An Impedance-Based Immunosensor for the Detection of Ovalbumin in White Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Antibody Production and Purification
2.3. Indirect ELISA
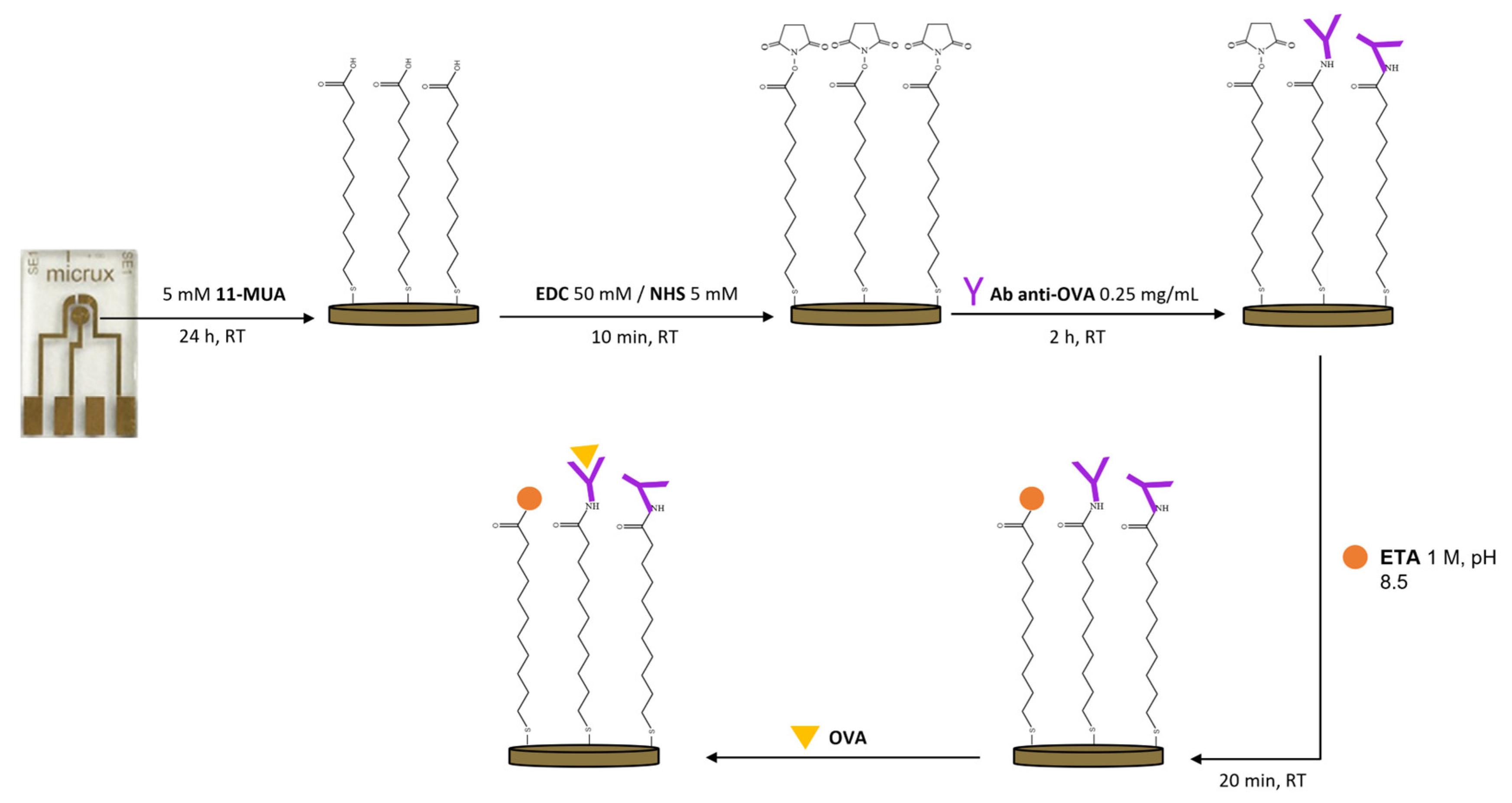
2.4. Immunosensor Development
2.5. Surface Characterisation by Atomic Force Microscopy
2.6. Impedimetric Measurements
2.7. Preparation of Wine Samples for Impedimetric Tests on Real Matrix
2.8. Statistical Analysis
3. Results and Discussion
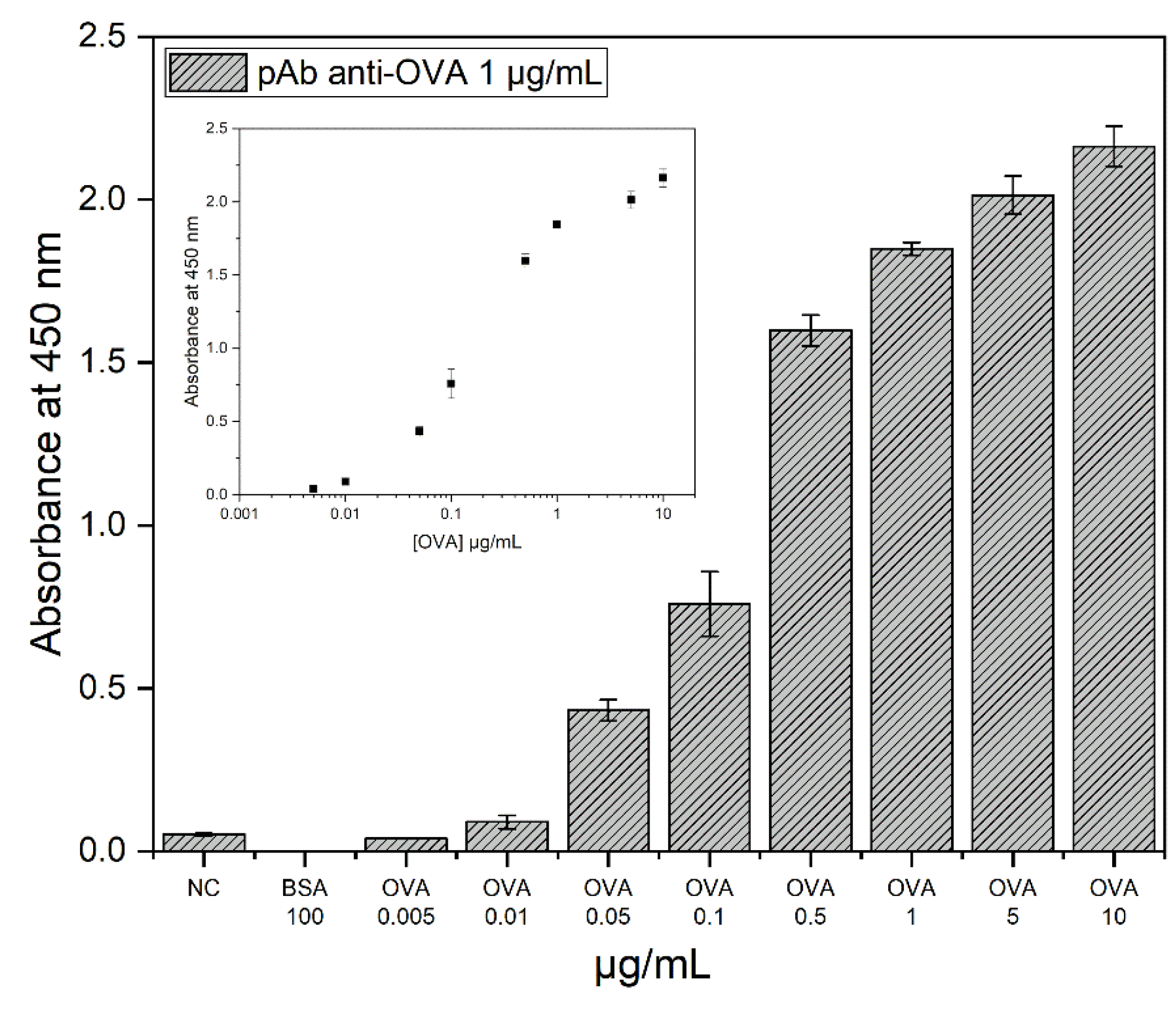
3.1. Evaluation of the Antibody-Binding Capacity by Indirect ELISA
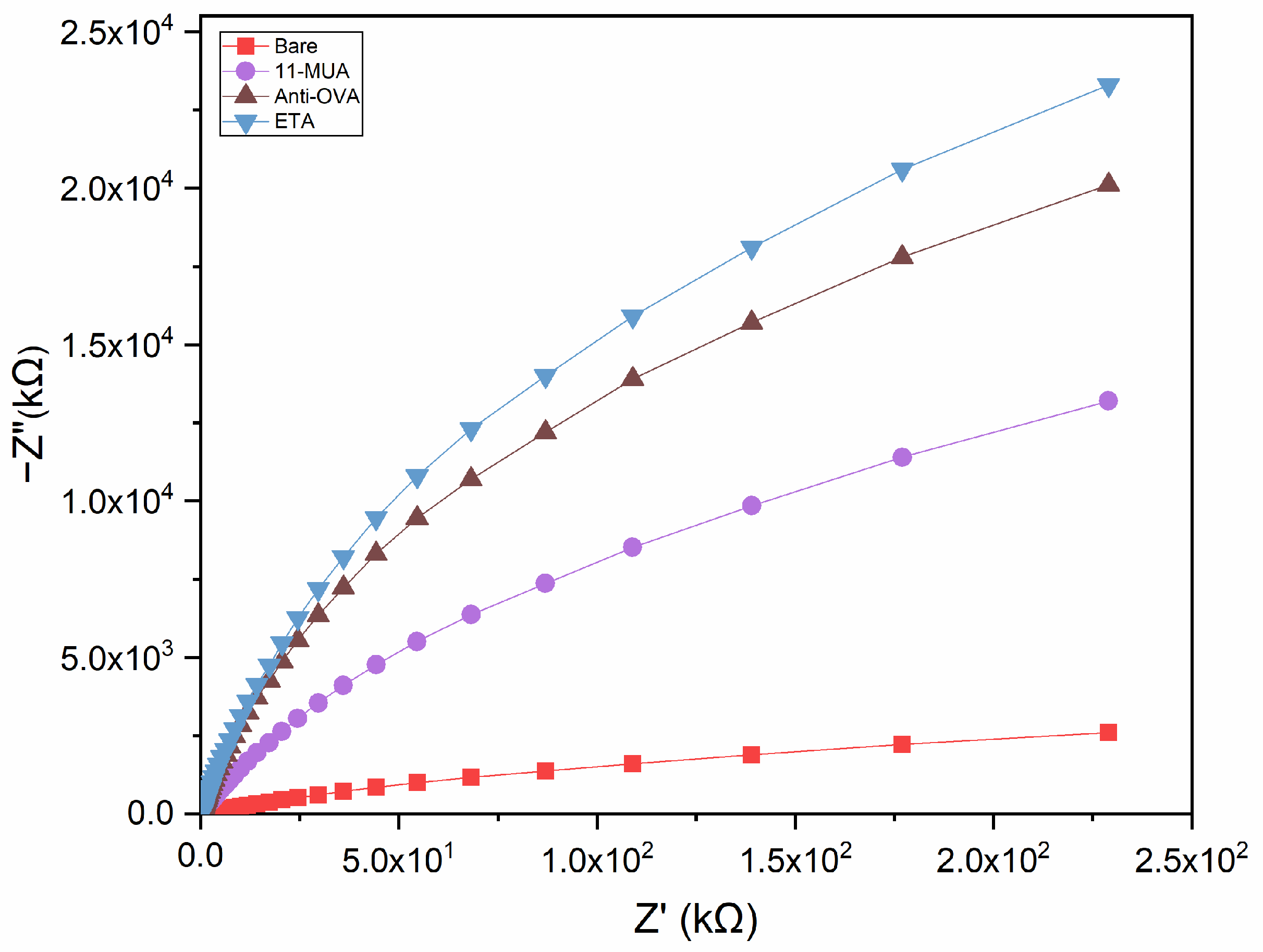
3.2. Electrochemical Characterisation of the Immunosensor Assembling
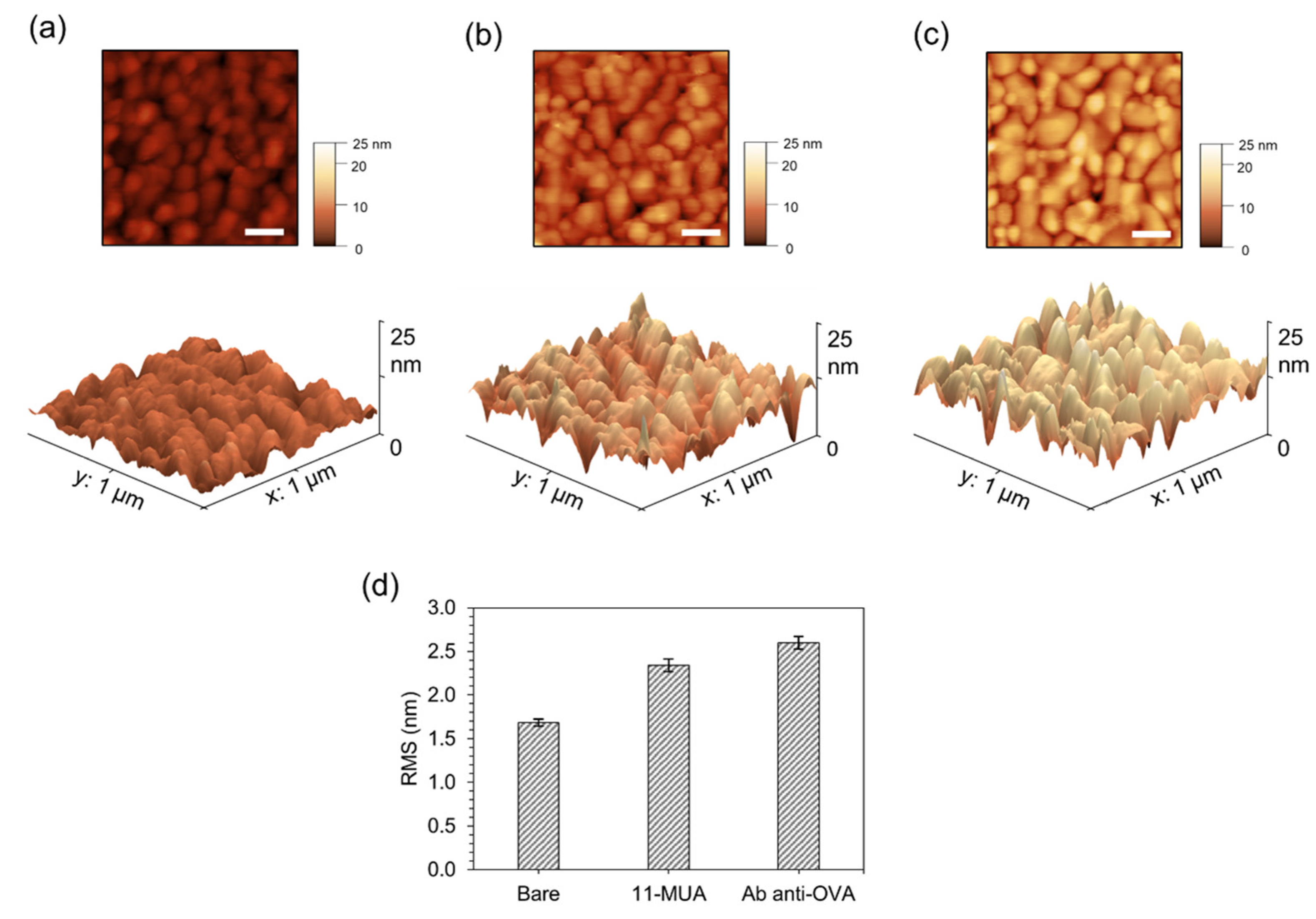
3.3. AFM Analysis
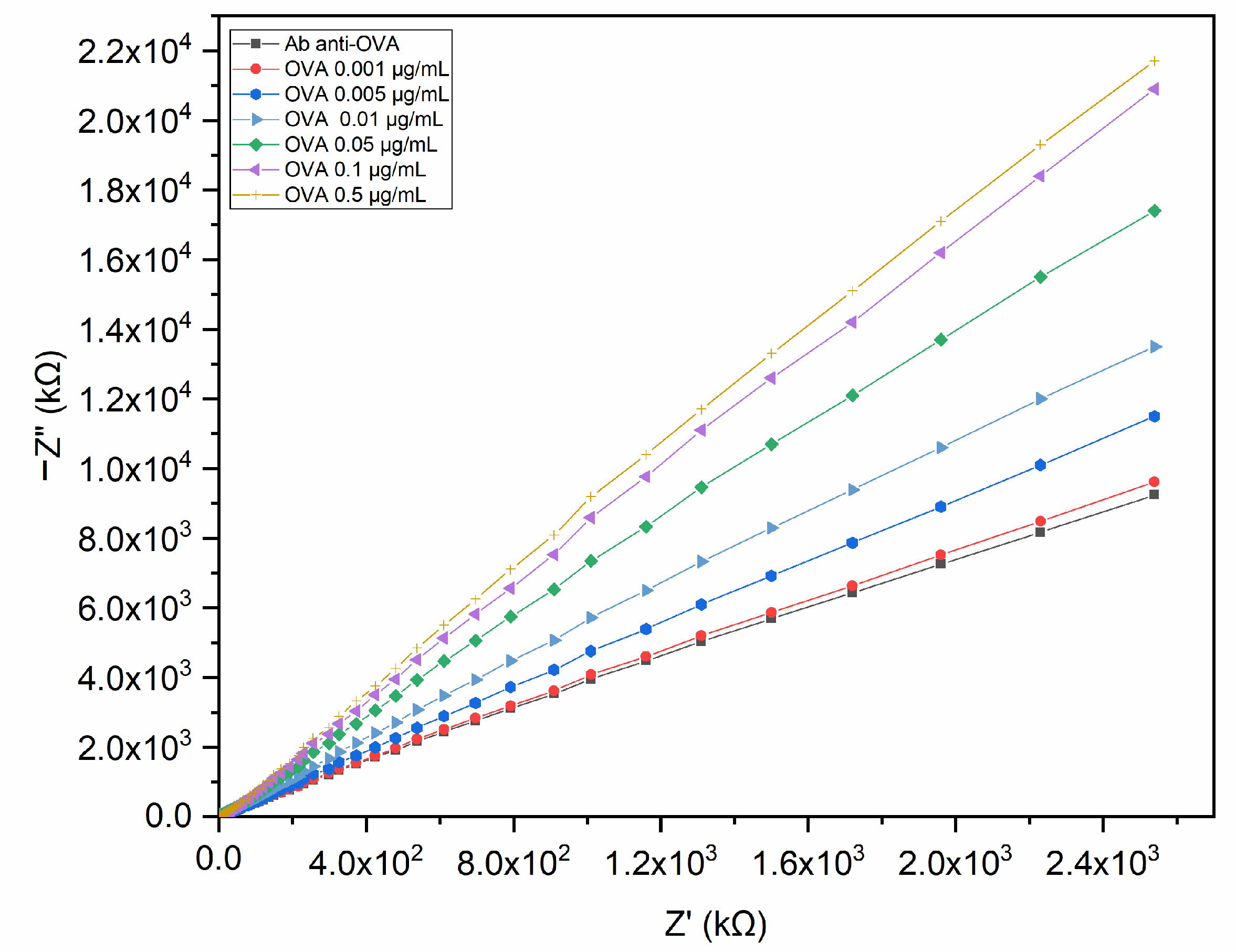
3.4. Electrochemical Characterisation of the Immunosensor Performance
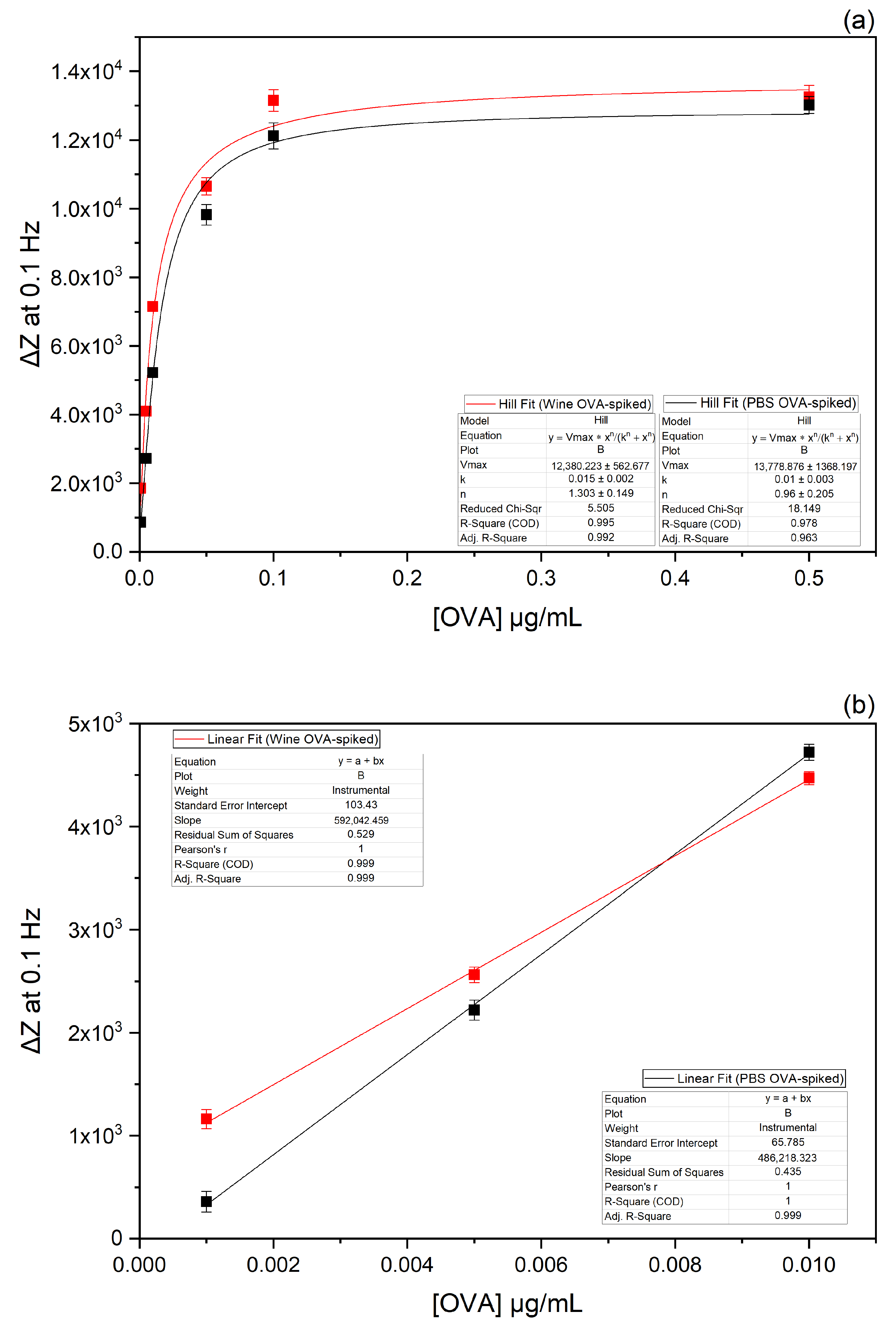
3.5. Electrochemical Characterisation of the Immunosensor Performance on Real Matrix
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mine, Y.; Yang, M. Recent advances in the understanding of egg allergens: Basic, industrial, and clinical perspectives. J. Agric. Food Chem. 2008, 56, 4874–4900. [Google Scholar] [CrossRef]
- Ghanem, C.; Taillandier, P.; Rizk, M.; Rizk, Z.; Nehme, N.; Souchard, J.P.; El Rayess, Y. Analysis of the impact of fining agents types, oenological tannins and mannoproteins and their concentrations on the phenolic composition of red wine. LWT Food Sci. Technol. 2017, 83, 101–109. [Google Scholar] [CrossRef]
- Weiss, K.; Bisson, L. Effect of Bentonite Treatment of Grape Juice on Yeast Fermentation. Am. J. Enol. Vitic. 2002, 53, 28–36. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the evaluation of allergenic foods and food ingredients for labelling purposes. EFSA J. 2014, 12, 3894. [Google Scholar] [CrossRef]
- Food and Drug Administration, U.S. Department of Health and Human Services. Egg Regulatory Program Standards (ERPS). Available online: https://www.fda.gov/federal-state-local-tribal-and-territorial-officials/regulatory-program-standards/egg-regulatory-program-standards-erps (accessed on 15 May 2023).
- European Commission. Commission Implementing Regulation (EU) no 579/2012. L171. Off. J. Eur. Union 2012, L171, 4–7. [Google Scholar]
- OIV. Criteria for the Quantification of Potentially Allergenic Residues of Fining Agent Proteins in Wine. OIV-OENO 427–2010 Modified by OIV–Comex 502–2012. 2012. Available online: https://www.oiv.int/public/medias/2154/oiv-comex-502-2012-en.pdf (accessed on 5 April 2023).
- Van Hengel, A.J. Food allergen detection methods and the challenge to protect food-allergic consumers. Anal. Bioanal. Chem. 2007, 389, 111–118. [Google Scholar] [CrossRef]
- Monaci, L.; Visconti, A. Mass spectrometry-based proteomics methods for analysis of food allergens. TrAC Trends Anal. Chem. 2009, 28, 581–591. [Google Scholar] [CrossRef]
- Johnson, P.E.; Baumgartner, S.; Aldick, T.; Bessant, C.; Giosafatto, V.; Heick, J.; Mamone, G.; O’Connor, G.; Poms, R.; Popping, B.; et al. Current perspectives and recommendations for the development of mass spectrometry methods for the determination of allergens in foods. J. AOAC Int. 2011, 94, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Cryar, A.; Pritchard, C.; Burkitt, W.; Walker, M.; O’Connor, G.; Burns, D.T.; Quaglia, M. Towards absolute quantification of allergenic proteins in food--lysozyme in wine as a model system for metrologically traceable mass spectrometric methods and certified reference materials. J. AOAC Int. 2013, 96, 1350–1361. [Google Scholar] [CrossRef]
- Monaci, L.; Pilolli, R.; De Angelis, E.; Godula, M.; Visconti, A. Multi-allergen detection in food by micro high-performance liquid chromatography coupled to a dual cell linear ion trap mass spectrometry. J. Chromatogr. A 2014, 1358, 136–144. [Google Scholar] [CrossRef]
- Shin, J.H.; Reddy, Y.V.M.; Park, T.J.; Park, J.P. Recent advances in analytical strategies and microsystems for food allergen detection. Food Chem. 2022, 371, 131120. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, C.; Hayat, A.; Mishra, R.; Vasilescu, A.; Del Valle, M.; Marty, J.-L. A novel electrochemical aptamer–antibody sandwich assay for lysozyme detection. Analyst 2015, 140, 4148–4153. [Google Scholar] [CrossRef]
- Eissa, S.; L’Hocine, L.; Siaj, M.; Zourob, M. A graphene-based label-free voltammetric immunosensor for sensitive detection of the egg allergen ovalbumin. Analyst 2013, 138, 4378–4384. [Google Scholar] [CrossRef] [PubMed]
- Pilolli, R.; Visconti, A.; Monaci, L. Rapid and label-free detection of egg allergen traces in wines by surface plasmon resonance biosensor. Anal. Bioanal. Chem. 2015, 407, 3787–3797. [Google Scholar] [CrossRef]
- Čadková, M.; Metelka, R.; Holubová, L.; Horák, D.; Dvořáková, V.; Bílková, Z.; Korecká, L. Magnetic beads-based electrochemical immunosensor for monitoring allergenic food proteins. Anal. Biochem. 2015, 484, 4–8. [Google Scholar] [CrossRef]
- Baldo, T.A.; Proença, C.D.A.; Felix, F.D.S.; Freitas, T.A.; Sakata, S.K.; Angnes, L.; Faria, R.C. Disposable electrochemical microfluidic device for ultrasensitive detection of egg allergen in wine samples. Talanta 2021, 232, 122447. [Google Scholar] [CrossRef] [PubMed]
- Titoiu, A.M.; Porumb, R.; Fanjul-Bolado, P.; Epure, P.; Zamfir, M.; Vasilescu, A. Detection of Allergenic Lysozyme during Winemaking with an Electrochemical Aptasensor. Electroanalysis 2019, 31, 2262–2273. [Google Scholar] [CrossRef]
- Daniels, J.S.; Pourmand, N. Label-Free Impedance Biosensors: Opportunities and Challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef]
- Qureshi, A.; Niazi, J.H.; Kallempudi, S.; Gurbuz, Y. Label-free capacitive biosensor for sensitive detection of multiple biomarkers using gold interdigitated capacitor arrays. Biosens. Bioelectron. 2010, 25, 2318–2323. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Varriale, A.; Bonnot, K.; Peransi, S.; Scala, A.; D’Auria, S. Self-oriented monolayer immobilization of ovalbumin and B. cereus antibody molecules on a chemically modified surface of silicon nitride fosters the enhancement of capture of bio-agents. Colloids Surf. B Biointerfaces 2016, 148, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Pennacchio, A.; Varriale, A.; Scala, A.; Marzullo, V.M.; Staiano, M.; D’Auria, S. A novel fluorescence polarization assay for determination of penicillin G in milk. Food Chem. 2016, 190, 381–385. [Google Scholar] [CrossRef]
- Capo, A.; Calabrese, A.; Frant, M.; Walczak, M.; Szczotka-Bochniarz, A.; Manessis, G.; Bossis, I.; Staiano, M.; D’Auria, S.; Varriale, A. SPR-Based Detection of ASF Virus in Cells. Int. J. Mol. Sci. 2022, 23, 7463. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Moore, E. Electrochemical immunosensor modified with self-assembled monolayer of 11-mercaptoundecanoic acid on gold electrodes for detection of benzo[a]pyrene in water. Analyst 2012, 137, 5839–5844. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochemist. Rev. 2008, 29 (Suppl. S1), S49–S52. [Google Scholar]
- Shrivastava, A. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive Biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Chen, D.; Li, J. Interfacial design and functionization on metal electrodes through self-assembled monolayers. Surf. Sci. Rep. 2006, 61, 445–463. [Google Scholar] [CrossRef]
- Tuttle, P.V.t.; Rundell, A.E.; Webster, T.J. Influence of biologically inspired nanometer surface roughness on antigen-antibody interactions for immunoassay-biosensor applications. Int. J. Nanomed. 2006, 1, 497–505. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G. Morphological Characteristics of Au Films Deposited on Ti: A Combined SEM-AFM Study. Coatings 2018, 8, 121. [Google Scholar] [CrossRef]
- Wang, S.; Esfahani, M.; Gurkan, U.A.; Inci, F.; Kuritzkes, D.R.; Demirci, U. Efficient on-chip isolation of HIV subtypes. Lab Chip 2012, 12, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Zorea, J.; Shukla, R.P.; Elkabets, M.; Ben-Yoav, H. Probing antibody surface density and analyte antigen incubation time as dominant parameters influencing the antibody-antigen recognition events of a non-faradaic and diffusion-restricted electrochemical immunosensor. Anal. Bioanal. Chem. 2020, 412, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Probing Biomolecular Interactions at Conductive and Semiconductive Surfaces by Impedance Spectroscopy: Routes to Impedimetric Immunosensors, DNA-Sensors, and Enzyme Biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]

| Sensing Substrate | Technique | Linear Range | LoD | Assay Time (min) | Number of Steps | References |
|---|---|---|---|---|---|---|
| GO/screen-printed carbon | Differential pulse voltammetry | 1 pg/mL–0.5 μg/mL | 0.83 pg/mL | 60 | 5 | Eissa et al. (2013) [15] |
| Dextran-coated sensor chips (CM5) | Surface plasmon resonance (SPR) | 0.03–0.2 μg/mL | 0.6 μg/mL | – | 4 | Pilolli et al. (2015) [16] |
| Screen-printed platinum | Linear sweep voltammetry | 0.5–9.5 μg/mL | 0.2 μg/mL | 60 | 4 | Čadková et al. (2015) [17] |
| Graphene/screen-printed carbon electrodes | Amperometry | 0.01–10 pg/mL | 0.2 fg/mL | 60 | 6 | Baldo et al. (2021) [18] |
| Thin-film gold single electrodes | Non-Faradaic impedance spectroscopy | 0.001–0.01 μg/mL | 0.2 µg/mL | 15 | 4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, A.; Capo, A.; Capaccio, A.; Agovino, E.; Varriale, A.; Pascale, M.; D’Auria, S.; Staiano, M. An Impedance-Based Immunosensor for the Detection of Ovalbumin in White Wine. Biosensors 2023, 13, 669. https://doi.org/10.3390/bios13070669
Calabrese A, Capo A, Capaccio A, Agovino E, Varriale A, Pascale M, D’Auria S, Staiano M. An Impedance-Based Immunosensor for the Detection of Ovalbumin in White Wine. Biosensors. 2023; 13(7):669. https://doi.org/10.3390/bios13070669
Chicago/Turabian StyleCalabrese, Alessia, Alessandro Capo, Angela Capaccio, Elettra Agovino, Antonio Varriale, Michelangelo Pascale, Sabato D’Auria, and Maria Staiano. 2023. "An Impedance-Based Immunosensor for the Detection of Ovalbumin in White Wine" Biosensors 13, no. 7: 669. https://doi.org/10.3390/bios13070669
APA StyleCalabrese, A., Capo, A., Capaccio, A., Agovino, E., Varriale, A., Pascale, M., D’Auria, S., & Staiano, M. (2023). An Impedance-Based Immunosensor for the Detection of Ovalbumin in White Wine. Biosensors, 13(7), 669. https://doi.org/10.3390/bios13070669









