Multisensory Evaluation of Muscle Activity and Human Manipulability during Upper Limb Motor Tasks
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
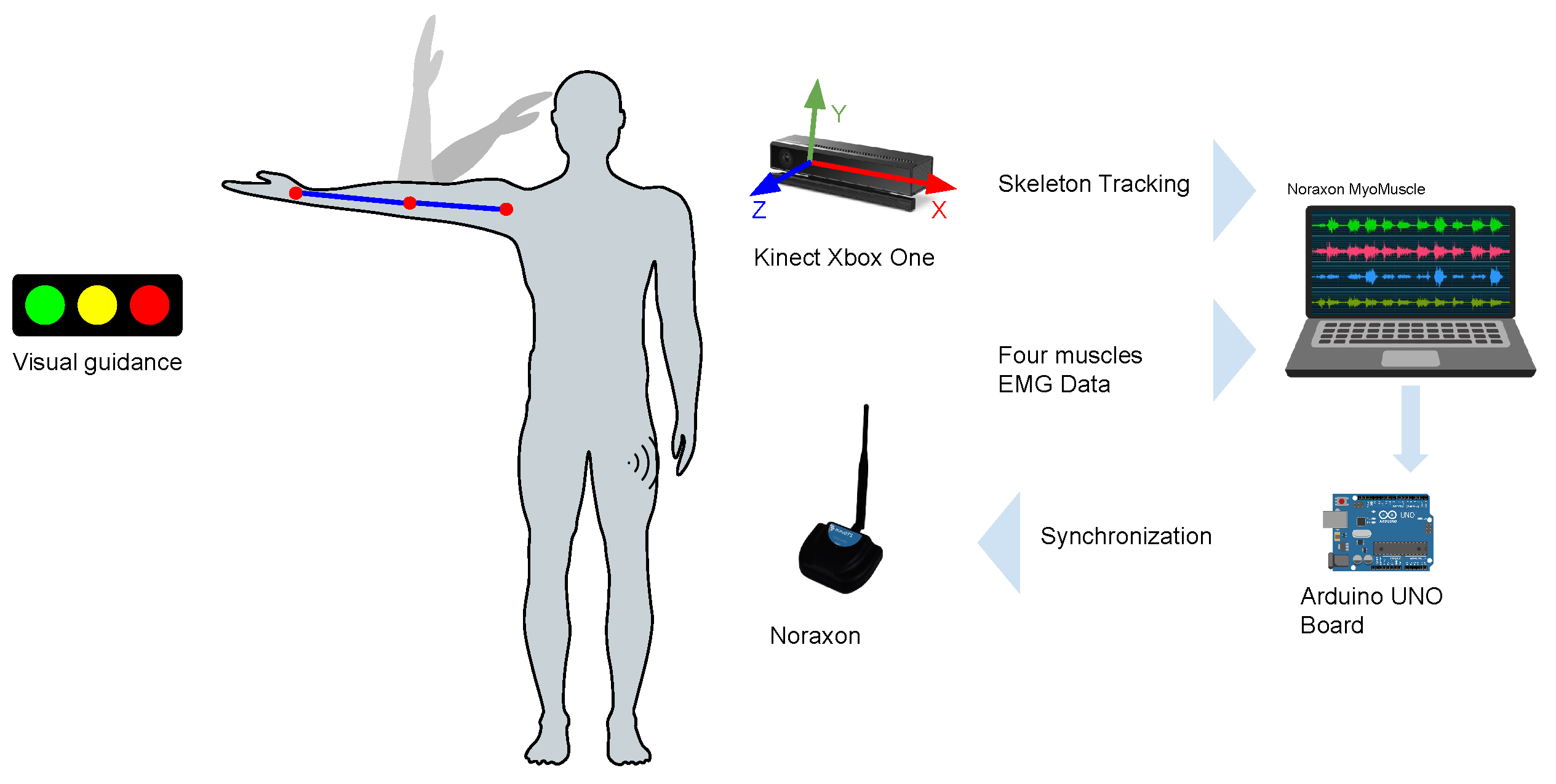
2.2. Setup and Equipment
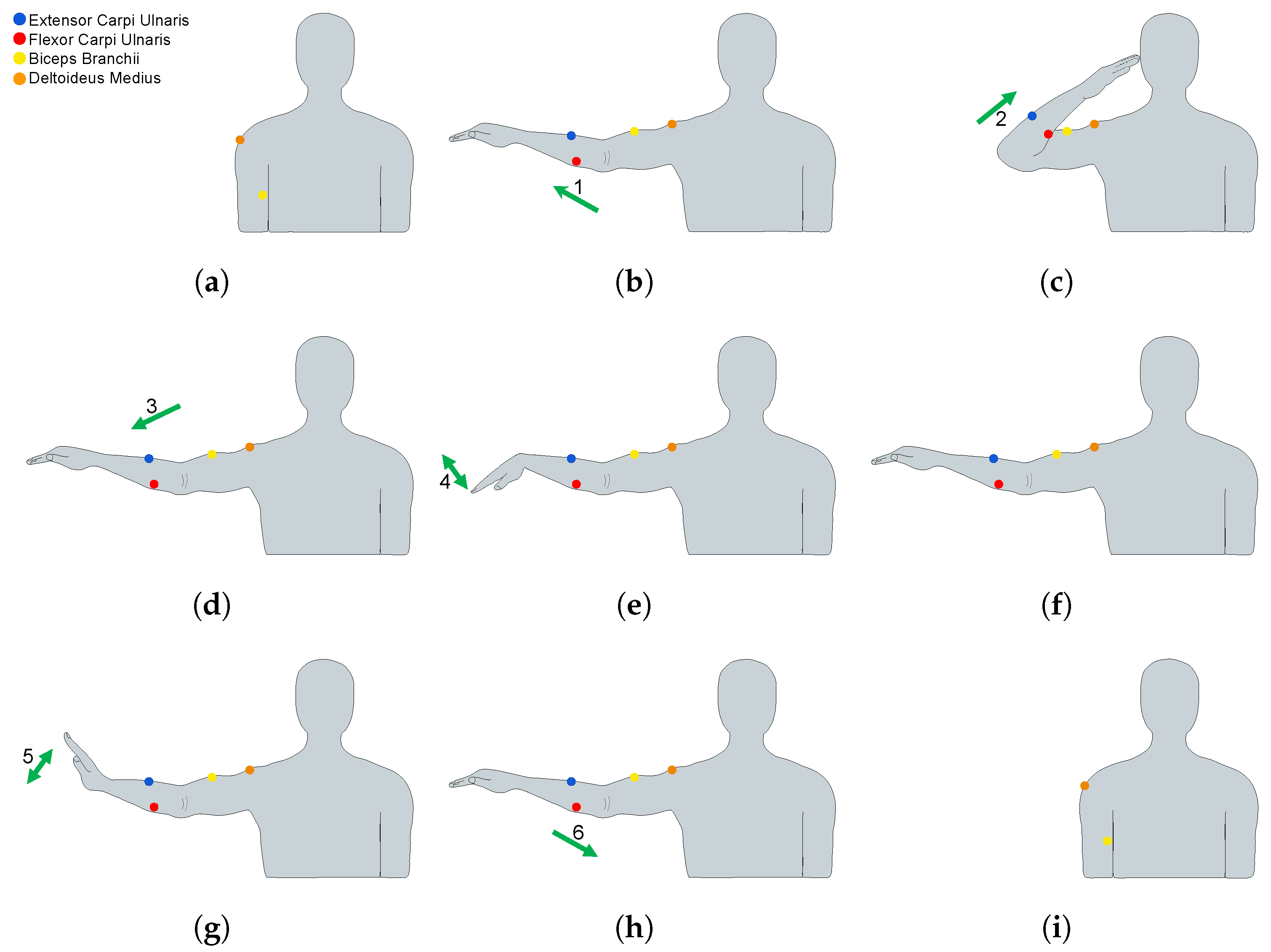
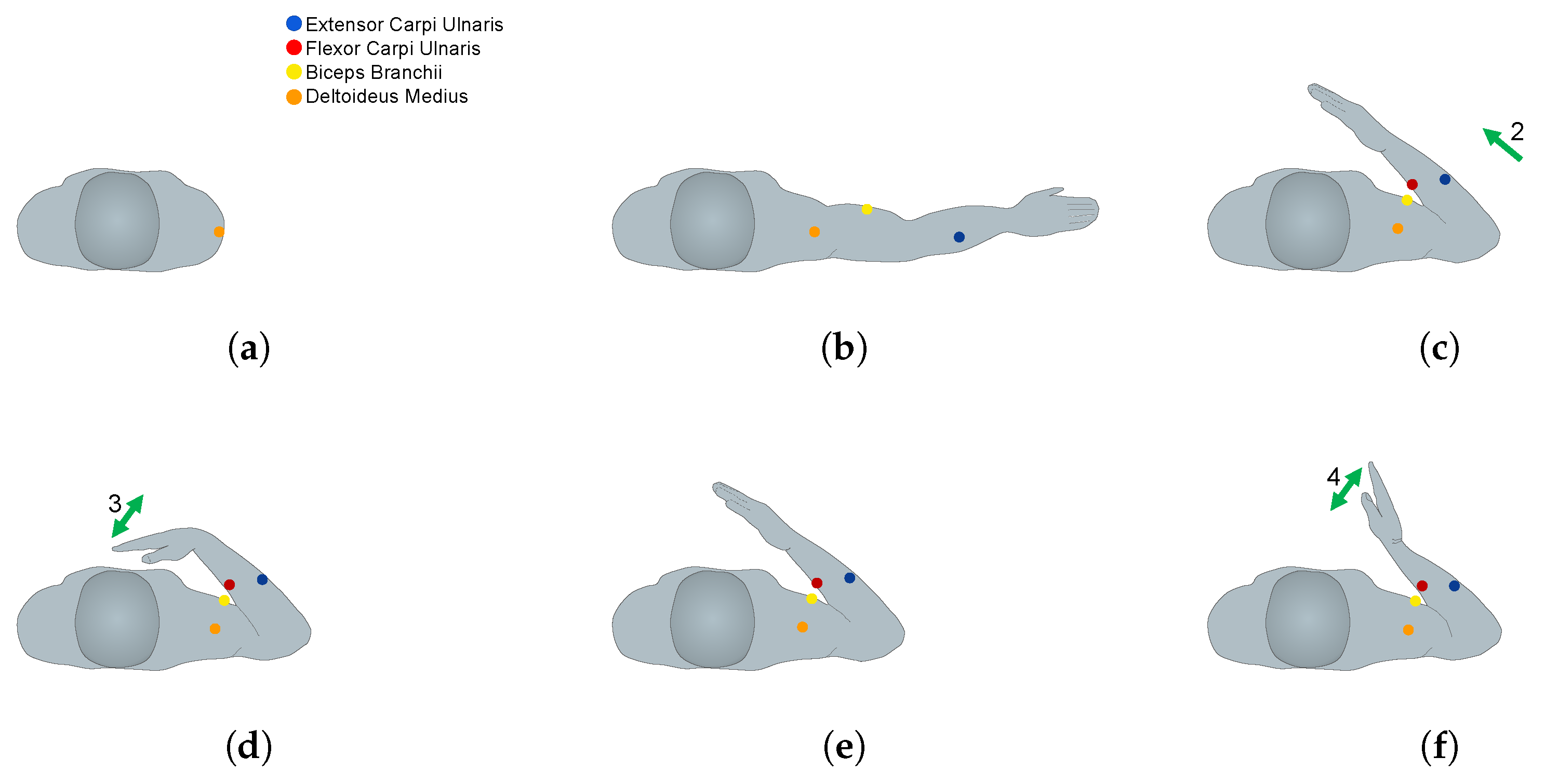
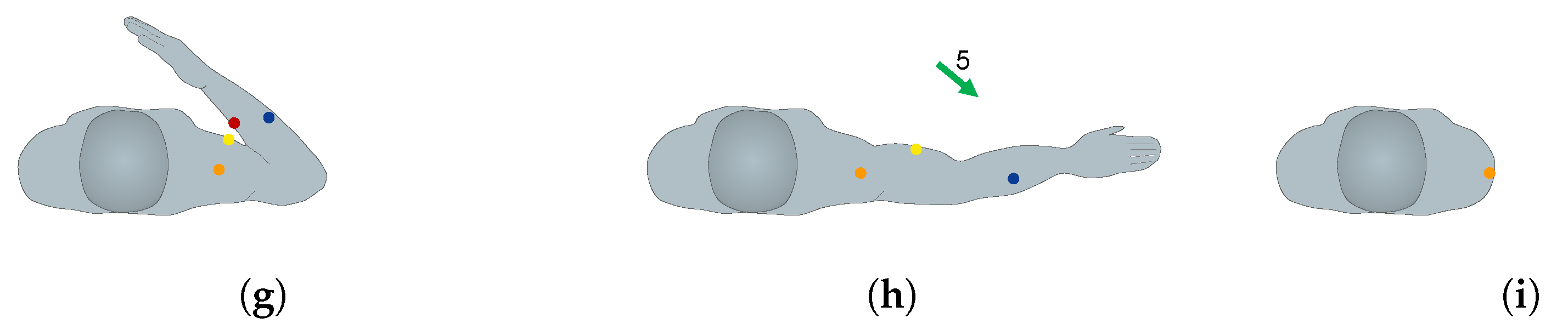
2.3. EMG Sensor Placement and MVC Procedures
2.4. Exercises and Data Acquisition
3. Data Analysis
3.1. Human Arm Kinematic Model
3.2. Human Manipulability
3.3. Data Synchronization
3.4. Analysis of EMG Factors
3.5. Statistical Analysis
4. Results
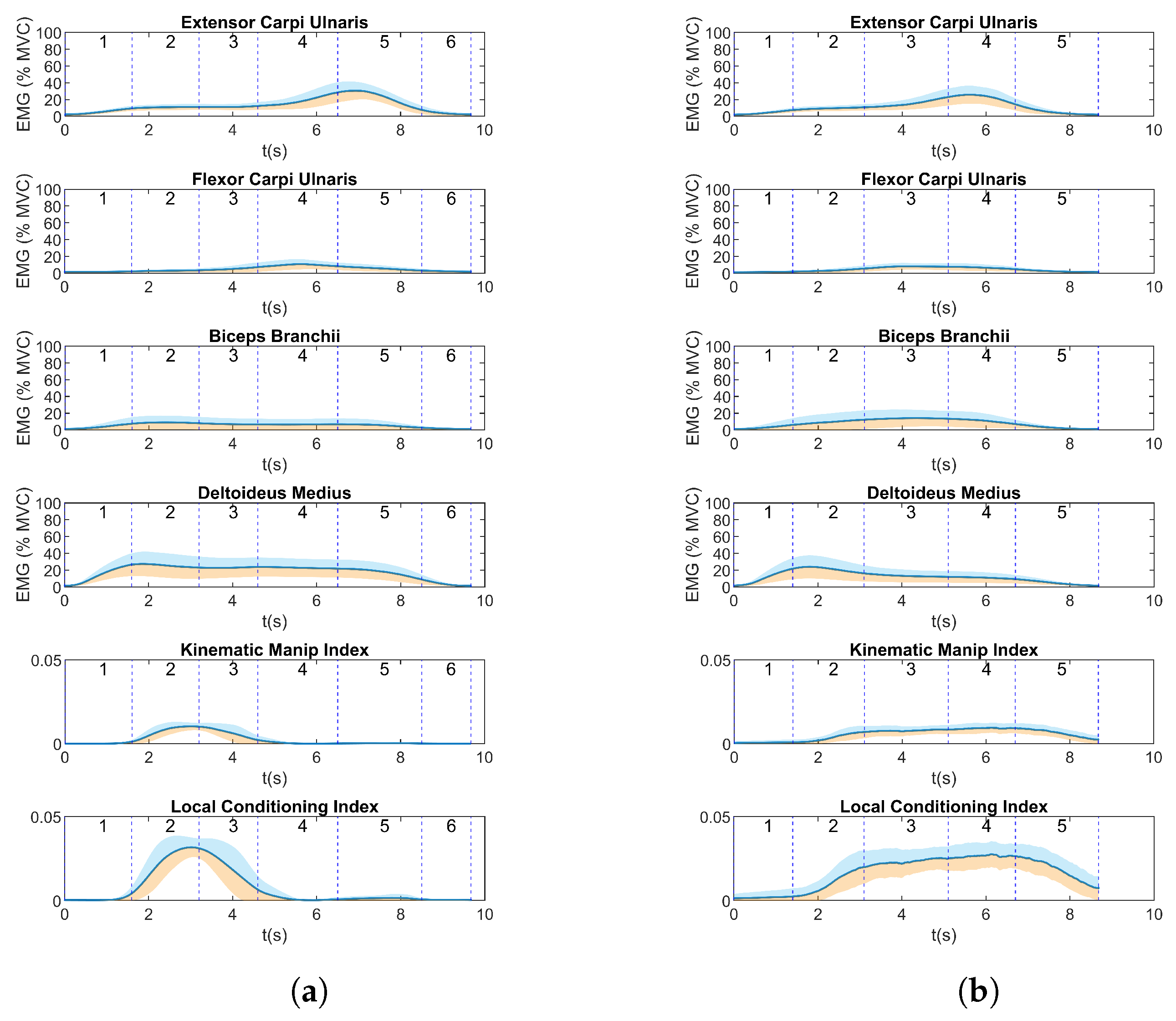
4.1. EMG and Manipulability
4.2. EMG Metrics
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez, A.; Miralles, D. Postural analysis and workspace design using an analytically derived inverse kinematics model. Theor. Issues Ergon. Sci. 2015, 16, 187–200. [Google Scholar] [CrossRef]
- McDonald, A.C.; Mulla, D.M.; Keir, P.J. Muscular and kinematic adaptations to fatiguing repetitive upper extremity work. Appl. Ergon. 2019, 75, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Menychtas, D.; Glushkova, A.; Manitsaris, S. Analyzing the kinematic and kinetic contributions of the human upper body’s joints for ergonomics assessment. J. Ambient. Intell. Humaniz. Comput. 2020, 11, 6093–6105. [Google Scholar] [CrossRef]
- Maillet, J.; Rossi, J.; Hug, F.; Proquez, J.J.; Nordez, A. Influence of experience on kinematics of upper limbs during sewing gesture. Appl. Ergon. 2022, 102, 103737. [Google Scholar] [CrossRef] [PubMed]
- Norheim, K.L.; Samani, A.; Madeleine, P. The effects of age on response time, accuracy, and shoulder/arm kinematics during hammering. Appl. Ergon. 2021, 90, 103157. [Google Scholar] [CrossRef] [PubMed]
- Harari, Y.; Bechar, A.; Riemer, R. Workers’ biomechanical loads and kinematics during multiple-task manual material handling. Appl. Ergon. 2020, 83, 102985. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, I.; Lencioni, T.; Bowman, T.; Bertoni, R.; Turolla, A.; Ferrarin, M.; Jonsdottir, J. Effects of robot therapy on upper body kinematics and arm function in persons post stroke: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2020, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, O.G.; Morell, V.; Ramon, J.L.; Jara, C.A. Human Pose Detection for Robotic-Assisted and Rehabilitation Environments. Appl. Sci. 2021, 11, 4183. [Google Scholar] [CrossRef]
- Thrane, G.; Thrane, G.; Sunnerhagen, K.S.; Murphy, M.A. Upper limb kinematics during the first year after stroke: The stroke arm longitudinal study at the University of Gothenburg (SALGOT). J. Neuroeng. Rehabil. 2020, 17, 76. [Google Scholar] [CrossRef]
- Chen, Y.W.; Liao, W.W.; Chen, C.L.; Wu, C.Y. Kinematic descriptions of upper limb function using simulated tasks in activities of daily living after stroke. Hum. Mov. Sci. 2021, 79, 102834. [Google Scholar] [CrossRef]
- Garcia, G.J.; Alepuz, A.; Balastegui, G.; Bernat, L.; Mortes, J.; Sanchez, S.; Vera, E.; Jara, C.A.; Morell, V.; Pomares, J.; et al. ARMIA: A Sensorized Arm Wearable for Motor Rehabilitation. Biosensors 2021, 12, 469. [Google Scholar] [CrossRef]
- Yoshikawa, T. Manipulability of Robotic Mechanisms. Int. J. Robot. Res. 1985, 4, 3–9. [Google Scholar] [CrossRef]
- Petrič, T.; Peternel, L.; Morimoto, J.; Babič, J. Assistive Arm-Exoskeleton Control Based on Human Muscular Manipulability. Front. Neurorobotics 2019, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, S.; Bingul, Z. Comparative study of performance indices for fundamental robot manipulators. Robot. Auton. Syst. 2006, 54, 567–573. [Google Scholar] [CrossRef]
- Jarque-Bou, N.J.; Sancho-Bru, J.L.; Vergara, M. A systematic review of EMG applications for the characterization of forearm and hand muscle activity during activities of daily living: Results, challenges, and open issues. Sensors 2021, 21, 3035. [Google Scholar] [CrossRef] [PubMed]
- Garro, F.; Chiappalone, M.; Buccelli, S.; Michieli, L.D.; Semprini, M. Neuromechanical Biomarkers for Robotic Neurorehabilitation. Front. Neurorobotics 2021, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; He, B.; Wang, Y.; Cao, J.; Gao, S. EMG-Centered Multisensory Based Technologies for Pattern Recognition in Rehabilitation: State of the Art and Challenges. Biosensors 2020, 10, 85. [Google Scholar] [CrossRef]
- Olmo, M.D.; Domingo, R. EMG characterization and processing in production engineering. Materials 2020, 13, 5815. [Google Scholar] [CrossRef] [PubMed]
- Shair, E.F.; Ahmad, S.A.; Marhaban, M.H.; Tamrin, S.B.; Abdullah, A.R. EMG processing based measures of fatigue assessment during manual lifting. Biomed Res. Int. 2017, 2017, 3937254. [Google Scholar] [CrossRef]
- Department of Rehabilitation. National University Hospital of Singapur. Arm Exercises for Stroke Patients. Available online: https://www.nuh.com.sg/our-services/Specialties/Rehabilitation/Documents/Patient%20and%20Caregiver%20Resources/Physiotherapy/Physiotherapy%20-%20Stroke%20-%20Arm%20Exercises.pdf (accessed on 2 May 2023).
- Yoshikawa, T. Dynamic Manipulability of Robot Manipulators; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 1985; pp. 1033–1038. [Google Scholar] [CrossRef]
- Denavit, J.; Hartenberg, R.S. A Kinematic Notation for Lower-Pair Mechanisms Based on Matrices. J. Appl. Mech. 2021, 22, 215–221. [Google Scholar] [CrossRef]
- Corke, P. Robotics, Vision and Control; Springer: Berlin/Heidelberg, Germany, 2011; Volume 73. [Google Scholar] [CrossRef]
- Vahrenkamp, N.; Asfour, T.; Metta, G.; Sandini, G.; Dillmann, R. Manipulability Analysis. In Proceedings of the 2012 12th IEEE-RAS International Conference on Humanoid Robots (Humanoids 2012), Osaka, Japan, 29 November–1 December 2012; pp. 568–573. [Google Scholar] [CrossRef]
- Pyo, Y.; Nakashima, K.; Kuwahata, S.; Kurazume, R.; Tsuji, T.; Morooka, K.; Hasegawa, T. Service robot system with an informationally structured environment. Robot. Auton. Syst. 2015, 74, 148–165. [Google Scholar] [CrossRef]
- Hernández, O.G.; Lopez-Castellanos, J.M.; Jara, C.A.; Garcia, G.J.; Ubeda, A.; Morell-Gimenez, V.; Gomez-Donoso, F. A kinematic, imaging and electromyography dataset for human muscular manipulability index prediction. Sci. Data 2023, 10, 132. [Google Scholar] [CrossRef] [PubMed]




| Sensor transmission range | 20 m |
| Selectable low-pass cutoff | 500/1000/1500 Hz |
| Wireless update rate | 100 Hz |
| Selectable sample rate | 3000/1500 Hz |
| Differential Input impedance | >10 Mohm |
| Baseline noise | 5 uV RMS |
| Electronic gain | 200 |
| Overall gain | 500 |
| 1 | q1 | 0 | 0 | |
| 2 | q3 | 0 | 0 | |
| 3 | q3 | 0 | ||
| 4 | q4 | − | 0 | 0 |
| 5 | q5 | − | 0 | |
| 6 | q6 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Castellanos, J.M.; Ramon, J.L.; Pomares, J.; Garcia, G.J.; Ubeda, A. Multisensory Evaluation of Muscle Activity and Human Manipulability during Upper Limb Motor Tasks. Biosensors 2023, 13, 697. https://doi.org/10.3390/bios13070697
Lopez-Castellanos JM, Ramon JL, Pomares J, Garcia GJ, Ubeda A. Multisensory Evaluation of Muscle Activity and Human Manipulability during Upper Limb Motor Tasks. Biosensors. 2023; 13(7):697. https://doi.org/10.3390/bios13070697
Chicago/Turabian StyleLopez-Castellanos, Jose M., Jose L. Ramon, Jorge Pomares, Gabriel J. Garcia, and Andres Ubeda. 2023. "Multisensory Evaluation of Muscle Activity and Human Manipulability during Upper Limb Motor Tasks" Biosensors 13, no. 7: 697. https://doi.org/10.3390/bios13070697
APA StyleLopez-Castellanos, J. M., Ramon, J. L., Pomares, J., Garcia, G. J., & Ubeda, A. (2023). Multisensory Evaluation of Muscle Activity and Human Manipulability during Upper Limb Motor Tasks. Biosensors, 13(7), 697. https://doi.org/10.3390/bios13070697








