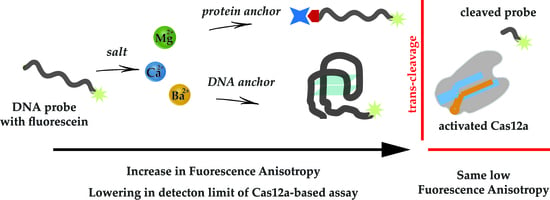
DNA Probes for Cas12a-Based Assay with Fluorescence Anisotropy Enhanced Due to Anchors and Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
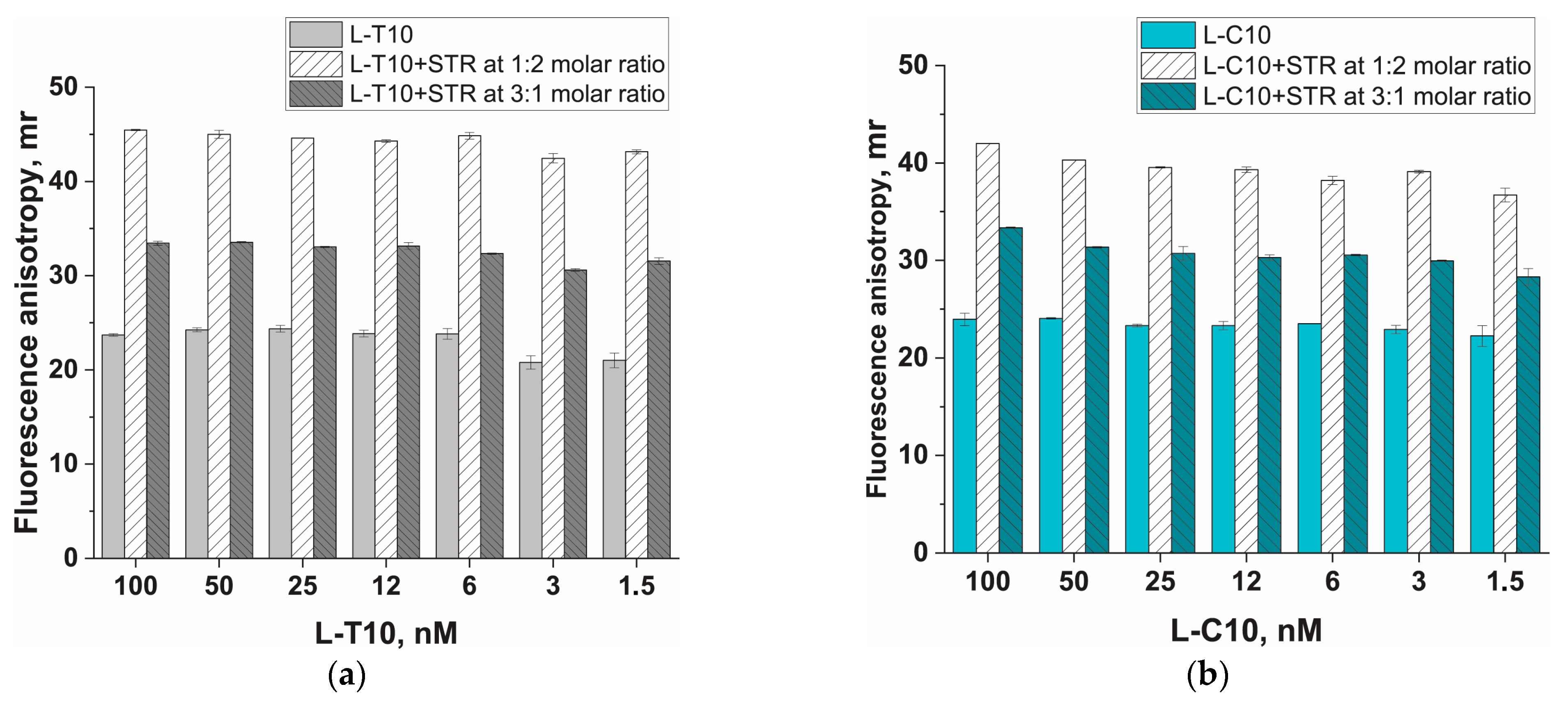
2.2. Estimation of Streptavidin Anchor Impact on Fluorescence Anisotropy of ssDNA Probes in the Absence of Metal Salts
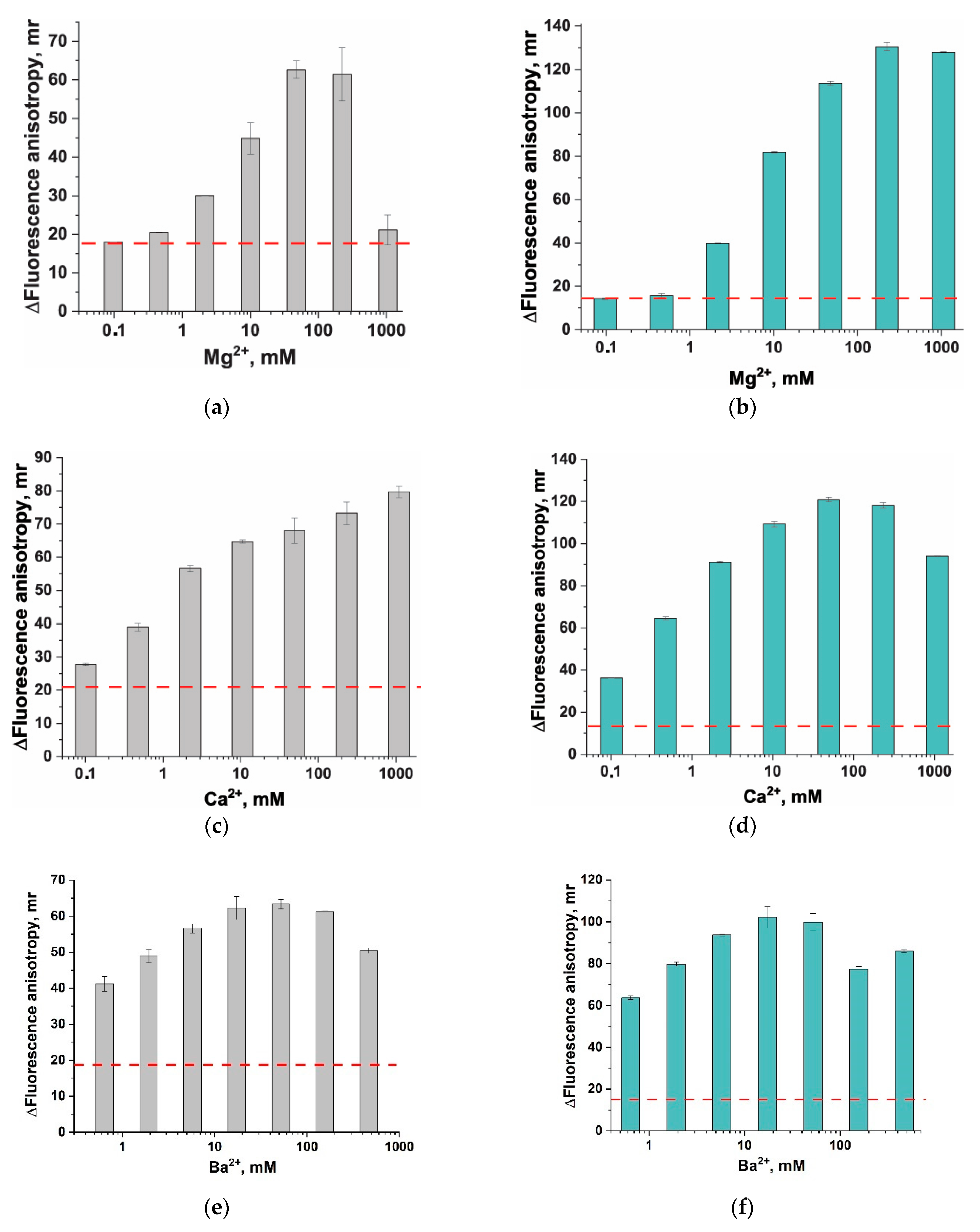
2.3. Estimation of Divalent Cation Salts’ Impact on Fluorescence Anisotropy of ssDNA Probes without and with Anchors
2.4. Circular Dichroism (CD) Spectra Measurements
2.5. Synthesis of dsDNA Cis-Target for Cas12a
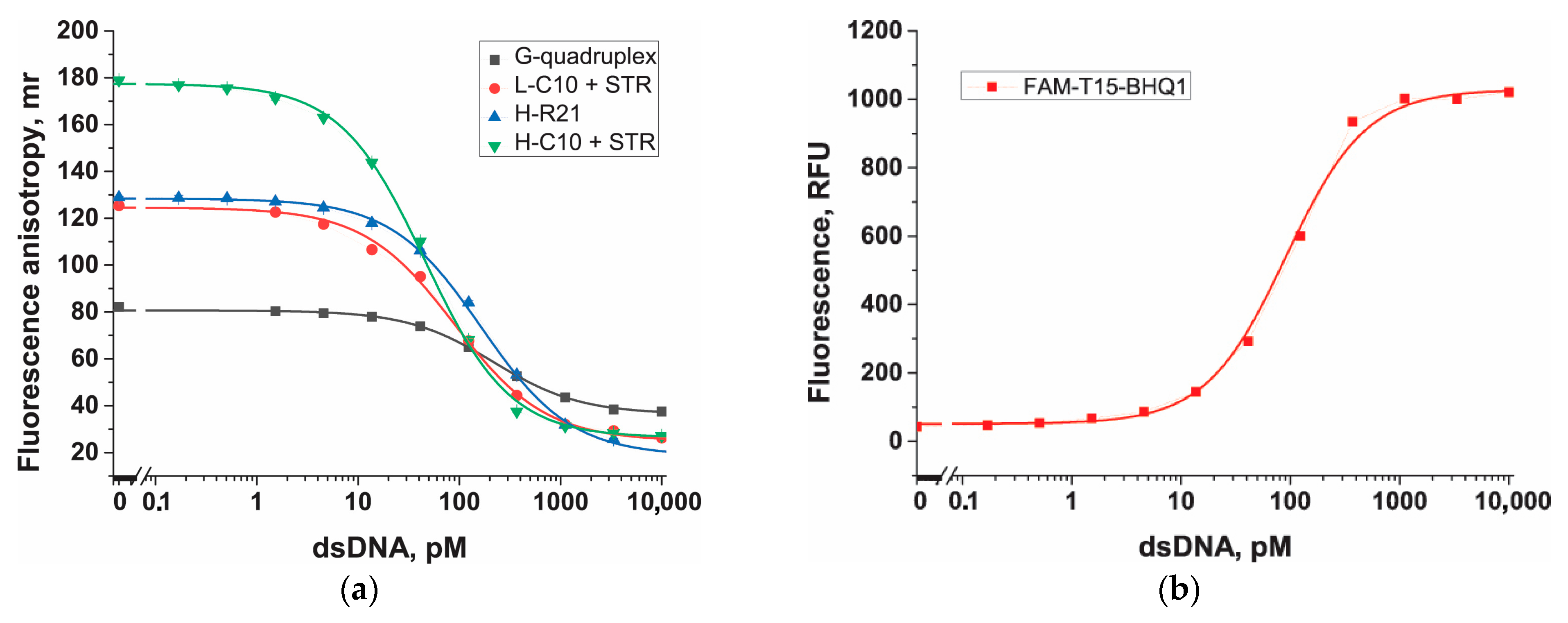
2.6. Comparison of Probes in the Cas12a-Based Assay Using the dsDNA Cis-Target
2.7. Estimation of Trans-Cleavage of the Probes Using Polyacrylamide Gel Electrophoresis
2.8. Cas12a-Based Assay of E. amylovora without Preamplification
2.9. Cas12a-Based Assay of E. amylovora Combined with RPA
3. Results and Discussion
3.1. Design of Experiments
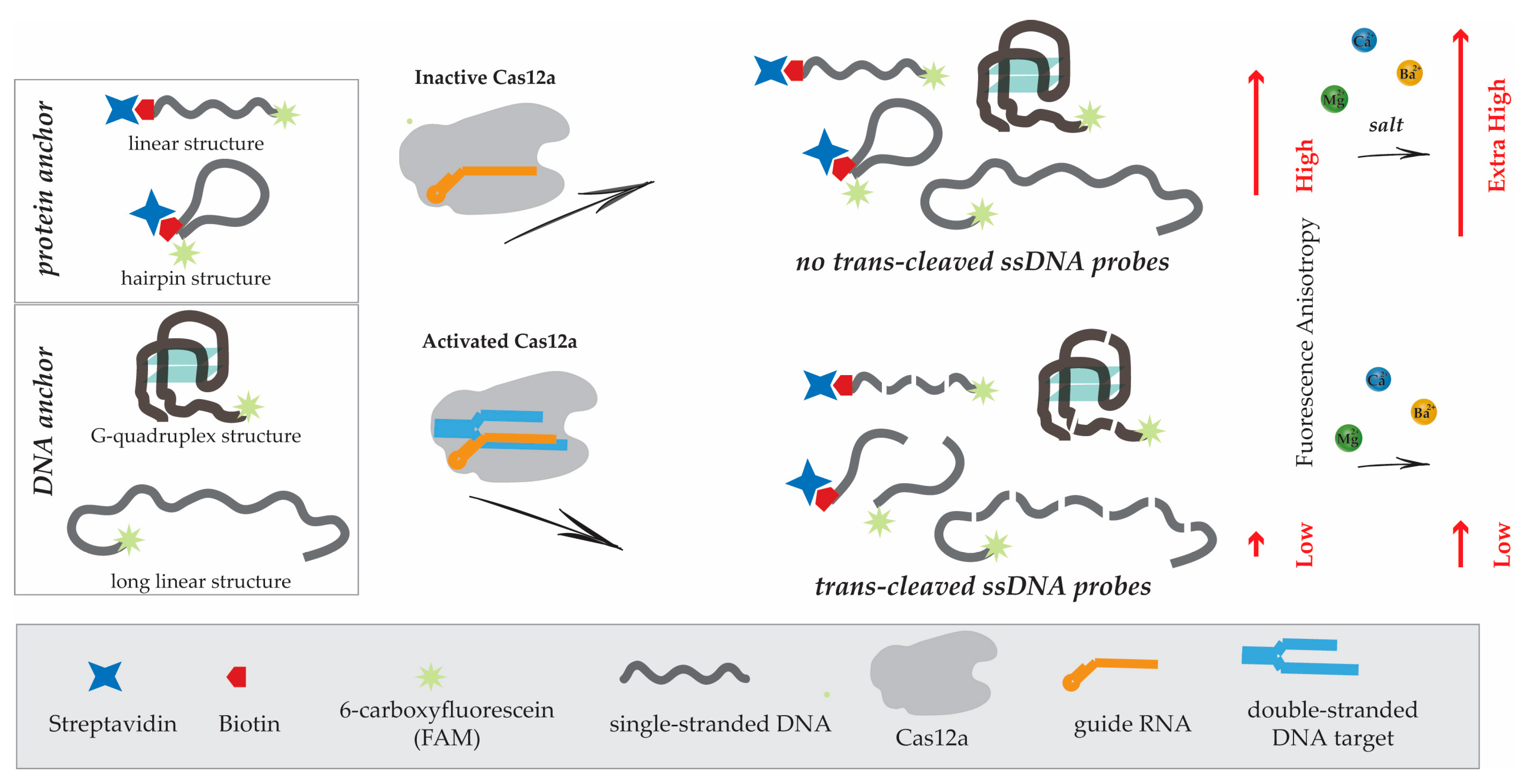
3.2. Protein Anchor, Its Impact on Fluorescent Anisotropy (FA), and Strategies to Increase FA
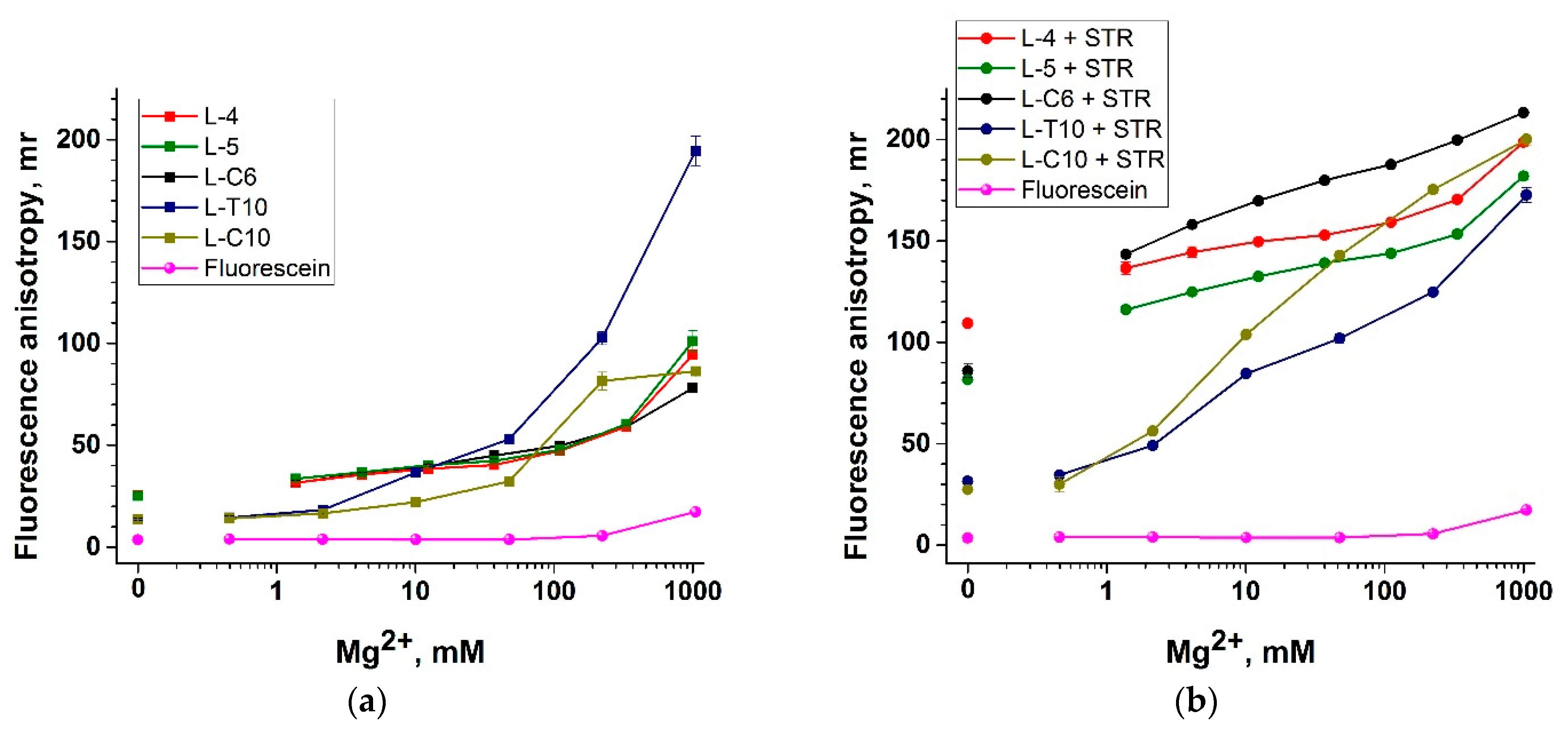
3.3. Divalent Metal Ions and Their Influence on Fluorescent Anisotropy
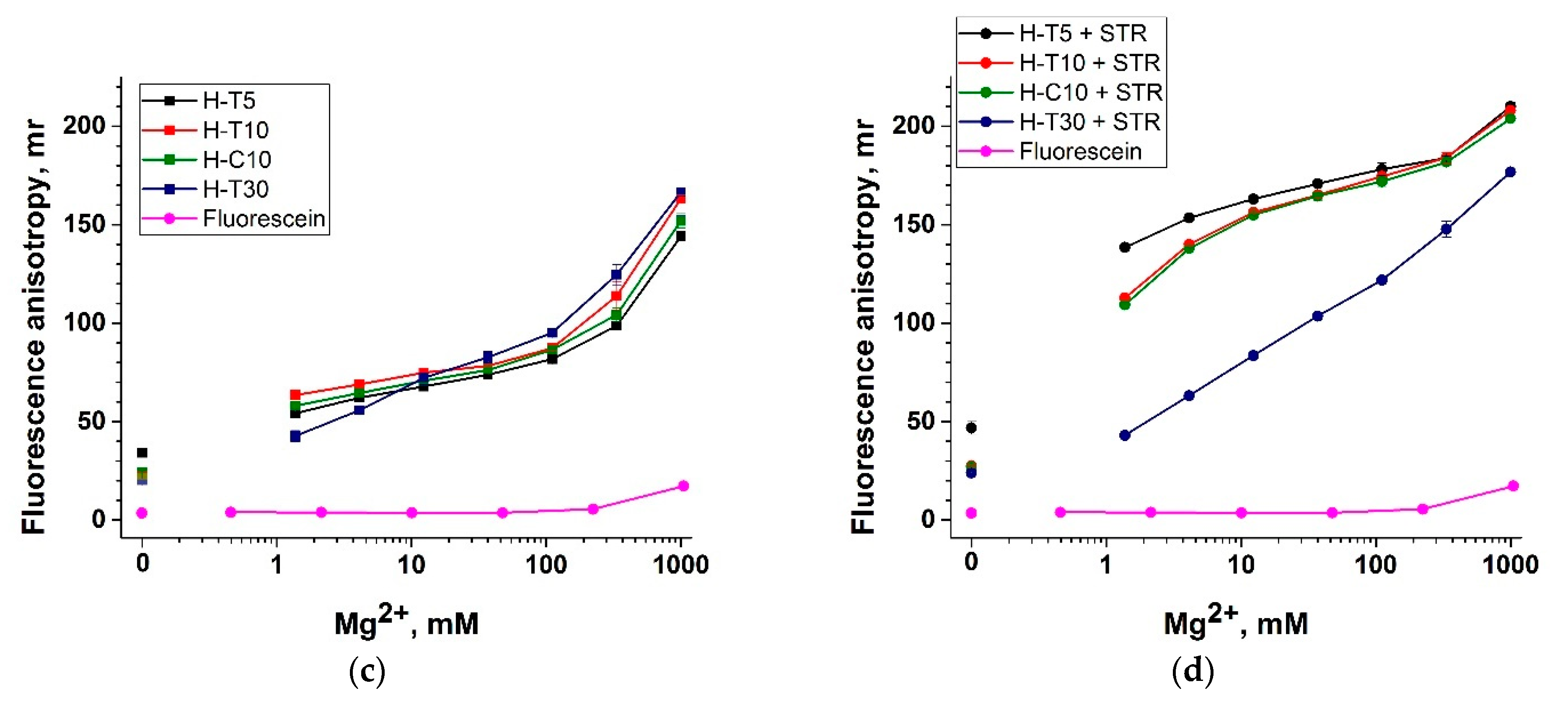
3.4. Selection of the ssDNA Probe Structure to Maximize Fluorescent Anisotropy in Combination with a Protein Anchor
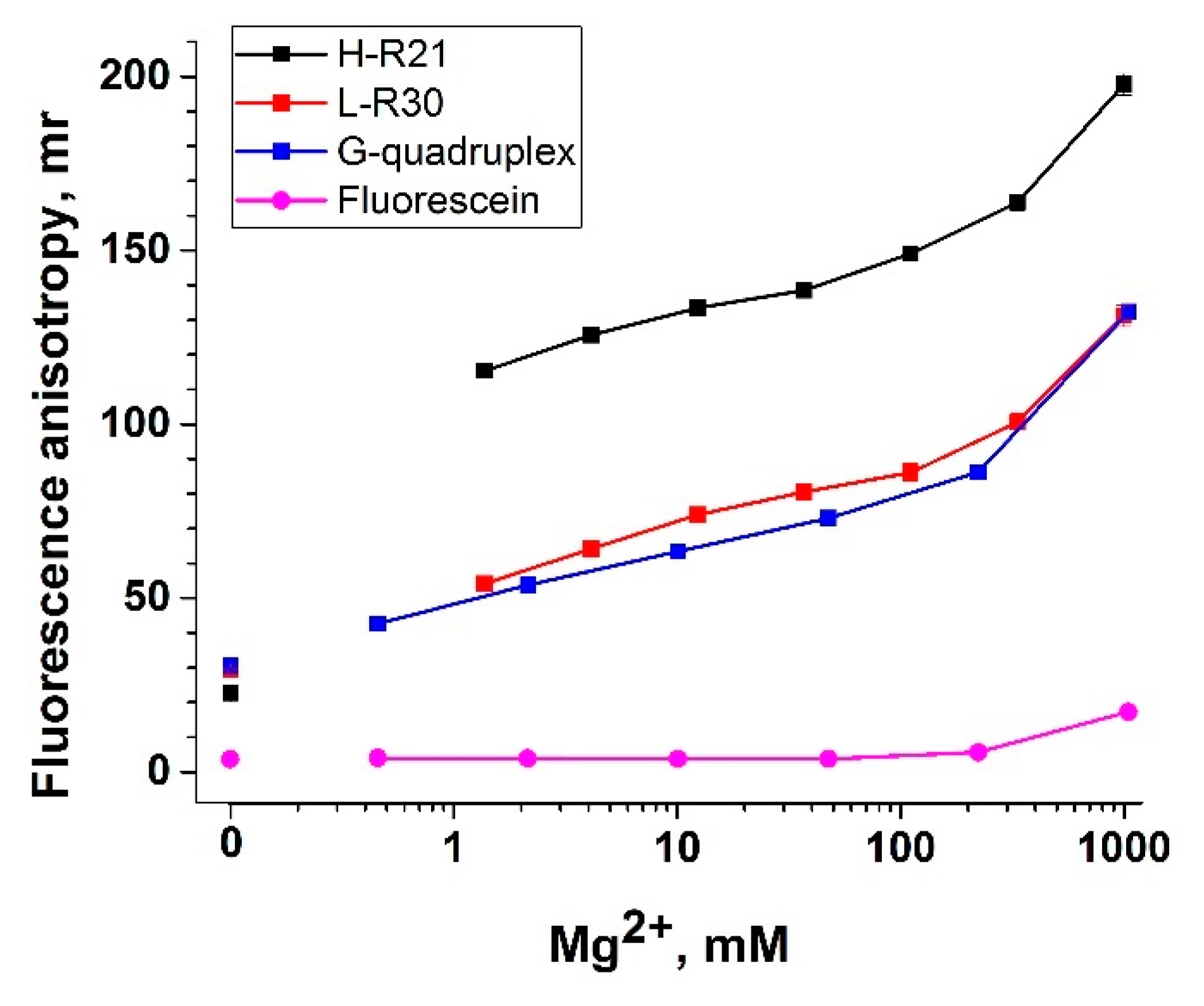
3.5. Nucleic Acid Anchors and Its Impact on Fluorescent Anisotropy
- (1)
- G-quadruplex with an extended end on the 3′-end ending with fluorescein (G-quadruplex). We used an aptamer specific to ochratoxin A (OTA) [47] since it has a tightly organized structure, which increases the anisotropy of labeled OTA.
- (2)
- A long (30 random nucleotides) DNA oligonucleotide that does not form any stems or other secondary structures (L-R30).
- (3)
- A 37-nucleotide hairpin, including 21 random nucleotides in the loop.
3.6. Comparison of Probes in Cas12a-Based Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wu, Y.; Wu, Y.; Chang, Y.; Liu, M. CRISPR-Cas systems: From gene scissors to programmable biosensors. TrAC Trends Anal. Chem. 2021, 137, 116210. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Du, Y.-C.; Wang, D.-X.; Ma, J.-Y.; Tang, A.-N.; Kong, D.-M. Signal amplification and output of CRISPR/Cas-based biosensing systems: A review. Anal. Chim. Acta 2021, 1185, 338882. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Newbigging, A.M.; Tao, J.; Cao, Y.; Peng, H.; Le, C.; Wu, J.; Pang, B.; Li, J.; Tyrrell, D.L.; et al. CRISPR technology incorporating amplification strategies: Molecular assays for nucleic acids, proteins, and small molecules. Chem. Sci. 2021, 12, 4683–4698. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.; Mesa, P.; Thomsen, J.; Paul, B.; Alcon, P.; Jensen, S.B.; Saligram, B.; Moses, M.E.; Hatzakis, N.S.; Montoya, G. Conformational activation promotes CRISPR-Cas12a catalysis and resetting of the endonuclease activity. Cell 2018, 175, 1856–1871.e21. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, H.; Le, X.C. Signal amplification by the trans-cleavage activity of CRISPR-Cas systems: Kinetics and performance. Anal. Chem. 2023, 95, 206–217. [Google Scholar] [CrossRef]
- Ghouneimy, A.; Mahas, A.; Marsic, T.; Aman, R.; Mahfouz, M. CRISPR-based diagnostics: Challenges and potential solutions toward point-of-care applications. ACS Synth. Biol. 2023, 12, 1–16. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Y.; Chen, G.; Wu, D.; Wu, Y.; Li, G. CRISPR/Cas systems-inspired nano/biosensors for detecting infectious viruses and pathogenic bacteria. Small Methods 2022, 6, 2200794. [Google Scholar] [CrossRef]
- Sohail, M.; Qin, L.; Li, S.; Chen, Y.; Zaman, M.H.; Zhang, X.; Li, B.; Huang, H. Molecular reporters for CRISPR/Cas: From design principles to engineering for bioanalytical and diagnostic applications. TrAC Trends Anal. Chem. 2022, 149, 116539. [Google Scholar] [CrossRef]
- Li, S.Y.; Cheng, Q.X.; Wang, J.M.; Li, X.Y.; Zhang, Z.L.; Gao, S.; Cao, R.B.; Zhao, G.P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef]
- Mukama, O.; Wu, J.; Li, Z.; Liang, Q.; Yi, Z.; Lu, X.; Liu, Y.; Liu, Y.; Hussain, M.; Makafe, G.G.; et al. An ultrasensitive and specific point-of-care CRISPR/Cas12 based lateral flow biosensor for the rapid detection of nucleic acids. Biosens. Bioelectron. 2020, 159, 112143. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. DIRECT2: A novel platform for a CRISPR–Cas12-based assay comprising universal DNA–IgG probe and a direct lateral flow test. Biosens. Bioelectron. 2022, 208, 114227. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qi, L.; Liu, Y.; Guo, L.; Zhao, R.; Yang, M.; Du, Y.; Li, B. CLIPON: A CRISPR-enabled strategy that turns commercial pregnancy test strips into general point-of-need test devices. Angew. Chem. Int. Ed. 2022, 61, e202115907. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Safenkova, I.V.; Zherdev, A.V.; Wan, Y.; Dzantiev, B.B. Comparison of single-stranded DNA probes conjugated with magnetic particles for trans-cleavage in Cas12a-based biosensors. Biosensors 2023, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Burkin, K.M.; Ivanov, A.V.; Zherdev, A.V.; Dzantiev, B.B.; Safenkova, I.V. A critical study on DNA probes attached to microplate for CRISPR/Cas12 trans-cleavage activity. Biosensors 2023, 13, 824. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Ebrahimi, S.B.; Ramani, N.; Mirkin, C.A. Enhancing CRISPR-Cas-mediated detection of nucleic acid and non-nucleic acid targets using enzyme-labeled reporters. J. Am. Chem. Soc. 2022, 144, 16310–16315. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lim, J.; Shin, M.; Paek, S.-H.; Choi, J.-W. CRISPR-Cas12a-based nucleic acid amplification-free DNA biosensor via Au nanoparticle-assisted metal-enhanced fluorescence and colorimetric analysis. Nano Lett. 2021, 21, 693–699. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Zhang, S.; Li, C.; Ma, J. Amplification-free, single-microbead-based Cas12a assay for one-step DNA detection at the single-molecule level. Anal. Chem. 2022, 94, 13076–13083. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2006; p. 980. [Google Scholar]
- Xiao, X.; Zhen, S. Recent advances in fluorescence anisotropy/polarization signal amplification. RSC Adv. 2022, 12, 6364–6376. [Google Scholar] [CrossRef]
- Hall, M.D.; Yasgar, A.; Peryea, T.; Braisted, J.C.; Jadhav, A.; Simeonov, A.; Coussens, N.P. Fluorescence polarization assays in high-throughput screening and drug discovery: A review. Methods Appl. Fluoresc. 2016, 4, 022001. [Google Scholar] [CrossRef]
- Yao, L.; Xu, J.; Qu, W.; Qiao, D.; Eremin, S.A.; Lu, J.; Chen, W.; Qin, P. Performance improved fluorescence polarization for easy and accurate authentication of chicken adulteration. Food Control 2022, 133, 108604. [Google Scholar] [CrossRef]
- Qin, P.; Li, Y.; Yao, B.; Zhu, Y.; Xu, J.; Yao, L.; Chen, W. Rational incorporating of loop-mediated isothermal amplification with fluorescence anisotropy for rapid, sensitive and on-site identification of pork adulteration. Food Control 2022, 137, 108863. [Google Scholar] [CrossRef]
- Lee, C.Y.; Degani, I.; Cheong, J.; Lee, J.-H.; Choi, H.-J.; Cheon, J.; Lee, H. Fluorescence polarization system for rapid COVID-19 diagnosis. Biosens. Bioelectron. 2021, 178, 113049. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Takeda, Y.; Takahashi, K.; Fukuyama, M.; Maeki, M.; Ishida, A.; Tani, H.; Shigemura, K.; Hibara, A.; Ogawa, H.; et al. Non-competitive fluorescence polarization immunoassay for detection of H5 avian influenza virus using a portable analyzer. Anal. Bioanal. Chem. 2021, 413, 4619–4623. [Google Scholar] [CrossRef]
- Wakao, O.; Satou, K.; Nakamura, A.; Galkina, P.A.; Nishiyama, K.; Sumiyoshi, K.; Kurosawa, F.; Maeki, M.; Ishida, A.; Tani, H.; et al. High-throughput fluorescence polarization immunoassay by using a portable fluorescence polarization imaging analyzer. Lab Chip 2019, 19, 2581–2588. [Google Scholar] [CrossRef]
- Zhao, Z.; Wei, L.; Cao, M.; Lu, M. A smartphone-based system for fluorescence polarization assays. Biosens. Bioelectron. 2019, 128, 91–96. [Google Scholar] [CrossRef]
- Dutta, S.; Paul, D. Smartphone “anatomy”: Features used for ubiquitous sensing. In Smartphones for Chemical Analysis: From Proof-of-Concept to Analytical Applications; Nelis, J.L.D., Tsagkaris, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 101, pp. 1–21. [Google Scholar]
- Xie, T.J.; Xie, J.L.; Luo, Y.J.; Mao, K.; Huang, C.Z.; Li, Y.F.; Zhen, S.J. CRISPR-Cas12a coupled with DNA nanosheet-amplified fluorescence anisotropy for sensitive detection of biomolecules. Anal. Chem. 2023, 95, 7237–7243. [Google Scholar] [CrossRef]
- Lipfert, J.; Doniach, S.; Das, R.; Herschlag, D. Understanding nucleic acid-ion interactions. Annu. Rev. Biochem. 2014, 83, 813–841. [Google Scholar] [CrossRef]
- Müller, J. Functional metal ions in nucleic acids. Metallomics 2010, 2, 318–327. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Safenkova, I.V.; Drenova, N.V.; Zherdev, A.V.; Dzantiev, B.B. Comparison of biosensing methods based on different isothermal amplification strategies: A case study with Erwinia amylovora. Biosensors 2022, 12, 1174. [Google Scholar] [CrossRef]
- Xia, N.; Sun, Z.; Ding, F.; Wang, Y.; Sun, W.; Liu, L. Protease Biosensor by Conversion of a Homogeneous Assay into a Surface-Tethered Electrochemical Analysis Based on Streptavidin–Biotin Interactions. ACS Sens. 2021, 6, 1166–1173. [Google Scholar] [CrossRef]
- Liu, L.; Deng, D.; Wu, D.; Hou, W.; Wang, L.; Li, N.; Sun, Z. Duplex-specific nuclease-based electrochemical biosensor for the detection of microRNAs by conversion of homogeneous assay into surface-tethered electrochemical analysis. Anal. Chim. Acta 2021, 1149, 338199. [Google Scholar] [CrossRef] [PubMed]
- Barshevskaya, L.V.; Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Modular Set of Reagents in Lateral Flow Immunoassay: Application for Antibiotic Neomycin Detection in Honey. Biosensors 2023, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, J.; Zhang, J.; Chen, Y.; Yin, L.; Jin, D.; Gu, D.; Zhao, H.; Xu, Y.; Wang, J. Definition of CRISPR Cas12a trans-cleavage units to facilitate CRISPR diagnostics. Front. Microbiol. 2021, 12, 766464. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, J.L. Fire Blight: The Disease and Its Causative Agent, Erwinia amylovora; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Luong, J.H.T.; Vashist, S.K. Chemistry of biotin–streptavidin and the growing concern of an emerging biotin interference in clinical immunoassays. ACS Omega 2020, 5, 10–18. [Google Scholar] [CrossRef]
- Zhao, Q.; Tao, J.; Uppal, J.S.; Peng, H.; Wang, H.; Le, X.C. Nucleic acid aptamers improving fluorescence anisotropy and fluorescence polarization assays for small molecules. TrAC Trends Anal. Chem. 2019, 110, 401–409. [Google Scholar] [CrossRef]
- Perrier, S.; Guieu, V.; Chovelon, B.; Ravelet, C.; Peyrin, E. Panoply of fluorescence polarization/anisotropy signaling mechanisms for functional nucleic acid-based sensing platforms. Anal. Chem. 2018, 90, 4236–4248. [Google Scholar] [CrossRef] [PubMed]
- Chovelon, B.; Fiore, E.; Faure, P.; Peyrin, E.; Ravelet, C. Kissing interactions for the design of a multicolour fluorescence anisotropy chiral aptasensor. Talanta 2019, 205, 120098. [Google Scholar] [CrossRef]
- Chen, H.; Meisburger, S.P.; Pabit, S.A.; Sutton, J.L.; Webb, W.W.; Pollack, L. Ionic strength-dependent persistence lengths of single-stranded RNA and DNA. Proc. Natl. Acad. Sci. USA 2012, 109, 799–804. [Google Scholar] [CrossRef]
- Ellouze, C.; Piot, F.; Takahashi, M. Use of fluorescein-labeled oligonucleotide for analysis of formation and dissociation kinetics of T:A:T triple-stranded DNA: Effect of divalent cations. J. Biochem. 1997, 121, 521–526. [Google Scholar] [CrossRef]
- Koculi, E.; Hyeon, C.; Thirumalai, D.; Woodson, S.A. charge density of divalent metal cations determines RNA stability. J. Am. Chem. Soc. 2007, 129, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Shu, B.; Tian, T.; Xiong, E.; Huang, M.; Zhu, D.; Sun, J.; Liu, Q.; Wang, S.; Li, Y.; et al. Droplet Cas12a Assay Enables DNA Quantification from Unamplified Samples at the Single-Molecule Level. Nano Lett. 2021, 21, 4643–4653. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, M.; Merlo, R.; Bagheri, N.; Moscone, D.; Valenti, A.; Saha, A.; Arantes Pablo, R.; Ippodrino, R.; Ricci, F.; Treglia, I.; et al. Enhancement of CRISPR/Cas12a trans-cleavage activity using hairpin DNA reporters. Nucleic Acids Res. 2022, 50, 8377–8391. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Aguado, J.A.; Penner, G. Determination of ochratoxin A with a DNA aptamer. J. Agric. Food Chem. 2008, 56, 10456–10461. [Google Scholar] [CrossRef]
- Phan, Q.A.; Truong, L.B.; Medina-Cruz, D.; Dincer, C.; Mostafavi, E. CRISPR/Cas-powered nanobiosensors for diagnostics. Biosens. Bioelectron. 2022, 197, 113732. [Google Scholar] [CrossRef]

| №. | Short Name | Sequence 5′-3′ | Structure and Purpose |
|---|---|---|---|
| 1 | L-4 | FAM-TTAT-Bio | Linear structure for protein anchor |
| 2 | L-5 | FAM-TTATT-Bio | |
| 3 | L-C6 | FAM-CCCCCC-Bio | |
| 4 | L-T10 | FAM-TTTTTTTTTT-Bio | |
| 5 | L-C10 | FAM-CCCCCCCCCC-Bio | |
| 6 | L-A10 | FAM-AAAAAAAAA-Bio | |
| 7 | H-T5 | FAM-CTCTCATTTTTAGAGAG-Bio | Hairpin structure for protein anchor * |
| 8 | H-T10 | FAM-CTCTCATTTTTTTTTTAGAGAG-Bio | |
| 9 | H-C10 | FAM-CTCTCACCCCCCCCCCAGAGAG-Bio | |
| 10 | H-T30 | FAM-CTCTCATTTTTTTTTTTTTTTTTTTTTTTTTTTTTTAGAGAG-Bio | |
| 11 | H-R21 | AAAAAAACCTCCAAGAGTTAGATCATACAGTTTTTTT-FAM | Hairpin structure as DNA anchor |
| 12 | G-quadruplex | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA-FAM | G-quadruplex structure as DNA anchor ** |
| 13 | L-R30 | FAM-GTTATTGCCGCCGCACAGCGTAAAGGTAAG | Linear structure as DNA anchor |
| 14 | A10 | AAAAAAAAAA | Linear structure to form double strand with L-T10 probe |
| 15 | FAM-T15-BHQ1 | FAM-TTTTTTTTTTTTTTT-BHQ1 | Linear structure for fluorescence detection after trans-cleavage |
| Probe/Parameter | H-C10 + STR | L-C10 + STR | H-R21 | G-quadruplex | FAM-T15-BHQ1 |
|---|---|---|---|---|---|
| A1 | 178.3 ± 0.9 | 125.0 ± 0.6 | 128.4 ± 0.5 | 81.0 ± 0.4 | 51.5 ± 1.0 |
| A2 | 26.7 ± 0.4 | 25.4 ± 0.9 | 21.7 ± 0.8 | 37.0 ± 0.4 | 1028.1 ± 20.1 |
| x0 | 44.5 ± 2.2 | 87.1 ± 5.3 | 150.0 ± 7.9 | 207.7 ± 9.9 | 91.1 ± 11.2 |
| p | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 |
| IC10 | 5.3 ± 0.5 | 9.1 ± 1.1 | 17.9 ± 1.7 | 24.5 ± 2.6 | 14.2 ± 1.8 |
| Reduced chi-sqr | 54.1 | 11.1 | 7.21 | 1.2 | 38.4 |
| Adj. R-square | 0.9996 | 0.9989 | 0.9993 | 0.9990 | 1.0 |
| LOD, pM | 0.8 ± 0.3 | 2.0 ± 0.4 | 5.1 ± 0.4 | 16.1 ± 1.3 | 6.7 ± 1.7 |
| LOQ, pM | 1.4 ± 0.2 | 3.5 ± 0.3 | 3.5 ± 0.3 | 27.9 ± 1.2 | 10.5 ± 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safenkova, I.V.; Samokhvalov, A.V.; Serebrennikova, K.V.; Eremin, S.A.; Zherdev, A.V.; Dzantiev, B.B. DNA Probes for Cas12a-Based Assay with Fluorescence Anisotropy Enhanced Due to Anchors and Salts. Biosensors 2023, 13, 1034. https://doi.org/10.3390/bios13121034
Safenkova IV, Samokhvalov AV, Serebrennikova KV, Eremin SA, Zherdev AV, Dzantiev BB. DNA Probes for Cas12a-Based Assay with Fluorescence Anisotropy Enhanced Due to Anchors and Salts. Biosensors. 2023; 13(12):1034. https://doi.org/10.3390/bios13121034
Chicago/Turabian StyleSafenkova, Irina V., Alexey V. Samokhvalov, Kseniya V. Serebrennikova, Sergei A. Eremin, Anatoly V. Zherdev, and Boris B. Dzantiev. 2023. "DNA Probes for Cas12a-Based Assay with Fluorescence Anisotropy Enhanced Due to Anchors and Salts" Biosensors 13, no. 12: 1034. https://doi.org/10.3390/bios13121034
APA StyleSafenkova, I. V., Samokhvalov, A. V., Serebrennikova, K. V., Eremin, S. A., Zherdev, A. V., & Dzantiev, B. B. (2023). DNA Probes for Cas12a-Based Assay with Fluorescence Anisotropy Enhanced Due to Anchors and Salts. Biosensors, 13(12), 1034. https://doi.org/10.3390/bios13121034