Flexible and Wearable Biosensors for Monitoring Health Conditions
Abstract
1. Introduction
2. Detection Objects
2.1. Saliva
2.2. Sweat
2.3. Tears
2.4. Interstitial Fluids (ISFs)
2.5. Respiration
2.6. Physiological Pressure
3. Preparation Methods
3.1. Surface Processing
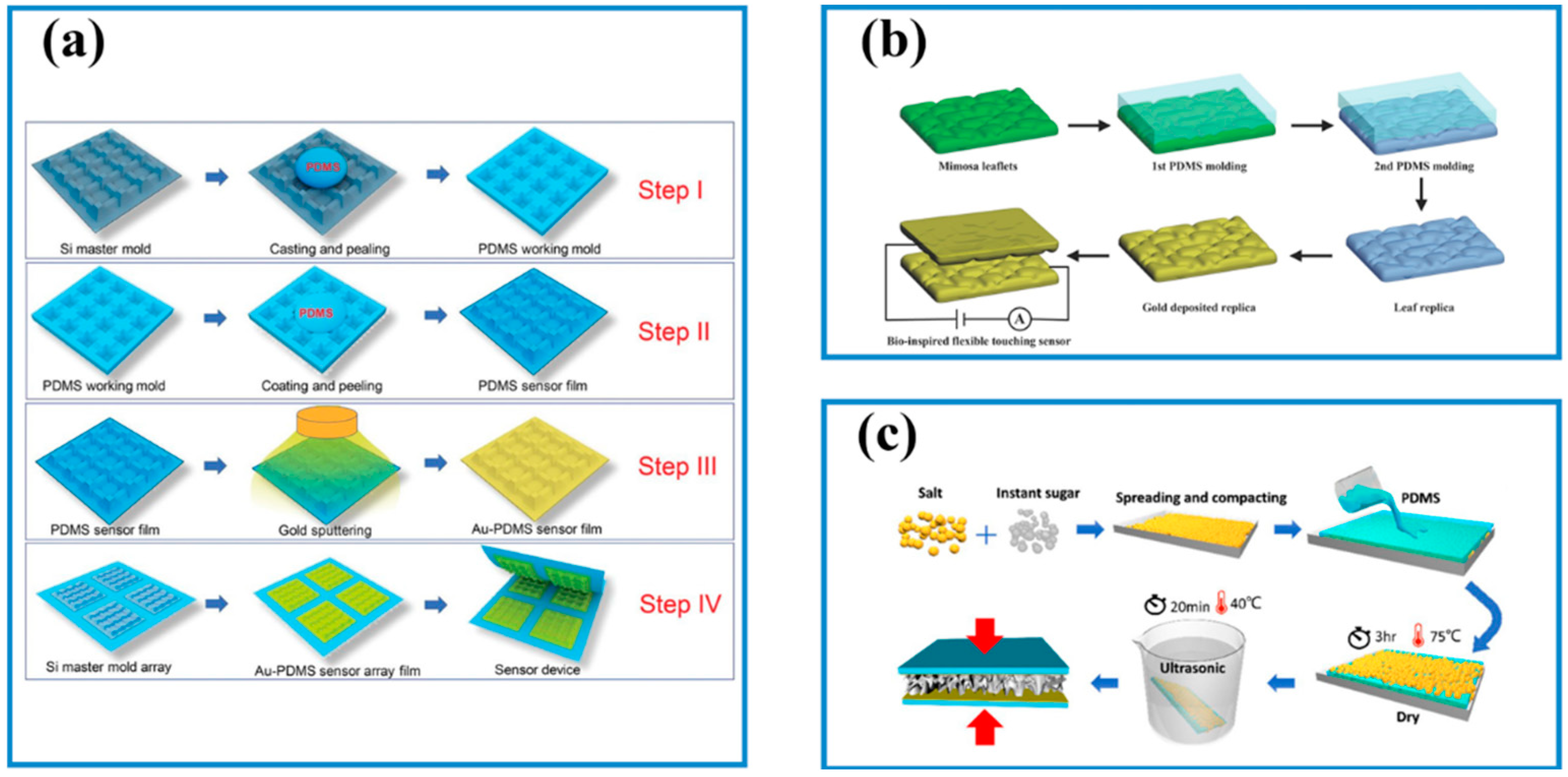
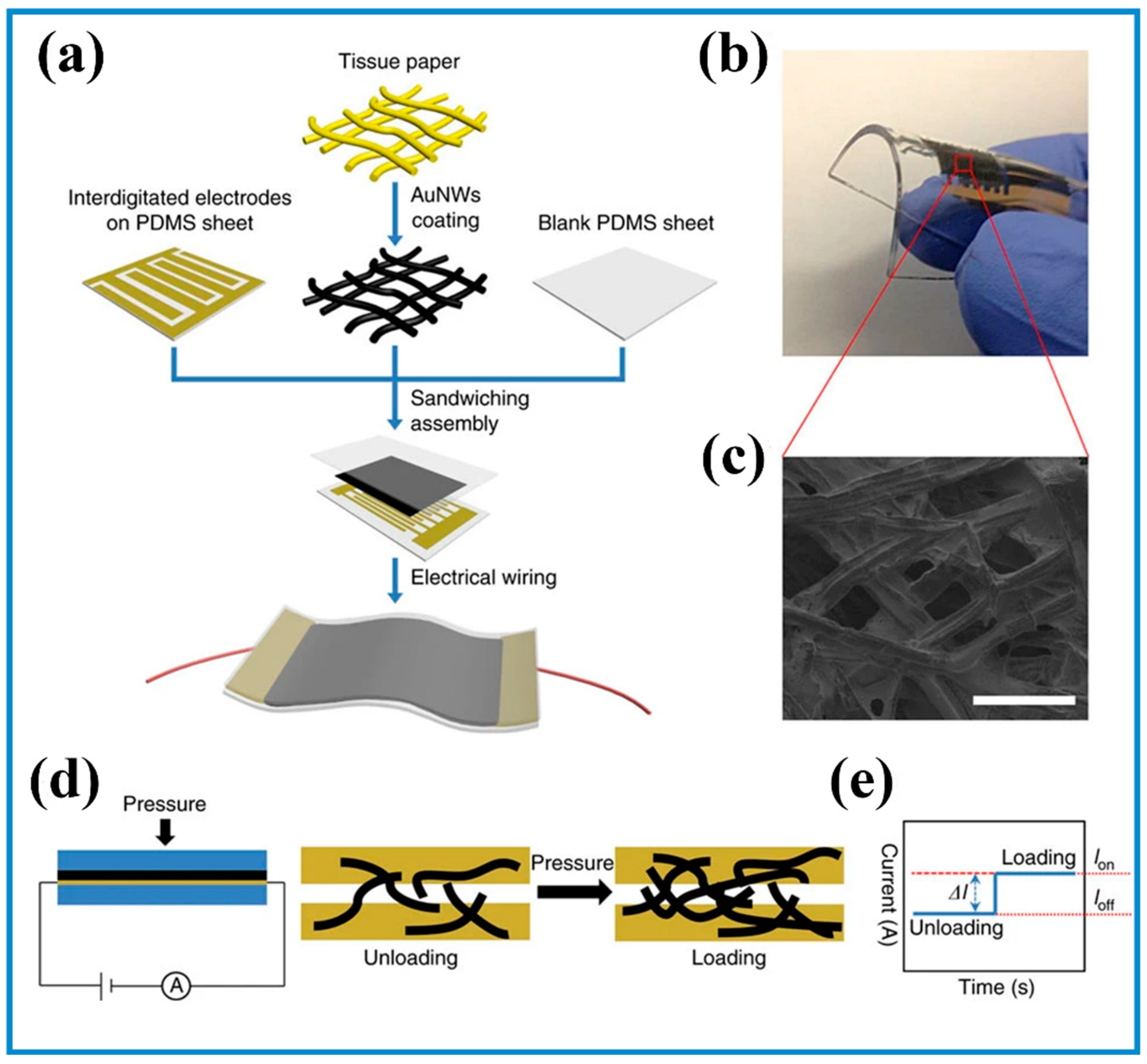
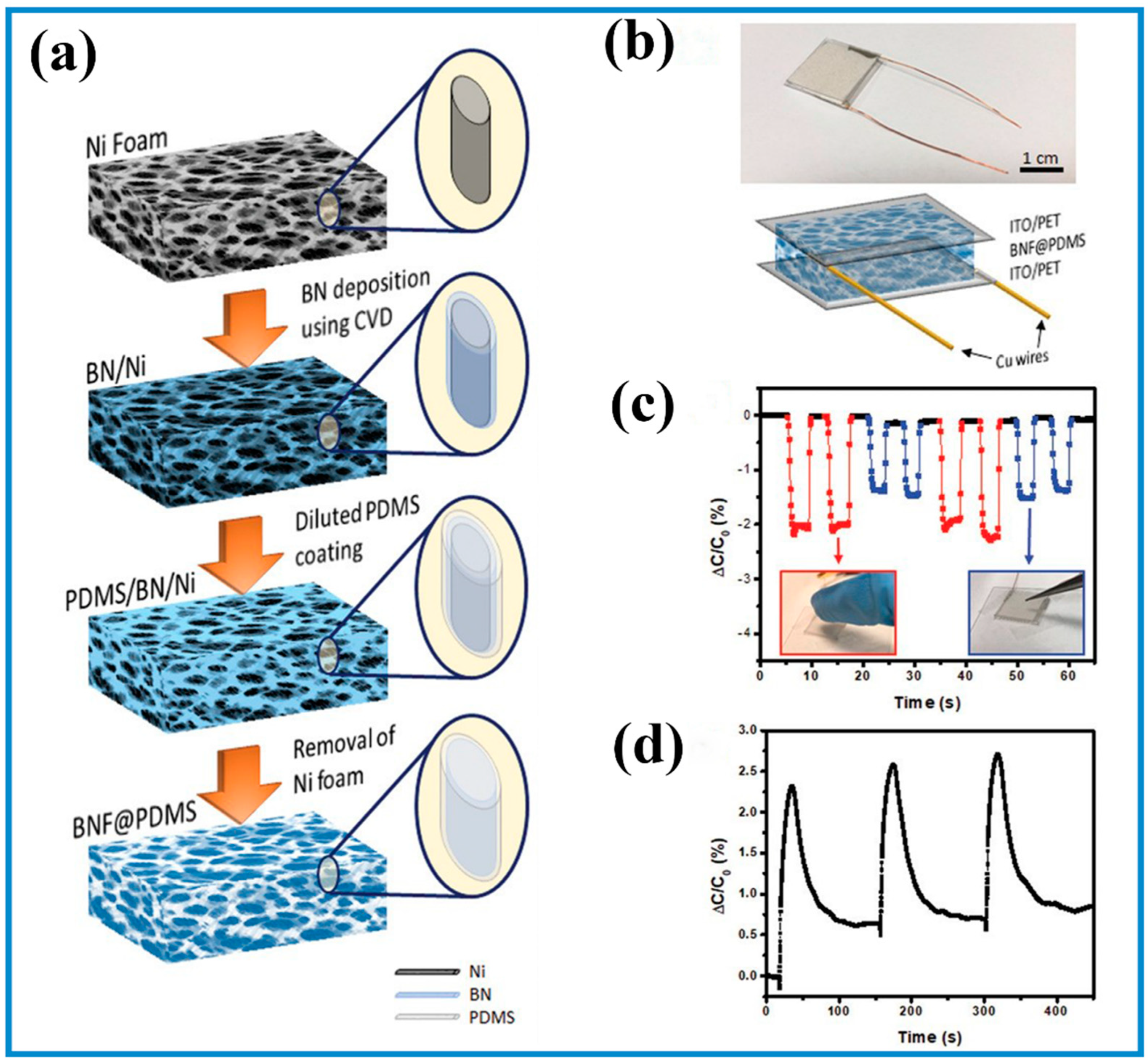
3.2. Micromanufacturing Technologies
4. Application Manners
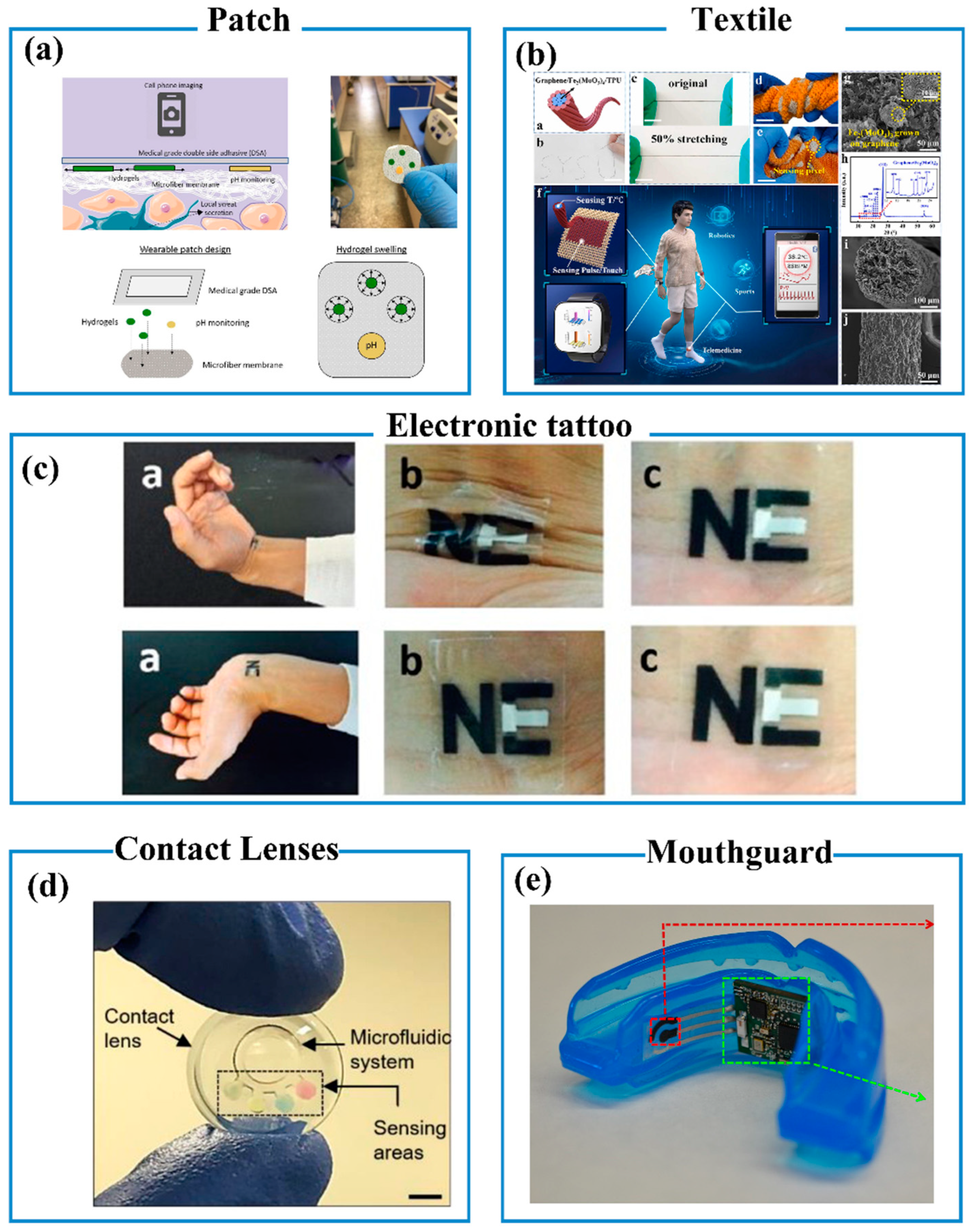
4.1. Patch
4.2. Textile
4.3. Wearable Tattoo Sensors
4.4. Microneedles-Based Sensors
4.5. Contact Lenses Sensors
4.6. Mouthguard Sensors
5. Signal Processing
6. Wearable Biosensors
6.1. Physiological Pressure Sensors
6.2. Wearable Sweat Sensors
6.2.1. Sweat Extraction Strategies
6.2.2. Common Sweat-Based Wearable Sensor
- pH and Ion Detection
- Glucose Detection
- Zn2+ Detection
- Urea Detection
- Levodopa Detection
- Vitamin C (VC) Detection
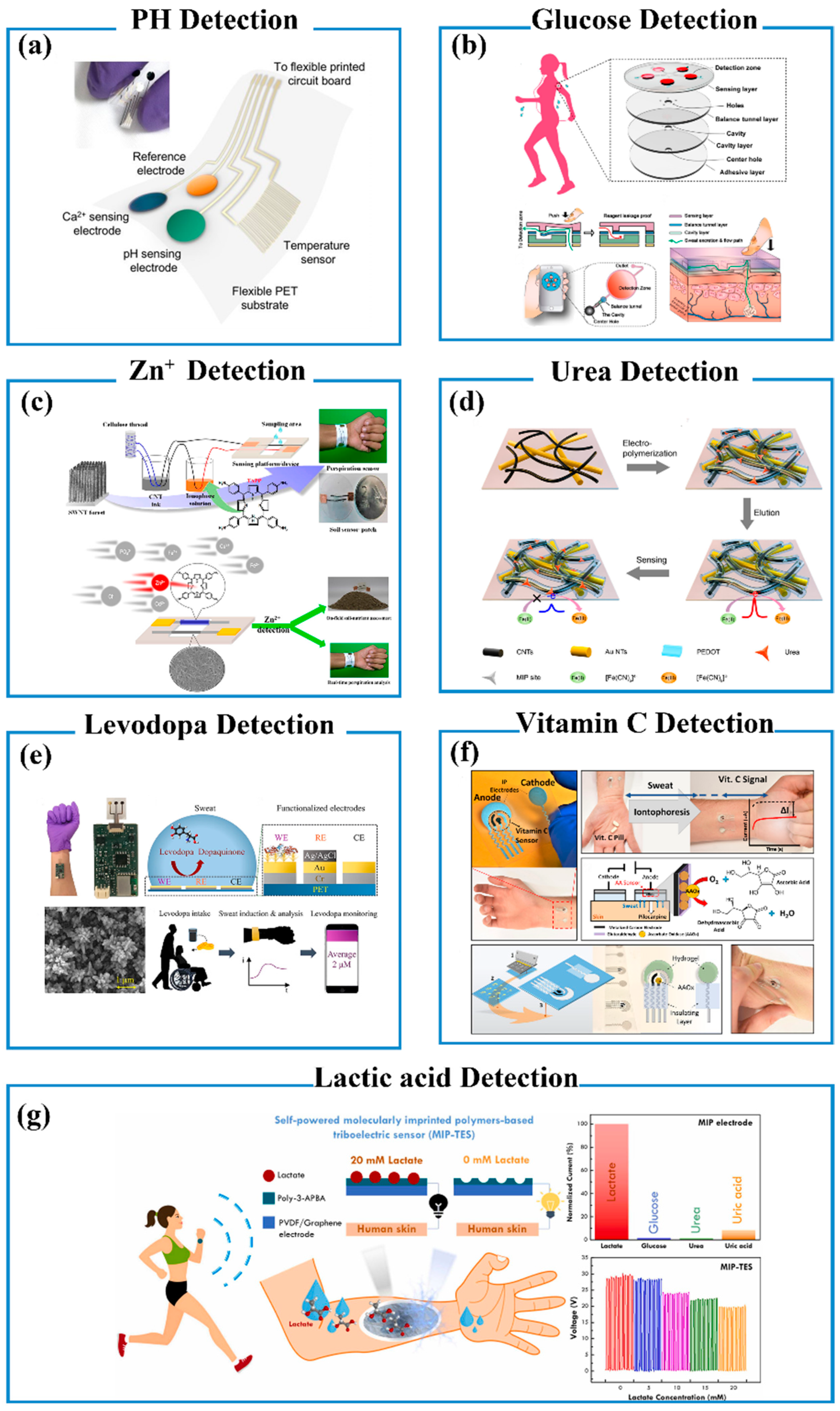
- Lactic Acid Detection
6.2.3. Problems That Occur When Analyzing Sweat
6.3. Wearable Self-Powered Biosensors
6.3.1. Piezoelectric Nanogenerators (PENGs)
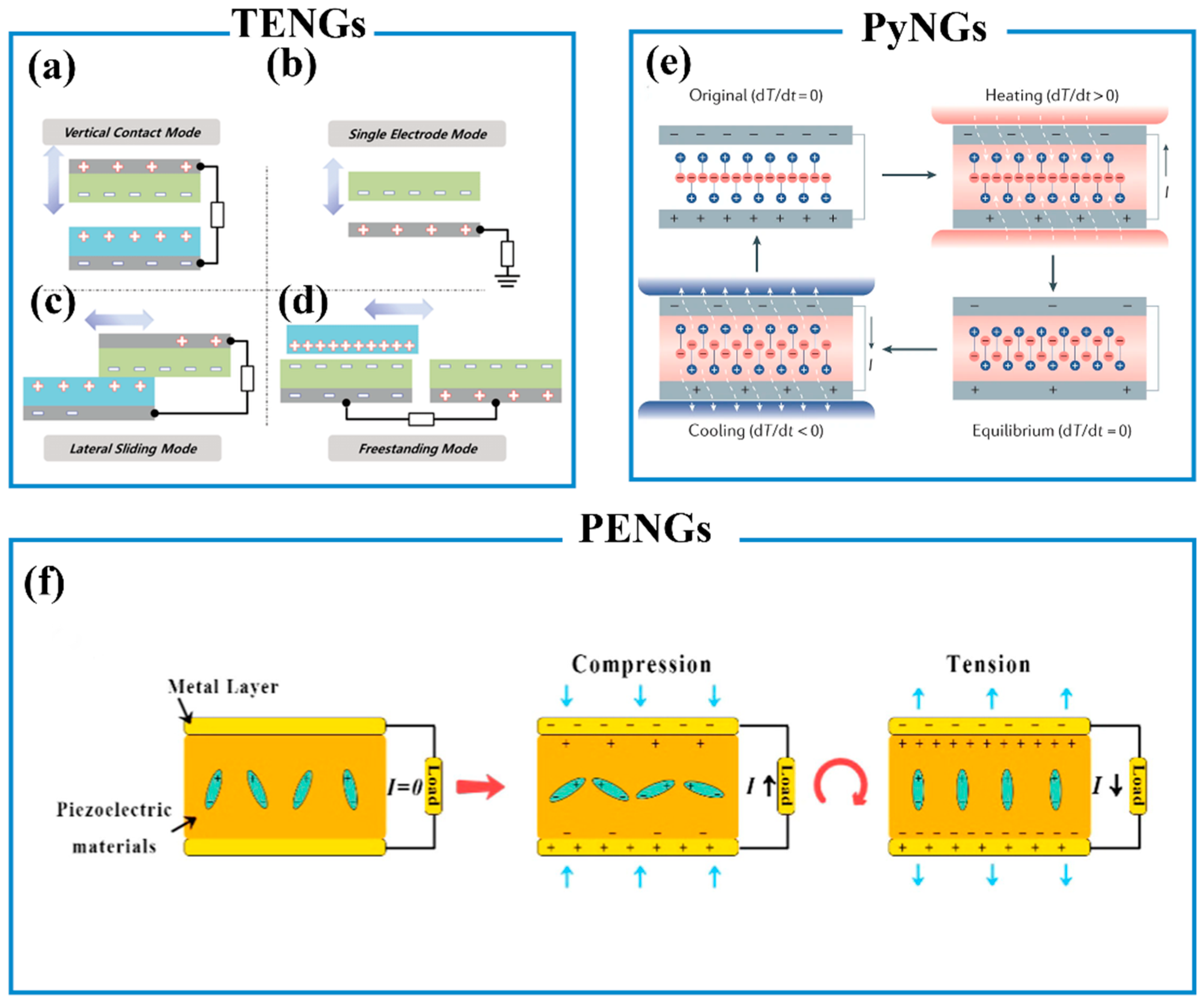
6.3.2. Triboelectric Nanogenerators (TENGs)
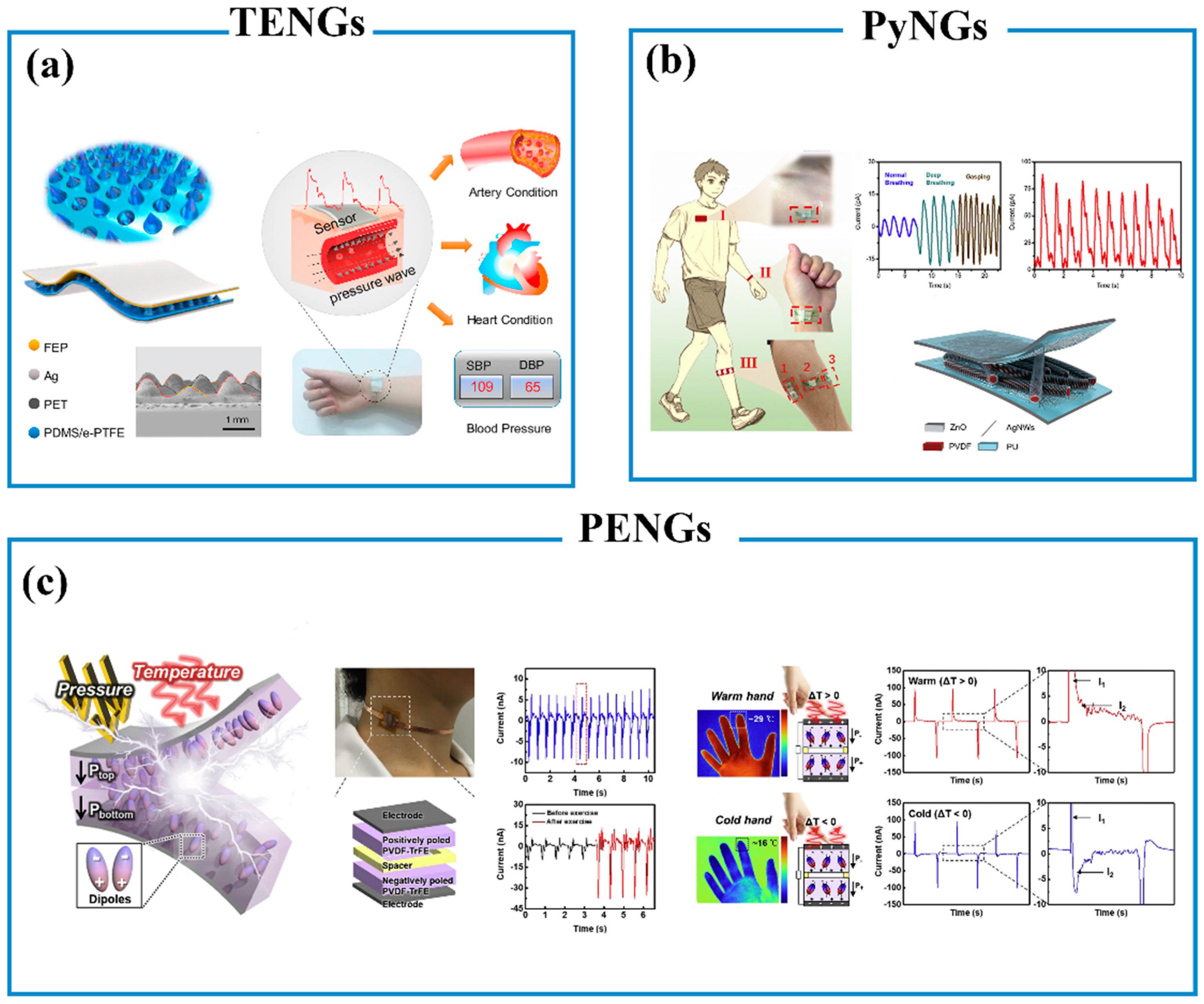
6.3.3. Pyroelectric Nanogenerators (PyNGs)
6.3.4. Biofuel Cell
7. Summary and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Gao, W. Wearable and Flexible Electronics for Continuous Molecular Monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Tang, Q.; Wang, Z.L.; Li, Z. Self-Powered Cardiovascular Electronic Devices and Systems. Nat. Rev. Cardiol. 2021, 18, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yu, L.; Yeo, J.C.; Lim, C.T. Flexible Hybrid Sensors for Health Monitoring: Materials and Mechanisms to Render Wearability. Adv. Mater. 2020, 32, 1902133. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, Z.; Park, S. Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics. Adv. Mater. 2019, 31, 1904765. [Google Scholar] [CrossRef]
- Chen, X.; Luo, F.; Yuan, M.; Xie, D.; Shen, L.; Zheng, K.; Wang, Z.; Li, X.; Tao, L. A Dual-Functional Graphene-Based Self-Alarm Health-Monitoring E-Skin. Adv. Funct. Mater. 2019, 29, 1904706. [Google Scholar] [CrossRef]
- Lin, W.; Wang, B.; Peng, G.; Shan, Y.; Hu, H.; Yang, Z. Skin-Inspired Piezoelectric Tactile Sensor Array with Crosstalk-Free Row+Column Electrodes for Spatiotemporally Distinguishing Diverse Stimuli. Adv. Sci. 2021, 8, 2002817. [Google Scholar] [CrossRef]
- Tai, L.; Gao, W.; Chao, M.; Bariya, M.; Ngo, Q.P.; Shahpar, Z.; Nyein, H.Y.Y.; Park, H.; Sun, J.; Jung, Y.; et al. Methylxanthine Drug Monitoring with Wearable Sweat Sensors. Adv. Mater. 2018, 30, 1707442. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Bae, G.Y.; Han, J.T.; Lee, G.; Lee, S.; Kim, S.W.; Park, S.; Kwon, J.; Jung, S.; Cho, K. Pressure/Temperature Sensing Bimodal Electronic Skin with Stimulus Discriminability and Linear Sensitivity. Adv. Mater. 2018, 30, 1803388. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical Bioadhesive Interface for Bioelectronics. Nat. Mater. 2021, 20, 229–236. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, H.; Shi, B.; Xue, X.; Liu, Z.; Jin, Y.; Ma, Y.; Zou, Y.; Wang, X.; An, Z.; et al. In Vivo Self-Powered Wireless Cardiac Monitoring via Implantable Triboelectric Nanogenerator. ACS Nano 2016, 10, 6510–6518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Nyein, H.Y.Y.; Hou, L.; Lin, Y.; Bariya, M.; Ahn, C.H.; Ji, W.; Fan, Z.; Javey, A. A Wearable Nutrition Tracker. Adv. Mater. 2021, 33, 2006444. [Google Scholar] [CrossRef] [PubMed]
- Parvez Mahmud, M.A.; Huda, N.; Farjana, S.H.; Asadnia, M.; Lang, C. Recent Advances in Nanogenerator-Driven Self-Powered Implantable Biomedical Devices. Adv. Energy Mater. 2018, 8, 1701210. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Barbee, M.H.; Cho, S.; Cho, S.; Shanker, R.; Kim, J.; Myoung, J.; Kim, M.P.; Baig, C.; et al. A Hierarchical Nanoparticle-in-Micropore Architecture for Enhanced Mechanosensitivity and Stretchability in Mechanochromic Electronic Skins. Adv. Mater. 2019, 31, 1808148. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qiao, X.; Tao, R.; Li, Y.; Zhao, S.; Cai, Y.; Luo, X. A Wearable Sensor Based on Multifunctional Conductive Hydrogel for Simultaneous Accurate pH and Tyrosine Monitoring in Sweat. Biosens. Bioelectron. 2023, 234, 115360. [Google Scholar] [CrossRef]
- Nishiyama, K.; Mizukami, R.; Kuki, S.; Ishida, A.; Chida, J.; Kido, H.; Maeki, M.; Tani, H.; Tokeshi, M. Electrochemical Enzyme-Based Blood ATP and Lactate Sensor for a Rapid and Straightforward Evaluation of Illness Severity. Biosens. Bioelectron. 2022, 198, 113832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yan, H.H.; Ru, C.; Zhu, F.; Zou, H.Y.; Gao, P.F.; Huang, C.Z.; Wang, J. Plasmonic Biosensor for the Highly Sensitive Detection of MicroRNA-21 via the Chemical Etching of Gold Nanorods under a Dark-Field Microscope. Biosens. Bioelectron. 2022, 201, 113942. [Google Scholar] [CrossRef]
- Bian, R.; Meng, L.; Guo, C.; Tang, Z.; Liu, H. A Facile One-Step Approach for Constructing Multidimensional Ordered Nanowire Micropatterns via Fibrous Elastocapillary Coalescence. Adv. Mater. 2019, 31, 1900534. [Google Scholar] [CrossRef]
- Jeong, C.; Lee, J.S.; Park, B.; Hong, C.S.; Kim, J.U.; Kim, T. Controllable Configuration of Sensing Band in a Pressure Sensor by Lenticular Pattern Deformation on Designated Electrodes. Adv. Mater. 2019, 31, 1902689. [Google Scholar] [CrossRef]
- Sharma, S.; Chhetry, A.; Zhang, S.; Yoon, H.; Park, C.; Kim, H.; Sharifuzzaman, M.; Hui, X.; Park, J.Y. Hydrogen-Bond-Triggered Hybrid Nanofibrous Membrane-Based Wearable Pressure Sensor with Ultrahigh Sensitivity over a Broad Pressure Range. ACS Nano 2021, 15, 4380–4393. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Zhai, W.; Zheng, G.; Ji, Y.; Dai, K.; Mi, L.; Zhang, D.; Liu, C.; Shen, C. Multifunctional Interlocked E-Skin Based on Elastic Micropattern Array Facilely Prepared by Hot-Air-Gun. Chem. Eng. J. 2021, 407, 127960. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable Flexible Sweat Sensors for Healthcare Monitoring: A Review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Bariya, M.; Javey, A. Wearable Biosensors for Body Computing. Adv. Funct. Mater. 2021, 31, 2008087. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.C.; Suryadevara, N.K.; Nag, A. Wearable Sensors for Healthcare: Fabrication to Application. Sensors 2022, 22, 5137. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-End Design of Wearable Sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Lima, D.P.; Diniz, D.G.; Moimaz, S.A.S.; Sumida, D.H.; Okamoto, A.C. Saliva: Reflection of the Body. Int. J. Infect. Dis. 2010, 14, e184–e188. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Liu, Y.; Qiao, Y.; Xia, H.; Zhou, J. Oral wearable sensors: Health management based on the oral cavity. Biosens. Bioelectron. X 2022, 10, 100135. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, F.; Wang, K.; Zhang, W.; Li, Y.; Sun, Y.; Sun, X.; Li, C.; Dong, B.; Wang, L.; et al. Smart Biosensors and Intelligent Devices for Salivary Biomarker Detection. Trends Anal. Chem. 2021, 140, 116281. [Google Scholar] [CrossRef]
- de Castro, L.F.; de Freitas, S.V.; Duarte, L.C.; de Souza, J.A.C.; Paixão, T.R.L.C.; Coltro, W.K.T. Salivary Diagnostics on Paper Microfluidic Devices and Their Use as Wearable Sensors for Glucose Monitoring. Anal. Bioanal. Chem. 2019, 411, 4919–4928. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Cheng, Y.; Huang, W.; Xiang, L. Electrochemical Biosensors Based on Saliva Electrolytes for Rapid Detection and Diagnosis. J. Mater. Chem. B 2023, 11, 33–54. [Google Scholar] [CrossRef]
- Carrero-Ferrer, I.; Molins-Legua, C.; Campíns-Falcó, P. Plasmonic Sensor for Hydrogen Sulphide in Saliva: Multisensor Platform and Bag Format. Talanta 2022, 245, 123449. [Google Scholar] [CrossRef]
- Wang, L.; Nie, Y.; Wang, P.; Li, Y.; Ma, Q.; Yu, D. A Novel Bacterial Imprinted Polymers-Electrochemiluminescent Sensor for Lactobacillus salivarius Detection. Sens. Actuators B Chem. 2022, 358, 131467. [Google Scholar] [CrossRef]
- Clingan, C.A.; Dittakavi, M.; Rozwadowski, M.; Gilley, K.N.; Cislo, C.R.; Barabas, J.; Sandford, E.; Olesnavich, M.; Flora, C.; Tyler, J.; et al. Monitoring Health Care Workers at Risk for COVID-19 Using Wearable Sensors and Smartphone Technology: Protocol for an Observational MHealth Study. JMIR Res. Protoc. 2021, 10, e29562. [Google Scholar] [CrossRef]
- Moon, J.M.; Del Caño, R.; Moonla, C.; Sakdaphetsiri, K.; Saha, T.; Francine Mendes, L.; Yin, L.; Chang, A.-Y.; Seker, S.; Wang, J. Self-Testing of Ketone Bodies, along with Glucose, Using Touch-Based Sweat Analysis. ACS Sens. 2022, 7, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Fiore, L.; Mazzaracchio, V.; Serani, A.; Fabiani, G.; Fabiani, L.; Volpe, G.; Moscone, D.; Bianco, G.M.; Occhiuzzi, C.; Marrocco, G.; et al. Microfluidic Paper-Based Wearable Electrochemical Biosensor for Reliable Cortisol Detection in Sweat. Sens. Actuators B Chem. 2023, 379, 133258. [Google Scholar] [CrossRef]
- Huangfu, X.; Guo, Y.; Mugo, S.M.; Zhang, Q. Hydrovoltaic Nanogenerators for Self-Powered Sweat Electrolyte Analysis. Small 2023, 19, 2207134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, G.; Mu, W.; Wan, X.; Lu, D.; Gao, J.; Wen, D. Nonenzymatic Sweat Wearable Uric Acid Sensor Based on N-Doped Reduced Graphene Oxide/Au Dual Aerogels. Anal. Chem. 2023, 95, 3864–3872. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, R.; Rogers, J.A.; Ray, T.R. Recent Progress, Challenges, and Opportunities for Wearable Biochemical Sensors for Sweat Analysis. Sens. Actuators B Chem. 2021, 332, 129447. [Google Scholar] [CrossRef]
- Ramachandran, B.; Liao, Y.C. Microfluidic Wearable Electrochemical Sweat Sensors for Health Monitoring. Biomicrofluidics 2022, 16, 051501. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, D.S.; Wang, S.; Luan, H.; Sekine, Y.; Model, J.B.; Aranyosi, A.J.; Ghaffari, R.; Rogers, J.A. Soft, Environmentally Degradable Microfluidic Devices for Measurement of Sweat Rate and Total Sweat Loss and for Colorimetric Analysis of Sweat Biomarkers. EcoMat 2023, 5, e12270. [Google Scholar] [CrossRef]
- Hagan, S.; Martin, E.; Enríquez-de-Salamanca, A. Tear Fluid Biomarkers in Ocular and Systemic Disease: Potential Use for Predictive, Preventive and Personalised Medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, N.; Bikkannavar, P.; Cordeiro, M.F.; Yetisen, A.K. Ophthalmic Sensing Technologies for Ocular Disease Diagnostics. Analyst 2021, 146, 6416–6444. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Cho, Y.C.; Koh, W.G.; Choy, Y.B. Preocular Sensor System for Concurrent Monitoring of Glucose Levels and Dry Eye Syndrome Using Tear Fluids. PLoS ONE 2020, 15, e0239317. [Google Scholar] [CrossRef]
- Friedel, M.; Thompson, I.A.P.; Kasting, G.; Polsky, R.; Cunningham, D.; Soh, H.T.; Heikenfeld, J. Opportunities and Challenges in the Diagnostic Utility of Dermal Interstitial Fluid. Nat. Biomed. Eng. 2023, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Dervisevic, E.; Esser, L.; Easton, C.D.; Cadarso, V.J.; Voelcker, N.H. Wearable Microneedle Array-Based Sensor for Transdermal Monitoring of pH Levels in Interstitial Fluid. Biosens. Bioelectron. 2023, 222, 114955. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, J.; Seth, A.; Liu, L.; You, M.; Gupta, P.; Rathi, P.; Wang, Y.; Cao, S.; Jiang, Q.; et al. Microneedle Patch for the Ultrasensitive Quantification of Protein Biomarkers in Interstitial Fluid. Nat. Biomed. Eng. 2021, 5, 64–76. [Google Scholar] [CrossRef]
- Taylor, R.M.; Baca, J.T. Feasibility of Interstitial Fluid Ketone Monitoring with Microneedles. Metabolites 2022, 12, 424. [Google Scholar] [CrossRef]
- Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Tangkuaram, T.; Mercier, P.P.; Dassau, E.; Wang, J. Microneedle-Based Detection of Ketone Bodies along with Glucose and Lactate: Toward Real-Time Continuous Interstitial Fluid Monitoring of Diabetic Ketosis and Ketoacidosis. Anal. Chem. 2020, 92, 2291–2300. [Google Scholar] [CrossRef]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A Review of the Volatiles from the Healthy Human Body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Saalberg, Y.; Wolff, M. VOC Breath Biomarkers in Lung Cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.T.; Tran, M.T.; Feller, J.F.; Castro, M.; Van Ngo, T.; Hassan, K.; Nine, M.J.; Losic, D. Graphene and Metal Organic Frameworks (MOFs) Hybridization for Tunable Chemoresistive Sensors for Detection of Volatile Organic Compounds (VOCs) Biomarkers. Carbon 2020, 159, 333–344. [Google Scholar] [CrossRef]
- Meng, K.; Xiao, X.; Wei, W.; Chen, G.; Nashalian, A.; Shen, S.; Xiao, X.; Chen, J. Wearable Pressure Sensors for Pulse Wave Monitoring. Adv. Mater. 2022, 34, 2109357. [Google Scholar] [CrossRef]
- Feng, T.; Chen, X.; Geng, J.; Nie, B. A Portable Applanation Tonometer for Accurate Intraocular Pressure Measurements. Sens. Actuators A Phys. 2022, 344, 113708. [Google Scholar] [CrossRef]
- Liu, C.; Kim, J.; Kwak, S.S.; Hourlier-Fargette, A.; Avila, R.; Vogl, J.; Tzavelis, A.; Chung, H.U.; Lee, J.Y.; Kim, D.H.; et al. Wireless, Skin-Interfaced Devices for Pediatric Critical Care: Application to Continuous, Noninvasive Blood Pressure Monitoring. Adv. Healthc. Mater. 2021, 10, 2100383. [Google Scholar] [CrossRef]
- Xu, F.; Li, X.; Shi, Y.; Li, L.; Wang, W.; He, L.; Liu, R. Recent Developments for Flexible Pressure Sensors: A Review. Micromachines 2018, 9, 580. [Google Scholar] [CrossRef]
- An, B.W.; Shin, J.H.; Kim, S.Y.; Kim, J.; Ji, S.; Park, J.; Lee, Y.; Jang, J.; Park, Y.G.; Cho, E.; et al. Smart Sensor Systems for Wearable Electronic Devices. Polymers 2017, 9, 303. [Google Scholar] [CrossRef]
- Cui, X.; Jiang, Y.; Hu, L.; Cao, M.; Xie, H.; Zhang, X.; Huang, F.; Xu, Z.; Zhu, Y. Synergistically Microstructured Flexible Pressure Sensors with High Sensitivity and Ultrawide Linear Range for Full-Range Human Physiological Monitoring. Adv. Mater.Technol. 2023, 8, 2200609. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Xu, M.; Dai, F.; Li, Z. Flat Silk Cocoon Pressure Sensor Based on a Sea Urchin-like Microstructure for Intelligent Sensing. ACS Sustain. Chem. Eng. 2022, 10, 17252–17260. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lasalde-Ramírez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable Chemical Sensors for Biomarker Discovery in the Omics Era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, C.; DeMas-Giménez, G.; Royo, S. Overview of Biofluids and Flow Sensing Techniques Applied in Clinical Practice. Sensors 2022, 22, 6836. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, H.; Li, J.; Bandodkar, A.J.; Rogers, J.A. Body-Interfaced Chemical Sensors for Noninvasive Monitoring and Analysis of Biofluids. Trends Chem. 2019, 1, 559–571. [Google Scholar] [CrossRef]
- Young, T.; Clark, V.; Arroyo-Currás, N.; Heikenfeld, J. Perspective—The Feasibility of Continuous Protein Monitoring in Interstitial Fluid. ECS Sens. Plus 2023, 2, 027001. [Google Scholar] [CrossRef] [PubMed]
- Sarcan, F. ZnO Nanoparticles-Based Vacuum Pressure Sensor. Nanotechnology 2020, 31, 435502. [Google Scholar] [CrossRef]
- Offenzeller, C.; Knoll, M.; Jakoby, B.; Hilber, W. Embedded, Fully Spray-Coated Pressure Sensor Using a Capacitive Transducing Mechanism. Polymers 2018, 10, 852. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Hou, J.; Li, Y.; Li, M.; Song, Y. Bioinspired Micropatterned Superhydrophilic Au-Areoles for Surface-Enhanced Raman Scattering (SERS) Trace Detection. Adv. Funct. Mater. 2018, 28, 1800448. [Google Scholar] [CrossRef]
- Park, H.; Masud, M.K.; Na, J.; Lim, H.; Phan, H.P.; Kaneti, Y.V.; Alothman, A.A.; Salomon, C.; Nguyen, N.T.; Hossain, M.S.A.; et al. Mesoporous Gold–Silver Alloy Films towards Amplification-Free Ultra-Sensitive MicroRNA Detection. J. Mater. Chem. B 2020, 8, 9512–9523. [Google Scholar] [CrossRef]
- Wang, Q.; Ling, S.; Liang, X.; Wang, H.; Lu, H.; Zhang, Y. Self-Healable Multifunctional Electronic Tattoos Based on Silk and Graphene. Adv. Funct. Mater. 2019, 29, 1808695. [Google Scholar] [CrossRef]
- Tay, R.Y.; Li, H.; Lin, J.; Wang, H.; Lim, J.S.K.; Chen, S.; Leong, W.L.; Tsang, S.H.; Teo, E.H.T. Lightweight, Superelastic Boron Nitride/Polydimethylsiloxane Foam as Air Dielectric Substitute for Multifunctional Capacitive Sensor Applications. Adv. Funct. Mater. 2020, 30, 1909604. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, J.; Tolle, C.R.; Zhu, Z. Flexible and Compressible PEDOT:PSS@Melamine Conductive Sponge Prepared via One-Step Dip Coating as Piezoresistive Pressure Sensor for Human Motion Detection. ACS Appl. Mater. Interfaces 2018, 10, 16077–16086. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, N.; Liu, W.; Li, M.; Ma, Y.; Luo, C.; Wang, S.; Rao, J.; Hu, X.; Su, J.; et al. 3D Hybrid Porous Mxene-Sponge Network and Its Application in Piezoresistive Sensor. Nano Energy 2018, 50, 79–87. [Google Scholar] [CrossRef]
- Li, T.; Chen, L.; Yang, X.; Chen, X.; Zhang, Z.; Zhao, T.; Li, X.; Zhang, J. A Flexible Pressure Sensor Based on an MXene–Textile Network Structure. J. Mater. Chem. C 2019, 7, 1022–1027. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, H.; Yue, W.; Gao, S.; Kan, H.; Zhang, C.; Zhang, C.; Pang, J.; Lou, Z.; Wang, L.; et al. Micro-Nano Processing of Active Layers in Flexible Tactile Sensors via Template Methods: A Review. Small 2021, 17, 2100804. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, D.; Li, C.; Liu, W.; Liu, H. Engineered Microstructure Derived Hierarchical Deformation of Flexible Pressure Sensor Induces a Supersensitive Piezoresistive Property in Broad Pressure Range. Adv. Sci. 2020, 7, 2000154. [Google Scholar] [CrossRef]
- Su, B.; Gong, S.; Ma, Z.; Yap, L.W.; Cheng, W. Mimosa-Inspired Design of a Flexible Pressure Sensor with Touch Sensitivity. Small 2015, 11, 1886–1891. [Google Scholar] [CrossRef]
- Wang, J.; Suzuki, R.; Shao, M.; Gillot, F.; Shiratori, S. Capacitive Pressure Sensor with Wide-Range, Bendable, and High Sensitivity Based on the Bionic Komochi Konbu Structure and Cu/Ni Nanofiber Network. ACS Appl. Mater. Interfaces 2019, 11, 11928–11935. [Google Scholar] [CrossRef]
- Song, Y.; Chen, H.; Su, Z.; Chen, X.; Miao, L.; Zhang, J.; Cheng, X.; Zhang, H. Highly Compressible Integrated Supercapacitor-Piezoresistance-Sensor System with CNT-PDMS Sponge for Health Monitoring. Small 2017, 13, 1702091. [Google Scholar] [CrossRef]
- Liu, Y.; Mo, J.; Fu, Q.; Lu, Y.; Zhang, N.; Wang, S.; Nie, S. Enhancement of Triboelectric Charge Density by Chemical Functionalization. Adv. Funct. Mater. 2020, 30, 2004714. [Google Scholar] [CrossRef]
- Xia, X.; Mugo, S.M.; Zhang, Q. Responsive Microgels-Based Wearable Devices for Sensing Multiple Health Signals. Chem. Eng. J. 2022, 427, 130903. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Li, M.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. MXene-Coated Air-Permeable Pressure-Sensing Fabric for Smart Wear. ACS Appl. Mater. Interfaces 2020, 12, 46446–46454. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, S.K.; Ormsby, C.; Neethirajan, S. Noninvasive Label-Free Detection of Cortisol and Lactate Using Graphene Embedded Screen-Printed Electrode. Nano-Micro Lett. 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, Y.; Wang, Y.; Mao, H.; Zhang, Q.; Deng, Y. Flexible 3D Architectured Piezo/Thermoelectric Bimodal Tactile Sensor Array for E-Skin Application. Adv. Energy Mater. 2020, 10, 2001945. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, N.; Cao, Y.; Wang, F.; Wang, P.; Ma, Y.; Lu, B.; Hou, G.; Fang, Z.; Liang, Z.; et al. Climbing-Inspired Twining Electrodes Using Shape Memory for Peripheral Nerve Stimulation and Recording. Sci. Adv. 2019, 5, eaaw1066. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A Laser-Engraved Wearable Sensor for Sensitive Detection of Uric Acid and Tyrosine in Sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef]
- Hua, Q.; Sun, J.; Liu, H.; Bao, R.; Yu, R.; Zhai, J.; Pan, C.; Wang, Z.L. Skin-Inspired Highly Stretchable and Conformable Matrix Networks for Multifunctional Sensing. Nat. Commun. 2018, 9, 244. [Google Scholar] [CrossRef]
- Oh, J.; Yang, J.C.; Kim, J.O.; Park, H.; Kwon, S.Y.; Lee, S.; Sim, J.Y.; Oh, H.W.; Kim, J.; Park, S. Pressure Insensitive Strain Sensor with Facile Solution-Based Process for Tactile Sensing Applications. ACS Nano 2018, 12, 7546–7553. [Google Scholar] [CrossRef]
- Liang, X.; Li, H.; Dou, J.; Wang, Q.; He, W.; Wang, C.; Li, D.; Lin, J.; Zhang, Y. Stable and Biocompatible Carbon Nanotube Ink Mediated by Silk Protein for Printed Electronics. Adv. Mater. 2020, 32, 2000165. [Google Scholar] [CrossRef]
- Jung, S.; Kim, J.H.; Kim, J.; Choi, S.; Lee, J.; Park, I.; Hyeon, T.; Kim, D.H. Reverse-Micelle-Induced Porous Pressure-Sensitive Rubber for Wearable Human-Machine Interfaces. Adv. Mater. 2014, 26, 4825–4830. [Google Scholar] [CrossRef]
- Shin, S.H.; Ji, S.; Choi, S.; Pyo, K.H.; Wan An, B.; Park, J.; Kim, J.; Kim, J.Y.; Lee, K.S.; Kwon, S.Y.; et al. Integrated Arrays of Air-Dielectric Graphene Transistors as Transparent Active-Matrix Pressure Sensors for Wide Pressure Ranges. Nat. Commun. 2017, 8, 14950. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Yu, W.; Wang, K.; Wei, J.; Wu, D.; Cao, A.; Li, Z.; Cheng, Y.; Zheng, Q.; et al. Stretchable and Highly Sensitive Graphene-on-Polymer Strain Sensors. Sci. Rep. 2012, 2, 870. [Google Scholar] [CrossRef]
- Conti, S.; Pimpolari, L.; Calabrese, G.; Worsley, R.; Majee, S.; Polyushkin, D.K.; Paur, M.; Pace, S.; Keum, D.H.; Fabbri, F.; et al. Low-Voltage 2D Materials-Based Printed Field-Effect Transistors for Integrated Digital and Analog Electronics on Paper. Nat. Commun. 2020, 11, 3566. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, X.; Serpe, M.J.; Zhang, Q. Microgel-Based Devices as Wearable Capacitive Electronic Skins for Monitoring Cardiovascular Risks. Adv. Mater. Technol. 2020, 5, 1900818. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A Wearable Chemical–Electrophysiological Hybrid Biosensing System for Real-Time Health and Fitness Monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, H.; Xiao, L.; Duan, Y.; Zhu, H.; Bian, J.; Ye, D.; Yin, Z. Assembly and Applications of 3D Conformal Electronics on Curvilinear Surfaces. Mater. Horiz. 2019, 6, 642–683. [Google Scholar] [CrossRef]
- Kim, E.; Lee, B.J.; Maleski, K.; Chae, Y.; Lee, Y.; Gogotsi, Y.; Ahn, C.W. Microsupercapacitor with a 500 Nm Gap between MXene/CNT Electrodes. Nano Energy 2021, 81, 105616. [Google Scholar] [CrossRef]
- Gao, Y.; Ota, H.; Schaler, E.W.; Chen, K.; Zhao, A.; Gao, W.; Fahad, H.M.; Leng, Y.; Zheng, A.; Xiong, F.; et al. Wearable Microfluidic Diaphragm Pressure Sensor for Health and Tactile Touch Monitoring. Adv. Mater. 2017, 29, 1701985. [Google Scholar] [CrossRef]
- Bouhamed, A.; Rajendran, D.; Frenzel, P.; Zubkova, T.; Al-Hamry, A.; Miesel, D.; Kamatchi, V.; Ramalingame, R.; Bautista-Quijano, J.R.; Lang, H.; et al. Customizing Hydrothermal Properties of Inkjet Printed Sensitive Films by Functionalization of Carbon Nanotubes. Nanotechnology 2021, 32, 105708. [Google Scholar] [CrossRef]
- Byun, J.; Lee, B.; Oh, E.; Kim, H.; Kim, S.; Lee, S.; Hong, Y. Fully Printable, Strain-Engineered Electronic Wrap for Customizable Soft Electronics. Sci. Rep. 2017, 7, 45328. [Google Scholar] [CrossRef]
- Byun, J.; Oh, E.; Lee, B.; Kim, S.; Lee, S.; Hong, Y. A Single Droplet-Printed Double-Side Universal Soft Electronic Platform for Highly Integrated Stretchable Hybrid Electronics. Adv. Funct. Mater. 2017, 27, 1701912. [Google Scholar] [CrossRef]
- Calamak, S. Sodium Polyacrylate Microparticle Containing Multifunctional Skin Patch for Sweat Analysis. Microchem. J. 2020, 159, 105473. [Google Scholar] [CrossRef]
- Shajari, S.; Salahandish, R.; Zare, A.; Hassani, M.; Moossavi, S.; Munro, E.; Rashid, R.; Rosenegger, D.; Bains, J.S.; Sanati Nezhad, A. MicroSweat: A Wearable Microfluidic Patch for Noninvasive and Reliable Sweat Collection Enables Human Stress Monitoring. Adv. Sci. 2022, 10, 2204171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, J.; Wang, F.; Kong, D. Stretchable and Superwettable Colorimetric Sensing Patch for Epidermal Collection and Analysis of Sweat. ACS Sens. 2021, 6, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, F.; Qiu, C.; Redoute, J.M.; Yuce, M.R. A Rigid-Flex Wearable Health Monitoring Sensor Patch for IoT-Connected Healthcare Applications. IEEE Internet Things J. 2020, 7, 6932–6945. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, S.; Ma, R.; Chen, Z.; Zhuo, J.; Cao, L.; Yang, J.; Yang, G.; Yi, F. High-Performance Multimodal Smart Textile for Artificial Sensation and Health Monitoring. Nano Energy 2022, 103, 107778. [Google Scholar] [CrossRef]
- Alshabouna, F.; Lee, H.S.; Barandun, G.; Tan, E.; Cotur, Y.; Asfour, T.; Gonzalez-Macia, L.; Coatsworth, P.; Núnez-Bajo, E.; Kim, J.S.; et al. PEDOT:PSS-Modified Cotton Conductive Thread for Mass Manufacturing of Textile-Based Electrical Wearable Sensors by Computerized Embroidery. Mater. Today 2022, 59, 56–67. [Google Scholar] [CrossRef]
- Gogurla, N.; Kim, Y.; Cho, S.; Kim, J.; Kim, S. Multifunctional and Ultrathin Electronic Tattoo for On-Skin Diagnostic and Therapeutic Applications. Adv. Mater. 2021, 33, 2008308. [Google Scholar] [CrossRef]
- Laurila, M.M.; Peltokangas, M.; Montero, K.L.; Verho, J.; Haapala, M.; Oksala, N.; Vehkaoja, A.; Mäntysalo, M. Self-Powered, High Sensitivity Printed e-Tattoo Sensor for Unobtrusive Arterial Pulse Wave Monitoring. Nano Energy 2022, 102, 107625. [Google Scholar] [CrossRef]
- Kim, J.; de Araujo, W.R.; Samek, I.A.; Bandodkar, A.J.; Jia, W.; Brunetti, B.; Paixão, T.R.L.C.; Wang, J. Wearable Temporary Tattoo Sensor for Real-Time Trace Metal Monitoring in Human Sweat. Electrochem. Commun. 2015, 51, 41–45. [Google Scholar] [CrossRef]
- Mishra, R.K.; Barfidokht, A.; Karajic, A.; Sempionatto, J.R.; Wang, J.; Wang, J. Wearable Potentiometric Tattoo Biosensor for On-Body Detection of G-Type Nerve Agents Simulants. Sens. Actuators B Chem. 2018, 273, 966–972. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, Y.; Shi, G.; Haick, H.; Zhang, M. Wearable Clinic: From Microneedle-Based Sensors to Next-Generation Healthcare Platforms. Small 2023, 2207539. [Google Scholar] [CrossRef]
- Wu, Y.; Tehrani, F.; Teymourian, H.; Mack, J.; Shaver, A.; Reynoso, M.; Kavner, J.; Huang, N.; Furmidge, A.; Duvvuri, A.; et al. Microneedle Aptamer-Based Sensors for Continuous, Real-Time Therapeutic Drug Monitoring. Anal. Chem. 2022, 94, 8335–8345. [Google Scholar] [CrossRef]
- Mishra, R.K.; Goud, K.Y.; Li, Z.; Moonla, C.; Mohamed, M.A.; Tehrani, F.; Teymourian, H.; Wang, J. Continuous Opioid Monitoring along with Nerve Agents on a Wearable Microneedle Sensor Array. J. Am. Chem. Soc. 2020, 142, 5991–5995. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Riley, P.R.; Mishra, R.; Azizi Machekposhti, S.; Narayan, R. Transdermal Polymeric Microneedle Sensing Platform for Fentanyl Detection in Biofluid. Biosensors 2022, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, X.; Gu, Z.; Xiu, G.; Xiao, X. Electrochemical Sensing in Contact Lenses. Electroanalysis 2022, 34, 227–236. [Google Scholar] [CrossRef]
- Moreddu, R.; Wolffsohn, J.S.; Vigolo, D.; Yetisen, A.K. Laser-Inscribed Contact Lens Sensors for the Detection of Analytes in the Tear Fluid. Sens. Actuators B Chem. 2020, 317, 128183. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.S.; Kim, K.; Ji, S.; Kim, Y.T.; Park, J.; Na, K.; Bae, K.H.; Kyun Kim, H.; et al. Wearable Smart Sensor Systems Integrated on Soft Contact Lenses for Wireless Ocular Diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, S.Y.; Cheong, W.H.; Jang, J.; Park, Y.G.; Na, K.; Kim, Y.T.; Heo, J.H.; Lee, C.Y.; et al. Soft, Smart Contact Lenses with Integrations of Wireless Circuits, Glucose Sensors, and Displays. Sci. Adv. 2018, 4, eaap9841. [Google Scholar] [CrossRef]
- Song, H.; Shin, H.; Seo, H.; Park, W.; Joo, B.J.; Kim, J.; Kim, J.; Kim, H.K.; Kim, J.; Park, J. Wireless Non-Invasive Monitoring of Cholesterol Using a Smart Contact Lens. Adv. Sci. 2022, 9, 2203597. [Google Scholar] [CrossRef]
- Li, X.; Luo, C.; Fu, Q.; Zhou, C.; Ruelas, M.; Wang, Y.; He, J.; Wang, Y.; Zhang, Y.S.; Zhou, J. A Transparent, Wearable Fluorescent Mouthguard for High-Sensitive Visualization and Accurate Localization of Hidden Dental Lesion Sites. Adv. Mater. 2020, 32, 2000060. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable Salivary Uric Acid Mouthguard Biosensor with Integrated Wireless Electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Tomoto, K.; Nitta, H.; Toma, K.; Takeuchi, S.; Sekita, T.; Minakuchi, S.; Mitsubayashi, K. A Wearable Cellulose Acetate-Coated Mouthguard Biosensor for In Vivo Salivary Glucose Measurement. Anal. Chem. 2020, 92, 12201–12207. [Google Scholar] [CrossRef]
- Sangsawang, R.; Buranachai, C.; Thavarungkul, P.; Kanatharana, P.; Jeerapan, I. Cavitas Electrochemical Sensors for the Direct Determination of Salivary Thiocyanate Levels. Microchim. Acta 2021, 188, 415. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, B.; Li, Y.; Pang, X.; Fu, Q.; Xiao, Z.; Shi, Z.; Li, X.; Luo, C.; Zhou, Z.; et al. Au@Ag Nanorods-PDMS Wearable Mouthguard as a Visualized Detection Platform for Screening Dental Caries and Periodontal Diseases. Adv. Healthc. Mater. 2022, 11, 2102682. [Google Scholar] [CrossRef] [PubMed]
- Ciui, B.; Tertis, M.; Feurdean, C.N.; Ilea, A.; Sandulescu, R.; Wang, J.; Cristea, C. Cavitas Electrochemical Sensor toward Detection of N-Epsilon (Carboxymethyl)lysine in Oral Cavity. Sens. Actuators B Chem. 2019, 281, 399–407. [Google Scholar] [CrossRef]
- Yin, Y.; Guo, C.; Li, H.; Yang, H.; Xiong, F.; Chen, D. The Progress of Research into Flexible Sensors in the Field of Smart Wearables. Sensors 2022, 22, 5089. [Google Scholar] [CrossRef]
- Madrid, R.E.; Ashur Ramallo, F.; Barraza, D.E.; Chaile, R.E. Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users. Bioengineering 2022, 9, 101. [Google Scholar] [CrossRef]
- Yin, J.; Li, J.; Reddy, V.S.; Ji, D.; Ramakrishna, S.; Xu, L. Flexible Textile-Based Sweat Sensors for Wearable Applications. Biosensors 2023, 13, 127. [Google Scholar] [CrossRef]
- Wong, S.H.D.; Deen, G.R.; Bates, J.S.; Maiti, C.; Lam, C.Y.K.; Pachauri, A.; AlAnsari, R.; Bělský, P.; Yoon, J.; Dodda, J.M. Smart Skin-Adhesive Patches: From Design to Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2213560. [Google Scholar] [CrossRef]
- Meng, L.; Ge, K.; Song, Y.; Yang, D.; Lin, Z. Long-Term Wearable Electrocardiogram Signal Monitoring and Analysis Based on Convolutional Neural Network. IEEE Trans. Instrum. Meas. 2021, 70, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Z.; Zhang, J.; Liu, Y.; Yu, D.; Guo, X. Wearable Ultraviolet Sensor Based on Convolutional Neural Network Image Processing Method. Sens. Actuators A Phys. 2022, 338, 113402. [Google Scholar] [CrossRef]
- Casanova, A.; Cuartero, M.; Alacid, Y.; Almagro, C.M.; García-Cánovas, F.; García, M.S.; Ortuño, J.A. A Sustainable Amperometric Biosensor for the Analysis of Ascorbic, Benzoic, Gallic and Kojic Acids through Catechol Detection. Innovation and Signal Processing. Analyst 2020, 145, 3645–3655. [Google Scholar] [CrossRef] [PubMed]
- McHenry, A.; Friedel, M.; Heikenfeld, J. Voltammetry Peak Tracking for Longer-Lasting and Reference-Electrode-Free Electrochemical Biosensors. Biosensors 2022, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Suresh, L.; Yang, J.; Zhang, X.; Tan, S.C. Augmenting Sensor Performance with Machine Learning Towards Smart Wearable Sensing Electronic Systems. Adv. Intell. Syst. 2022, 4, 2100194. [Google Scholar] [CrossRef]
- Santucci, F.; Lo Presti, D.; Massaroni, C.; Schena, E.; Setola, R. Precordial Vibrations: A Review of Wearable Systems, Signal Processing Techniques, and Main Applications. Sensors 2022, 22, 5805. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A Wearable and Highly Sensitive Pressure Sensor with Ultrathin Gold Nanowires. Nat. Commun. 2014, 5, 3132. [Google Scholar] [CrossRef]
- Ho, M.D.; Ling, Y.; Yap, L.W.; Wang, Y.; Dong, D.; Zhao, Y.; Cheng, W. Percolating Network of Ultrathin Gold Nanowires and Silver Nanowires toward “Invisible” Wearable Sensors for Detecting Emotional Expression and Apexcardiogram. Adv. Funct. Mater. 2017, 27, 1700845. [Google Scholar] [CrossRef]
- Ji, B.; Zhou, Q.; Wu, J.; Gao, Y.; Wen, W.; Zhou, B. Synergistic Optimization toward the Sensitivity and Linearity of Flexible Pressure Sensor via Double Conductive Layer and Porous Microdome Array. ACS Appl. Mater. Interfaces 2020, 12, 31021–31035. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, C.; Xia, Z.; Chen, M.; Jia, Y.; Tao, B.; Li, S.; Cai, K. A Facile and Novel Design of Multifunctional Electronic Skin Based on Polydimethylsiloxane with Micropillars for Signal Monitoring. J. Mater. Chem. B 2020, 8, 8315–8322. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, Q.; Wu, Y.; Wang, Y.; Qing, X.; Li, M.; Liu, K.; Wang, W.; Wang, D. A Nanofiber Based Artificial Electronic Skin with High Pressure Sensitivity and 3D Conformability. Nanoscale 2016, 8, 12105–12112. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, J.; Kim, Y.; Choi, H.B.; Yang, J.C.; Lee, S.; Pyatykh, M.; Kim, J.; Sim, J.Y.; Park, S. Highly Uniform and Low Hysteresis Piezoresistive Pressure Sensors Based on Chemical Grafting of Polypyrrole on Elastomer Template with Uniform Pore Size. Small 2019, 15, 1901744. [Google Scholar] [CrossRef] [PubMed]
- Ruth, S.R.A.; Feig, V.R.; Tran, H.; Bao, Z. Microengineering Pressure Sensor Active Layers for Improved Performance. Adv. Funct. Mater. 2020, 30, 2003491. [Google Scholar] [CrossRef]
- Ge, G.; Zhang, Y.; Shao, J.; Wang, W.; Si, W.; Huang, W.; Dong, X. Stretchable, Transparent, and Self-Patterned Hydrogel-Based Pressure Sensor for Human Motions Detection. Adv. Funct. Mater. 2018, 28, 1802576. [Google Scholar] [CrossRef]
- Feng, S.; Li, Q.; Wang, S.; Wang, B.; Hou, Y.; Zhang, T. Tunable Dual Temperature–Pressure Sensing and Parameter Self-Separating Based on Ionic Hydrogel via Multisynergistic Network Design. ACS Appl. Mater. Interfaces 2019, 11, 21049–21057. [Google Scholar] [CrossRef]
- Wang, Z.; Cong, Y.; Fu, J. Stretchable and Tough Conductive Hydrogels for Flexible Pressure and Strain Sensors. J. Mater. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, F.; Handschuh-Wang, S.; Gan, T.; Zhou, X.; Zhou, X. Biomimetic Extreme-Temperature- and Environment-Adaptable Hydrogels. ChemPhysChem 2019, 20, 2139–2154. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, W.; Jung, K.; Park, B.; Park, J.; Ko, J.; Cho, H. A Highly Sensitive and Flexible Capacitive Pressure Sensor Based on a Porous Three-Dimensional PDMS/Microsphere Composite. Polymers 2020, 12, 1412. [Google Scholar] [CrossRef]
- Zhou, Q.; Ji, B.; Wei, Y.; Hu, B.; Gao, Y.; Xu, Q.; Zhou, J.; Zhou, B. A Bio-Inspired Cilia Array as the Dielectric Layer for Flexible Capacitive Pressure Sensors with High Sensitivity and a Broad Detection Range. J. Mater. Chem. A 2019, 7, 27334–27346. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Sun, H.; Qing, X. A Flexible Ionic Liquid-Polyurethane Sponge Capacitive Pressure Sensor. Sens. Actuators A Phys. 2019, 285, 67–72. [Google Scholar] [CrossRef]
- Hu, W.; Niu, X.; Zhao, R.; Pei, Q. Elastomeric Transparent Capacitive Sensors Based on an Interpenetrating Composite of Silver Nanowires and Polyurethane. Appl. Phys. Lett. 2013, 102, 083303. [Google Scholar] [CrossRef]
- Vandeparre, H.; Watson, D.; Lacour, S.P. Extremely Robust and Conformable Capacitive Pressure Sensors Based on Flexible Polyurethane Foams and Stretchable Metallization. Appl. Phys. Lett. 2013, 103, 204103. [Google Scholar] [CrossRef]
- Dong, W.; Yao, D.; Yang, L. Soft Bimodal Sensor Array Based on Conductive Hydrogel for Driving Status Monitoring. Sensors 2020, 20, 1641. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, S.; Wang, N.; Ye, Z.; Qi, H.; Guo, X.; Jin, Y. Low-Temperature and Solution-Processed Indium Tin Oxide Films and Their Applications in Flexible Transparent Capacitive Pressure Sensors. Appl. Phys. A 2016, 122, 424. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Miao, Y.; Yang, H.; Chen, X.; Xiao, X.; Jiang, Z.; Chen, X.; Nie, B.; Liu, J. Highly Stretchable and Sensitive Pressure Sensor Array Based on Icicle-Shaped Liquid Metal Film Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 27961–27970. [Google Scholar] [CrossRef]
- Xiong, Y.; Shen, Y.; Tian, L.; Hu, Y.; Zhu, P.; Sun, R.; Wong, C.P. A Flexible, Ultra-Highly Sensitive and Stable Capacitive Pressure Sensor with Convex Microarrays for Motion and Health Monitoring. Nano Energy 2020, 70, 104436. [Google Scholar] [CrossRef]
- Qiu, Z.; Wan, Y.; Zhou, W.; Yang, J.; Yang, J.; Huang, J.; Zhang, J.; Liu, Q.; Huang, S.; Bai, N.; et al. Ionic Skin with Biomimetic Dielectric Layer Templated from Calathea zebrine Leaf. Adv. Funct. Mater. 2018, 28, 1802343. [Google Scholar] [CrossRef]
- Cao, Z.; He, K.; Xiong, W.; Chen, Y.; Qiu, X.; Yu, D.; Guo, X. Flexible Micropillar Array for Pressure Sensing in High Density Using Image Sensor. Adv. Mater. Interfaces 2020, 7, 1902205. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, T.; Li, Q.; Chen, Y.; Qiu, X.; Liu, J.; Bian, Y.; Xuan, F. Flexible Microstructured Pressure Sensors: Design, Fabrication and Applications. Nanotechnology 2022, 33, 322002. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.; Tang, W.; Chen, J.; Zhu, Z.; Li, L.; Zhou, N.; Kang, X.; Xu, D.; Wang, L.; et al. Flexible Pressure Sensors Based on Bionic Microstructures: From Plants to Animals. Adv. Mater. Inter. 2022, 9, 2101312. [Google Scholar] [CrossRef]
- Basarir, F.; Madani, Z.; Vapaavuori, J. Recent Advances in Silver Nanowire Based Flexible Capacitive Pressure Sensors: From Structure, Fabrication to Emerging Applications. Adv. Mater. Interface 2022, 9, 2200866. [Google Scholar] [CrossRef]
- Saha, T.; Fang, J.; Mukherjee, S.; Dickey, M.D.; Velev, O.D. Wearable Osmotic-Capillary Patch for Prolonged Sweat Harvesting and Sensing. ACS Appl. Mater. Interfaces 2021, 13, 8071–8081. [Google Scholar] [CrossRef]
- Weng, X.; Fu, Z.; Zhang, C.; Jiang, W.; Jiang, H. A Portable 3D Microfluidic Origami Biosensor for Cortisol Detection in Human Sweat. Anal. Chem. 2022, 94, 3526–3534. [Google Scholar] [CrossRef]
- Chen, Y.C.; Shan, S.S.; Liao, Y.T.; Liao, Y.C. Bio-Inspired Fractal Textile Device for Rapid Sweat Collection and Monitoring. Lab Chip 2021, 21, 2524–2533. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Dong, Y.; Gong, J.; Li, Z.; Zhang, J. Core-Shell Structured Gold Nanorods on Thread-Embroidered Fabric-Based Microfluidic Device for Ex Situ Detection of Glucose and Lactate in Sweat. Sens. Actuators B Chem. 2022, 353, 131154. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Arie, T.; Akita, S.; Takei, K. Wearable, Flexible, and Multifunctional Healthcare Device with an ISFET Chemical Sensor for Simultaneous Sweat pH and Skin Temperature Monitoring. ACS Sens. 2017, 2, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cordero, E.; Bellando, F.; Zhang, J.; Wildhaber, F.; Longo, J.; Guérin, H.; Ionescu, A.M. Three-Dimensional Integrated Ultra-Low-Volume Passive Microfluidics with Ion-Sensitive Field-Effect Transistors for Multiparameter Wearable Sweat Analyzers. ACS Nano 2018, 12, 12646–12656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhai, Q.; Dong, D.; An, T.; Gong, S.; Shi, Q.; Cheng, W. Highly Stretchable and Strain-Insensitive Fiber-Based Wearable Electrochemical Biosensor to Monitor Glucose in the Sweat. Anal. Chem. 2019, 91, 6569–6576. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Y.; Su, L.; Zhao, D.; Zhao, L.; Zhang, X. Microfluidic Chip-Based Wearable Colorimetric Sensor for Simple and Facile Detection of Sweat Glucose. Anal. Chem. 2019, 91, 14803–14807. [Google Scholar] [CrossRef]
- He, X.; Yang, S.; Pei, Q.; Song, Y.; Liu, C.; Xu, T.; Zhang, X. Integrated Smart Janus Textile Bands for Self-Pumping Sweat Sampling and Analysis. ACS Sens. 2020, 5, 1548–1554. [Google Scholar] [CrossRef]
- Li, G.; Mo, X.; Law, W.C.; Chan, K.C. Wearable Fluid Capture Devices for Electrochemical Sensing of Sweat. ACS Appl. Mater. Interfaces 2019, 11, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Subramaniam, C. Point-of-Care, Cable-Type Electrochemical Zn2+ Sensor with Ultrahigh Sensitivity and Wide Detection Range for Soil and Sweat Analysis. ACS Sustain. Chem. Eng. 2019, 7, 14569–14579. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liu, R.; Qin, Y.; Qiu, Q.F.; Chen, Z.; Cheng, S.B.; Huang, W.H. Flexible Electrochemical Urea Sensor Based on Surface Molecularly Imprinted Nanotubes for Detection of Human Sweat. Anal. Chem. 2018, 90, 13081–13087. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.C.; Liaw, T.S.; Lin, Y.; Nyein, H.Y.Y.; Bariya, M.; Ji, W.; Hettick, M.; Zhao, C.; Zhao, J.; Hou, L.; et al. Wearable Sweat Band for Noninvasive Levodopa Monitoring. Nano Lett. 2019, 19, 6346–6351. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Tran, B.; Ahn, C.H.; Brown, B.J.; Ji, W.; Davis, N.; Javey, A. A Wearable Patch for Continuous Analysis of Thermoregulatory Sweat at Rest. Nat. Commun. 2021, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; De Loyola e Silva, A.N.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; May, J.; et al. Epidermal Enzymatic Biosensors for Sweat Vitamin C: Toward Personalized Nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef]
- Kim, S.; Lee, B.; Reeder, J.T.; Seo, S.H.; Lee, S.U.; Hourlier-Fargette, A.; Shin, J.; Sekine, Y.; Jeong, H.; Oh, Y.S.; et al. Soft, Skin-Interfaced Microfluidic Systems with Integrated Immunoassays, Fluorometric Sensors, and Impedance Measurement Capabilities. Proc. Natl. Acad. Sci. USA 2020, 117, 27906–27915. [Google Scholar] [CrossRef]
- Kanokpaka, P.; Chang, L.Y.; Wang, B.C.; Huang, T.H.; Shih, M.J.; Hung, W.S.; Lai, J.Y.; Ho, K.C.; Yeh, M.H. Self-Powered Molecular Imprinted Polymers-Based Triboelectric Sensor for Noninvasive Monitoring Lactate Levels in Human Sweat. Nano Energy 2022, 100, 107464. [Google Scholar] [CrossRef]
- Xuan, X.; Pérez-Ràfols, C.; Chen, C.; Cuartero, M.; Crespo, G.A. Lactate Biosensing for Reliable On-Body Sweat Analysis. ACS Sens. 2021, 6, 2763–2771. [Google Scholar] [CrossRef]
- Xiao, G.; He, J.; Qiao, Y.; Wang, F.; Xia, Q.; Wang, X.; Yu, L.; Lu, Z.; Li, C.M. Facile and Low-Cost Fabrication of a Thread/Paper-Based Wearable System for Simultaneous Detection of Lactate and pH in Human Sweat. Adv. Fiber Mater. 2020, 2, 265–278. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, J.; Wu, Z.; Li, R.; Wang, Y.; Gong, Z.; Zhao, X.; Sun, C.; Guo, S. Enhanced Performance of Piezoelectric Nanogenerator Based on Aligned Nanofibers and Three-Dimensional Interdigital Electrodes. Nano Energy 2019, 65, 103924. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Jin, X.; Sun, Y.; Lu, B.; Wang, H.; Fang, J.; Shao, H.; Lin, T. Unexpectedly High Piezoelectricity of Electrospun Polyacrylonitrile Nanofiber Membranes. Nano Energy 2019, 56, 588–594. [Google Scholar] [CrossRef]
- Khadtare, S.; Ko, E.J.; Kim, Y.H.; Lee, H.S.; Moon, D.K. A Flexible Piezoelectric Nanogenerator Using Conducting Polymer and Silver Nanowire Hybrid Electrodes for Its Application in Real-Time Muscular Monitoring System. Sens. Actuators A Phys. 2019, 299, 111575. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Z.; Zheng, X.; Zhao, X.; Wang, H.; Liang, F.; Guan, S.; Wang, Y.; Zhao, Y.; Chen, A.; et al. A Contact-Sliding-Triboelectrification-Driven Dynamic Optical Transmittance Modulator for Self-Powered Information Covering and Selective Visualization. Adv. Mater. 2020, 32, 1904988. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Yang, G.; Hao, X.; Lv, X.; Wu, F.; Liu, J.; Zhang, Y. Wearable and Self-Powered Sensors Made by Triboelectric Nanogenerators Assembled from Antibacterial Bromobutyl Rubber. Nano Energy 2021, 82, 105769. [Google Scholar] [CrossRef]
- Qiao, G.; Wang, J.; Yu, X.; Jia, R.; Cheng, T.; Wang, Z.L. A Bidirectional Direct Current Triboelectric Nanogenerator with the Mechanical Rectifier. Nano Energy 2021, 79, 105408. [Google Scholar] [CrossRef]
- Sun, P.; Jiang, S.; Huang, Y. Nanogenerator as Self-Powered Sensing Microsystems for Safety Monitoring. Nano Energy 2021, 81, 105646. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Chernozem, R.V.; Pariy, I.O.; Surmeneva, M.A. A Review on Piezo- and Pyroelectric Responses of Flexible Nano- and Micropatterned Polymer Surfaces for Biomedical Sensing and Energy Harvesting Applications. Nano Energy 2021, 79, 105442. [Google Scholar] [CrossRef]
- Sathiyanathan, P.; Dhevi, D.M.; Prabu, A.A.; Kim, K.J. Electrospun Polyvinylidene Fluoride-Polyoctafluoropentyl Acrylate-Hydroxyapatite Blend Based Piezoelectric Pressure Sensors. Macromol. Res. 2019, 27, 743–749. [Google Scholar] [CrossRef]
- Wang, A.; Hu, M.; Zhou, L.; Qiang, X. Self-Powered Wearable Pressure Sensors with Enhanced Piezoelectric Properties of Aligned P(VDF-TrFE)/MWCNT Composites for Monitoring Human Physiological and Muscle Motion Signs. Nanomaterials 2018, 8, 1021. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.S.; Huang, Y.; Damadoran, A.R.; Xia, J.; Martin, L.W.; et al. Conformable Amplified Lead Zirconate Titanate Sensors with Enhanced Piezoelectric Response for Cutaneous Pressure Monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.I.; Chang, Y.L.; Chen, J.; Barbee, T.; Wang, W.; Kim, J.Y.; Kwon, M.K.; Shervin, S.; Moradnia, M.; Pouladi, S.; et al. Piezoelectric Pressure Sensor Based on Flexible Gallium Nitride Thin Film for Harsh-Environment and High-Temperature Applications. Sens. Actuators A Phys. 2020, 305, 111940. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Lu, L.; Wang, X.; Li, X.; Chen, X.; Liu, J. Flexible and Lead-Free Piezoelectric Nanogenerator as Self-Powered Sensor Based on Electrospinning BZT-BCT/P(VDF-TrFE) Nanofibers. Sens. Actuators A Phys. 2020, 303, 111796. [Google Scholar] [CrossRef]
- Yang, T.; Pan, H.; Tian, G.; Zhang, B.; Xiong, D.; Gao, Y.; Yan, C.; Chu, X.; Chen, N.; Zhong, S.; et al. Hierarchically Structured PVDF/ZnO Core-Shell Nanofibers for Self-Powered Physiological Monitoring Electronics. Nano Energy 2020, 72, 104706. [Google Scholar] [CrossRef]
- Yan, C.; Deng, W.; Jin, L.; Yang, T.; Wang, Z.; Chu, X.; Su, H.; Chen, J.; Yang, W. Epidermis-Inspired Ultrathin 3D Cellular Sensor Array for Self-Powered Biomedical Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 41070–41075. [Google Scholar] [CrossRef]
- Kwak, S.S.; Yoon, H.J.; Kim, S.W. Textile-Based Triboelectric Nanogenerators for Self-Powered Wearable Electronics. Adv. Funct. Mater. 2019, 29, 1804533. [Google Scholar] [CrossRef]
- Fan, F.R.; Tian, Z.Q.; Lin Wang, Z. Flexible Triboelectric Generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Wu, C.; Wang, A.C.; Ding, W.; Guo, H.; Wang, Z.L. Triboelectric Nanogenerator: A Foundation of the Energy for the New Era. Adv. Energy Mater. 2019, 9, 1802906. [Google Scholar] [CrossRef]
- Khandelwal, G.; Maria Joseph Raj, N.P.; Kim, S.J. Triboelectric Nanogenerator for Healthcare and Biomedical Applications. Nano Today 2020, 33, 100882. [Google Scholar] [CrossRef]
- Hinchet, R.; Seung, W.; Kim, S.W. Recent Progress on Flexible Triboelectric Nanogenerators for Self-Powered Electronics. ChemSusChem 2015, 8, 2327–2344. [Google Scholar] [CrossRef]
- Chen, S.; Wu, N.; Lin, S.; Duan, J.; Xu, Z.; Pan, Y.; Zhang, H.; Xu, Z.; Huang, L.; Hu, B.; et al. Hierarchical Elastomer Tuned Self-Powered Pressure Sensor for Wearable Multifunctional Cardiovascular Electronics. Nano Energy 2020, 70, 104460. [Google Scholar] [CrossRef]
- Shin, Y.E.; Sohn, S.D.; Han, H.; Park, Y.; Shin, H.J.; Ko, H. Self-Powered Triboelectric/Pyroelectric Multimodal Sensors with Enhanced Performances and Decoupled Multiple Stimuli. Nano Energy 2020, 72, 104671. [Google Scholar] [CrossRef]
- Yi, F.; Zhang, Z.; Kang, Z.; Liao, Q.; Zhang, Y. Recent Advances in Triboelectric Nanogenerator-Based Health Monitoring. Adv. Funct. Mater. 2019, 29, 1808849. [Google Scholar] [CrossRef]
- Mathew, A.A.; Chandrasekhar, A.; Vivekanandan, S. A Review on Real-Time Implantable and Wearable Health Monitoring Sensors Based on Triboelectric Nanogenerator Approach. Nano Energy 2021, 80, 105566. [Google Scholar] [CrossRef]
- Fan, F.R.; Lin, L.; Zhu, G.; Wu, W.; Zhang, R.; Wang, Z.L. Transparent Triboelectric Nanogenerators and Self-Powered Pressure Sensors Based on Micropatterned Plastic Films. Nano Lett. 2012, 12, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Tcho, I.W.; Kim, W.G.; Jeon, S.B.; Park, S.J.; Lee, B.J.; Bae, H.K.; Kim, D.; Choi, Y.K. Surface Structural Analysis of a Friction Layer for a Triboelectric Nanogenerator. Nano Energy 2017, 42, 34–42. [Google Scholar] [CrossRef]
- Ke, K.; Chung, C. High-Performance Al/PDMS TENG with Novel Complex Morphology of Two-Height Microneedles Array for High-Sensitivity Force-Sensor and Self-Powered Application. Small 2020, 16, 2001209. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.; Bhatta, T.; Salauddin, M.; Rasel, M.S.; Rahman, M.T.; Rana, S.M.S.; Park, J.Y. A Human Skin-Inspired Self-Powered Flex Sensor with Thermally Embossed Microstructured Triboelectric Layers for Sign Language Interpretation. Nano Energy 2020, 76, 105071. [Google Scholar] [CrossRef]
- Zhao, T.; Fu, Y.; Sun, C.; Zhao, X.; Jiao, C.; Du, A.; Wang, Q.; Mao, Y.; Liu, B. Wearable Biosensors for Real-Time Sweat Analysis and Body Motion Capture Based on Stretchable Fiber-Based Triboelectric Nanogenerators. Biosens. Bioelectron. 2022, 205, 114115. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Bowen, C.R. Multiscale-Structuring of Polyvinylidene Fluoride for Energy Harvesting: The Impact of Molecular-, Micro- and Macro-Structure. J. Mater. Chem. A 2017, 5, 3091–3128. [Google Scholar] [CrossRef]
- Bowen, C.R.; Kim, H.A.; Weaver, P.M.; Dunn, S. Piezoelectric and Ferroelectric Materials and Structures for Energy Harvesting Applications. Energy Environ. Sci. 2014, 7, 25–44. [Google Scholar] [CrossRef]
- Karan, S.K.; Maiti, S.; Lee, J.H.; Mishra, Y.K.; Khatua, B.B.; Kim, J.K. Recent Advances in Self-Powered Tribo-/Piezoelectric Energy Harvesters: All-In-One Package for Future Smart Technologies. Adv. Funct. Mater. 2020, 30, 2004446. [Google Scholar] [CrossRef]
- Kolubah, P.D.; Mohamed, H.O.; Ayach, M.; Rao Hari, A.; Alshareef, H.N.; Saikaly, P.; Chae, K.J.; Castaño, P. W2N-MXene Composite Anode Catalyst for Efficient Microbial Fuel Cells Using Domestic Wastewater. Chem. Eng. J. 2023, 461, 141821. [Google Scholar] [CrossRef]
- Huang, L.; Zhong, K.; Wu, Y.; Wu, Y.; Liu, X.; Huang, L.; Yan, J.; Zhang, H. Facile Synthesis of Hollow Carbon Spheres by Gas-Steamed Bifunctional NH4F for Efficient Cathodes in Microbial Fuel Cells. Carbon 2023, 207, 86–94. [Google Scholar] [CrossRef]
- Kižys, K.; Zinovičius, A.; Jakštys, B.; Bružaitė, I.; Balčiūnas, E.; Petrulevičienė, M.; Ramanavičius, A.; Morkvėnaitė-Vilkončienė, I. Microbial Biofuel Cells: Fundamental Principles, Development and Recent Obstacles. Biosensors 2023, 13, 221. [Google Scholar] [CrossRef]
- Xie, L.; Chen, Y.; Zhao, Y.; Zhou, G.; Nötzel, R. InN/InGaN Quantum Dot Abiotic One-Compartment Glucose Photofuel Cell: Power Supply and Sensing. ACS Omega 2022, 7, 1437–1443. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Yan, K.; Yao, X.; Liu, Y.; Zhang, J. A Light-Induced Self-Powered Competitive Immunosensor for the Detection of Platelet Derived Growth Factor-BB via an Elaborately Assembled Bioconjugate. Sens. Actuators B Chem. 2020, 316, 128130. [Google Scholar] [CrossRef]
- Hartel, M.C.; Lee, D.; Weiss, P.S.; Wang, J.; Kim, J. Resettable Sweat-Powered Wearable Electrochromic Biosensor. Biosens. Bioelectron. 2022, 215, 114565. [Google Scholar] [CrossRef]
- Franco, J.H.; Minteer, S.D.; De Andrade, A.R. Ethanol Biofuel Cells: Hybrid Catalytic Cascades as a Tool for Biosensor Devices. Biosensors 2021, 11, 41. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Zhang, Y.; Lv, P.; Feng, Q.; Wei, Q. Encapsulation of Enzyme by Metal-Organic Framework for Single-Enzymatic Biofuel Cell-Based Self-Powered Biosensor. Nano Energy 2020, 68, 104308. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, S. Bioelectricity Production from Sweat-Activated Germination of Bacterial Endospores. Biosens. Bioelectron. 2021, 186, 113293. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Pei, X.; Das, P.; Qin, H.; Lee, S.W.; Esfandyarpour, R. A Self-Powered Triboelectric MXene-Based 3D-Printed Wearable Physiological Biosignal Sensing System for on-Demand, Wireless, and Real-Time Health Monitoring. Nano Energy 2022, 101, 107511. [Google Scholar] [CrossRef]
- Sunitha, K.A.; Dixit, S.; Singh, P. Design and Development of a Self-Powered Wearable Device for a Tele-Medicine Application. Wirel. Pers. Commun. 2019, 108, 175–186. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, J.; Xu, W.; Xiong, R.; Huang, C. Cellulose-Based Fibrous Materials for Self-Powered Wearable Pressure Sensor: A Mini Review. Cellulose 2023, 30, 1981–1998. [Google Scholar] [CrossRef]
- Mondal, R.; Hasan, M.A.M.; Zhang, R.; Olin, H.; Yang, Y. Nanogenerators-Based Self-Powered Sensors. Adv. Mater. Technol. 2022, 7, 2200282. [Google Scholar] [CrossRef]
- Xue, Z.; Wu, L.; Yuan, J.; Xu, G.; Wu, Y. Self-Powered Biosensors for Monitoring Human Physiological Changes. Biosensors 2023, 13, 236. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Zhou, S.; Qin, Y.; Xia, X.; Sun, Y.; Han, G.; Shu, T.; Hu, L.; Zhang, Q. Flexible and Wearable Biosensors for Monitoring Health Conditions. Biosensors 2023, 13, 630. https://doi.org/10.3390/bios13060630
Song Z, Zhou S, Qin Y, Xia X, Sun Y, Han G, Shu T, Hu L, Zhang Q. Flexible and Wearable Biosensors for Monitoring Health Conditions. Biosensors. 2023; 13(6):630. https://doi.org/10.3390/bios13060630
Chicago/Turabian StyleSong, Zhimin, Shu Zhou, Yanxia Qin, Xiangjiao Xia, Yanping Sun, Guanghong Han, Tong Shu, Liang Hu, and Qiang Zhang. 2023. "Flexible and Wearable Biosensors for Monitoring Health Conditions" Biosensors 13, no. 6: 630. https://doi.org/10.3390/bios13060630
APA StyleSong, Z., Zhou, S., Qin, Y., Xia, X., Sun, Y., Han, G., Shu, T., Hu, L., & Zhang, Q. (2023). Flexible and Wearable Biosensors for Monitoring Health Conditions. Biosensors, 13(6), 630. https://doi.org/10.3390/bios13060630





