A Label-Free, Mix-and-Detect ssDNA-Binding Assay Based on Cationic Conjugated Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. CCP-Based Binding Assay
2.3. SGI and SYBR Gold-Based Fluorescence Assay
2.4. Quantification of Unlabeled ssDNA on AuNPs
2.5. Quantification of Thiolated ssDNA on AuNPs
3. Results
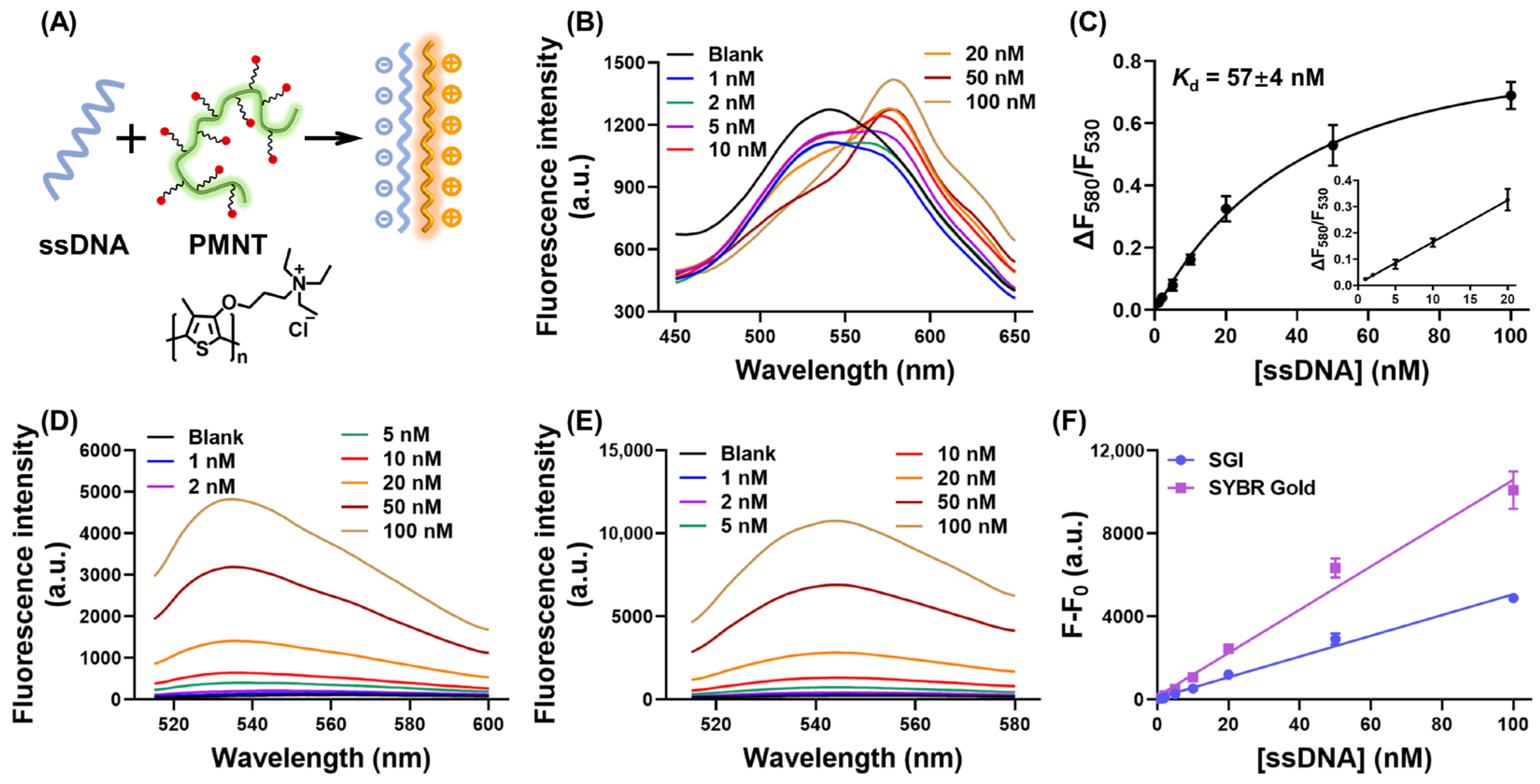
3.1. The Mechanism of Our CCP-Based ssDNA-Binding Assay
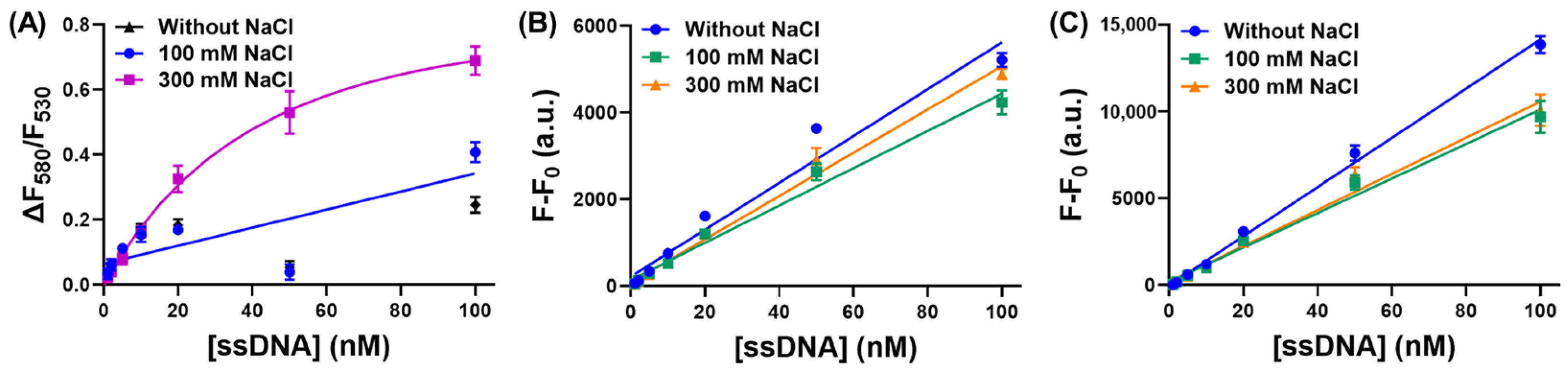
3.2. The Influence of Salt on Our CCP-Based ssDNA-Binding Assay
3.3. Effect of CCP Concentration
3.4. Effect of pH
3.5. Effect of DNA Sequence
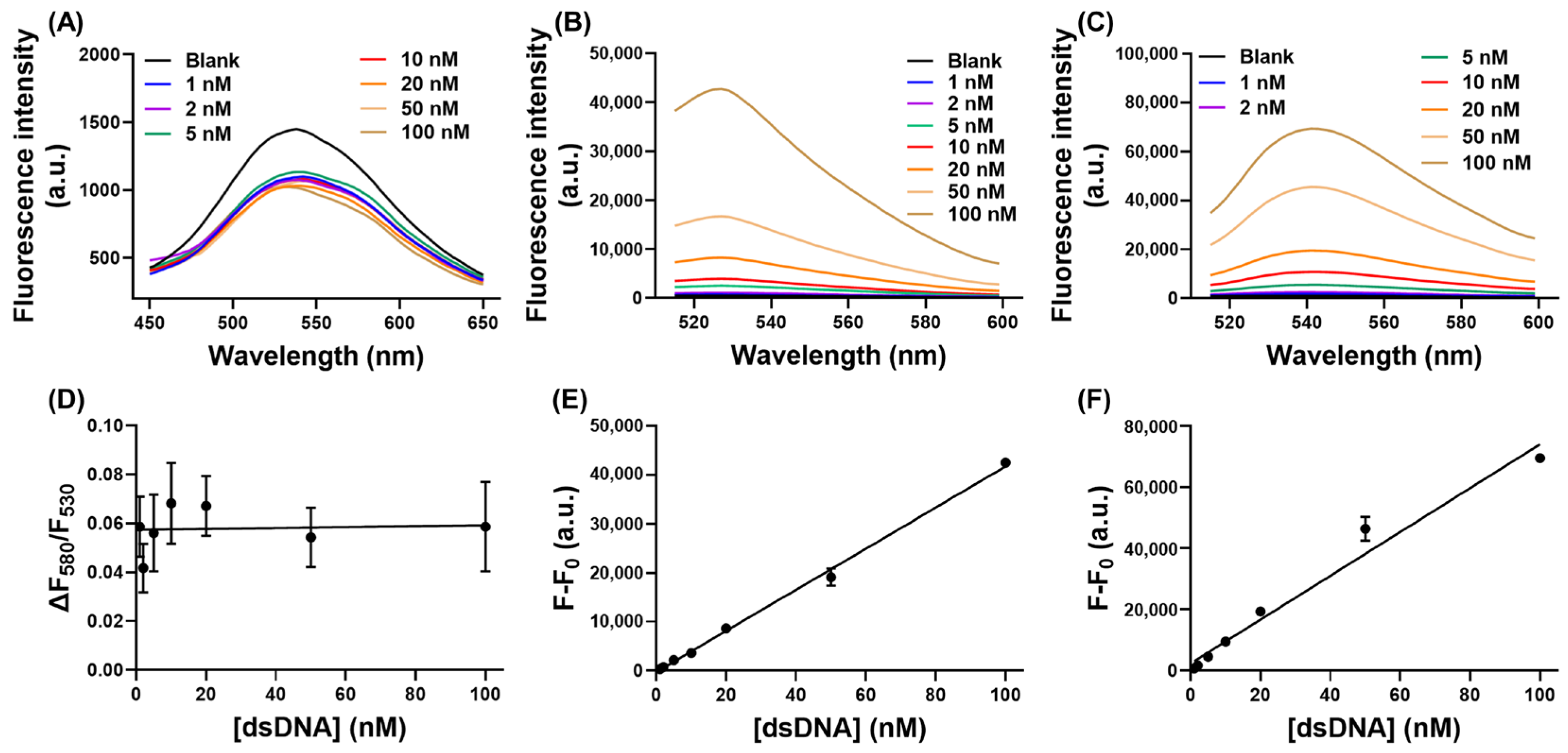
3.6. Effect of dsDNA
3.7. Detection of ssDNA on AuNPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierce, K.E.; Sanchez, J.A.; Rice, J.E.; Wangh, L.J. Linear-After-The-Exponential (LATE)-PCR: Primer design criteria for high yields of specific single-stranded DNA and improved real-time detection. Proc. Natl. Acad. Sci. USA 2005, 102, 8609–8614. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, N.R.; Bolene, M.A.E.; Jaspan, M.; Ayers, A.G.; Zarrandikoetxea, S.; Freudman, J.; Shah, N.; Tolwani, A.M.; Hu, Y.; Chern, T.L.; et al. Multiplexed reverse-transcriptase quantitative polymerase chain reaction using plasmonic nanoparticles for point-of-care COVID-19 diagnosis. Nat. Nanotechnol. 2022, 17, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Smyrlaki, I.; Ekman, M.; Lentini, A.; Rufino de Sousa, N.; Papanicolaou, N.; Vondracek, M.; Aarum, J.; Safari, H.; Muradrasoli, S.; Rothfuchs, A.G.; et al. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. Nat. Commun. 2020, 11, 4812. [Google Scholar] [CrossRef] [PubMed]
- Zipper, H.; Brunner, H.; Bernhagen, J.; Vitzthum, F. Investigations on DNA intercalation and surface binding by SYBR Green I, its structure determination and methodological implications. Nucleic Acids Res. 2004, 32, e103. [Google Scholar] [CrossRef]
- Kolbeck, P.J.; Vanderlinden, W.; Gemmecker, G.; Gebhardt, C.; Lehmann, M.; Lak, A.; Nicolaus, T.; Cordes, T.; Lipfert, J. Molecular structure, DNA binding mode, photophysical properties and recommendations for use of SYBR Gold. Nucleic Acids Res. 2021, 49, 5143–5158. [Google Scholar] [CrossRef]
- Han, X.; Wang, E.; Cui, Y.; Lin, Y.; Chen, H.; An, R.; Liang, X.; Komiyama, M. The staining efficiency of cyanine dyes for single-stranded DNA is enormously dependent on nucleotide composition. Electrophoresis 2019, 40, 1708–1714. [Google Scholar] [CrossRef]
- Tatulli, G.; Pompa, P.P. An amplification-free colorimetric test for sensitive DNA detection based on the capturing of gold nanoparticle clusters. Nanoscale 2020, 12, 15604–15610. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Wang, Y.; Lei, F.; Li, L.; Liu, J.; Wu, P. Highly Stable Colorimetric Sensing by Assembly of Gold Nanoparticles with SYBR Green I: From charge screening to charge neutralization. Anal. Chem. 2019, 92, 1455–1462. [Google Scholar] [CrossRef]
- Wang, L.; Song, S.; Pan, D.; Li, D.; Fan, C. Gold nanoparticle-based sensing strategies for biomolecular detection. Pure Appl. Chem. 2010, 82, 81–89. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar]
- Yazdian-Robati, R.; Hedayati, N.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Colorimetric gold nanoparticles-based aptasensors. Nanomed. J. 2018, 5, 1–5. [Google Scholar]
- Liu, B.; Liu, J. Interface-driven hybrid materials based on DNA-functionalized gold nanoparticles. Matter 2019, 1, 825–847. [Google Scholar] [CrossRef]
- Li, H.; Rothberg, L. Colorimetric detection of DNA se-quences based on electrostatic interactions with unmodified gold nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 14036–14039. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rothberg, L.J. Label-Free Colorimetric Detection of Specific Sequences in Genomic DNA Amplified by the Polymerase Chain Reaction. J. Am. Chem. Soc. 2004, 126, 10958–10961. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J. Label-Free Colorimetric Biosensors Based on Aptamers and Gold Nanoparticles: A Critical Review. Anal. Sens. 2021, 1, 30–43. [Google Scholar] [CrossRef]
- He, H.; Dai, J.; Duan, Z.; Zheng, B.; Meng, Y.; Guo, Y.; Xiao, D. Unusual sequence length-dependent gold nanoparticles aggregation of the ssDNA sticky end and its application for enzyme-free and signal amplified colorimetric DNA detection. Sci. Rep. 2016, 6, 30878. [Google Scholar] [CrossRef]
- Huang, P.-J.J.J.; Yang, J.; Chong, K.; Ma, Q.; Li, M.; Zhang, F.; Moon, W.J.; Zhang, G.; Liu, J. Good’s Buffers Have Various Affinities on Gold Nanoparticles Regulating Fluorescent and Colorimetric DNA Sensing. Chem. Sci. 2020, 11, 6795–6804. [Google Scholar] [CrossRef]
- Feng, X.; Liu, L.; Wang, S.; Zhu, D. Water-soluble fluorescent conjugated polymers and their interactions with biomacromolecules for sensitive biosensors. Chem. Soc. Rev. 2010, 39, 2411–2419. [Google Scholar] [CrossRef]
- Wang, J.; Lv, F.; Liu, L.; Ma, Y.; Wang, S. Strategies to design conjugated polymer based materials for biological sensing and imaging. Coord. Chem. Rev. 2018, 354, 135–154. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, F.; Liu, L.; Wang, S. Water-soluble conjugat-ed organic molecules as optical and electrochemical materials for interdisciplinary biological applications. Acc. Chem. Res. 2019, 52, 3211–3222. [Google Scholar] [CrossRef]
- Gaylord, B.S.; Heeger, A.J.; Bazan, G.C. DNA detection using water-soluble conjugated polymers and peptide nucleic acid probes. Proc. Natl. Acad. Sci. USA 2002, 99, 10954–10957. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Liu, L.; Wang, S. Fluorescent conjugated polymer-based FRET technique for detection of DNA methylation of cancer cells. Nat. Protoc. 2010, 5, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qiu, T.; Liu, L.; Lv, F.; Li, Z.; Ying, J.; Wang, S. Conjoint analysis of DNA methylation for tumor differentiation using cationic conjugated polymers. ACS Appl. Bio Mater. 2020, 3, 2867–2872. [Google Scholar] [CrossRef] [PubMed]
- Sinsinbar, G.; Palaniappan, A.; Yildiz, U.H.; Liedberg, B. A Perspective on Polythiophenes as Conformation Dependent Optical Reporters for Label-Free Bioanalytics. ACS Sens. 2022, 7, 686–703. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Yousefi, K.; Behbudi, G.; Babapoor, A.; Omidifar, N.; Lai, C.W.; Gholami, A.; Chiang, W.H. Recent advancements in polythiophene-based materials and their biomedical, geno sensor and DNA detection. Int. J. Mol. Sci. 2021, 22, 6850. [Google Scholar] [CrossRef]
- Ho, H.A.; Leclerc, M. Optical sensors based on hybrid aptamer/conjugated polymer complexes. J. Am. Chem. Soc. 2004, 126, 1384–1387. [Google Scholar] [CrossRef]
- Ho, H.A.; Boissinot, M.; Bergeron, M.G.; Corbeil, G.; Doré, K.; Boudreau, D.; Leclerc, M. Colorimetric and fluorometric detection of nucleic acids using cationic polythiophene derivatives. Angew. Chem., Int. Ed. 2002, 114, 1618–1621. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, C.; Niu, C.; Wang, X.; Li, Z.; Liu, J. Binding Studies of Cationic Conjugated Polymers and DNA for Label-Free Fluorescent Biosensors. ACS Appl. Polym. Mater. 2022, 4, 6211–6218. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Li, B. A colorimetric aptamer biosensor based on cationic polythiophene derivative as peroxidase mimetics for the ultrasensitive detection of thrombin. Talanta 2017, 175, 224–228. [Google Scholar] [CrossRef]
- Ho, H.A.; Najari, A.; Leclerc, M. Optical detection of DNA and proteins with cationic polythiophenes. Acc. Chem. Res. 2008, 41, 168–178. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, K.; Lopez, A.; Li, Z.; Liu, J. General Label-Free Fluorescent Aptamer Binding Assay Using Cationic Conjugated Polymers. Anal. Chem. 2022, 94, 15456–15463. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tang, Y.; Wang, S.; Li, Y.; Zhu, D. Fluorescent amplifying recognition for DNA G-quadruplex folding with a cationic conju-gated polymer: A platform for homogeneous potassium detection. J. Am. Chem. Soc. 2005, 127, 12343–12346. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Feng, F.; He, F.; Wang, S.; Li, Y.; Zhu, D. Direct visualization of enzymatic cleavage and oxidative damage by hydroxyl radicals of single-stranded DNA with a cationic polythiophene derivative. J. Am. Chem. Soc. 2006, 128, 14972–14976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ong, S.; Chen, Y.; Jimmy Huang, P.J.; Liu, J. Label-Free and Dye-Free Fluorescent Sensing of Tetracyclines Using a Capture-Selected DNA Aptamer. Anal. Chem. 2022, 94, 10175–10182. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Jimmy Huang, P.J.; Zheng, J.; Liu, J. 2-Aminopurine Fluorescence Spectroscopy for Probing a Glucose Binding Aptamer. ChemBioChem 2022, 23, e202200127. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Freezing Directed Construction of Bio/Nano Interfaces: Reagentless Conjugation, Denser Spherical Nucleic Acids, and Better Nanoflares. J. Am. Chem. Soc. 2017, 139, 9471–9474. [Google Scholar] [CrossRef]
- Jarmoskaite, I.; AlSadhan, I.; Vaidyanathan, P.P.; Herschlag, D. How to Measure and Evaluate Binding Affinities. eLife 2020, 9, e57264. [Google Scholar] [CrossRef]
- Zandieh, M.; Patel, K.; Liu, J. Adsorption of Linear and Spherical DNA Oligonucleotides onto Microplastics. Langmuir 2022, 38, 1915–1922. [Google Scholar] [CrossRef]


| DNA Type | Method | Binding Efficiency (3 Trials) | Average | ||
|---|---|---|---|---|---|
| Unlabeled DNA | CCP-based ssDNA-binding assay | 56.0% | 59.3% | 54.4% | 57 ± 3% |
| Fluorescence-dye-based method | 59.0% | 59.1% | 59.3% | 59.1 ± 0.2% | |
| Thiolated DNA | CCP-based ssDNA-binding assay | 52.2% | 46.4% | 43.6% | 47 ± 4% |
| Fluorescence-dye-based method | 46.6% | 47.1% | 47.3% | 47.0 ± 0.4% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zandieh, M.; Ding, Y.; Wu, L.; Wang, X.; Liu, J.; Li, Z. A Label-Free, Mix-and-Detect ssDNA-Binding Assay Based on Cationic Conjugated Polymers. Biosensors 2023, 13, 122. https://doi.org/10.3390/bios13010122
Zhang P, Zandieh M, Ding Y, Wu L, Wang X, Liu J, Li Z. A Label-Free, Mix-and-Detect ssDNA-Binding Assay Based on Cationic Conjugated Polymers. Biosensors. 2023; 13(1):122. https://doi.org/10.3390/bios13010122
Chicago/Turabian StyleZhang, Pengbo, Mohamad Zandieh, Yuzhe Ding, Lyuyuan Wu, Xiaoyu Wang, Juewen Liu, and Zhengping Li. 2023. "A Label-Free, Mix-and-Detect ssDNA-Binding Assay Based on Cationic Conjugated Polymers" Biosensors 13, no. 1: 122. https://doi.org/10.3390/bios13010122
APA StyleZhang, P., Zandieh, M., Ding, Y., Wu, L., Wang, X., Liu, J., & Li, Z. (2023). A Label-Free, Mix-and-Detect ssDNA-Binding Assay Based on Cationic Conjugated Polymers. Biosensors, 13(1), 122. https://doi.org/10.3390/bios13010122





