Liu, T.-C.; Lee, Y.-C.; Ko, C.-Y.; Liu, R.-S.; Ke, C.-C.; Lo, Y.-C.; Hong, P.-S.; Chu, C.-Y.; Chang, C.-W.; Wu, P.-W. Highly sensitive/selective 3D nanostructured immunoparticle-based interface on a multichannel sensor array for detecting amyloid-beta in Alzheimer’s disease. Theranostics 2018, 8, 4210. [Google Scholar] [CrossRef]
- Huang, Z.; Li, M.; Zhang, L.; Liu, Y. Electrochemical immunosensor based on superwettable microdroplet array for detecting multiple Alzheimer’s disease biomarkers. Front. Bioeng. Biotechnol. 2022, 10, 1029428. [Google Scholar] [CrossRef]
- Ke, H.; Sha, H.; Wang, Y.; Guo, W.; Zhang, X.; Wang, Z.; Huang, C.; Jia, N. Electrochemiluminescence resonance energy transfer system between GNRs and Ru(bpy)32+: Application in magnetic aptasensor for β-amyloid. Biosens. Bioelectron. 2018, 100, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Palley, B.F.; Artur, J.C.; De Arruda, M.N.; De Souza, G.F.; Graves, D.A.; de Carvalho Bovolato, A.L.; Deffune, E.; Schelp, A.O.; Gonçalves, E.S.; De Moraes, M.L. Screen-Printed Electrodes on Tyvek Substrate as Low-Cost Device to Applications in Alzheimer’s Disease Detection. J. Electrochem. Soc. 2022, 169, 037505. [Google Scholar] [CrossRef]
- Sharma, A.; Piplani, P. Acridine: A Scaffold for the Development of Drugs for Alzheimer’s Disease. In Current Topics in Medicinal Chemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023. [Google Scholar]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Ding, S.; Du, D.; Wang, X.; Hu, X.; Guan, P.; Lyu, Z.; Lin, Y. Recent advances in electrochemical biosensors for the detection of Aβ42, a biomarker for Alzheimer disease diagnosis. TrAC Trends Anal. Chem. 2023, 164, 117087. [Google Scholar] [CrossRef]
- Serafín, V.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Razzino, C.; Yáñez-Sedeño, P.; Barderas, R.; Campuzano, S.; Pingarrón, J. Enlightening the advancements in electrochemical bioanalysis for the diagnosis of Alzheimer’s disease and other neurodegenerative disorders. J. Pharm. Biomed. Anal. 2020, 189, 113437. [Google Scholar] [CrossRef]
- Miku, E. Recent advancements in electrochemical biosensors for Alzheimer’s disease biomarkers detection. Curr. Med. Chem. 2021, 28, 4049–4073. [Google Scholar] [CrossRef]
- Le, H.T.N.; Cho, S. Sensitive Electrochemical Detection of Phosphorylated-Tau Threonine 231 in Human Serum Using Interdigitated Wave-Shaped Electrode. Biomedicines 2022, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hu, J.; Mao, Z.; Koh, K.; Chen, H. Loach mucus-like guanosine-based hydrogel as an antifouling coating for electrochemical detection of tau protein. Sens. Actuators B Chem. 2022, 370, 132419. [Google Scholar] [CrossRef]
- Yola, B.B.; Karaman, C.; Özcan, N.; Atar, N.; Polat, İ.; Yola, M.L. Electrochemical Tau Protein Immunosensor Based on MnS/GO/PANI and Magnetite-incorporated Gold Nanoparticles. Electroanalysis 2022, 34, 1519–1528. [Google Scholar] [CrossRef]
- Eduarda Schneider, M.; Guillade, L.; Correa-Duarte, M.A.; Moreira, F.T.C. Development of a biosensor for phosphorylated Tau 181 protein detection in Early-Stage Alzheimer’s disease. Bioelectrochemistry 2022, 145, 108057. [Google Scholar] [CrossRef]
- Nur Sonuç Karaboğa, M.; Kemal Sezgintürk, M. A Practical Approach for the Detection of Protein Tau with a Portable Potentiostat. Electroanalysis 2023, 35, e202200072. [Google Scholar] [CrossRef]
- Khetani, S.; Singh, A.; Besler, B.; Butterworth, S.; Lijnse, T.; Loughery, K.; Smith, K.; Hosseini, E.; Narang, R.; Karan, K.; et al. μDrop: Multi-analyte portable electrochemical-sensing device for blood-based detection of cleaved tau and neuron filament light in traumatic brain injury patients. Biosens. Bioelectron. 2021, 178, 113033. [Google Scholar] [CrossRef]
- Hun, X.; Kong, X. An enzyme linked aptamer photoelectrochemical biosensor for Tau-381 protein using AuNPs/MoSe2 as sensing material. J. Pharm. Biomed. Anal. 2021, 192, 113666. [Google Scholar] [CrossRef]
- Sonuç Karaboga, M.N.; Sezgintürk, M.K. Analysis of Tau-441 protein in clinical samples using rGO/AuNP nanocomposite-supported disposable impedimetric neuro-biosensing platform: Towards Alzheimer’s disease detection. Talanta 2020, 219, 121257. [Google Scholar] [CrossRef]
- Li, X.; Jiang, M.; Cheng, J.; Ye, M.; Zhang, W.; Jaffrezic-Renault, N.; Guo, Z. Signal multi-amplified electrochemical biosensor for voltammetric determination of tau-441 protein in biological samples using carbon nanomaterials and gold nanoparticles to hint dementia. Microchim. Acta 2020, 187, 302. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chiu, L.-Y.; Chen, Y.; Qin, S.; Wu, X.; Liu, C.C. Neutral Charged Immunosensor Platform for Protein-based Biomarker Analysis with Enhanced Sensitivity. ACS Sens. 2019, 4, 161–169. [Google Scholar] [CrossRef]
- Carlin, N.; Martic-Milne, S. Anti-tau antibodies based electrochemical sensor for detection of tau protein biomarkers. J. Electrochem. Soc. 2018, 165, G3018. [Google Scholar] [CrossRef]
- Shiravandi, A.; Yari, F.; Tofigh, N.; Kazemi Ashtiani, M.; Shahpasand, K.; Ghanian, M.-H.; Shekari, F.; Faridbod, F. Earlier Detection of Alzheimer’s Disease Based on a Novel Biomarker cis P-tau by a Label-Free Electrochemical Immunosensor. Biosensors 2022, 12, 879. [Google Scholar] [PubMed]
- Shui, B.; Tao, D.; Cheng, J.; Mei, Y.; Jaffrezic-Renault, N.; Guo, Z. A novel electrochemical aptamer–antibody sandwich assay for the detection of tau-381 in human serum. Analyst 2018, 143, 3549–3554. [Google Scholar] [CrossRef]
- Singh, P. Electrochemical Biosensors: Applications in Diagnostics, Therapeutics, Environment, and Food Management; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Chen, H.; Zhang, J.; Huang, R.; Wang, D.; Deng, D.; Zhang, Q.; Luo, L. The Applications of Electrochemical Immunosensors in the Detection of Disease Biomarkers: A Review. Molecules 2023, 28, 3605. [Google Scholar] [CrossRef]
- Hanif, S.; Muhammad, P.; Niu, Z.; Ismail, M.; Morsch, M.; Zhang, X.; Li, M.; Shi, B. Nanotechnology-based strategies for early diagnosis of central nervous system disorders. Adv. NanoBiomed Res. 2021, 1, 2100008. [Google Scholar] [CrossRef]
- Malaiya, A.; Singhai, M.; Singh, M.; Prajapati, S.K.; Choudhury, H.; Fatima, M.; Alexander, A.; Dubey, S.K.; Greish, K.; Kesharwani, P. Recent Update on the Alzheimer’s Disease Progression, Diagnosis and Treatment Approaches. Curr. Drug Targets 2022, 23, 978–1001. [Google Scholar]
- Jellinger, K.A. Neuropathology of the Alzheimer’s continuum: An update. Free. Neuropathol. 2020, 1, 32. [Google Scholar]
- Abdullah, S.A.; Najm, L.; Ladouceur, L.; Ebrahimi, F.; Shakeri, A.; Al-Jabouri, N.; Didar, T.F.; Dellinger, K. Functional Nanomaterials for the Diagnosis of Alzheimer’s Disease: Recent Progress and Future Perspectives. Adv. Funct. Mater. 2023, 2302673. [Google Scholar] [CrossRef]
- Khizar, S.; Elaissari, A.; Al-Dossary, A.A.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A. Advancement in Nanoparticle-based Biosensors for Point-of-care In vitro Diagnostics. Curr. Top. Med. Chem. 2022, 22, 807–833. [Google Scholar] [CrossRef]
- Li, B.; Tan, H.; Jenkins, D.; Raghavan, V.S.; Rosa, B.G.; Güder, F.; Pan, G.; Yeatman, E.; Sharp, D.J. Clinical detection of neurodegenerative blood biomarkers using graphene immunosensor. Carbon 2020, 168, 144–162. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Rosa, B.G.; Akingbade, O.E.; Guo, X.; Gonzalez-Macia, L.; Crone, M.A.; Cameron, L.P.; Freemont, P.; Choy, K.-L.; Güder, F.; Yeatman, E. Multiplexed immunosensors for point-of-care diagnostic applications. Biosens. Bioelectron. 2022, 203, 114050. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Z.; Xie, L.; Luo, S.; Zeng, T.; Zhang, Y.; Zhang, M.; Wang, S.; Li, M.; Wei, Y.; et al. Explore how immobilization strategies affected immunosensor performance by comparing four methods for antibody immobilization on electrode surfaces. Sci. Rep. 2022, 12, 22444. [Google Scholar] [CrossRef] [PubMed]
- Putzbach, W.; Ronkainen, N.J. Immobilization Techniques in the Fabrication of Nanomaterial-Based Electrochemical Biosensors: A Review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef]
- Kim, J.; Park, M. Recent Progress in Electrochemical Immunosensors. Biosensors 2021, 11, 360. [Google Scholar] [CrossRef]
- Adesina, A.; Mashazi, P. Oriented Antibody Covalent Immobilization for Label-Free Impedimetric Detection of C-Reactive Protein via Direct and Sandwich Immunoassays. Front. Chem. 2021, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guisán, J.M.; Rocha-Martin, J. Oriented immobilization of antibodies onto sensing platforms—A critical review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Agrawal, M.; Srivastava, A. Signal amplification strategies in electrochemical biosensors via antibody immobilization and nanomaterial-based transducers. Mater. Adv. 2022, 3, 8864–8885. [Google Scholar] [CrossRef]
- Gajos, K.; Szafraniec, K.; Petrou, P.; Budkowski, A. Surface density dependent orientation and immunological recognition of antibody on silicon: TOF-SIMS and surface analysis of two covalent immobilization methods. Appl. Surf. Sci. 2020, 518, 146269. [Google Scholar] [CrossRef]
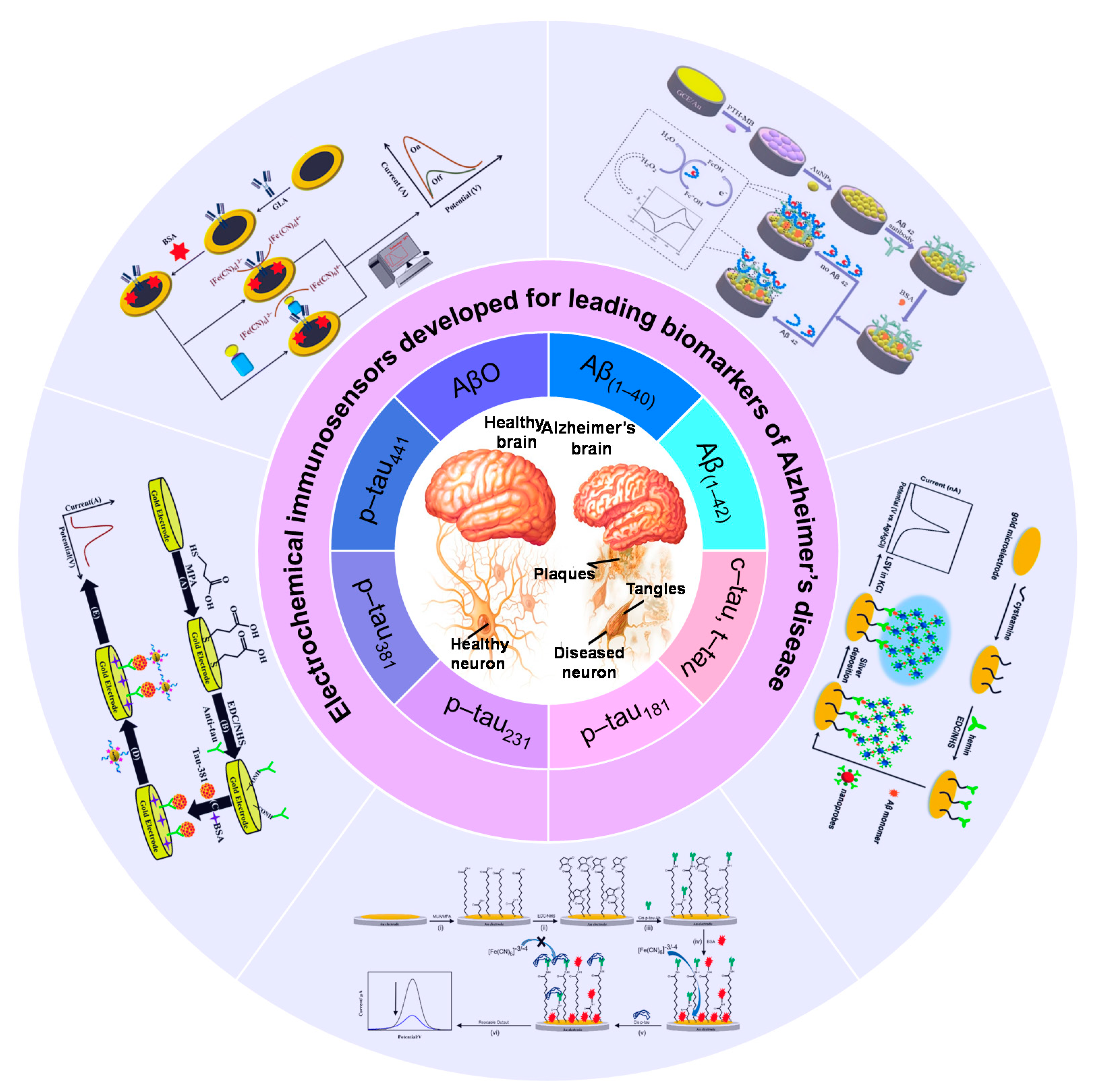

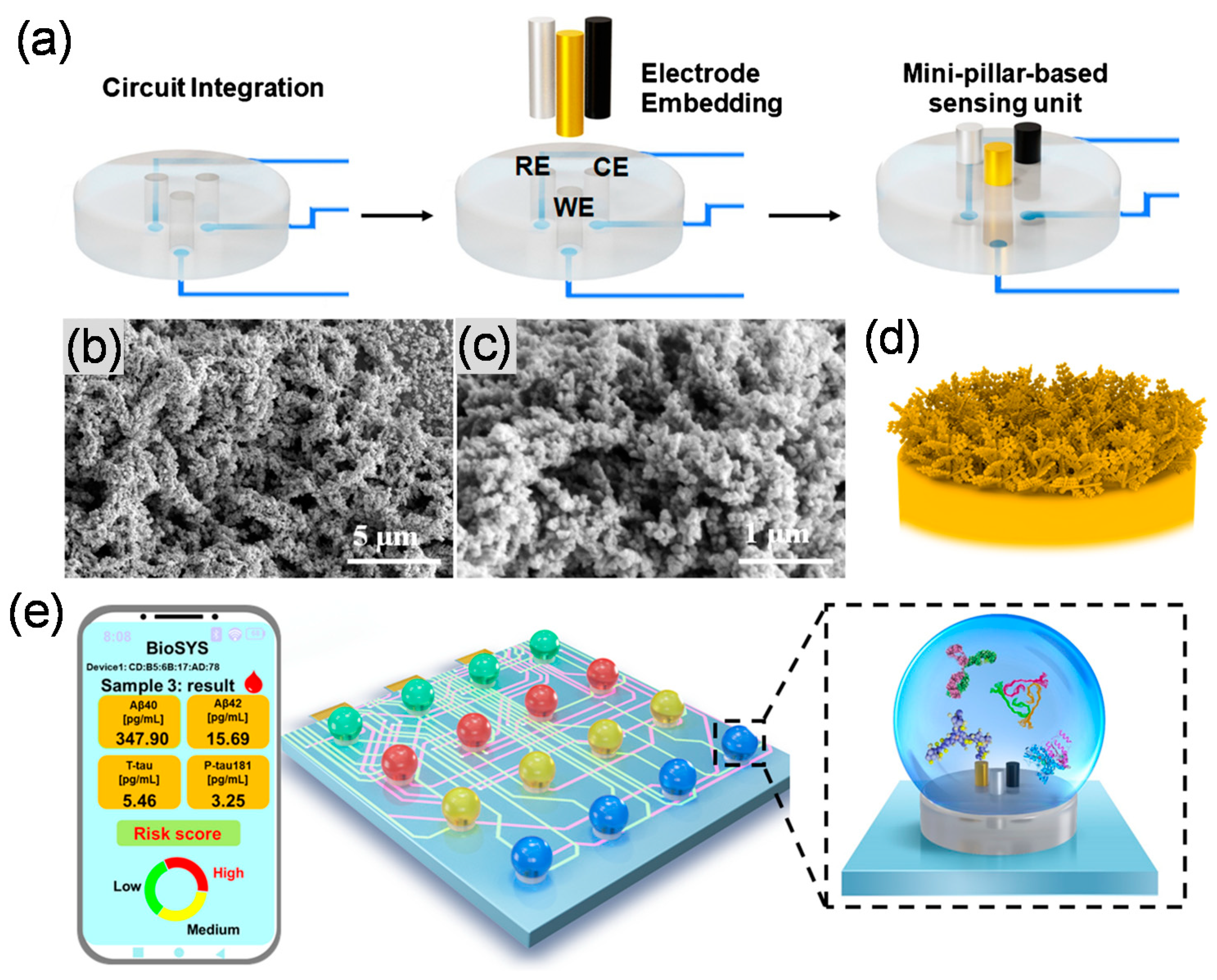

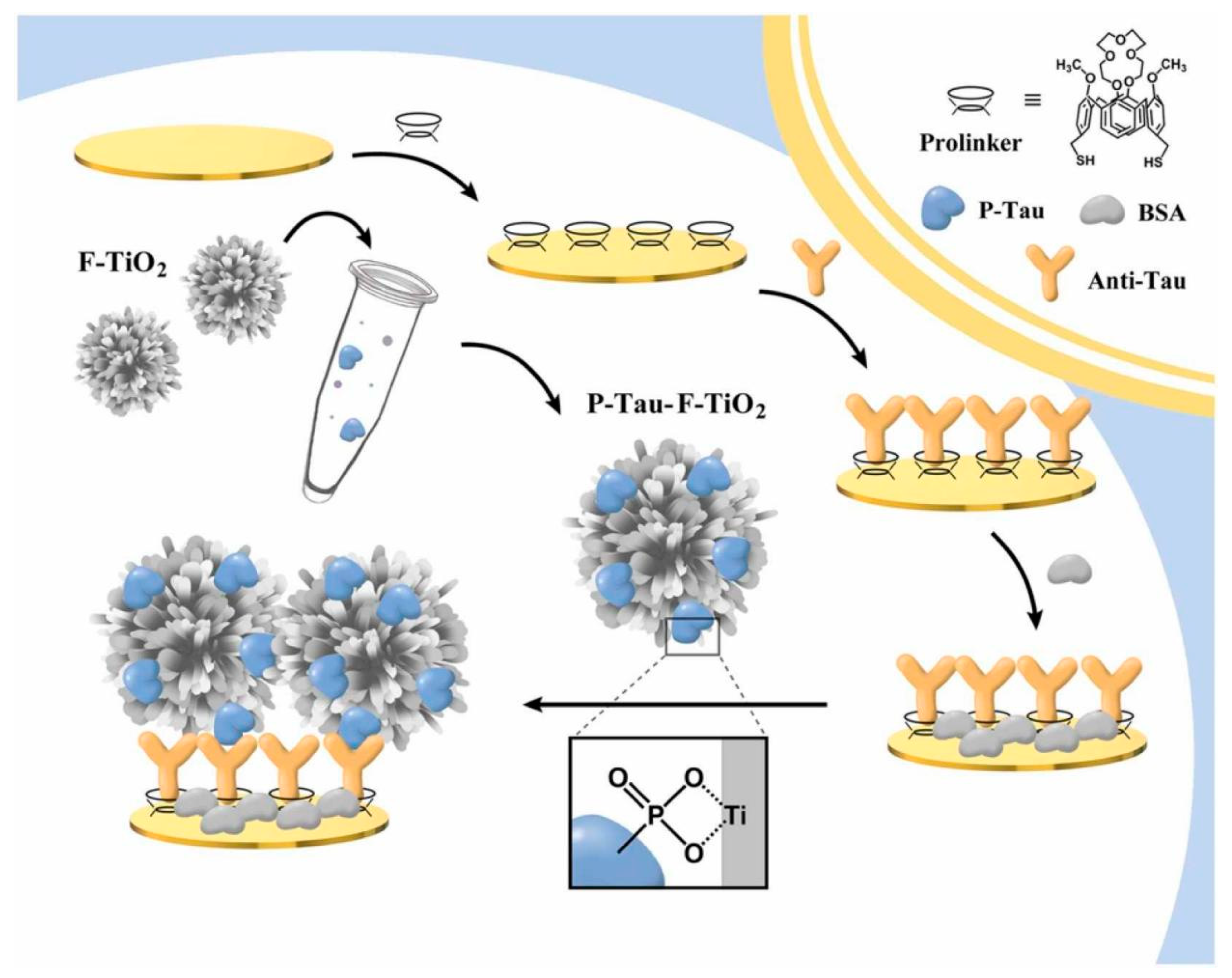

| Analytes | Signal Transducer | Nanomaterials | Bioreceptors | Detection Techniques | Signal Marker | Real Sample | Detection Range | LOD | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Aβ | GE | AuNPs | Ab | EIS | [Fe(CN)6]3−/4− | Tear/Blood | 1 pg mL−1–1 ng mL−1 | 1 pg mL−1 | [71] |
| Aβ | Interdigitated microelectrode | - * | Ab | EIS | [Fe(CN)6]3−/4− | - ♣ | 0.1–100 pg mL−1 | 0.1 pg mL−1 | [80] |
| Aβ | GCE | AuNPs-graphitic carbon nitride nanosheets (g-C3N4) nanocomposite/PdNPs | Ab | Electrochemiluminescence (ECL) | PdNPs@MOFs/K2S2O8 | Serum | 10 fg mL−1–50 ng mL−1 | 3.4 fg mL−1 | [81] |
| Aβ | GE | AuNPs | Ab | Linear square voltammetry (LSV) | [Fe(CN)6]3−/4− | CSF | 1 pM–50 nM | 0.2 pM | [82] |
| Aβ | GE | Gold nanodendrites | Ab | Square-wave voltammetry (SWV) | Ru(NH3)63+ | Serum | 10−10–10−7 mg mL−1 | 8.6 × 10−12 mg mL−1 | [83] |
| Aβ | Gold nanopillar array | Nanopillar array | Ab | SWV | HRP/[Fe(CN)6]3−/4− | Tear | 0.1–1 ng mL−1 | 0.14 ng mL−1 | [84] |
| Aβ | GCE | CuSNPs@COFs nanocomposite/AuNPs | Aptamer/Ab | DPV | Thi-AuNPs/[Fe(CN)6]3−/4− | CSF | 1 pM–1 μM | 0.4 pM | [77] |
| Aβ(1-40) | Pt/Ir microelectrodes | - | Ab | EIS | - ♠ | Brain tissue lysate samples | 1–104 pg mL−1 | 4.81 pg mL−1 | [85] |
| Aβ(1-42) | GE | ZnO nanoarrays | Ab | DPV | [Fe(CN)6]3−/4− | Plasma | 0.5–100 pg mL−1 | 62.3 fg mL−1 | [68] |
| Aβ(1-42) | GE | Tantalum (Ta) nanolayer | Ab | DPV | [Fe(CN)6]3−/4− | - | 2.2 pM–22 μM | 1.62 pM | [75] |
| Aβ(1-42) | GCE | Highly ordered pyrolytic graphite nanoarray | Ab | DPV | FCA | - | 10–200 ng mL−1 | 10 ng mL−1 | [74] |
| Aβ(1-42) | SPGE | - | Ab | DPV | [Fe(CN)6]3−/4− | Plasma | 1–1000 pg mL−1 | 1.4 pg mL−1 | [70] |
| Aβ(1-42) | GCE | AuNPs | Ab | CV | FcOH/H2O2 | Serum/Saliva | Serum: 0.056–13.7 nM/ Saliva: 0.056–41.2 nM | Serum: 25.2 pM/ Saliva: 23.8 pM | [76] |
| Aβ(1-42) | GCE | SnO2 nanofibers | Ab | EIS | [Fe(CN)6]3−/4− | Plasma | 1 fg mL−1–1 ng mL−1 | 0.638 fg mL−1 | [78] |
| Aβ(1-42) | 3D nanostructure PC substrate | AuNPs | Ab | EIS | [Fe(CN)6]3−/4− | Plasma | 10 pg mL−1–100 ng mL−1 | 113 fg mL−1 | [73] |
| Aβ(1-42) | Glass/Ti/Au Interdigitated chain-shaped electrode | Gold nanoarray | Ab | EIS | [Fe(CN)6]3–/4– | Serum/CSF | 10−3–103 ng mL−1 | 100 pg mL−1 (serum)/ ∼500 pg mL−1 (CSF) | [86] |
| Aβ(1-42) | GCE | Fe3O4@polypyrrole-AuNPs nanocomposite/Flower-like Co-MOFs nanocomposite | Ab | ECL | Cobalt-based MOFs/N-(aminobutyl)-N-(ethylisoluminol) (ABEI)/[Fe(CN)6]3–/4– | Serum | 10 fg mL−1–100 ng mL−1 | 3 fg mL−1 | [87] |
| Aβ(1-42) | SPGE | Reduced graphene oxide (rGO) | Ab | DPV | [Fe(CN)6]3−/4− | Plasma | 11 pM–55 nM | 2.398 pM | [88] |
| Aβ(1-42) | GE | - | Ab | Capacitive | [Fe(CN)6]3−/4− | Serum | 10–104 pg mL−1 | 7.5 pg mL−1 | [89] |
| Aβ(1-40)/Aβ(1-42) | GE | Conductive silk fibroin 3D nanostructure | Ab | LSV | Methionine (35) | Serum | 2 pM–5 nM | Aβ(1-40): 6.63 pg mL−1/ Aβ(1-42): 3.74 pg mL−1 | [90] |
| Aβ(1-40)/Aβ(1-42) | Au wire | Gold nanoarray | Ab | Chronoamperometry (CA)/DPV | [Fe(CN)6]3−/4− | Serum | 0.1–1000 pg mL−1 | Aβ(1-40): 0.125 pg mL−1/Aβ(1-42): 0.089 pg mL−1 | [79] |
| Aβ(1-40)/Aβ(1-42) | Superwettable microchips | Vertical graphene@AuNPs nanocomposite | Ab | DPV | [Fe(CN)6]3−/4−/Ferrocene | Serum | 0.1 pg mL−1–10 ng mL−1 | Aβ(1-40): 0.064 pg mL−1/Aβ(1-42): 0.012 pg mL−1 | [91] |
| Aβ | Magnetic glass carbon electrode | Fe3O4 NPs/ Mesoporous carbon nanospheres/Gold nanorods | Ab/Aptamer | ECL | Ru(bpy)32+/gold nanorods | Serum | 1 × 10−5–100 ng mL−1 | 4.2 × 10−6 ng mL−1 | [92] |
| Anti Aβ(1-40) Ab | SPCE | - | Aβ(1-40) antigen | CV | - | Serum/CSF | 1 ng mL−1–10 μg mL−1 | 1 ng mL−1 | [93] |
| Analytes | Signal Transducer | Nanomaterials | Bioreceptors | Detection Techniques | Signal Marker | Real Sample | Detection Range | LOD | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| p-tau181 | Au wire | Gold nanoarray | Ab | CA/DPV | [Fe(CN)6]3−/4− | Serum | 0.1–1000 pg mL−1 | 0.176 pg mL−1 | [79] |
| p-tau181/t-tau | Superwettable microchips | Vertical graphene@AuNPs nanocomposite | Ab | DPV | [Fe(CN)6]3−/4−/Ferrocene | Serum | 0.1 pg mL−1–10 ng mL−1 | p-tau181: 0.041 pg mL−1/ t-tau: 0.039 pg mL−1 | [91] |
| p-tau441 | ITO | rGO-AuNPs nanocomposite | Ab | EIS | [Fe(CN)6]3−/4− | Serum/CSF | 1–500 pg mL−1 | 0.091 pg mL−1 | [106] |
| p-tau441 | GE | MWCNTs-rGO nanocomposite | Ab | DPV | [Fe(CN)6]3−/4− | Serum | 0.5–80 fM | 0.46 fM | [107] |
| t-tau | GE | - * | Ab | DPV | [Ru(NH3)6]2+/3+, [Fe(CN)6]3−/4− | Serum | 0.968–454 pM | 0.968 pM | [108] |
| p-tau441 | GE | - | Ab | EIS | [Fe(CN)6]3−/4− | - ♣ | 10–100 μg mL−1 | 10 μg mL−1 | [109] |
| p-tau441 | SPCE | - | Ab | CV | [Fe(CN)6]3−/4− | CSF | 0.0064–0.8 ng mL−1 | 0.0053 ng mL−1 | [103] |
| p-tau | GE | Flower-shaped TiO2 nanostructure | Ab | EIS | [Fe(CN)6]3−/4− | Serum | 1–200 ng mL−1 | 1.774 pg mL−1 | [31] |
| tau | GCE | MnS-GO-PANI nanocomposite/AuNPs@Fe3O4 nanocomposite | Ab | DPV | [Fe(CN)6]3−/4− | Plasma | 1 × 10−13–1 × 10−6 M | 1 × 10−14 M | [101] |
| p-tau | GE | - | Ab | DPV | [Fe(CN)6]3−/4− | CSF/ Serum | 0.05–3000 pM | 0.02 pM | [110] |
| p-tau181 | SPCE | MWCNTs-PtNPs nanocomposite | Ab | SWV | [Fe(CN)6]3−/4− | Serum | 8.6–1100 pg mL−1 | 0.24 pg mL−1 | [102] |
| p-tau231 | GE | Gold nanoarray | Ab | EIS | [Fe(CN)6]3−/4− | Serum | 10−4–101 ng mL−1 | 140 pg mL−1 | [99] |
| tau | GCE | PDDA nanolayer | Ab | EIS | [Fe(CN)6]3−/4− | Serum | 0.01–100 ng mL−1 | 1.31 pg mL−1 | [100] |
| c-tau | GE | - | Ab | DPV | [Fe(CN)6]3−/4− | Serum | 10 pg mL−1–100 ng mL−1 | 0.1 pg mL−1 | [104] |
| p-tau381 | GE | AuNPs | Ab | DPV | [Fe(CN)6]3−/4− | Serum | 0.5–100 pM | 0.42 pM | [111] |
| tau | GE | Gold nanodendrites | Ab | SWV | Ru(NH3)63+ | Serum | 10−10–10−7 mg mL−1 | 5.91 × 10−11 mg mL−1 | [83] |
| p-tau381 | CE | AuNPs-MoSe2 NSs nanocomposite | Aptamer/Ab | PEC | [Fe(CN)6]3−/4− | Serum | 0.5 fM–1 nM | 0.3 fM | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Angnes, L.; Sattarahmady, N.; Negahdary, M.; Heli, H. Electrochemical Immunosensors Developed for Amyloid-Beta and Tau Proteins, Leading Biomarkers of Alzheimer’s Disease. Biosensors 2023, 13, 742. https://doi.org/10.3390/bios13070742
Sharma A, Angnes L, Sattarahmady N, Negahdary M, Heli H. Electrochemical Immunosensors Developed for Amyloid-Beta and Tau Proteins, Leading Biomarkers of Alzheimer’s Disease. Biosensors. 2023; 13(7):742. https://doi.org/10.3390/bios13070742
Chicago/Turabian StyleSharma, Abhinav, Lúcio Angnes, Naghmeh Sattarahmady, Masoud Negahdary, and Hossein Heli. 2023. "Electrochemical Immunosensors Developed for Amyloid-Beta and Tau Proteins, Leading Biomarkers of Alzheimer’s Disease" Biosensors 13, no. 7: 742. https://doi.org/10.3390/bios13070742
APA StyleSharma, A., Angnes, L., Sattarahmady, N., Negahdary, M., & Heli, H. (2023). Electrochemical Immunosensors Developed for Amyloid-Beta and Tau Proteins, Leading Biomarkers of Alzheimer’s Disease. Biosensors, 13(7), 742. https://doi.org/10.3390/bios13070742








