Non-Destructive Screening of Sodium Metabisulfite Residue on Shrimp by SERS with Copy Paper Loaded with AgNP
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Synthesis of AgNPs
2.3. Fabrication of AgNP−CP
2.4. Preparation of Standard Solutions
2.5. Preparation of Shrimp Samples
2.6. SERS Sampling and Measurement
3. Results and Discussion
3.1. Fabrication and Characterization of AgNP-CP
3.2. Usability Evaluation of the SERS Substrate
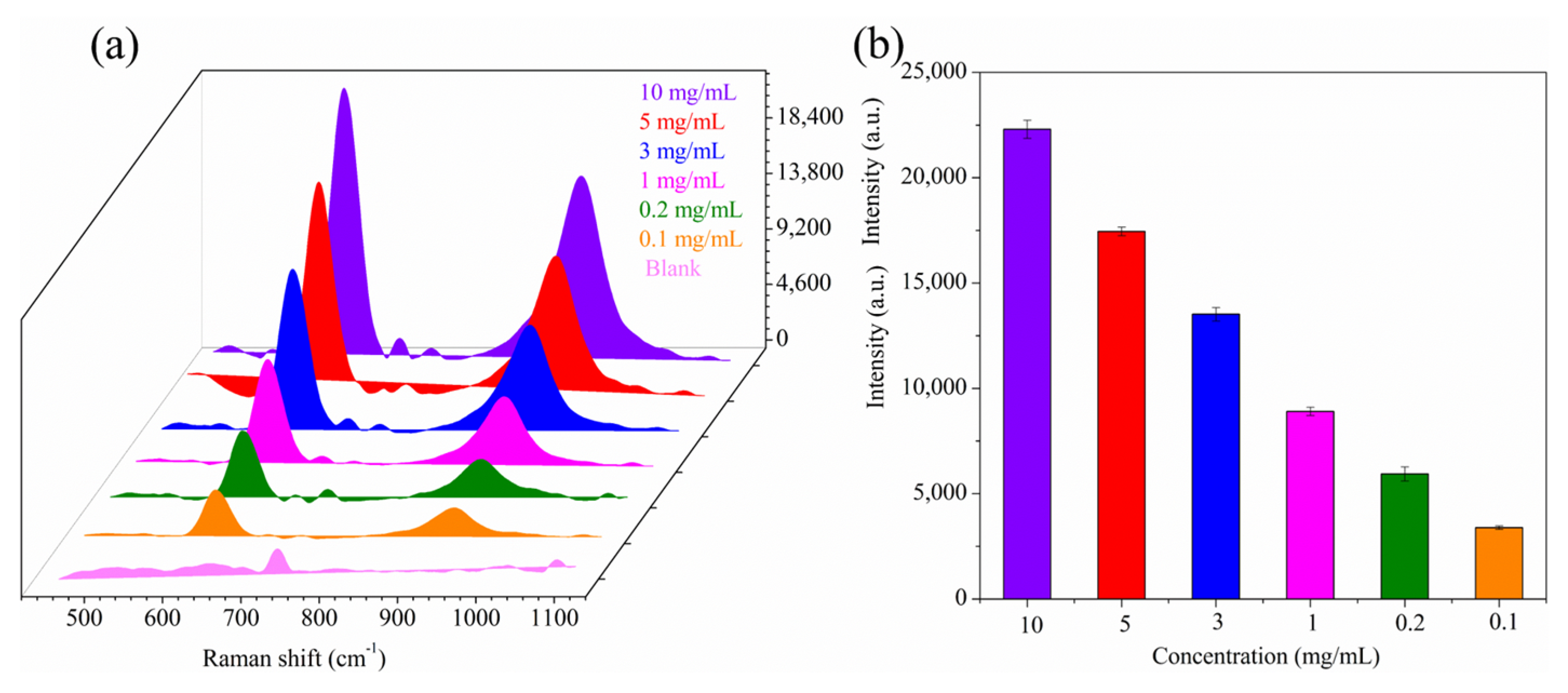
3.3. Analysis Sensitivity
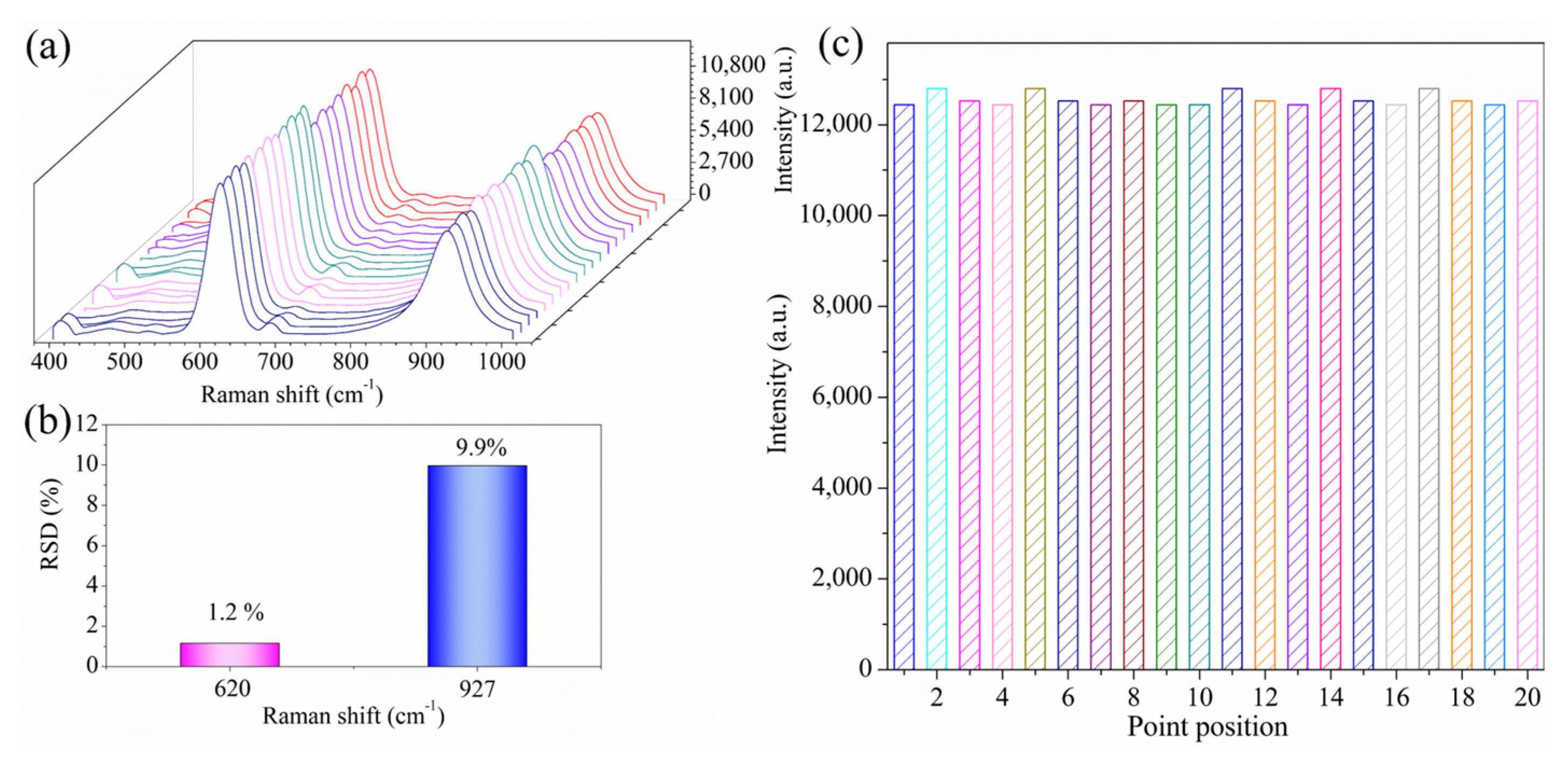
3.4. Precision Evaluation of the Analysis
3.5. Stability Evaluation of the Analysis
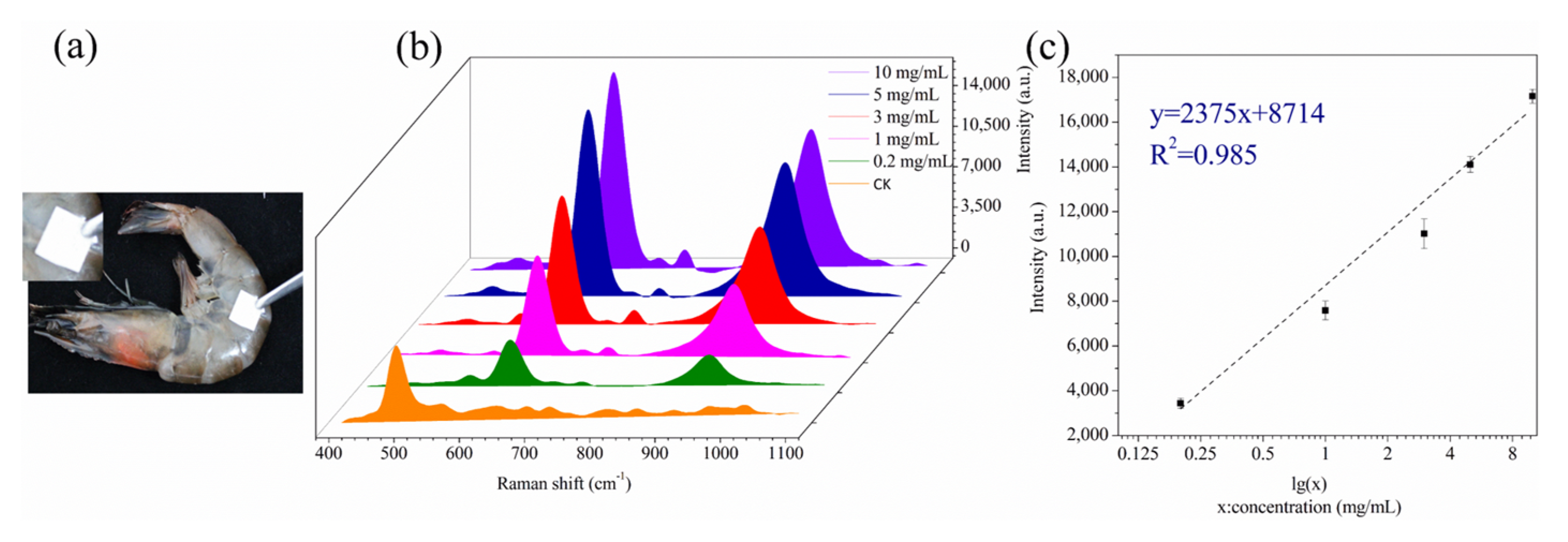
3.6. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robbins, K.S.; Shah, R.; MacMahon, S.; de Jager, L.S. Development of a Liquid Chromatography–Tandem Mass Spectrometry Method for the Determination of Sulfite in Food. J. Agric. Food Chem. 2015, 63, 5126–5132. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, H.; Guan, X.L.; Long, L.H.; Hu, Z.L.; Ni, L.; Wang, F.; Chen, J.G.; Wu, P.F. Sulfite triggers sustained calcium overload in cultured cortical neurons via a redox-dependent mechanism. Toxicol. Lett. 2016, 258, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhu, F.; Chen, L.; Wu, H.; Wang, T.; Chen, K. Mechanism analysis of toxicity of sodium sulfite to human hepatocytes L02. Mol. Cell. Biochem. 2020, 473, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.Y.; Yun, Y.; Sang, N. Differential Effects Between One Week and Four Weeks Exposure to Same Mass of SO2 on Synaptic Plasticity in Rat Hippocampus. Environ. Toxicol. 2016, 31, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Yue, H.; Yun, Y.; Sang, N. Chronic SO2 inhalation above environmental standard impairs neuronal behavior and represses glutamate receptor gene expression and memory-related kinase activation via neuroinflammation in rats. Environ. Res. 2015, 137, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Pizzoferrato, L.; Quattrucci, E.; Di Lullo, G. Evaluation of an HPLC method for the determination of sulphiting agents in foods. Food Addit. Contam. 1990, 7, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Theisen, S.; Hansch, R.; Kothe, L.; Leist, U.; Galensa, R. A fast and sensitive HPLC method for sulfite analysis in food based on a plant sulfite oxidase biosensor. Biosens. Bioelectron. 2010, 26, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.C.; Chan, B.T.-P.; Chan, A. Determination of free and reversibly-bound sulfite in selected foods by high-performance liquid chromatography with fluorometric detection. J. AOAC Int. 2008, 91, 98–102. [Google Scholar]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, C.; Jin, Z.; Xu, X.; Cai, Y.; Bai, Y. HPTLC-bioautography/SERS screening nifedipine adulteration in food supplement based on Ginkgo biloba. Microchem. J. 2020, 154, 104647. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, C.; Hellmann, B.; Xu, X. HPTLC-Densitometry Determination of Riboflavin Fortified in Rice Noodle: Confirmed by SERS-Fingerprint. Food Anal. Methods 2020, 13, 718–725. [Google Scholar] [CrossRef]
- Pan, C.; Chen, H.J.; Lin, Q.; Luo, S.H.; Gu, J.L.; Ye, S.Q.; Zeng, Y.M.; Ren, B.; Tian, Z.Q.; Xue, W.D.; et al. Evaluation of the SERS-based strategy in fast and on-site food safety inspection: Qualitative and quantitative analysis of trace unexpected herbicide in complicated herbicide matrix. J. Raman Spectrosc. 2020, 51, 2562–2567. [Google Scholar] [CrossRef]
- Xu, M.L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of Pesticide Residues in Food Using Surface-Enhanced Raman Spectroscopy: A Review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef]
- Xin, H.; Namgung, B.; Lee, L.P. Nanoplasmonic optical antennas for life sciences and medicine. Nat. Rev. Mater. 2018, 3, 228–243. [Google Scholar] [CrossRef]
- Ma, H.; Liu, S.; Liu, Y.; Zhu, J.; Han, X.X.; Ozaki, Y.; Zhao, B. In-situ fingerprinting phosphorylated proteins via surface-enhanced Raman spectroscopy: Single-site discrimination of Tau biomarkers in Alzheimer’s disease. Biosens. Bioelectron. 2021, 171, 112748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Z.; Zou, Y.; Farooq, S.; Li, Y.; Zhang, H. Novel Ag-coated nanofibers prepared by electrospraying as a SERS platform for ultrasensitive and selective detection of nitrite in food. Food Chem. 2023, 412, 135563. [Google Scholar] [CrossRef]
- Bhaskar, S.; Srinivasan, V.; Ramamurthy, S.S. Nd2O3-Ag Nanostructures for Plasmonic Biosensing, Antimicrobial, and Anticancer Applications. ACS Appl. Nano Mater. 2023, 6, 1129–1145. [Google Scholar] [CrossRef]
- Xiong, Y.; Shepherd, S.; Tibbs, J.; Bacon, A.; Liu, W.; Akin, L.D.; Ayupova, T.; Bhaskar, S.; Cunningham, B.T. Photonic Crystal Enhanced Fluorescence: A Review on Design Strategies and Applications. Micromachines 2023, 14, 668. [Google Scholar] [CrossRef]
- Beeram, R.; Vepa, K.R.; Soma, V.R. Recent Trends in SERS-Based Plasmonic Sensors for Disease Diagnostics, Biomolecules Detection, and Machine Learning Techniques. Biosensors 2023, 13, 328. [Google Scholar] [CrossRef]
- Zhang, D.; Pu, H.; Huang, L.; Sun, D.-W. Advances in flexible surface-enhanced Raman scattering (SERS) substrates for nondestructive food detection: Fundamentals and recent applications. Trends Food Sci. Technol. 2021, 109, 690–701. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Chen, Y. HPTLC+SRES screening of pesticide for point-of-care application as shown with thiram in juice. Food Chem. X 2023, 18, 100670. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hao, Q.; Zhao, Y.; Chen, Y. Two-Dimensional Printed AgNPs@Paper Swab for SERS Screening of Pesticide Residues on Apples and Pears. J. Agric. Food Chem. 2023, 71, 4982–4989. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R. SERS detection of food contaminants by means of portable Raman instruments. J. Raman Spectrosc. 2018, 49, 954–981. [Google Scholar] [CrossRef]
- Restaino, S.M.; White, I.M. A critical review of flexible and porous SERS sensors for analytical chemistry at the point-of-sample. Anal. Chim. Acta 2019, 1060, 17–29. [Google Scholar] [CrossRef]
- Xie, J.; Li, L.; Khan, I.M.; Wang, Z.; Ma, X. Flexible paper-based SERS substrate strategy for rapid detection of methyl parathion on the surface of fruit. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118104. [Google Scholar] [CrossRef]
- Villa, J.E.L.; Quiñones, N.R.; Fantinatti-Garboggini, F.; Poppi, R.J. Fast discrimination of bacteria using a filter paper–based SERS platform and PLS-DA with uncertainty estimation. Anal. Bioanal. Chem. 2019, 411, 705–713. [Google Scholar] [CrossRef]
- Maddipatla, D.; Narakathu, B.B.; Atashbar, M. Recent Progress in Manufacturing Techniques of Printed and Flexible Sensors: A Review. Biosensors 2020, 10, 199. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Chen, M.; Yang, H.; Rong, L.; Chen, X. A gas-diffusion microfluidic paper-based analytical device (μPAD) coupled with portable surface-enhanced Raman scattering (SERS): Facile determination of sulphite in wines. Analyst 2016, 141, 5511–5519. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, X.X.; Wang, Y.R.; Fang, E.H.; Zhang, Z.G.; Chen, X. Headspace Thin-Film Microextraction Coupled with Surface-Enhanced Raman Scattering as a Facile Method for Reproducible and Specific Detection of Sulfur Dioxide in Wine. Anal. Chem. 2015, 87, 633–640. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, Q.; Tan, L.L.; Liu, C.G.; Shang, L. Metal-Organic Frameworks-Mediated Assembly of Gold Nanoclusters for Sensing Applications. J. Anal. Test. 2022, 6, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Soni, R.K. Silver nanodendrites for ultralow detection of thiram based on surface-enhanced Raman spectroscopy. Nanotechnology 2019, 30, 385502. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; He, M.; Ran, J.; Cai, G.; Wu, J.; Wang, X. Depositing a flexible substrate of triangular silver nanoplates onto cotton fabrics for sensitive SERS detection. Sens. Actuators B Chem. 2018, 270, 508–517. [Google Scholar] [CrossRef]
- Bai, X.; Xiao, Q.; Zhou, L.; Tang, Y.; He, Y. Detection of Sulfite Dioxide Residue on the Surface of Fresh-Cut Potato Slices Using Near-Infrared Hyperspectral Imaging System and Portable Near-Infrared Spectrometer. Molecules 2020, 25, 1651. [Google Scholar] [CrossRef] [PubMed]
- Xiang, K.; Chang, S.; Feng, J.; Li, C.; Ming, W.; Liu, Z.; Liu, Y.; Tian, B.; Zhang, J. A colorimetric and ratiometric fluorescence probe for rapid detection of SO2 derivatives bisulfite and sulfite. Dye. Pigment. 2016, 134, 190–197. [Google Scholar] [CrossRef]
- Ivković, B.; Brborić, J.; Dobričić, V.; Čudina, O. Development and validation of a new isocratic RP-HPLC method for simultaneous determination of sodium metabisulfite and sodium benzoate in pharmaceutical formulation. Acta Chromatogr. 2019, 31, 133–137. [Google Scholar] [CrossRef]




| Fingerprint Peak (cm−1) | Intensity | Signal Assignment |
|---|---|---|
| 620 | Strong | Symmetrical bending vibrations of O-S-O |
| 927 | Medium | Symmetrical and asymmetric S-O stretching vibrations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Zhao, Y.; Xi, X.; Chen, Y. Non-Destructive Screening of Sodium Metabisulfite Residue on Shrimp by SERS with Copy Paper Loaded with AgNP. Biosensors 2023, 13, 575. https://doi.org/10.3390/bios13060575
Yuan C, Zhao Y, Xi X, Chen Y. Non-Destructive Screening of Sodium Metabisulfite Residue on Shrimp by SERS with Copy Paper Loaded with AgNP. Biosensors. 2023; 13(6):575. https://doi.org/10.3390/bios13060575
Chicago/Turabian StyleYuan, Chao, Yanan Zhao, Xingjun Xi, and Yisheng Chen. 2023. "Non-Destructive Screening of Sodium Metabisulfite Residue on Shrimp by SERS with Copy Paper Loaded with AgNP" Biosensors 13, no. 6: 575. https://doi.org/10.3390/bios13060575
APA StyleYuan, C., Zhao, Y., Xi, X., & Chen, Y. (2023). Non-Destructive Screening of Sodium Metabisulfite Residue on Shrimp by SERS with Copy Paper Loaded with AgNP. Biosensors, 13(6), 575. https://doi.org/10.3390/bios13060575





