Microfluidic Platform Integrated with Carbon Nanofibers-Decorated Gold Nanoporous Sensing Device for Serum PSA Quantification
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Microfluidic Device Fabrication
2.3. CNFs/GNP Electrode Modification
2.4. Antibodies Immobilization
2.5. Analytical Procedure for PSA Quantification
2.6. Serum Sample Collection
2.7. Commercial ELISA Kit
3. Results and Discussion
3.1. CNFs/GNP Characterization
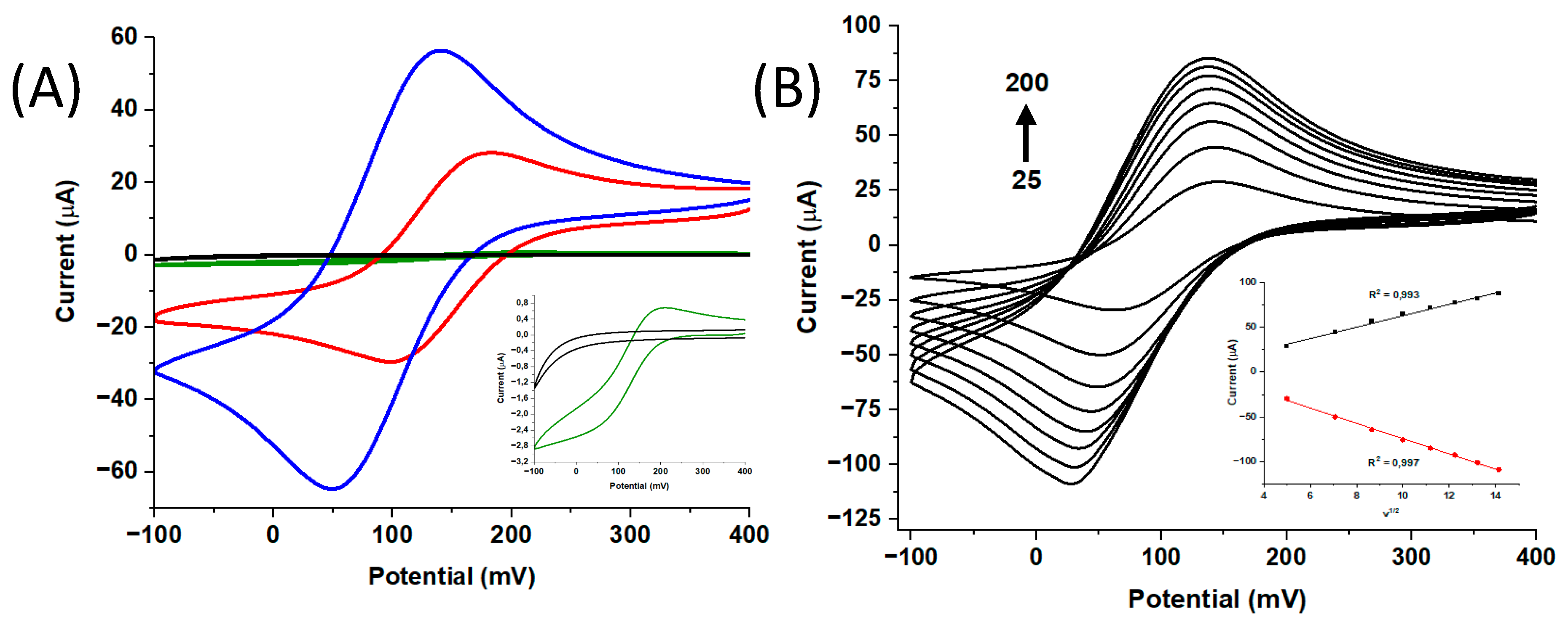
3.2. Optimization of Experimental Parameters
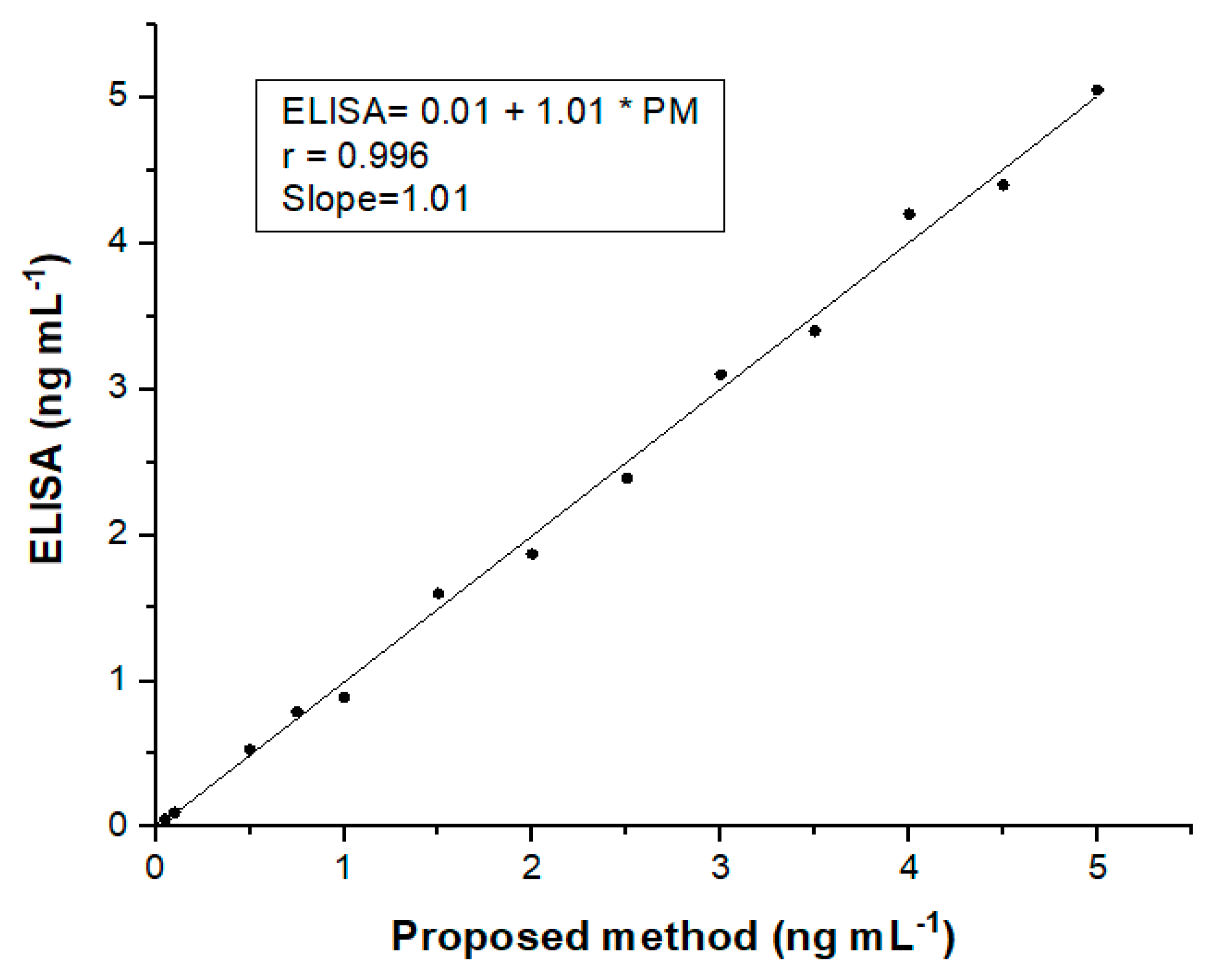
3.3. Analytical Performance of the Electrochemical Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krześniak, A.; Gabler, T.; Janik, M.; Koba, M.; Jönsson-Niedziółka, M.; Śmietana, M. A Microfluidic System for Analysis of Electrochemical Processing Using a Highly Sensitive Optical Fiber Microcavity. Opt. Lasers Eng. 2022, 158, 107173. [Google Scholar] [CrossRef]
- Gharib, G.; Bütün, İ.; Muganlı, Z.; Kozalak, G.; Namlı, İ.; Sarraf, S.S.; Ahmadi, V.E.; Toyran, E.; van Wijnen, A.J.; Koşar, A. Biomedical Applications of Microfluidic Devices: A Review. Biosensors 2022, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, Y.; Chen, Y.; Yang, M.; Zhao, S.; Qi, N.; Wang, Y.; Huo, D.; Hou, C. Hemin-Functionalized Microfluidic Chip with Dual-Electric Signal Outputs for Accurate Determination of Uric Acid. ACS Appl. Mater. Interfaces 2022, 14, 41369–41378. [Google Scholar] [CrossRef]
- Uliana, C.V.; Peverari, C.R.; Afonso, A.S.; Cominetti, M.R.; Faria, R.C. Fully Disposable Microfluidic Electrochemical Device for Detection of Estrogen Receptor Alpha Breast Cancer Biomarker. Biosens. Bioelectron. 2018, 99, 156–162. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Yang, J.; He, Y.; Li, Y. Application of Multiplex Microfluidic Electrochemical Sensors in Monitoring Hematological Tumor Biomarkers. Anal. Chem. 2020, 92, 11981–11986. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, H.; Wang, D.; Zhang, M.; Yang, Y.; Ren, T. Recent Advances in Microfluidic Sensors for Nutrients Detection in Water. TrAC—Trends Anal. Chem. 2023, 158, 116790. [Google Scholar] [CrossRef]
- Jannah, F.; Park, S.; Heo, J.M.; Choi, N.; Choo, J.; Kim, J.M. Rapid and Sensitive Detection of Lysophosphatidylcholine Using Zwitterionic Polydiacetylene Vesicles and a Microfluidic Gradient Sensor. Sens. Actuators B Chem. 2022, 371, 132528. [Google Scholar] [CrossRef]
- Hardeep Kaur, S.; Kumari, N.; Sharma, A.; Sachdeva, M.; Mutreja, V. Optical and Electrochemical Microfluidic Sensors for Water Contaminants: A Short Review. Mater. Today Proc. 2021, 48, 1673–1679. [Google Scholar] [CrossRef]
- Alnaimi, A.; Al-Hamry, A.; Makableh, Y.; Adiraju, A.; Kanoun, O. Gold Nanoparticles-MWCNT Based Aptasensor for Early Diagnosis of Prostate Cancer. Biosensors 2022, 12, 1130. [Google Scholar] [CrossRef]
- Garg, M.; Christensen, M.G.; Iles, A.; Sharma, A.L.; Singh, S.; Pamme, N. Microfluidic-Based Electrochemical Immunosensing of Ferritin. Biosensors 2020, 10, 91. [Google Scholar] [CrossRef]
- Lai, Z.-X.; Wu, C.-C.; Huang, N.-T. A Microfluidic Platform with an Embedded Miniaturized Electrochemical Sensor for On-Chip Plasma Extraction Followed by In Situ High-Sensitivity C-Reactive Protein (Hs-CRP) Detection. Biosensors 2022, 12, 1163. [Google Scholar] [CrossRef] [PubMed]
- Regiart, M.; Gimenez, A.M.; Marques, R.F.; Soares, I.S.; Bertotti, M. Microfluidic Device Based on Electrodeposited Nanoporous Gold/Carbon Nanotubes for Plasmodium Vivax Detection. Sens. Actuators B Chem. 2021, 340, 129961. [Google Scholar] [CrossRef]
- Scala-Benuzzi, M.L.; Soler-Illia, G.J.A.A.; Raba, J.; Battaglini, F.; Schneider, R.J.; Pereira, S.V.; Messina, G.A. Immunosensor Based on Porous Gold and Reduced Graphene Platform for the Determination of EE2 by Electrochemical Impedance Spectroscopy. J. Electroanal. Chem. 2021, 897, 115604. [Google Scholar] [CrossRef]
- Rajaji, U.; Kumar, Y.; Chen, S.M.; Raghu, M.S.; Parashuram, L.; Alzahrani, F.M.; Alsaiari, N.S.; Ouladsmane, M. Deep Eutectic Solvent Synthesis of Iron Vanadate-Decorated Sulfur-Doped Carbon Nanofiber Nanocomposite: Electrochemical Sensing Tool for Doxorubicin. Microchem. Acta 2021, 188, 303. [Google Scholar] [CrossRef]
- Kim, G.; Kim, B.H. One-Dimensional Hierarchical Porous Carbon Nanofibers with Cobalt Oxide in a Hollow Channel for Electrochemical Applications. J. Alloys Compd. 2022, 910, 164886. [Google Scholar] [CrossRef]
- Sun, L.; Sun, Y.; Fu, Q.; Pan, C. Facile Preparation of NiO Nanoparticles Anchored on N/P-Codoped 3D Carbon Nanofibers Network for High-Performance Asymmetric Supercapacitors. J. Alloys Compd. 2021, 888, 161488. [Google Scholar] [CrossRef]
- Verma, V.; Kala, D.; Gupta, S.; Kaushal, A.; Kumar, D. AuNs-GO Nanocomposite Modified Paper-Based Amperometric Biosensor as an Alternative Approach for Early Investigation of Leptospirosis. Biointerface Res. Appl. Chem. 2023, 13, 242. [Google Scholar] [CrossRef]
- Sadeghi, M.; Shabani-Nooshabadi, M.; Ansarinejad, H. A Nanoporous Gold Film Sensor Modified with Polypyrrole/CuO Nanocomposite for Electrochemical Determination of Piroxicam and Tramadole. Environ. Res. 2023, 216, 114633. [Google Scholar] [CrossRef]
- Siqueira, G.P.; de Faria, L.V.; Rocha, R.G.; Matias, T.A.; Richter, E.M.; Muñoz, R.A.A.; da Silva, I.S.; Dantas, L.M.F. Nanoporous Gold Microelectrode Arrays Using Microchips: A Highly Sensitive and Cost-Effective Platform for Electroanalytical Applications. J. Electroanal. Chem. 2022, 925, 116880. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Zhang, S.; Zeng, H.; Xia, C.; Zuo, T.; Yang, Z.; Zou, X.; He, J. Cancer Incidence and Mortality in China, 2013. Cancer Lett. 2017, 401, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, S.M.; Sheta, S.M.; Salem, S.R.; Abd-Elzaher, M.M.; Basaleh, A.S.; Labib, A.A. Prostate-Specific Antigen Monitoring Using Nano Zinc(II) Metal–Organic Framework-Based Optical Biosensor. Biosensors 2022, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.E.; Çakirsoy Çakar, G.; Azimjonov, J.; Kösem, M.; Cedi̇moğlu, İ.H. Automated Prostate Cancer Grading and Diagnosis System Using Deep Learning-Based Yolo Object Detection Algorithm. Expert Syst. Appl. 2022, 201, 117148. [Google Scholar] [CrossRef]
- Bilal, M.; Javaid, A.; Amjad, F.; Youssif, T.A.; Afzal, S. An Overview of Prostate Cancer (PCa) Diagnosis: Potential Role of MiRNAs. Transl. Oncol. 2022, 26, 101542. [Google Scholar] [CrossRef]
- Hu, Q.; Gan, S.; Bao, Y.; Zhang, Y.; Han, D.; Niu, L. Electrochemically Controlled ATRP for Cleavage-Based Electrochemical Detection of the Prostate-Specific Antigen at Femtomolar Level Concentrations. Anal. Chem. 2020, 92, 15982–15988. [Google Scholar] [CrossRef]
- Neeli, S.; Sharma, M.; Musale, A. Prostate-Specific Antigen: An Overview and Its Current Status in the Diagnosis of Prostate Cancer. Indian J. Health Sci. Biomed. Res. 2021, 14, 22–30. [Google Scholar] [CrossRef]
- Milligan, K.; Deng, X.; Ali-Adeeb, R.; Shreeves, P.; Punch, S.; Costie, N.; Crook, J.M.; Brolo, A.G.; Lum, J.J.; Andrews, J.L.; et al. Prediction of Disease Progression Indicators in Prostate Cancer Patients Receiving HDR-Brachytherapy Using Raman Spectroscopy and Semi-Supervised Learning: A Pilot Study. Sci. Rep. 2022, 12, 15104. [Google Scholar] [CrossRef]
- Marín-Barroso, E.; Messina, G.A.; Bertolino, F.A.; Raba, J.; Pereira, S.V. Electrochemical Immunosensor Modified with Carbon Nanofibers Coupled to a Paper Platform for the Determination of Gliadins in Food Samples. Anal. Methods 2019, 11, 2170–2178. [Google Scholar] [CrossRef]
- Regiart, M.; Ledo, A.; Fernandes, E.; Messina, G.A.; Brett, C.M.A.; Bertotti, M.; Barbosa, R.M. Highly Sensitive and Selective Nanostructured Microbiosensors for Glucose and Lactate Simultaneous Measurements in Blood Serum and in Vivo in Brain Tissue. Biosens. Bioelectron. 2022, 199, 113874. [Google Scholar] [CrossRef]
- Regiart, M.; Gimenez, A.; Lopes, A.; Carreño, M.; Bertotti, M. Ultrasensitive microfluidic electrochemical immunosensor based on electrodeposited nanoporous gold for SOX-2 determination. Anal. Chim. Acta 2020, 1127, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.G.; Gómez, G.E.; González-Martinez, C.; Valero, T.; Expósito-Hernández, J.; Puche, I.; Rodriguez-Martinez, A.; Serrano, M.J.; Lorente, J.A.; Fernández-Baldo, M.A. A Novel, Quick, and Reliable Smartphone-Based Method for Serum PSA Quantification: Original Design of a Portable Microfluidic Immunosensor-Based System. Cancers 2022, 14, 4483. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.G.; Gomez, G.E.; Boni, C.; Cañas García, I.; Garrido Navas, C.; D’vries, R.F.; Molina Vallejos, M.P.; Serrano, M.J.; Messina, G.A.; Expósito Hernández, J.; et al. Microfluidic amperometric immunosensor based on porous nanomaterial towards claudin7 determination for colorectal cancer diagnosis. Talanta 2023, 251, 123766. [Google Scholar] [CrossRef]
- Takita, S.; Nabok, A.; Lishchuk, A.; Mussa, M.H.; Smith, D. Detection of Prostate Cancer Biomarker PCA3 with Electrochemical Apta-Sensor. Eng. Proc. 2022, 16, 8. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, C.; Chang, H.; Jiang, M.; Sun, X.; Jing, W.; Huang, H.; Huang, D.; Kong, L.; Chen, Z.; et al. A Label-Free Photoelectrochemical Immunosensor for Prostate Specific Antigen Detection Based on Ag2S Sensitized Ag/AgBr/BiOBr Heterojunction by in-Situ Growth Method. Bioelectrochemistry 2021, 142, 107928. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, E.; Ehzari, H.; Shahlaei, M.; Behbood, L.; Arkan, E. The Electrochemical Immunosensor for Detection of Prostatic Specific Antigen Using Quince Seed Mucilage-GNPs-SNPs as a Green Composite. Bioelectrochemistry 2021, 139, 107744. [Google Scholar] [CrossRef]
- Assari, P.; Rafati, A.A.; Feizollahi, A.; Joghani, R.A. Fabrication of a Sensitive Label Free Electrochemical Immunosensor for Detection of Prostate Specific Antigen Using Functionalized Multi-Walled Carbon Nanotubes/Polyaniline/AuNPs. Mater. Sci. Eng. C 2020, 115, 111066. [Google Scholar] [CrossRef]
- Shamsazar, A.; Asadi, A.; Seifzadeh, D.; Mahdavi, M. A Novel and Highly Sensitive Sandwich-Type Immunosensor for Prostate-Specific Antigen Detection Based on MWCNTs-Fe3O4 Nanocomposite. Sens. Actuators B Chem. 2021, 346, 130459. [Google Scholar] [CrossRef]
- Chen, S.; Xu, L.; Sheng, K.; Zhou, Q.; Dong, B.; Bai, X.; Lu, G.; Song, H. A Label-Free Electrochemical Immunosensor Based on Facet-Controlled Au Nanorods/Reduced Graphene Oxide Composites for Prostate Specific Antigen Detection. Sens. Actuators B Chem. 2021, 336, 129748. [Google Scholar] [CrossRef]
- Medetalibeyoglu, H.; Kotan, G.; Atar, N.; Yola, M.L. A Novel and Ultrasensitive Sandwich-Type Electrochemical Immunosensor Based on Delaminated MXene@AuNPs as Signal Amplification for Prostate Specific Antigen (PSA) Detection and Immunosensor Validation. Talanta 2020, 220, 121403. [Google Scholar] [CrossRef]
- Ehzari, H.; Amiri, M.; Safari, M. Enzyme-Free Sandwich-Type Electrochemical Immunosensor for Highly Sensitive Prostate Specific Antigen Based on Conjugation of Quantum Dots and Antibody on Surface of Modified Glassy Carbon Electrode with Core–Shell Magnetic Metal-Organic Frameworks. Talanta 2020, 210, 120641. [Google Scholar] [CrossRef] [PubMed]
- Choosang, J.; Khumngern, S.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. An Ultrasensitive Label-Free Electrochemical Immunosensor Based on 3D Porous Chitosan–Graphene–Ionic Liquid–Ferrocene Nanocomposite Cryogel Decorated with Gold Nanoparticles for Prostate-Specific Antigen. Talanta 2021, 224, 121787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, X.; Mei, L.; Zhang, L.; Wang, X.; Liao, X.; Qiao, X.; Hong, C. PSA Detection Electrochemical Immunosensor Based on MOF-235 Nanomaterial Adsorption Aggregation Signal Amplification Strategy. Microchem. J. 2021, 171, 106870. [Google Scholar] [CrossRef]



| Method | Time (min) | CV % a Within-Assay | CV % a Between-Assay | CV% a | Linear Range | LOD |
|---|---|---|---|---|---|---|
| ELISA | 270 | 7.20 | 8.23 | 6.45 | 0.05–5 b | 45 c |
| MI d | 21 | 4.40 | 6.15 | 3.85 | 0.01–50 b | 5 c |
| Samples | Addition b | ELISA | Recovery% | MI d | Recovery% |
|---|---|---|---|---|---|
| − a | 0 | 0 | - | 0 | - |
| − | 0.1 | 0.089 + 0.002 c | 89 | 0.097 + 0.001 | 97 |
| − | 1 | 1.11 + 0.04 | 111 | 1.04 + 0.02 | 104 |
| − | 5 | 4.91 + 0.07 | 98.2 | 5.04 + 0.04 | 100.8 |
| − | 10 | 9.71 + 0.09 | 97.1 | 9.95 + 0.06 | 99.5 |
| − | 25 | 24.1 + 0.15 | 96.4 | 25.3 + 0.07 | 101.2 |
| − | 50 | 47.7 + 0.21 | 95.4 | 49.7 + 0.09 | 99.4 |
| + e | 0 | 0.25 + 0.02 | - | 0.25 + 0.01 | - |
| + | 0 | 0.98 + 0.04 | - | 0.97 + 0.03 | - |
| + | 0 | 1.63 + 0.08 | - | 1.65 + 0.04 | - |
| + | 0 | 3.45 + 0.11 | - | 3.43+ 0.06 | - |
| + | 0 | 5.15 + 0.16 | - | 5.18 + 0.09 | - |
| Assay Type | Platform | Detection Technique | Dynamic Range | LOD | Ref |
|---|---|---|---|---|---|
| Immunomagnetic assay | Microfluidic device | Amperometry | 10 pg mL−1 to 1500 pg mL−1 | 2 pg mL−1 | [32] |
| Label-free immunosensing | Ab/Ag2S/BiOBr/AgBr/Ag/ITO | Photo-electrochemistry | 0.001 to 50 ng mL−1 | 0.25 pg mL−1 | [35] |
| Label-free immunosensing | Ab/mucilage-GNPs-SNPs/GCE | DPV | 0.1 pg mL−1 to 100 ng mL−1 | 0.078 pg mL−1 | [36] |
| Label-free immunosensing | anti-PSA/AuNPs/PANI/MWCNTs-COOH/GCE | DPV | 1.66 pg·mL−1 to 1.3 ng·mL−1 | 0.5 pg·mL−1 | [37] |
| Sandwich-type immunoassay | Ab/Fe3O4/MWCNT/GCE | DPV | 2.5 pg mL−1 to 100 ng mL−1 | 0.39 pg mL−1 | [38] |
| Label-free immunosensing | Ab-Cs-rGO/AuNRs-FTO | DPV | 0.1 to 150 ng mL−1 | 16 pg mL−1 | [39] |
| Sandwich-type immunoassay | Ab/AuNPs-ATPGO/GCE and d-Ti3C2TX MXene@AuNPs as label of Ab2 | DPV | 0.01 to 1.0 pg mL−1 | 3 × 103 pg mL−1 | [40] |
| Sandwich-type immunoassay | Ab/CHIT-MOF/GCE and QDs as label of Ab2 | DPV | 1 pg mL−1 to 100 ng mL−1 | 0.45 pg mL−1 | [41] |
| Label-free immunosensing | Ab/AuNPs/CS–GR–IL–Fc cry/SPCE | DPV | 1.0 × 10−7 to 1.0 × 10−1 ng mL−1 | 4.8 × 10−5 pg mL−1 | [42] |
| Sandwich-type immunoassay | Ab/AuNPs/GCE and MOF-235/MB as label of Ab2 | DPV | 10 to 1200 pg⋅mL−1 | 3 pg⋅mL−1 | [43] |
| Sandwich-type immunoassay | Microfluidic device with Ab/CNFs/GNP/Pt electrode | Amperometry | 0.01 to 50 ng mL−1 | 5 pg mL−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felici, E.; Regiart, M.D.; Pereira, S.V.; Ortega, F.G.; Angnes, L.; Messina, G.A.; Fernández-Baldo, M.A. Microfluidic Platform Integrated with Carbon Nanofibers-Decorated Gold Nanoporous Sensing Device for Serum PSA Quantification. Biosensors 2023, 13, 390. https://doi.org/10.3390/bios13030390
Felici E, Regiart MD, Pereira SV, Ortega FG, Angnes L, Messina GA, Fernández-Baldo MA. Microfluidic Platform Integrated with Carbon Nanofibers-Decorated Gold Nanoporous Sensing Device for Serum PSA Quantification. Biosensors. 2023; 13(3):390. https://doi.org/10.3390/bios13030390
Chicago/Turabian StyleFelici, Emiliano, Matías D. Regiart, Sirley V. Pereira, Francisco G. Ortega, Lúcio Angnes, Germán A. Messina, and Martín A. Fernández-Baldo. 2023. "Microfluidic Platform Integrated with Carbon Nanofibers-Decorated Gold Nanoporous Sensing Device for Serum PSA Quantification" Biosensors 13, no. 3: 390. https://doi.org/10.3390/bios13030390
APA StyleFelici, E., Regiart, M. D., Pereira, S. V., Ortega, F. G., Angnes, L., Messina, G. A., & Fernández-Baldo, M. A. (2023). Microfluidic Platform Integrated with Carbon Nanofibers-Decorated Gold Nanoporous Sensing Device for Serum PSA Quantification. Biosensors, 13(3), 390. https://doi.org/10.3390/bios13030390







