Carbon Electrode Sensor for the Measurement of Cortisol with Fast-Scan Cyclic Voltammetry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. CFME Fabrication
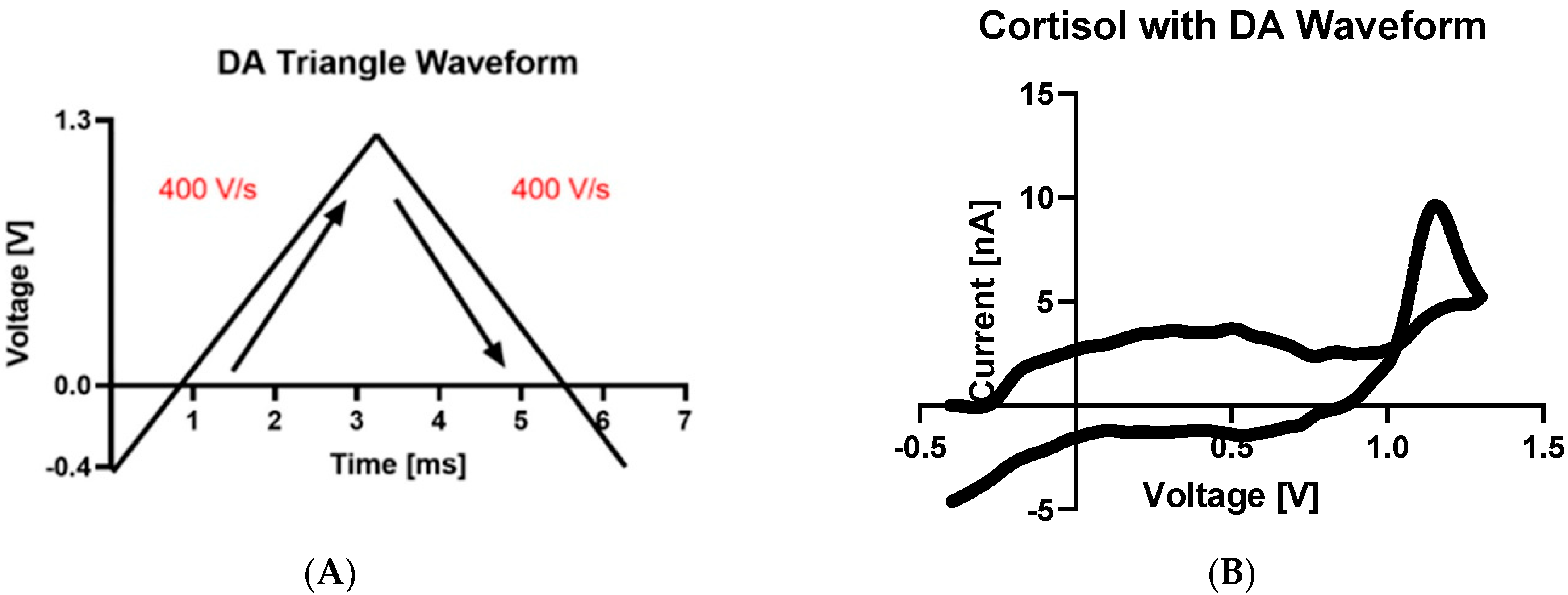
2.3. Fast-Scan Cyclic Voltammetry
2.4. Simulated Urine Measurement
2.5. Statistical Analysis
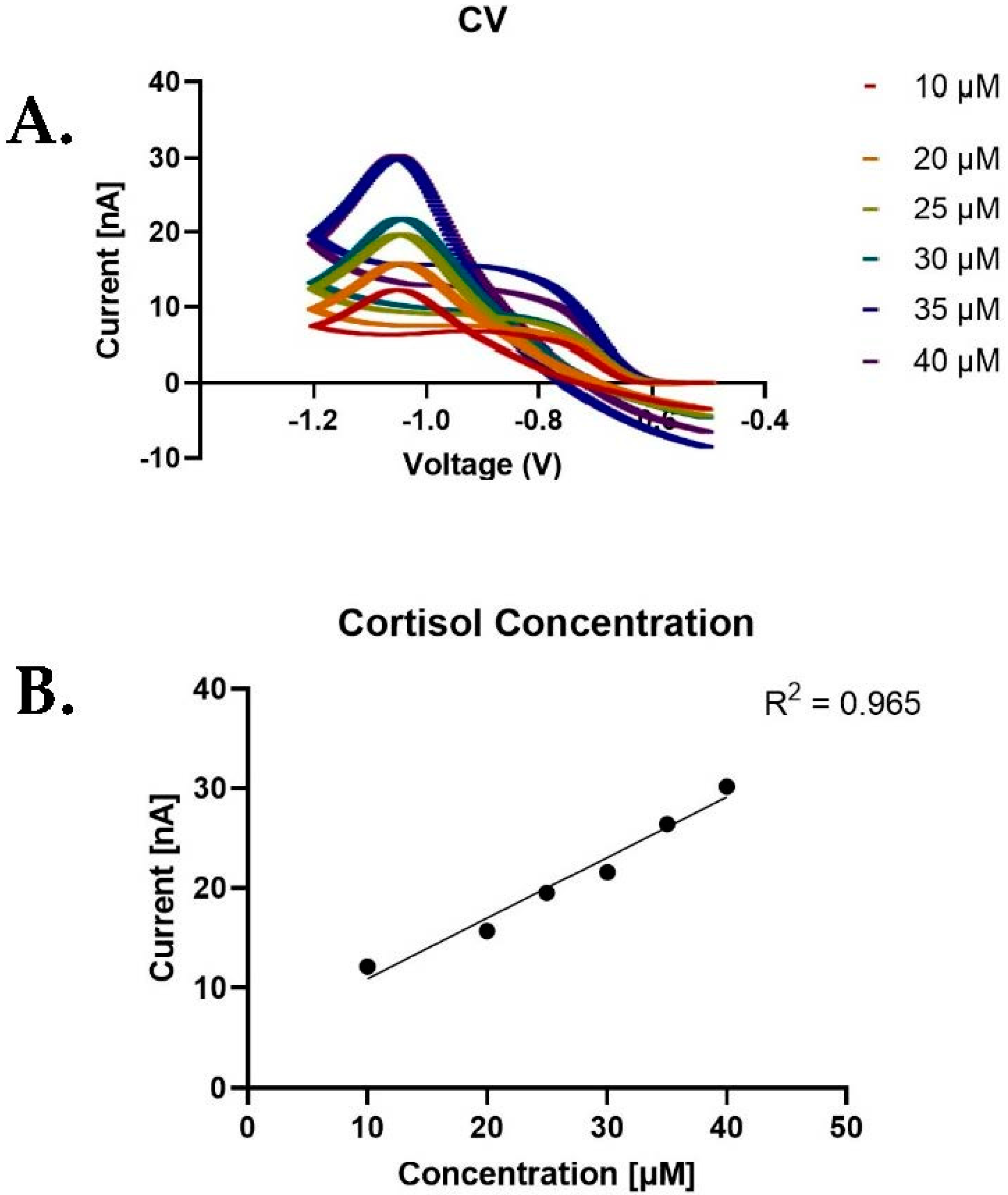
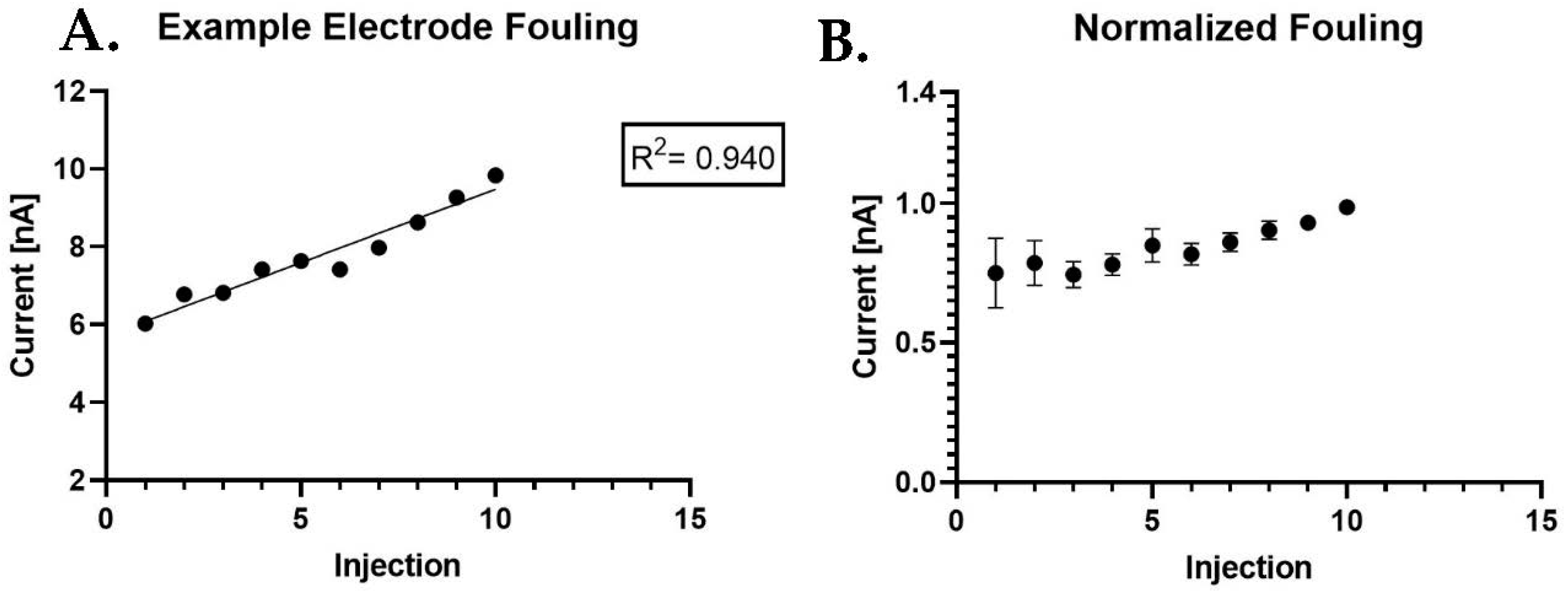
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Kaushik, A.; Kumar, R.; Nair, M.; Bhansali, S. Electrochemical sensing of cortisol: A recent update. Appl. Biochem. Biotechnol. 2014, 174, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Zorn, J.V.; Schür, R.R.; Boks, M.P.; Kahn, R.S.; Joëls, M.; Vinkers, C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 77, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Hucklebridge, F.; Clow, A.; Abeyguneratne, T.; Huezo-Diaz, P.; Evans, P. The awakening cortisol response and blood glucose levels. Life Sci. 1999, 64, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Husebye, E.; Allolio, B.; Arlt, W.; Badenhoop, K.; Bensing, S.; Betterle, C.; Falorni, A.; Gan, E.; Hulting, A.L.; Kasperlik-Zaluska, A. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J. Intern. Med. 2014, 275, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Casals, G.; Hanzu, F.A. Cortisol measurements in Cushing's syndrome: Immunoassay or mass spectrometry? Ann. Lab. Med. 2020, 40, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S.H. The detection of cortisol in human sweat: Implications for measurement of cortisol in hair. Ther. Drug Monit. 2014, 36, 30–34. [Google Scholar] [CrossRef]
- Du, X.; Zhai, J.; Li, X.; Zhang, Y.; Li, N.; Xie, X. Hydrogel-based optical ion sensors: Principles and challenges for point-of-care testing and environmental monitoring. ACS Sens. 2021, 6, 1990–2001. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Yu, M.; Hou, M.; Gong, G.; Tan, H.; Li, N.; Xu, J. NIR light-induced rapid self-healing hydrogel toward multifunctional applications in sensing. Nano Energy 2023, 107, 108119. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Guo, Z.; Zhang, C.; Tan, H.; Gong, G.; Yu, M.; Xu, L. Fully physical crosslinked BSA-based conductive hydrogels with high strength and fast self-recovery for human motion and wireless electrocardiogram sensing. Int. J. Biol. Macromol. 2023, 230, 123195. [Google Scholar] [CrossRef]
- Huang, Q.-D.; Lv, C.-H.; Yuan, X.-L.; He, M.; Lai, J.-P.; Sun, H. A novel fluorescent optical fiber sensor for highly selective detection of antibiotic ciprofloxacin based on replaceable molecularly imprinted nanoparticles composite hydrogel detector. Sens. Actuators B Chem. 2021, 328, 129000. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, M.; Zhang, Y.; Wang, Y.; Long, L.; Tan, H.; Li, N.; Xu, L.; Xu, J. Highly sensitive strain sensor and self-powered triboelectric nanogenerator using a fully physical crosslinked double-network conductive hydrogel. Nano Energy 2022, 104, 107955. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Moore, J.A.; Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N.; Chou, C.-F.; Swami, N.S. Aptamer-functionalized nanoparticles for surface immobilization-free electrochemical detection of cortisol in a microfluidic device. Biosens. Bioelectron. 2016, 78, 244–252. [Google Scholar] [CrossRef]
- Gonzalez, D.; Jacobsen, D.; Ibar, C.; Pavan, C.; Monti, J.; Fernandez Machulsky, N.; Balbi, A.; Fritzler, A.; Jamardo, J.; Repetto, E.M. Hair cortisol measurement by an automated method. Sci. Rep. 2019, 9, 8213. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.; Pandiaraj, M.; Bhansali, S.; Ponpandian, N.; Viswanathan, C. Carbon fiber based electrochemical sensor for sweat cortisol measurement. Sci. Rep. 2019, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Kinnamon, D.; Ghanta, R.; Lin, K.-C.; Muthukumar, S.; Prasad, S. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep. 2017, 7, 13312. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, C.; Wang, Z.; Yang, K.-A.; Cheng, X.; Liu, W.; Yu, W.; Lin, S.; Zhao, Y.; Cheung, K.M.; et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 2022, 8, eabk0967. [Google Scholar] [CrossRef]
- Huffman, M.L.; Venton, B.J. Electrochemical Properties of Different Carbon-Fiber Microelectrodes Using Fast-Scan Cyclic Voltammetry. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 2422–2428. [Google Scholar] [CrossRef]
- Huffman, M.L.; Venton, B.J. Carbon-fiber microelectrodes for in vivo applications. Analyst 2009, 134, 18–24. [Google Scholar] [CrossRef]
- Troyer, K.P.; Heien, M.L.; Venton, B.J.; Wightman, R.M. Neurochemistry and electroanalytical probes. Curr. Opin. Chem. Biol. 2002, 6, 696–703. [Google Scholar] [CrossRef]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.A.V.; Wightman, R.M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.N.; Tan, S.-Y.; Miller, T.S.; Macpherson, J.V.; Unwin, P.R. Comparison and reappraisal of carbon electrodes for the voltammetric detection of dopamine. Anal. Chem. 2013, 85, 11755–11764. [Google Scholar] [CrossRef] [PubMed]
- Rafi, H.; Zestos, A.G. Recent advances in FSCV detection of neurochemicals via waveform and carbon microelectrode modification. J. Electrochem. Soc. 2021, 168, 057520. [Google Scholar] [CrossRef]
- Vreeland, R.F.; Atcherley, C.W.; Russell, W.S.; Xie, J.Y.; Lu, D.; Laude, N.D.; Porreca, F.; Heien, M.L. Biocompatible PEDOT:Nafion composite electrode coatings for selective detection of neurotransmitters in vivo. Anal. Chem. 2015, 87, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pathirathna, P.; Siriwardhane, T.; McElmurry, S.P.; Hashemi, P. Real-time subsecond voltammetric analysis of Pb in aqueous environmental samples. Anal. Chem. 2013, 85, 7535–7541. [Google Scholar] [CrossRef]
- Yang, Y.; Ibrahim, A.A.; Hashemi, P.; Stockdill, J.L. Real-Time, Selective Detection of Copper (II) Using Ionophore-Grafted Carbon-Fiber Microelectrodes. Anal. Chem. 2016, 88, 6962–6966. [Google Scholar] [CrossRef] [PubMed]
- Pathirathna, P.; Siriwardhane, T.; McElmurry, S.P.; Morgan, S.L.; Hashemi, P. Fast voltammetry of metals at carbon-fiber microelectrodes: Towards an online speciation sensor. Analyst 2016, 141, 6432–6437. [Google Scholar] [CrossRef]
- Schmidt, A.C.; Wang, X.; Zhu, Y.; Sombers, L.A. Carbon nanotube yarn electrodes for enhanced detection of neurotransmitter dynamics in live brain tissue. ACS Nano 2013, 7, 7864–7873. [Google Scholar] [CrossRef]
- Calhoun, S.; Meunier, C.; Lee, C.; McCarty, G.; Sombers, L. Characterization of a multiple-scan-rate voltammetric waveform for real-time detection of met-enkephalin. ACS Chem. Neurosci. 2018, 10, 2022–2032. [Google Scholar] [CrossRef]
- Liu, F.; Ghasem Ardabili, N.; Brown, I.; Rafi, H.; Cook, C.; Nikopoulou, R.; Lopez, A.; Zou, S.; Hartings, M.R.; Zestos, A. Modified Sawhorse Waveform for the Voltammetric Detection of Oxytocin. J. Electrochem. Soc. 2022, 169, 017512. [Google Scholar] [CrossRef]
- Cho, W.; Liu, F.; Hendrix, A.; Asrat, T.; Connaughton, V.; Zestos, A.G. Timed Electrodeposition of PEDOT: Nafion onto Carbon Fiber-Microelectrodes Enhances Dopamine Detection in Zebrafish Retina. J. Electrochem. Soc. 2020, 167, 115501. [Google Scholar] [CrossRef]
- Michael, A.E.; Thurston, L.M.; Rae, M.T. Glucocorticoid metabolism and reproduction: A tale of two enzymes. Reprod. Camb. 2003, 126, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Takmakov, P.; Zachek, M.K.; Keithley, R.B.; Walsh, P.L.; Donley, C.; McCarty, G.S.; Wightman, R.M. Carbon microelectrodes with a renewable surface. Anal. Chem. 2010, 82, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Bath, B.D.; Michael, D.J.; Trafton, B.J.; Joseph, J.D.; Runnels, P.L.; Wightman, R.M. Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes. Anal. Chem. 2000, 72, 5994–6002. [Google Scholar] [CrossRef]
- Bath, B.D.; Martin, H.B.; Wightman, R.M.; Anderson, M.R. Dopamine adsorption at surface modified carbon-fiber electrodes. Langmuir 2001, 17, 7032–7039. [Google Scholar] [CrossRef]
- Zestos, A.G.; Nguyen, M.D.; Poe, B.L.; Jacobs, C.B.; Venton, B.J. Epoxy insulated carbon fiber and carbon nanotube fiber microelectrodes. Sens. Actuators B Chem. 2013, 182, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Heien, M.L.; Phillips, P.E.; Stuber, G.D.; Seipel, A.T.; Wightman, R.M. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 2003, 128, 1413–1419. [Google Scholar] [CrossRef]
- Roberts, J.G.; Moody, B.P.; McCarty, G.S.; Sombers, L.A. Specific oxygen-containing functional groups on the carbon surface underlie an enhanced sensitivity to dopamine at electrochemically pretreated carbon fiber microelectrodes. Langmuir 2010, 26, 9116–9122. [Google Scholar] [CrossRef]
- Zestos, A.G.; Jacobs, C.B.; Trikantzopoulos, E.; Ross, A.E.; Venton, B.J. Polyethylenimine Carbon Nanotube Fiber Electrodes for Enhanced Detection of Neurotransmitters. Anal. Chem. 2014, 86, 8568–8575. [Google Scholar] [CrossRef]
- Hashemi, P.; Dankoski, E.C.; Petrovic, J.; Keithley, R.B.; Wightman, R. Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal. Chem. 2009, 81, 9462–9471. [Google Scholar] [CrossRef]
- Wonnenberg, P.; Cho, W.; Liu, F.; Asrat, T.; Zestos, A.G. Polymer Modified Carbon Fiber Microelectrodes for Precision Neurotransmitter Metabolite Measurements. J. Electrochem. Soc. 2020, 167, 167507. [Google Scholar] [CrossRef]
- Rafi, H.; Zestos, A.G. Multiplexing neurochemical detection with carbon fiber multielectrode arrays using fast-scan cyclic voltammetry. Anal. Bioanal. Chem. 2021, 413, 6715–6726. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Z.; Ren, T.; Halpern, J.M. Nanostructured cyclodextrin-mediated surface for capacitive determination of cortisol in multiple biofluids. ACS Appl. Mater. Interfaces 2022, 14, 42374–42387. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Stine, J.M.; Chapin, A.; Ghodssi, R. A Portable Electrochemical Sensing Platform for Serotonin Detection Based on Surface-Modified Carbon Fiber Microelectrode. Anal. Methods 2023, 15, 1096–1104. [Google Scholar] [CrossRef]
- Cho, W.; Rafi, H.; Cho, S.; Balijepalli, A.; Zestos, A.G. High resolution voltammetric and field-effect transistor readout of carbon fiber microelectrode biosensors. Sens. Diagn. 2022, 1, 460–464. [Google Scholar] [CrossRef] [PubMed]





| Cortisol (μM) in Dilution | Percent Recovery of Cortisol in Urine |
|---|---|
| 1 μM | 53.1% |
| 5 μM | 98.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadad, M.; Hadad, N.; Zestos, A.G. Carbon Electrode Sensor for the Measurement of Cortisol with Fast-Scan Cyclic Voltammetry. Biosensors 2023, 13, 626. https://doi.org/10.3390/bios13060626
Hadad M, Hadad N, Zestos AG. Carbon Electrode Sensor for the Measurement of Cortisol with Fast-Scan Cyclic Voltammetry. Biosensors. 2023; 13(6):626. https://doi.org/10.3390/bios13060626
Chicago/Turabian StyleHadad, Michelle, Nadine Hadad, and Alexander G. Zestos. 2023. "Carbon Electrode Sensor for the Measurement of Cortisol with Fast-Scan Cyclic Voltammetry" Biosensors 13, no. 6: 626. https://doi.org/10.3390/bios13060626
APA StyleHadad, M., Hadad, N., & Zestos, A. G. (2023). Carbon Electrode Sensor for the Measurement of Cortisol with Fast-Scan Cyclic Voltammetry. Biosensors, 13(6), 626. https://doi.org/10.3390/bios13060626