Enhanced Coloration Time of Electrochromic Device Using Integrated WO3@PEO Electrodes for Wearable Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
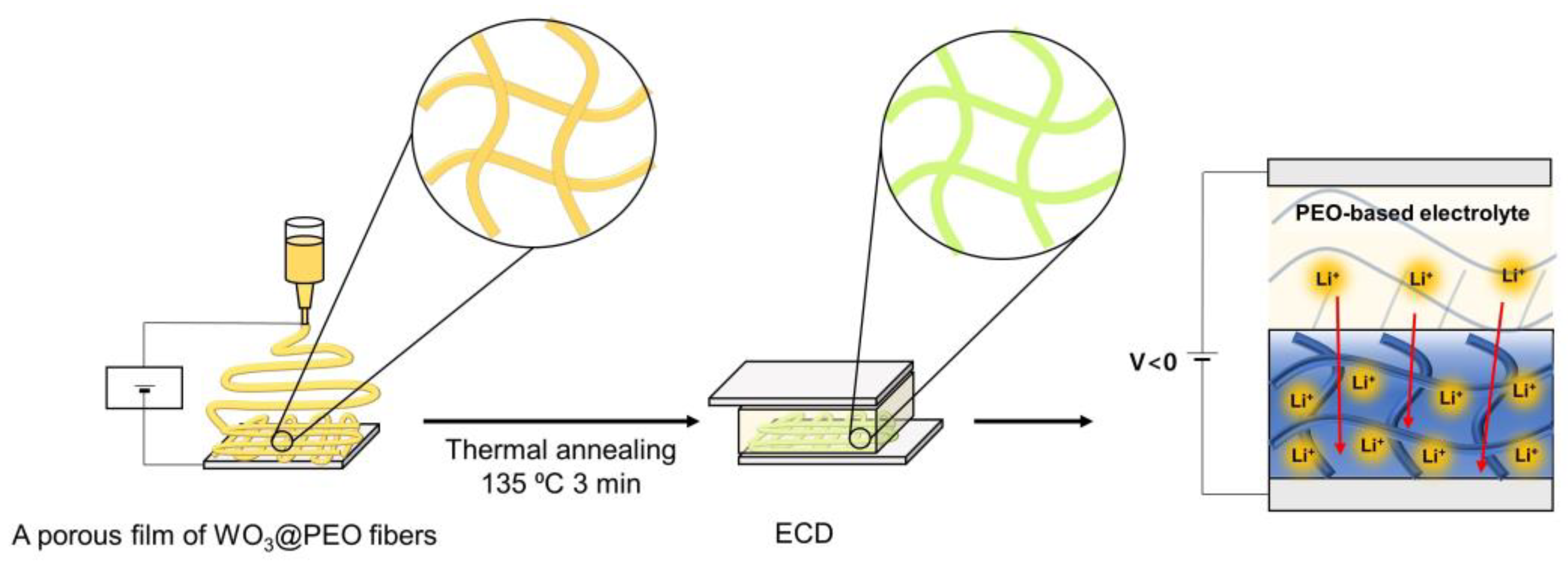
2.2. Preparation of Electrochromic Films
2.3. Fabrication of ECDs with Liquid Electrolytes
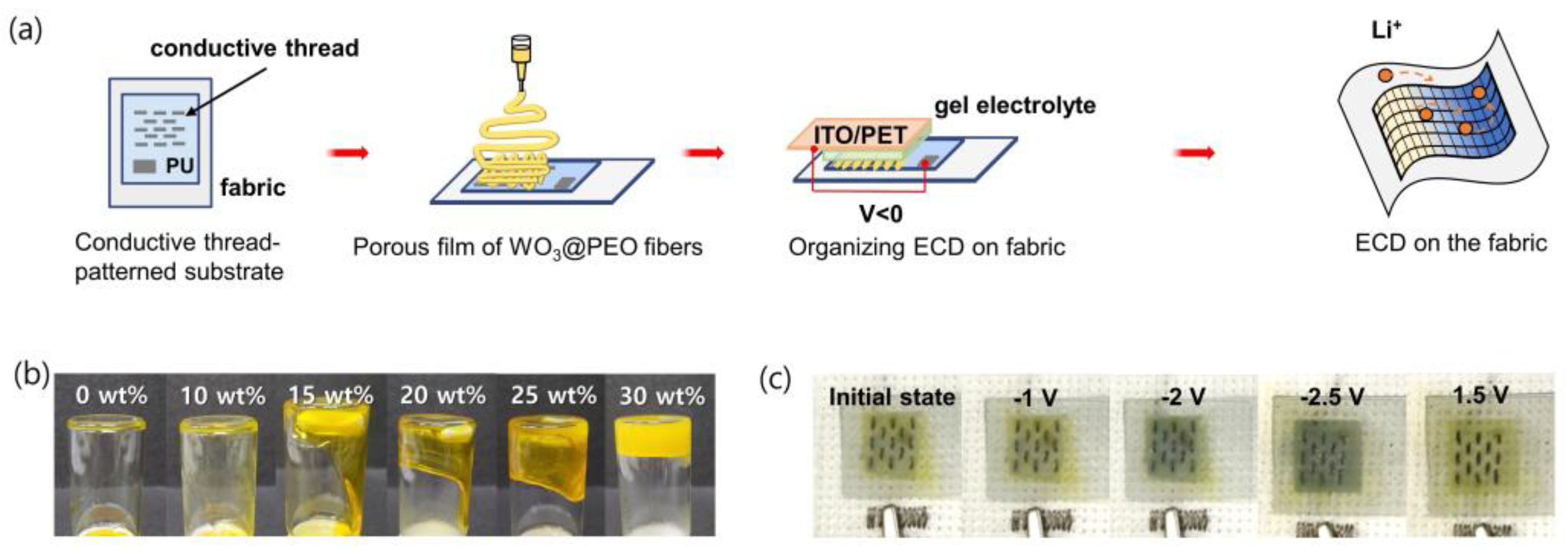
2.4. Preparation of an ECD with Gel Electrolytes on the Fabric
2.5. Characterizations
3. Results and Discussion
3.1. Electrochromic Characteristics of Nonporous and Porous Films of WO3@PEO
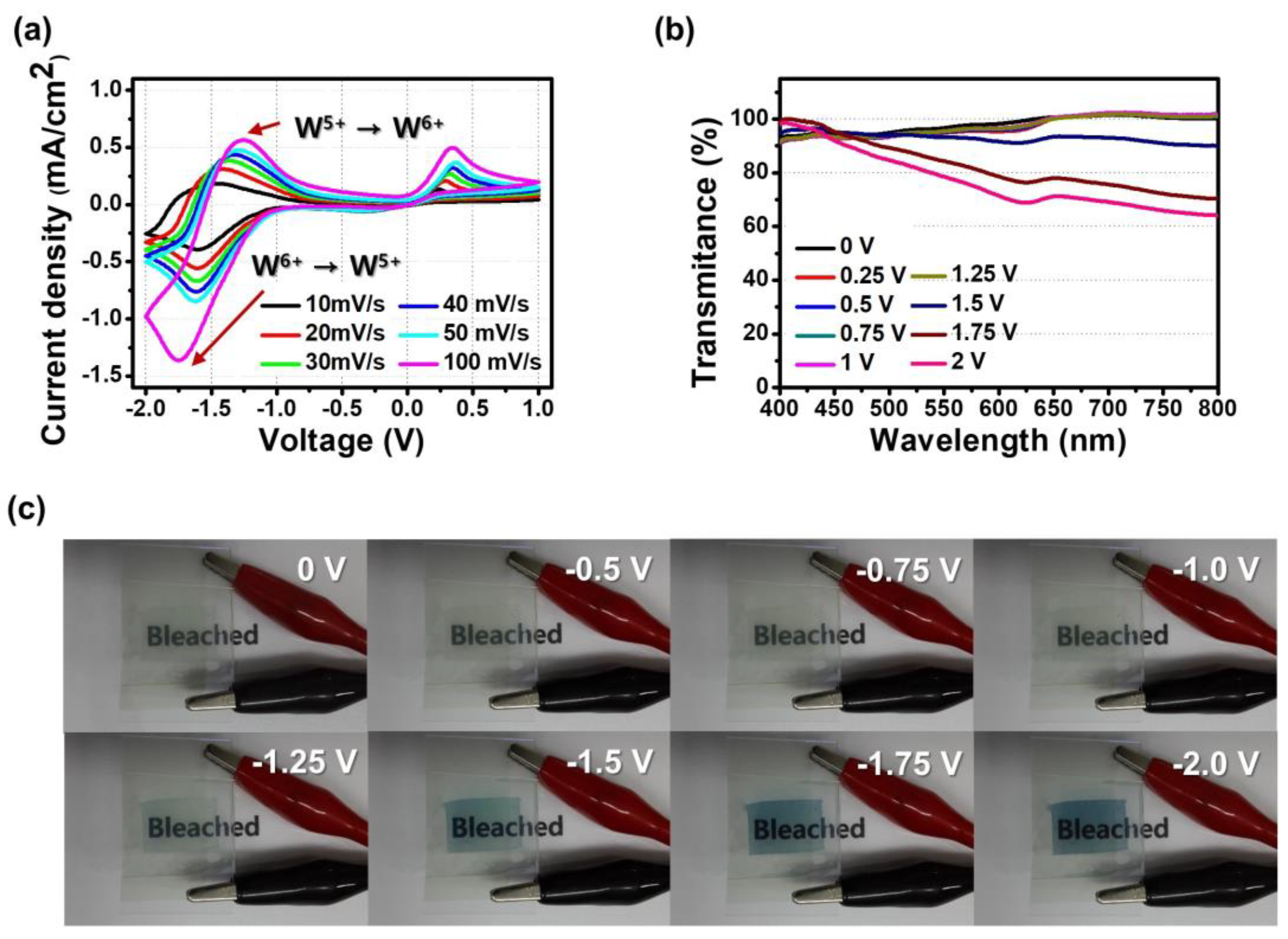
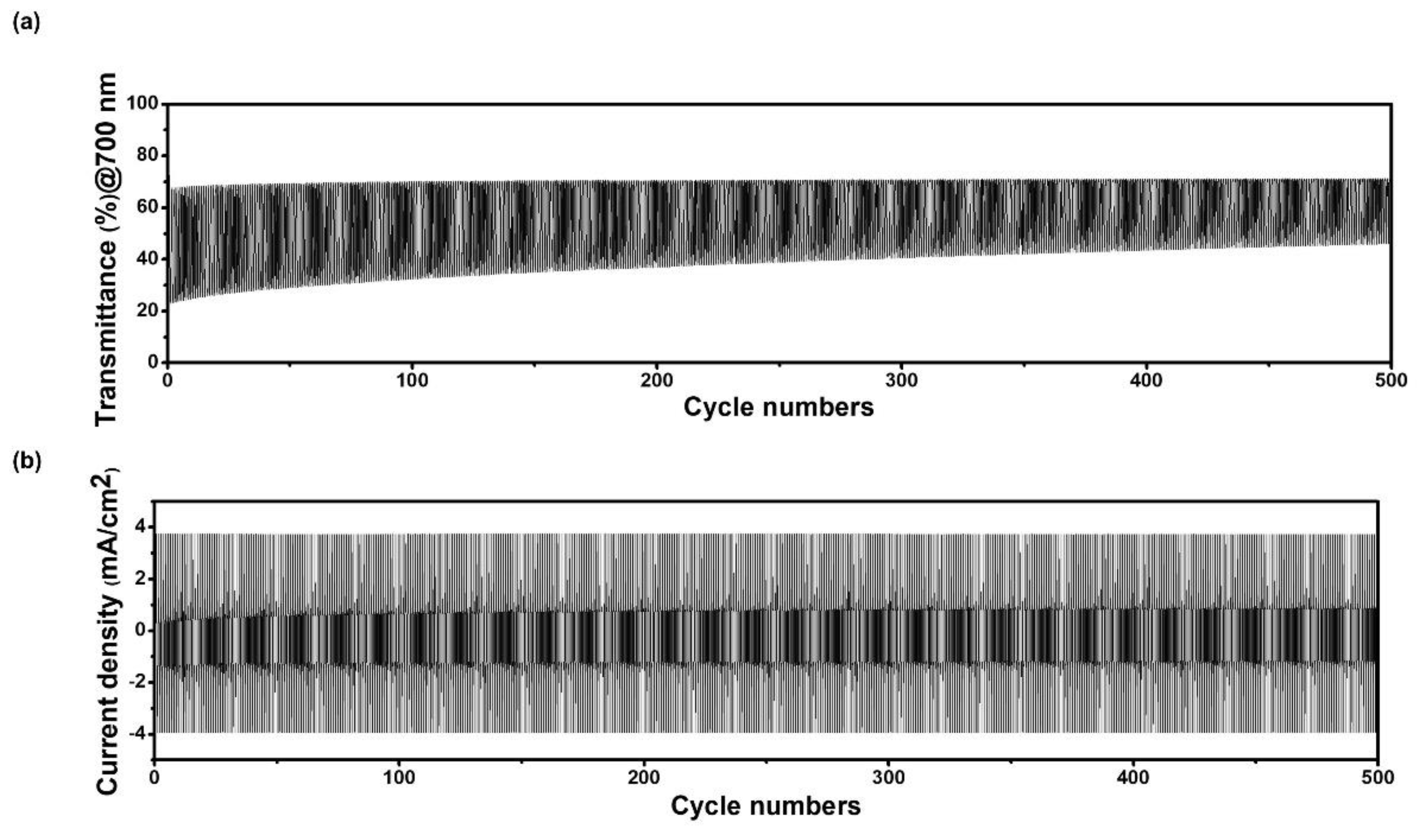
3.2. Electrochromic Characteristics of the Porous Film of WO3@PEO Fibers Based ECD
3.3. Demonstration of an ECD on the Fabric
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, D.; Kim, H.; Lee, M.; Son, M.; Ahn, S.-H.; Lee, C.S. Low-voltage modulated inorganic smart windows using solid polymer electrolyte. Sol. Energy Mater. Sol. Cells 2019, 200, 109966. [Google Scholar] [CrossRef]
- Buch, V.R.; Chawla, A.K.; Rawal, S.K. Review on electrochromic property for WO3 thin films using different deposition techniques. Mater. Today Proc. 2016, 3, 1429–1437. [Google Scholar] [CrossRef]
- Sun, X.W.; Wang, J.X. Fast Switching Electrochromic Display Using a Viologen-Modified ZnO Nanowire Array Electrode. Nano Lett. 2008, 8, 1884–1889. [Google Scholar] [CrossRef]
- Koo, B.-R.; Ahn, H.-J. Fast-switching electrochromic properties of mesoporous WO3 films with oxygen vacancy defects. Nanoscale 2017, 9, 17788–17793. [Google Scholar] [CrossRef]
- Gicevicius, M.; Cechanaviciute, I.A.; Ramanavicius, A. Electrochromic textile composites based on polyaniline-coated metallized conductive fabrics. J. Electrochem. Soc. 2020, 167, 155515. [Google Scholar] [CrossRef]
- Kang, J.-H.; Oh, Y.-J.; Paek, S.-M.; Hwang, S.-J.; Choy, J.-H. Electrochromic device of PEDOT–PANI hybrid system for fast response and high optical contrast. Sol. Energy Mater. Sol. Cells 2009, 93, 2040–2044. [Google Scholar] [CrossRef]
- Levasseur, D.; Mjejri, I.; Rolland, T.; Rougier, A. Color Tuning by Oxide Addition in PEDOT:PSS-Based Electrochromic Devices. Polymers 2019, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Kao, S.-Y.; Chang, T.-H.; Kung, C.-W.; Ho, K.-C. An electrochromic device based on Prussian blue, self-immobilized vinyl benzyl viologen, and ferrocene. Sol. Energy Mater. Sol. Cells 2016, 147, 75–84. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, Y.; Zhu, X.; Xing, Z.; Qi, S.; Wang, S.; Han, Y.; Chen, Z. Carbazole-Functionalized Poly(phenyl isocyanide)s: Synergistic Electrochromic Behaviors in the Visible Light Near-Infrared Region. Macromolecules 2021, 54, 5249–5259. [Google Scholar] [CrossRef]
- Chen, C.C. Characterization of Porous WO3 Electrochromic Device by Electrochemical Impedance Spectroscopy. J. Nanomater. 2013, 2013, 785023. [Google Scholar]
- Choi, D.; Son, M.; Im, T.; Ahn, S.-H.; Lee, C.S. Microstructure control of NiO-based ion storage layer with various sized NiO particles to evaluate the electrochromic performance. Mater. Chem. Phys. 2020, 249, 123121. [Google Scholar] [CrossRef]
- Gillaspie, D.T.; Tenent, R.C.; Dillon, A.C. Metal-oxide films for electrochromic applications: Present technology and future directions. J. Mater. Chem. 2010, 20, 9585–9592. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Tam, B.; Pei, P.; Li, F.; Xie, A.; Cheng, W. Macroporous Vanadium Oxide Ion Storage Films Enable Fast Switching Speed and High Cycling Stability of Electrochromic Devices. ACS Appl. Mater. Interfaces 2022, 14, 30021–30028. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, H.; Moon, H.C.; Kim, S.H. Low-voltage, simple WO3-based electrochromic devices by directly incorporating an anodic species into the electrolyte. J. Mater. Chem. C 2016, 4, 10887–10892. [Google Scholar] [CrossRef]
- Tuna, Ö.; Sezgin, A.; Budakoğlu, R.; Türküz, S.; Parlar, H. Electrochromic properties of tungsten trioxide (WO3) layers grown on ITO/glass substrates by magnetron sputtering. Vacuum 2015, 120, 28–31. [Google Scholar] [CrossRef]
- Blanchard, F.; Baloukas, B.; Martinu, L. Highly durable electrochromic tungsten oxide thin films prepared by high rate bias-enhanced sputter deposition. Appl. Mater. Today 2018, 12, 235–243. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Chen, X.; Li, W.; Wang, L.; Ren, F.; Zhao, J.; Endres, F.; Li, Y. Preparation of WO3 Films with Controllable Crystallinity for Improved Near-Infrared Electrochromic Performances. ACS Sustain. Chem. Eng. 2020, 8, 11658–11666. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, H.; Zhang, Y.; Liu, J.; Yan, H. Understand the Degradation Mechanism of Electrochromic WO3 Films by Double-step Chronoamperometry and Chronocoulometry Techniques Combined with in situ Spectroelectrochemical Study. Electroanalysis 2017, 29, 1573–1585. [Google Scholar] [CrossRef]
- Kim, J.J.; Zhou, C.; Mane, A.U.; Suh, H.S.; Kim, S.; Shi, B.; Fenter, P.; Elam, J.W.; Nealey, P.F.; Lee, B.; et al. Structural Changes during the Conversion Reaction of Tungsten Oxide Electrodes with Tailored, Mesoscale Porosity. ACS Nano 2022, 16, 5384–5392. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Q.; Wang, X.-L.; Xia, X.-H.; Yao, Z.-J.; Zhong, Y.; Tu, J.-P. Enhanced electrochromic and energy storage performance in mesoporous WO3 film and its application in a bi-functional smart window. Nanoscale 2018, 10, 8162–8169. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Yun, T.Y.; You, S.-H.; Tang, X.; Lee, J.; Seo, Y.; Kim, Y.-T.; Kim, S.H.; Moon, H.C.; Kim, J.K. Extremely fast electrochromic supercapacitors based on mesoporous WO3 prepared by an evaporation-induced self-assembly. NPG Asia Mater. 2020, 12, 84. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Huynh, K.A.; Le, Q.V.; Kim, H.; Ahn, S.H.; Kim, S.Y. Highly stable electrochromic cells based on amorphous tungsten oxides prepared using a solution-annealing process. Int. J. Energy Res. 2021, 45, 8061–8072. [Google Scholar] [CrossRef]
- Tajima, K.; Watanabe, H.; Nishino, M.; Kawamoto, T. Green fabrication of a complementary electrochromic device using water-based ink containing nanoparticles of WO3 and Prussian blue. RSC Adv. 2020, 10, 2562–2565. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.; Lee, S.-H.; Mahan, A.; Parilla, P.; Jones, K.; Norman, A.; To, B.; Blackburn, J.; Mitra, S.; Dillon, A. Optimization of crystalline tungsten oxide nanoparticles for improved electrochromic applications. Solid State Ion. 2007, 178, 895–900. [Google Scholar] [CrossRef]
- Monk, P.; Mortimer, R.; Rosseinsky, D. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Sirc, J.; Hobzova, R.; Kostina, N.; Munzarová, M.; Juklíčková, M.; Lhotka, M.; Kubinova, S.; Zajicova, A.; Michalek, J. Morphological Characterization of Nanofibers: Methods and Application in Practice. J. Nanomater. 2012, 2012, 121. [Google Scholar] [CrossRef]
- Bouvard, O.; Lagier, M.; Burnier, L.; Krammer, A.; Schüler, A. Strong coloration of nanoporous tungsten oxides by in-vacuo lithiation for all-solid-state electrochromic devices. Thin Solid Film. 2021, 730, 138700. [Google Scholar] [CrossRef]
- Li, X.; Yun, T.Y.; Kim, K.-W.; Kim, S.H.; Moon, H.C. Voltage-Tunable Dual Image of Electrostatic Force-Assisted Dispensing Printed, Tungsten Trioxide-Based Electrochromic Devices with a Symmetric Configuration. ACS Appl. Mater. Interfaces 2020, 12, 4022–4030. [Google Scholar] [CrossRef]
- Yun, T.Y.; Li, X.; Bae, J.; Kim, S.H.; Moon, H.C. Non-volatile, Li-doped ion gel electrolytes for flexible WO3-based electrochromic devices. Mater. Des. 2019, 162, 45–51. [Google Scholar] [CrossRef]
- Švec, P.; Petrov, O.V.; Lang, J.; Štěpnička, P.; Groborz, O.; Dunlop, D.; Blahut, J.; Kolouchová, K.; Loukotová, L.; Sedláček, O.; et al. Fluorinated Ferrocene Moieties as a Platform for Redox-Responsive Polymer 19F MRI Theranostics. Macromolecules 2022, 55, 658–671. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Zhou, K. The Mechanism of Trapped Ions Eroding the Electrochromic Performances of WO3 Thin Films. Int. J. Electrochem. Sci. 2018, 13, 7335–7346. [Google Scholar] [CrossRef]
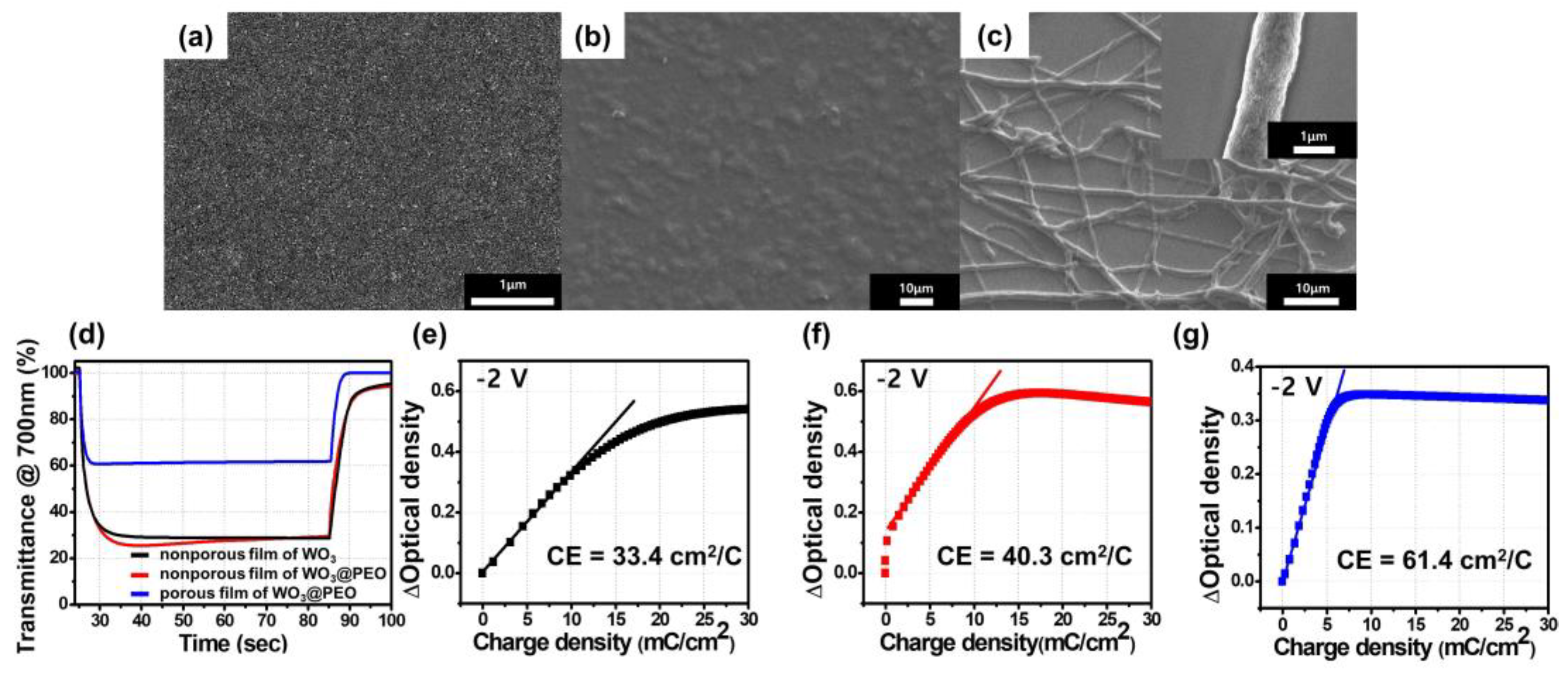
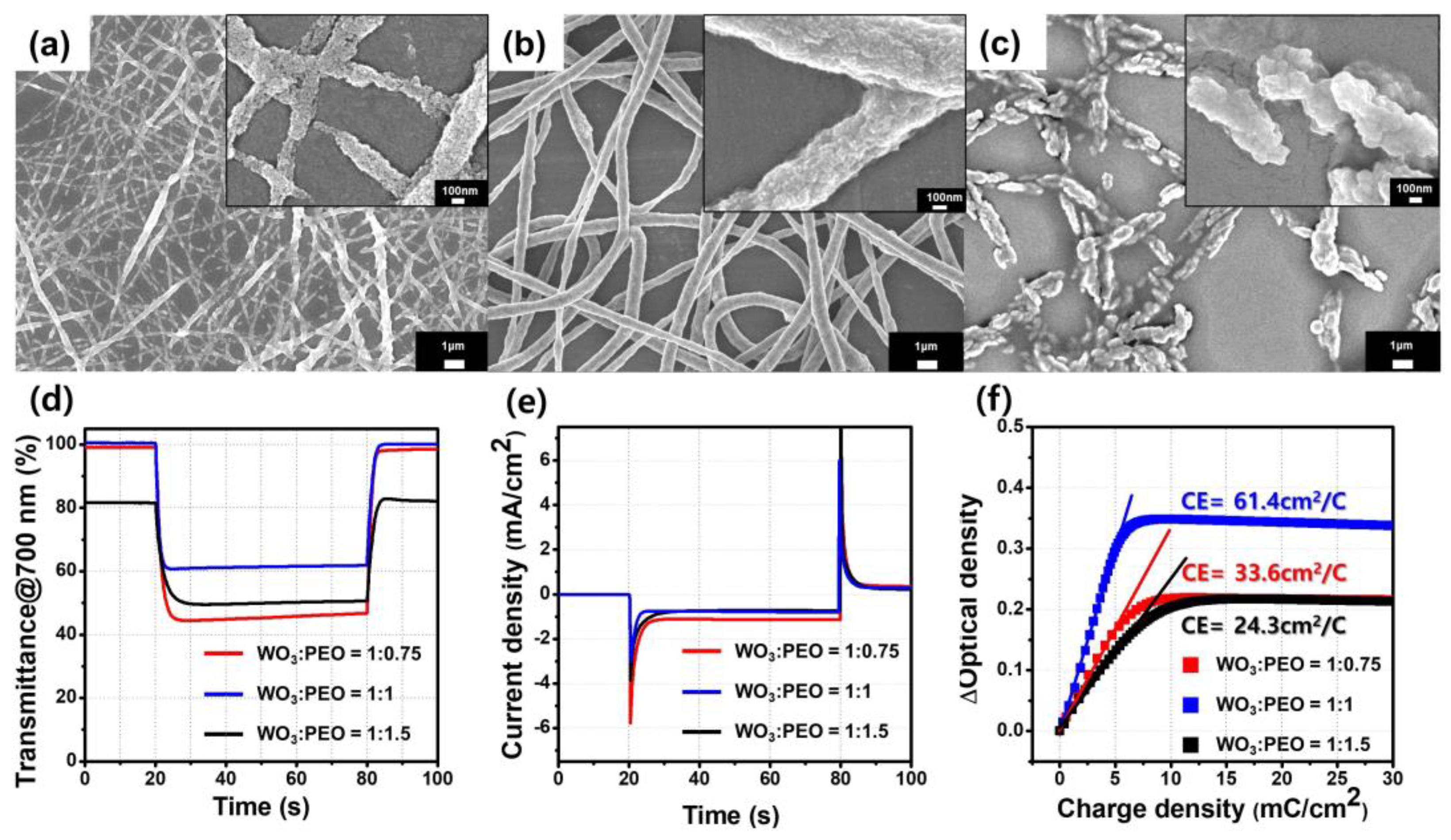
| η (cm2/C) | η/m (cm2/mg C) | Coloration Time (%90) (s) | ΔT (%) | WO3 (mg) | |
|---|---|---|---|---|---|
| A nonporous WO3 film | 22.3 | 69.7 | 4.0 | 73 | 0.32 |
| A nonporous film of WO3:PEO (1:1, w/w) | 41.2 | 258.0 | 4.7 | 74 | 0.16 |
| A porous film of WO3:PEO fibers (1:1, w/w) | 47.2 | 2770 | 1.6 | 40 | 0.017 |
| WO3: PEO (w/w) | Fiber Thickness (nm) | η (cm2/C) | η/m (cm2/mg C) | Coloration Time (%90) (s) | ΔT (%) |
|---|---|---|---|---|---|
| 1: 0.75 | 115 ± 60 | 33.6 | 259 | 3.0 | 54 |
| 1: 1 | 658 ± 54 | 61.4 | 3610 | 1.6 | 40 |
| 1:1.5 | 952 ± 192 | 24.3 | 101 | 4.5 | 32 |
| Coloration Time (s) | Applied Voltage (V) | η (cm2/C) | ΔT(%) | Ref. | |
|---|---|---|---|---|---|
| WO3 microparticle film | 12.5 | −0.9–0 | 58.2 | 76.1 | [14] |
| WO3 nanoparticle film | 10 | −1.5–0 | 34.3 | 52 | [20] |
| Mesoporous WO3 film | 2.4 | −0.6–0.6 | 79.7 | 75.6 | [28] |
| Amorphous WO3 film | 17 | −2.5–2.5 | 63 | 45.3 | [29] |
| WO3 dispersed film | 15 | −0.9–0.9 | 62.1 | 77.8 | [30] |
| a porous film of WO3@PEO fibers | 1.6 | −2–1 | 61.4 | 39.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.; Kim, S.; Ham, M.; Park, Y.; Kim, H.; Lee, W.; Lee, H. Enhanced Coloration Time of Electrochromic Device Using Integrated WO3@PEO Electrodes for Wearable Devices. Biosensors 2023, 13, 194. https://doi.org/10.3390/bios13020194
Kwon H, Kim S, Ham M, Park Y, Kim H, Lee W, Lee H. Enhanced Coloration Time of Electrochromic Device Using Integrated WO3@PEO Electrodes for Wearable Devices. Biosensors. 2023; 13(2):194. https://doi.org/10.3390/bios13020194
Chicago/Turabian StyleKwon, Haneul, Soohyun Kim, Mirim Ham, Yewon Park, Haekyoung Kim, Wonmok Lee, and Hyunjung Lee. 2023. "Enhanced Coloration Time of Electrochromic Device Using Integrated WO3@PEO Electrodes for Wearable Devices" Biosensors 13, no. 2: 194. https://doi.org/10.3390/bios13020194
APA StyleKwon, H., Kim, S., Ham, M., Park, Y., Kim, H., Lee, W., & Lee, H. (2023). Enhanced Coloration Time of Electrochromic Device Using Integrated WO3@PEO Electrodes for Wearable Devices. Biosensors, 13(2), 194. https://doi.org/10.3390/bios13020194






