Enzyme Cascade Electrode Reactions with Nanomaterials and Their Applicability towards Biosensor and Biofuel Cells
Abstract
1. Introduction
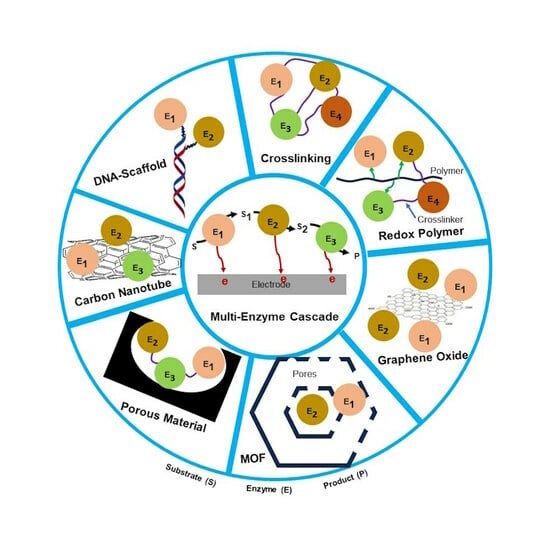
2. Nanomaterials—An Emerging Tool for Enzyme Cascade Reactions
2.1. Porous Carbon

2.2. Carbon Nanomaterials
2.3. Graphene and Graphene Oxide (GO/rGO)
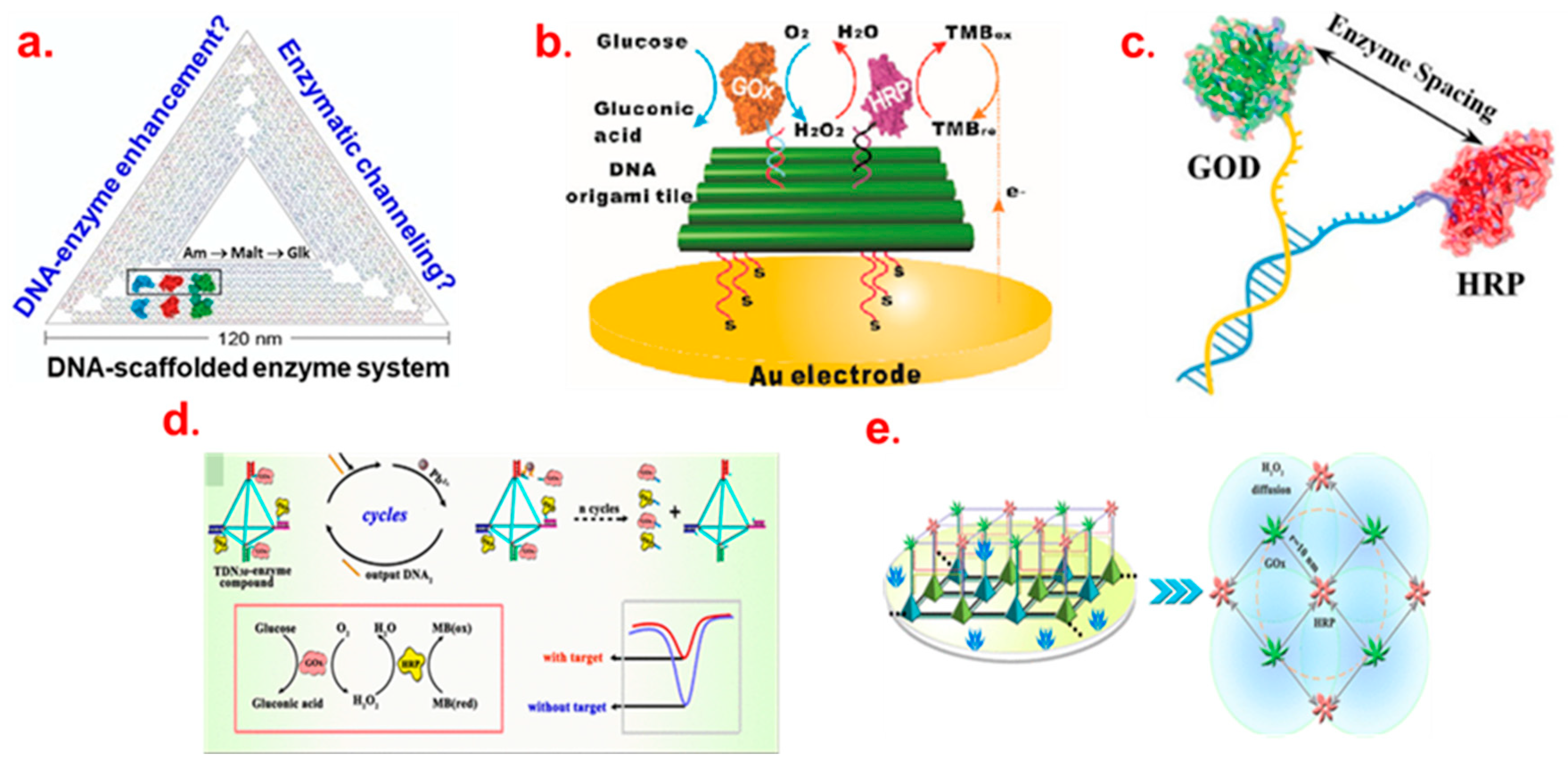
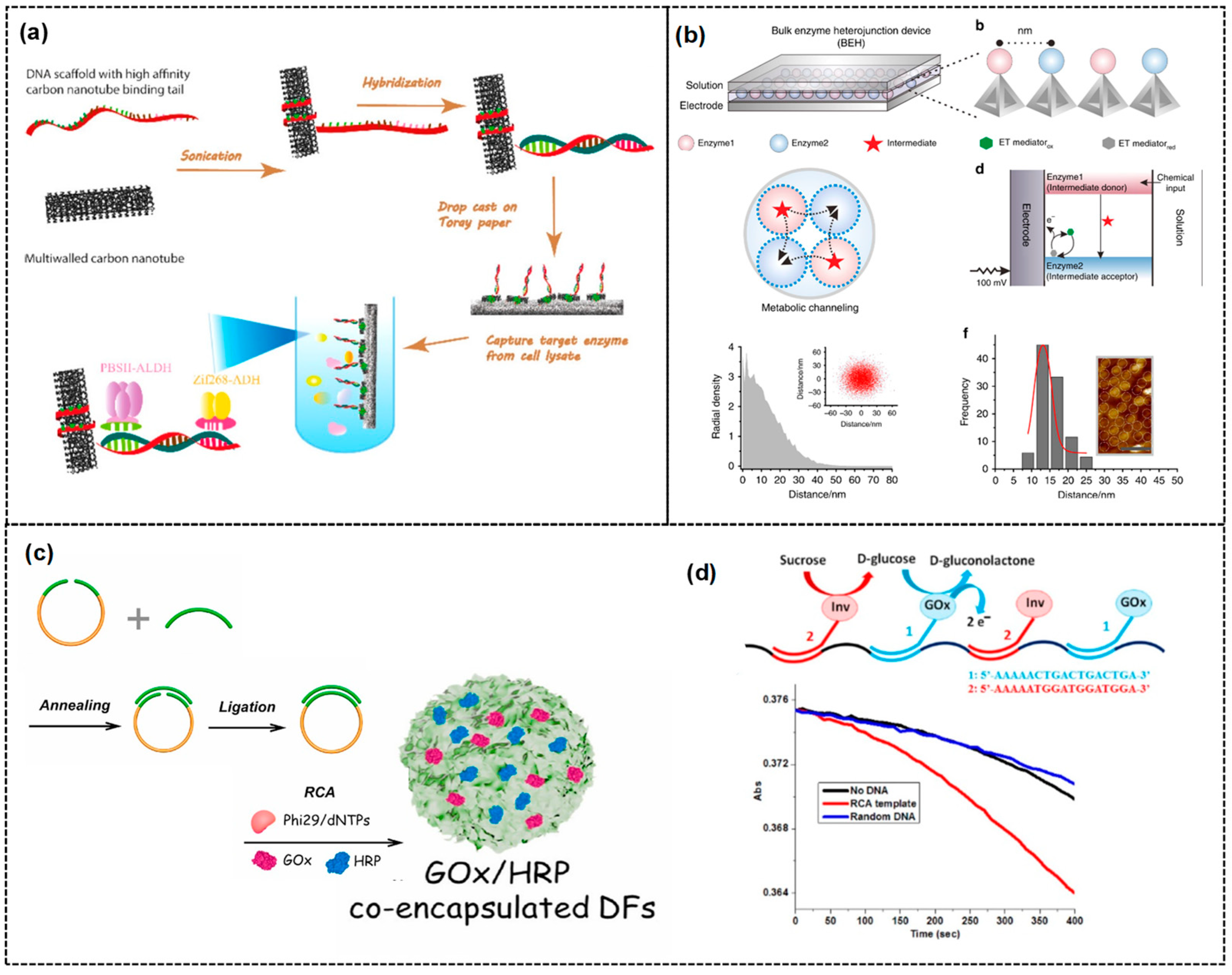
3. DNA Scaffold for Multi-Enzyme Cascade System
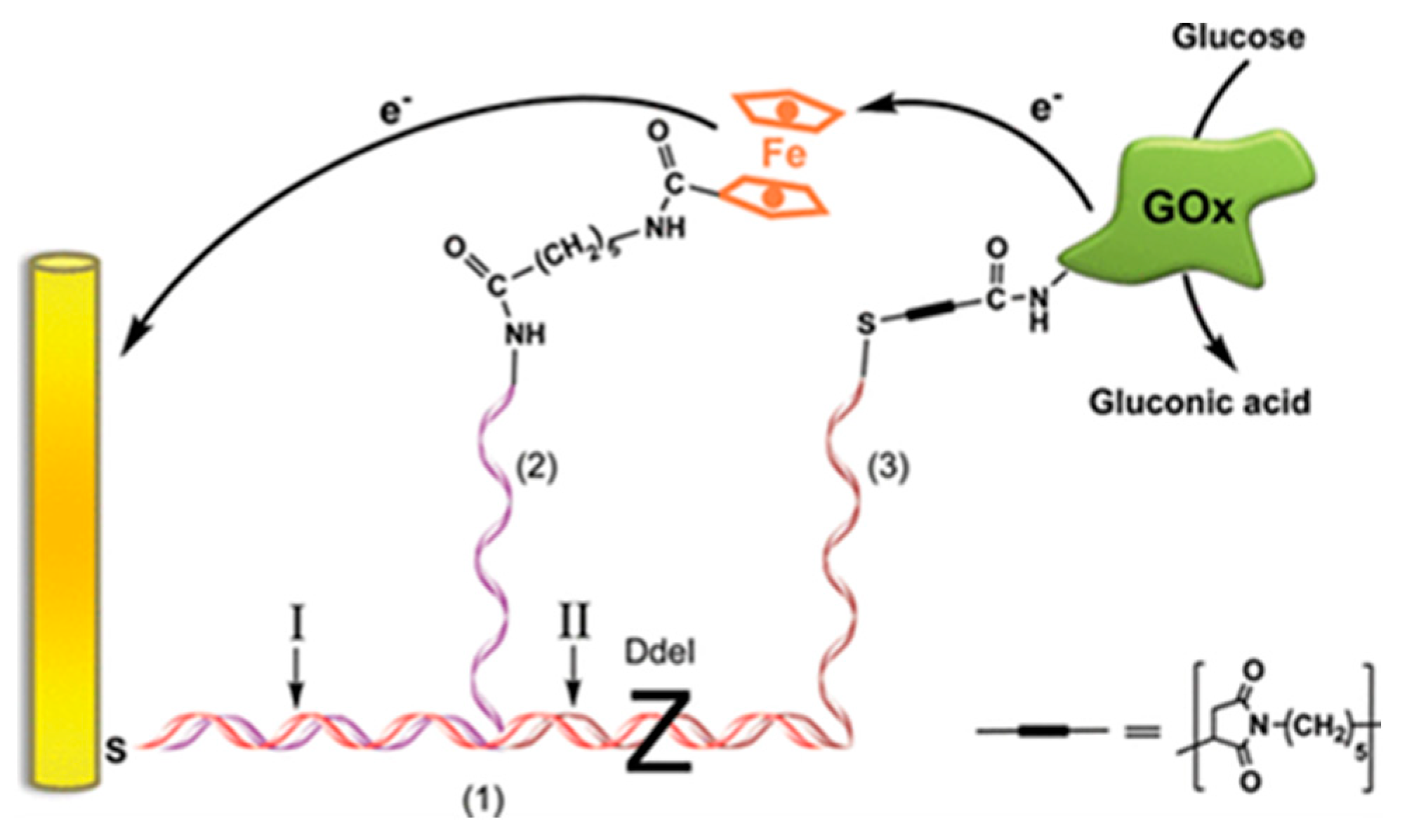
DNA Scaffold for the Multi-Enzyme BFC and Biosensor
4. Involvement of MOF Nanomaterial in Enzymes Cascade Reaction
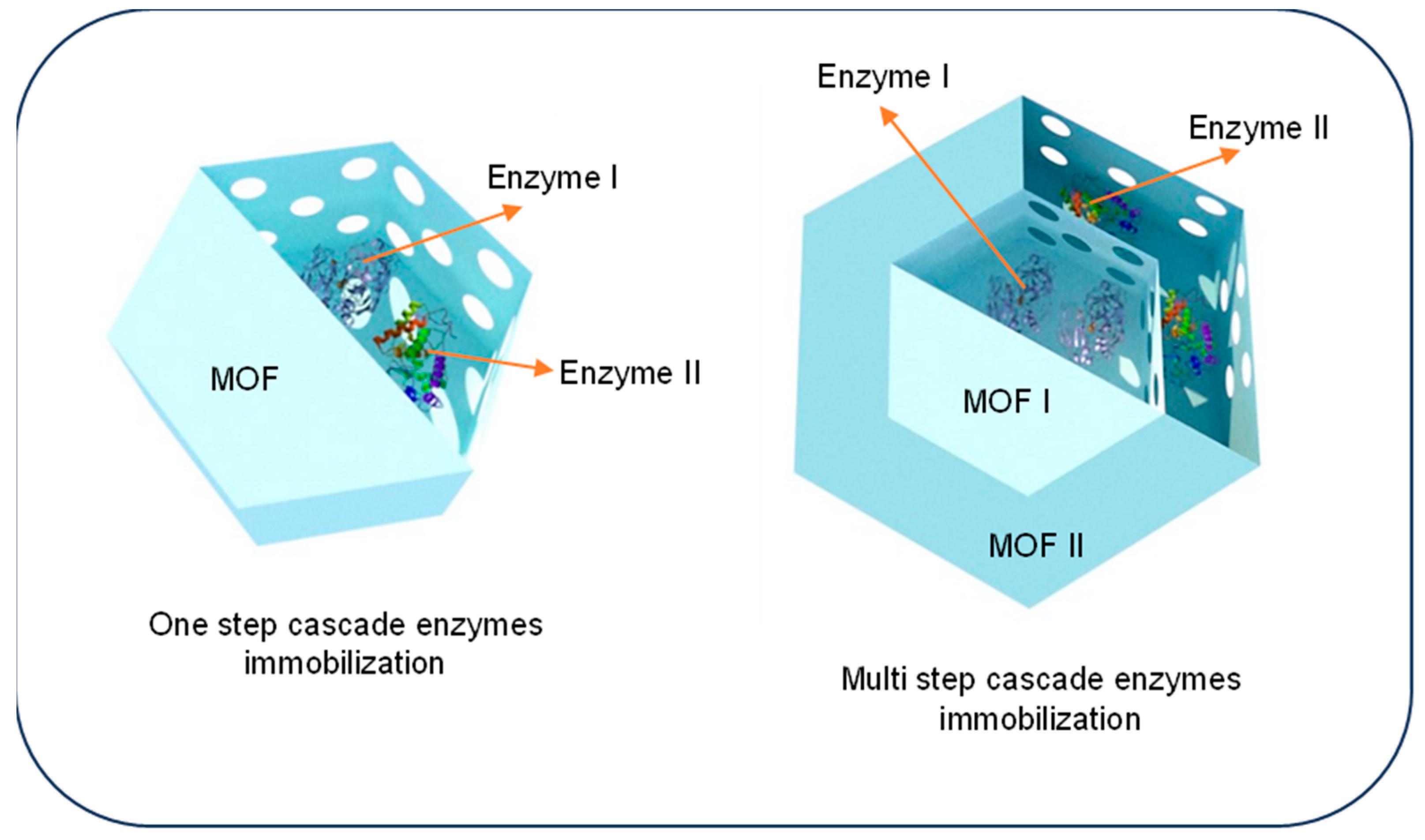
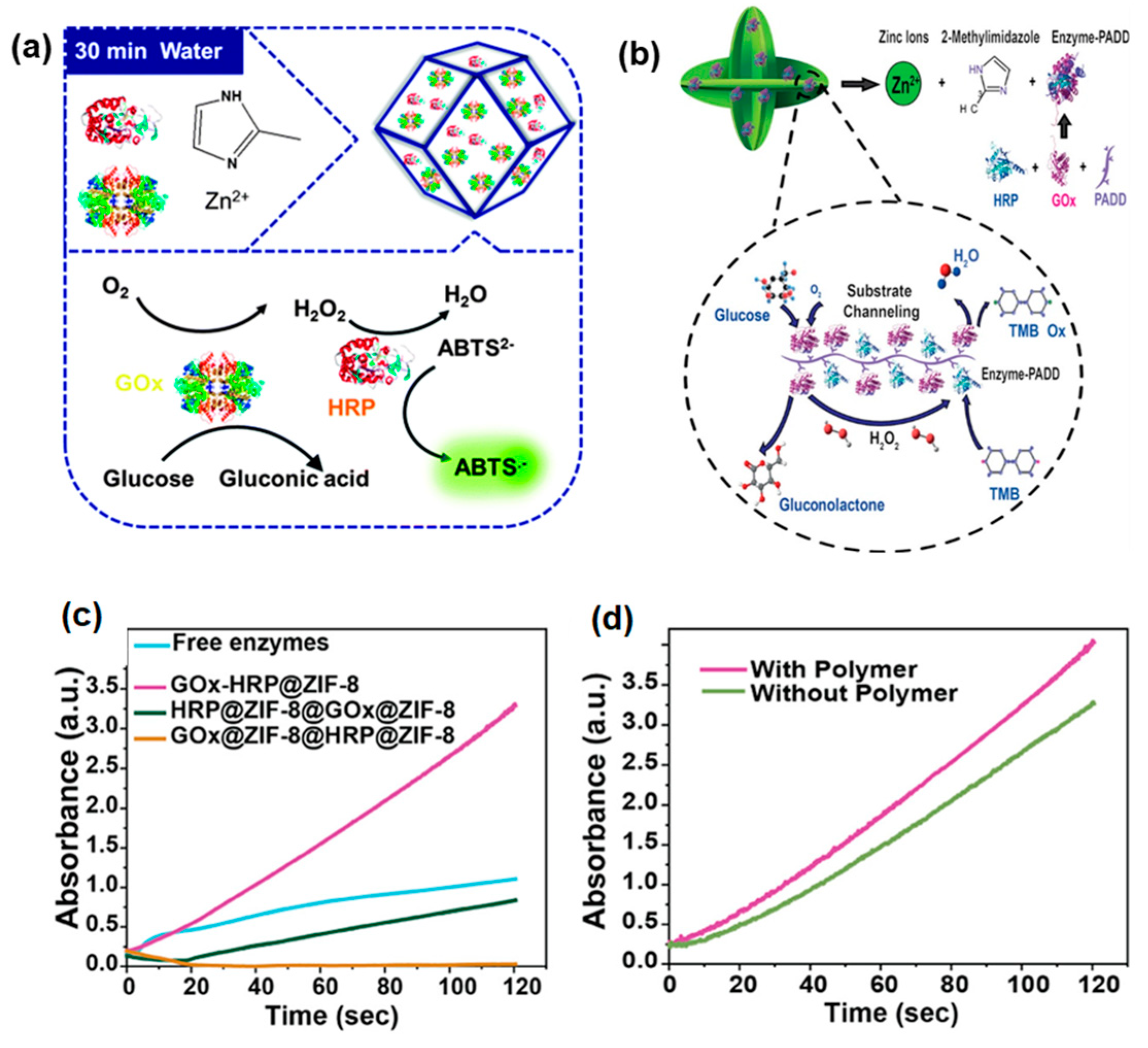
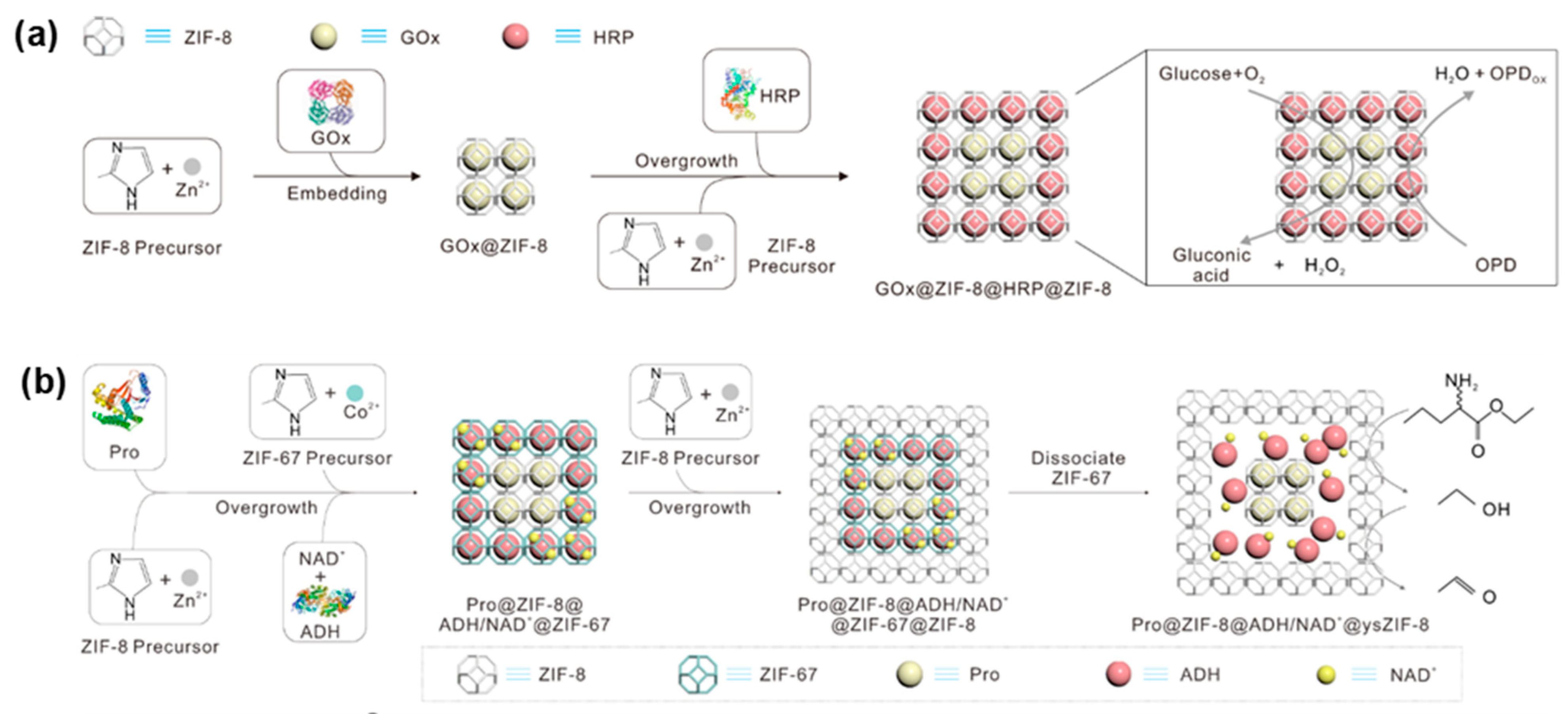
4.1. One-Step Co-Immobilization of Enzymes Using MOF
4.2. Multi-Step Co-Immobilization of Enzymes Using MOF
4.3. Progress in MOF Cascade Enzyme in Electrochemical BFCs and Biosensor Applications
5. Crosslinked Support
6. Polymeric Matrix/Hydrogel
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | α-amylase. |
| ABTS | (2:2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)). |
| ADH | alcohol dehydrogenase. |
| ALDH | aldehyde dehydrogenase. |
| BDC | benzene dicarboxylic acid. |
| BFCs | biofuel devices. |
| BOD | bilirubin oxidase. |
| BSA | bovine serum albumin. |
| CC | carbon cloth. |
| CEL | cellulase. |
| CMC | carboxymethyl cellulose. |
| CNH | creatinine amidohydrolase. |
| CNT | carbon nanotubes. |
| CRGO | chemically reduced graphene oxide. |
| ChOx | cholesterol oxidase. |
| DAP | 2:3-diaminophenazine. |
| DET | direct electron transfer. |
| DF | DNA flower. |
| DMS | dimethyl suberimidate. |
| DNA | deoxyribonucleic acid. |
| EGDGE | ethylene glycol diglycidyl ether. |
| FAD | flavin adenine dinucleotide. |
| FAD-GDH | flavin adenine dinucleotide-dependent glucose dehydrogenase. |
| FDH | fructose dehydrogenase. |
| ForDH | formate dehydrogenase. |
| GA | glutaraldehyde. |
| GCE | glassy carbon electrode. |
| GDH | glucose dehydrogenase. |
| GO | graphene oxide. |
| GOD/GOx | glucose oxidase. |
| GluA/GAL | glucoamylase. |
| H3TATB | 2:4,6-triyl-tribenzoic acid. |
| HRP | horseradish peroxidase. |
| INV | invertase. |
| LOD | limit of detection. |
| LOx | lactate oxidase. |
| LDH | lactate dehydrogenase. |
| MAF-7 | zinc and 3-methyl-1,2,4-triazole (Hmtz)). |
| MAL | maltase. |
| MG | methylene green. |
| MOF | metal–organic frameworks. |
| MUT | mutarotase. |
| MWCNT | multiwalled carbon nanotubes. |
| MgOC | magnesium oxide templated carbon. |
| NADC | sodium deoxycholate. |
| NADH | nicotinamide adenine dinucleotide. |
| NiPdNPs | nickel palladium nanoparticles. |
| NiR | nitrite reductase. |
| OCV | open circuit voltage. |
| OPD | o-phenylenediamine. |
| ORR | oxygen reduction reaction. |
| PADD | poly-(acrylamide-co-diallyl dimethylammonium chloride). |
| PC | porous carbon. |
| PDC | pyruvate decarboxylase. |
| PDH | pyranose dehydrogenase. |
| PEDGE | poly(ethylene glycol) diglycidyl ether. |
| PMMA | polymethyl methacrylate/poly(methyl 2-methylpropenoate). |
| PNP | purine nucleoside phosphorylase. |
| POD | peroxidase. |
| Pox | pyruvate oxidase. |
| PPy | polypyrrole. |
| RCA | rolling circle amplification. |
| RmBgl3B | Rhodothermus marinus β-glucosidase. |
| SOx/SOD | sarcosine oxidase. |
| SWCNT | single-walled carbon nanotubes. |
| TDN | tetrahedron. |
| TMB | 3:3′:5,5′-tetramethylbenzidine. |
| XOx | xanthine oxidase. |
| ZIF | zeolitic imidazolate framework. |
| β-Gal | β-galactosidase. |
References
- Armstrong, F.A.; Cheng, B.; Herold, R.A.; Megarity, C.F.; Siritanaratkul, B. From Protein Film Electrochemistry to Nanoconfined Enzyme Cascades and the Electrochemical Leaf. Chem. Rev. 2023, 123, 5421–5458. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Buller, R. Linear Enzyme Cascade for the Production of (–)-Iso-Isopulegol. Z. Für Naturforschung C 2019, 74, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial Biocatalytic Linear Cascades for Preparation of Organic Molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef] [PubMed]
- Rioz-Martínez, A.; Bisogno, F.R.; Rodríguez, C.; de Gonzalo, G.; Lavandera, I.; Pazmiño, D.E.T.; Fraaije, M.W.; Gotor, V. Biocatalysed Concurrent Production of Enantioenriched Compounds through Parallel Interconnected Kinetic Asymmetric Transformations. Org. Biomol. Chem. 2010, 8, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Subrizi, F.; Carter, E.M.; Sheppard, T.D.; Ward, J.M.; Hailes, H.C. Enzymatic Synthesis of Benzylisoquinoline Alkaloids Using a Parallel Cascade Strategy and Tyrosinase Variants. Nat. Commun. 2022, 13, 5436. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Dong, J.; Willner, I. Dynamic DNA Networks-Guided Directional and Orthogonal Transient Biocatalytic Cascades. J. Am. Chem. Soc. 2023, 145, 22135–22149. [Google Scholar] [CrossRef]
- Wang, C.; Yue, L.; Willner, I. Controlling Biocatalytic Cascades with Enzyme–DNA Dynamic Networks. Nat. Catal. 2020, 3, 941–950. [Google Scholar] [CrossRef]
- Sehl, T.; Hailes, H.C.; Ward, J.M.; Wardenga, R.; von Lieres, E.; Offermann, H.; Westphal, R.; Pohl, M.; Rother, D. Two Steps in One Pot: Enzyme Cascade for the Synthesis of nor(Pseudo)Ephedrine from Inexpensive Starting Materials. Angew. Chem. Int. Ed. 2013, 52, 6772–6775. [Google Scholar] [CrossRef]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-Enzymatic Cascade Reactions: Overview and Perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Blank, S.; Nguyen, Z.; Boucher, D.G.; Minteer, S.D. Electrochemical Cascade Reactions for Electro-Organic Synthesis. Curr. Opin. Electrochem. 2022, 35, 101049. [Google Scholar] [CrossRef]
- Devi, K.S.; Mahalakshmi, V.; Ghosh, A.R.; Kumar, A.S. Unexpected Co-Immobilization of Lactoferrin and Methylene Blue from Milk Solution on a Nafion/Mwcnt Modified Electrode and Application to Hydrogen Peroxide and Lactoferrin Biosensing. Electrochimica Acta 2017, 244, 26–37. [Google Scholar] [CrossRef]
- Hyeon, J.E.; Shin, S.K.; Han, S.O. Design of Nanoscale Enzyme Complexes Based on Various Scaffolding Materials for Biomass Conversion and Immobilization. Biotechnol. J. 2016, 11, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bao, T.; Zeng, X.; Xiong, H.; Wen, W.; Zhang, X.; Wang, S. Ultrasensitive Electrochemical DNA Biosensor Based on Functionalized Gold Clusters/Graphene Nanohybrids Coupling with Exonuclease Iii-Aided Cascade Target Recycling. Biosens. Bioelectron. 2017, 91, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Furusawa, H.; Nagamine, K.; Tokito, S. Constructive Optimization of a Multienzymatic Film Based on a Cascade Reaction for Electrochemical Biosensors. ACS Omega 2020, 5, 32844–32851. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lim, K.; Minteer, S.D. Cascaded Biocatalysis and Bioelectrocatalysis: Overview and Recent Advances. Annu. Rev. Phys. Chem. 2021, 72, 467–488. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.H.; Minteer, S.D.; De Andrade, A.R. Ethanol Biofuel Cells: Hybrid Catalytic Cascades as a Tool for Biosensor Devices. Biosensors 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Macazo, F.C.; Minteer, S.D. Enzyme Cascades in Biofuel Cells. Curr. Opin. Electrochem. 2017, 5, 114–120. [Google Scholar] [CrossRef]
- Palmore, G.R.; Bertschy, H.; Bergens, S.H.; Whitesides, G.M. A Methanol/Dioxygen Biofuel Cell That Uses Nad+-Dependent Dehydrogenases as Catalysts: Application of an Electro-Enzymatic Method to Regenerate Nicotinamide Adenine Dinucleotide at Low Overpotentials. J. Electroanal. Chem. 1998, 443, 155–161. [Google Scholar] [CrossRef]
- Shitanda, I.; Ozone, Y.; Morishita, Y.; Matsui, H.; Loew, N.; Motosuke, M.; Mukaimoto, T.; Kobayashi, M.; Mitsuhara, T.; Sugita, Y.; et al. Air-Bubble-Insensitive Microfluidic Lactate Biosensor for Continuous Monitoring of Lactate in Sweat. ACS Sens. 2023, 8, 2368–2374. [Google Scholar] [CrossRef]
- Olloqui-Sariego, J.L.; Calvente, J.J.; Andreu, R. Immobilizing Redox Enzymes at Mesoporous and Nanostructured Electrodes. Curr. Opin. Electrochem. 2021, 26, 100658. [Google Scholar] [CrossRef]
- Morishita, T.; Tsumura, T.; Toyoda, M.; Przepiórski, J.; Morawski, A.W.; Konno, H.; Inagaki, M. A Review of the Control of Pore Structure in Mgo-Templated Nanoporous Carbons. Carbon 2010, 48, 2690–2707. [Google Scholar] [CrossRef]
- Mazurenko, I.; Clément, R.; Byrne-Kodjabachian, D.; De Poulpiquet, A.; Tsujimura, S.; Lojou, E. Pore Size Effect of Mgo-Templated Carbon on Enzymatic H2 Oxidation by the Hyperthermophilic Hydrogenase from Aquifex Aeolicus. J. Electroanal. Chem. 2018, 812, 221–226. [Google Scholar] [CrossRef]
- Niiyama, A.; Murata, K.; Shigemori, Y.; Zebda, A.; Tsujimura, S. High-Performance Enzymatic Biofuel Cell Based on Flexible Carbon Cloth Modified with Mgo-Templated Porous Carbon. J. Power Sources 2019, 427, 49–55. [Google Scholar] [CrossRef]
- Shitanda, I.; Tsunaga, M.; Watanabe, H.; Itagaki, M.; Tsujimura, S.; Mikawa, T. High Capacity Lactate Biofuel Cell Using Enzyme Cascade without Nad. Chem. Lett. 2021, 50, 1160–1163. [Google Scholar] [CrossRef]
- Shitanda, I.; Hirano, K.; Loew, N.; Watanabe, H.; Itagaki, M.; Mikawa, T. High-Performance, Two-Step/Bi-Enzyme Lactate Biofuel Cell with Lactate Oxidase and Pyruvate Oxidase. J. Power Sources 2021, 498, 229935. [Google Scholar] [CrossRef]
- Kawai, H.; Kitazumi, Y.; Shirai, O.; Kano, K. Performance Analysis of an Oxidase/Peroxidase-Based Mediatorless Amperometric Biosensor. J. Electroanal. Chem. 2019, 841, 73–78. [Google Scholar] [CrossRef]
- Zor, C.; Reeve, H.A.; Quinson, J.; Thompson, L.A.; Lonsdale, T.H.; Dillon, F.; Grobert, N.; Vincent, K.A. H2-Driven Biocatalytic Hydrogenation in Continuous Flow Using Enzyme-Modified Carbon Nanotube Columns. Chem. Commun. 2017, 53, 9839–9841. [Google Scholar] [CrossRef]
- Adachi, T.; Miyata, T.; Makino, F.; Tanaka, H.; Namba, K.; Kano, K.; Sowa, K.; Kitazumi, Y.; Shirai, O. Experimental and Theoretical Insights into Bienzymatic Cascade for Mediatorless Bioelectrochemical Ethanol Oxidation with Alcohol and Aldehyde Dehydrogenases. ACS Catal. 2023, 13, 7955–7965. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Du, K.; Sun, J.; Zhou, X.; Feng, W.; Jiang, X.; Ji, P. A Two-Enzyme Immobilization Approach Using Carbon Nanotubes/Silica as Support. Biotechnol. Prog. 2015, 31, 42–47. [Google Scholar] [CrossRef]
- Lang, Q.; Yin, L.; Shi, J.; Li, L.; Xia, L.; Liu, A. Co-Immobilization of Glucoamylase and Glucose Oxidase for Electrochemical Sequential Enzyme Electrode for Starch Biosensor and Biofuel Cell. Biosens. Bioelectron. 2014, 51, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Arugula, M.A.; Williams, S.T.; Minteer, S.D.; Simonian, A.L. Layer-by-Layer Assembly of Carbon Nanotubes Modified with Invertase/Glucose Dehydrogenase Cascade for Sucrose/O2 Biofuel Cell. J. Electrochem. Soc. 2016, 163, F449–F454. [Google Scholar] [CrossRef]
- Contaldo, U.; Padrosa, D.R.; Jamet, H.; Albrecht, M.; Paradisi, F.; Le Goff, A. Optimising Electrical Interfacing between the Trimeric Copper Nitrite Reductase and Carbon Nanotubes. Chem.–A Eur. J. 2023, 29, e202301351. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.H.; Minteer, S.D.; de Andrade, A.R. Product Analysis of Operating an Ethanol/O2 Biofuel Cell Shows the Synergy between Enzymes within an Enzymatic Cascade. J. Electrochem. Soc. 2018, 165, H575–H579. [Google Scholar] [CrossRef]
- Chansaenpak, K.; Kamkaew, A.; Lisnund, S.; Prachai, P.; Ratwirunkit, P.; Jingpho, T.; Blay, V.; Pinyou, P. Development of a Sensitive Self-Powered Glucose Biosensor Based on an Enzymatic Biofuel Cell. Biosensors 2021, 11, 16. [Google Scholar] [CrossRef]
- Handa, Y.; Yamagiwa, K.; Ikeda, Y.; Yanagisawa, Y.; Watanabe, S.; Yabuuchi, N.; Komaba, S. Fabrication of Carbon-Felt-Based Multi-Enzyme Immobilized Anodes to Oxidize Sucrose for Biofuel Cells. ChemPhysChem 2014, 15, 2145–2151. [Google Scholar] [CrossRef]
- Wu, G.; Gao, Y.; Zhao, D.; Ling, P.; Gao, F. Methanol/Oxygen Enzymatic Biofuel Cell Using Laccase and Nad+-Dependent Dehydrogenase Cascades as Biocatalysts on Carbon Nanodots Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 40978–40986. [Google Scholar] [CrossRef]
- Yasujima, R.; Yasueda, K.; Horiba, T.; Komaba, S. Multi-Enzyme Immobilized Anodes Utilizing Maltose Fuel for Biofuel Cell Applications. ChemElectroChem 2018, 5, 2271–2278. [Google Scholar] [CrossRef]
- Adeel, M.; Bilal, M.; Rasheed, T.; Sharma, A.; Iqbal, H.M. Graphene and Graphene Oxide: Functionalization and Nano-Bio-Catalytic System for Enzyme Immobilization and Biotechnological Perspective. Int. J. Biol. Macromol. 2018, 120, 1430–1440. [Google Scholar] [CrossRef]
- Zore, O.V.; Pattammattel, A.; Gnanaguru, S.; Kumar, C.V.; Kasi, R.M. Bienzyme–Polymer–Graphene Oxide Quaternary Hybrid Biocatalysts: Efficient Substrate Channeling under Chemically and Thermally Denaturing Conditions. ACS Catal. 2015, 5, 4979–4988. [Google Scholar] [CrossRef]
- Zhao, F.; Li, H.; Jiang, Y.; Wang, X.; Mu, X. Co-Immobilization of Multi-Enzyme on Control-Reduced Graphene Oxide by Non-Covalent Bonds: An Artificial Biocatalytic System for the One-Pot Production of Gluconic Acid from Starch. Green Chem. 2014, 16, 2558–2565. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, S.; Zhang, L. Co-Immobilization of Cellulase and Glucose Oxidase on Graphene Oxide by Covalent Bonds: A Biocatalytic System for One-Pot Conversion of Gluconic Acid from Carboxymethyl Cellulose. J. Chem. Technol. Biotechnol. 2020, 95, 1116–1125. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, S. Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Ren, S.; Li, C.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent Progress in Multienzymes Co-Immobilization and Multienzyme System Applications. Chem. Eng. J. 2019, 373, 1254–1278. [Google Scholar] [CrossRef]
- Song, J.; He, W.; Shen, H.; Zhou, Z.; Li, M.; Su, P.; Yang, Y. Construction of Multiple Enzyme Metal–Organic Frameworks Biocatalyst Via DNA Scaffold: A Promising Strategy for Enzyme Encapsulation. Chem. Eng. J. 2019, 363, 174–182. [Google Scholar] [CrossRef]
- Maffeis, V.; Belluati, A.; Craciun, I.; Wu, D.; Novak, S.; Schoenenberger, C.A.; Palivan, C.G. Clustering of Catalytic Nanocompartments for Enhancing an Extracellular Non-Native Cascade Reaction. Chem. Sci. 2021, 12, 12274–12285. [Google Scholar] [CrossRef]
- Ngo, T.A.; Dinh, H.; Nguyen, T.M.; Liew, F.F.; Nakata, E.; Morii, T. Protein Adaptors Assemble Functional Proteins on DNA Scaffolds. Chem. Commun. 2019, 55, 12428–12446. [Google Scholar] [CrossRef]
- Dey, S.; Fan, C.; Gothelf, K.V.; Li, J.; Lin, C.; Liu, L.; Liu, N.; Nijenhuis, M.A.; Saccà, B.; Simmel, F.C.; et al. DNA Origami. Nat. Rev. Methods Primers 2021, 1, 13. [Google Scholar] [CrossRef]
- Engelhardt, F.A.S.; Praetorius, F.; Wachauf, C.H.; Brüggenthies, G.; Kohler, F.; Kick, B.; Kadletz, K.L.; Pham, P.N.; Behler, K.L.; Gerling, T.; et al. Custom-Size, Functional, and Durable DNA Origami with Design-Specific Scaffolds. ACS Nano 2019, 13, 5015–5027. [Google Scholar] [CrossRef]
- Lin, P.; Yang, H.; Nakata, E.; Morii, T. Mechanistic Aspects for the Modulation of Enzyme Reactions on the DNA Scaffold. Molecules 2022, 27, 6309. [Google Scholar] [CrossRef]
- Lin, P.; Dinh, H.; Morita, Y.; Nakata, E.; Morii, T. Dynamic Assembly of Cascade Enzymes by the Shape Transformation of a DNA Scaffold. Adv. Funct. Mater. 2023, 33, 2215023. [Google Scholar] [CrossRef]
- Du, C.; Hu, P.; Ren, L. Nucleic Acid-Based Scaffold Systems and Application in Enzyme Cascade Catalysis. Appl. Microbiol. Biotechnol. 2023, 107, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Kostrz, D.; Wayment-Steele, H.K.; Wang, J.L.; Follenfant, M.; Pande, V.S.; Strick, T.R.; Gosse, C. A Modular DNA Scaffold to Study Protein–Protein Interactions at Single-Molecule Resolution. Nat. Nanotechnol. 2019, 14, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.S.; Xiong, Y.; Huang, J.; Gang, O. Cascaded Enzyme Reactions over a Three-Dimensional, Wireframe DNA Origami Scaffold. JACS Au 2022, 2, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Kou, B.-B.; Chai, Y.-Q.; Yuan, Y.-L.; Yuan, R. A DNA Nanopillar as a Scaffold to Regulate the Ratio and Distance of Mimic Enzymes for an Efficient Cascade Catalytic Platform. Chem. Sci. 2021, 12, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Ke, Y.; Wang, Q. Selective in situ Assembly of Viral Protein onto DNA Origami. J. Am. Chem. Soc. 2018, 140, 8074–8077. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, Y.; Mao, C.; Xie, X.; Lin, Y. The Biological Applications of DNA Nanomaterials: Current Challenges and Future Directions. Signal Transduct. Target. Ther. 2021, 6, 351. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, C.; Zhang, J.; Wang, Z.; Wei, Y.; Li, Y.; Dai, S.; Tay, F.R.; Niu, L. DNA-Based Materials Inspired by Natural Extracellular DNA. Adv. Funct. Mater. 2023, 33, 2211669. [Google Scholar] [CrossRef]
- Wilner, O.I.; Weizmann, Y.; Gill, R.; Lioubashevski, O.; Freeman, R.; Willner, I. Enzyme Cascades Activated on Topologically Programmed DNA Scaffolds. Nat. Nanotechnol. 2009, 4, 249–254. [Google Scholar] [CrossRef]
- Douglas, S.M.; Bachelet, I.; Church, G.M. A Logic-Gated Nanorobot for Targeted Transport of Molecular Payloads. Science 2012, 335, 831–834. [Google Scholar] [CrossRef]
- Nakata, E.; Liew, F.F.; Uwatoko, C.; Kiyonaka, S.; Mori, Y.; Katsuda, Y.; Endo, M.; Sugiyama, H.; Morii, T. Zinc-Finger Proteins for Site-Specific Protein Positioning on DNA-Origami Structures. Angew. Chem. Int. Ed. 2012, 51, 2421–2424. [Google Scholar] [CrossRef]
- Praetorius, F.; Dietz, H. Self-Assembly of Genetically Encoded DNA-Protein Hybrid Nanoscale Shapes. Science 2017, 355, eaam5488. [Google Scholar] [CrossRef]
- Chhabra, R.; Sharma, J.; Ke, Y.; Liu, Y.; Rinker, S.; Lindsay, S.; Yan, H. Spatially Addressable Multiprotein Nanoarrays Templated by Aptamer-Tagged DNA Nanoarchitectures. J. Am. Chem. Soc. 2007, 129, 10304–10305. [Google Scholar] [CrossRef]
- Fu, J.; Yang, Y.R.; Dhakal, S.; Zhao, Z.; Liu, M.; Zhang, T.; Walter, N.G.; Yan, H. Assembly of Multienzyme Complexes on DNA Nanostructures. Nat. Protoc. 2016, 11, 2243–2273. [Google Scholar] [CrossRef]
- Kou, B.-B.; Chai, Y.-Q.; Yuan, Y.-L.; Yuan, R. PtNPs as Scaffolds to Regulate Interenzyme Distance for Construction of Efficient Enzyme Cascade Amplification for Ultrasensitive Electrochemical Detection of MMP-2. Anal. Chem. 2017, 89, 9383–9387. [Google Scholar] [CrossRef]
- Krall, A.S.; Christofk, H.R. Division Enzyme Regulates Metabolism. Nature 2017, 546, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.P.; Thomsen, R.P.; Turner, K.B.; Walper, S.A.; Vranish, J.; Kjems, J.; Ancona, M.G.; Medintz, I.L. Enhanced Catalysis from Multienzyme Cascades Assembled on a DNA Origami Triangle. ACS Nano 2019, 13, 13677–13689. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Fu, J.; Liu, M.; Jiang, S.; Andreoni, A.; Zuo, X.; Liu, Y.; Yan, H.; Fan, C. Constructing Submonolayer DNA Origami Scaffold on Gold Electrode for Wiring of Redox Enzymatic Cascade Pathways. ACS Appl. Mater. Interfaces 2019, 11, 13881–13887. [Google Scholar] [CrossRef]
- Chu, G.B.; Li, W.Y.; Han, X.X.; Sun, H.H.; Han, Y.; Zhi, G.Y.; Zhang, D.H. Co-Immobilization of God & Hrp on Y-Shaped DNA Scaffold and the Regulation of Inter-Enzyme Distance. Small 2023, 19, 2301413. [Google Scholar]
- Wang, D.; Chai, Y.; Yuan, Y.; Yuan, R. Precise Regulation of Enzyme Cascade Catalytic Efficiency with DNA Tetrahedron as Scaffold for Ultrasensitive Electrochemical Detection of DNA. Anal. Chem. 2019, 91, 3561–3566. [Google Scholar] [CrossRef]
- Wang, D.; Chai, Y.; Yuan, Y.; Yuan, R. Lattice-Like DNA Tetrahedron Nanostructure as Scaffold to Locate Gox and Hrp Enzymes for Highly Efficient Enzyme Cascade Reaction. ACS Appl. Mater. Interfaces 2020, 12, 2871–2877. [Google Scholar] [CrossRef] [PubMed]
- Morshed, J.; Hossain, M.M.; Zebda, A.; Tsujimura, S. A Disposable Enzymatic Biofuel Cell for Glucose Sensing Via Short-Circuit Current. Biosens. Bioelectron. 2023, 230, 115272. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.T.; Minteer, S.D. Biofuel Cells: Enhanced Enzymatic Bioelectrocatalysis. Annu. Rev. Anal. Chem. 2012, 5, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Piperberg, G.; Wilner, O.I.; Yehezkeli, O.; Tel-Vered, R.; Willner, I. Control of Bioelectrocatalytic Transformations on DNA Scaffolds. J. Am. Chem. Soc. 2009, 131, 8724–8725. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Van Nguyen, K.; Holade, Y.; Han, H.; Dooley, K.; Atanassov, P.; Banta, S.; Minteer, S.D. Improving the Performance of Methanol Biofuel Cells Utilizing an Enzyme Cascade Bioanode with DNA-Bridged Substrate Channeling. ACS Energy Lett. 2017, 2, 1435–1438. [Google Scholar] [CrossRef]
- Song, P.; Shen, J.; Ye, D.; Dong, B.; Wang, F.; Pei, H.; Wang, J.; Shi, J.; Wang, L.; Xue, W.; et al. Programming Bulk Enzyme Heterojunctions for Biosensor Development with Tetrahedral DNA Framework. Nat. Commun. 2020, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Qiao, Z.; Hai, X.; Song, W.; Bi, S. Versatile Electrochemical Biosensor Based on Bi-Enzyme Cascade Biocatalysis Spatially Regulated by DNA Architecture. Biosens. Bioelectron. 2021, 174, 112827. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, K.; Giroud, F.; Minteer, S.D. Improved Bioelectrocatalytic Oxidation of Sucrose in a Biofuel Cell with an Enzyme Cascade Assembled on a DNA Scaffold. J. Electrochem. Soc. 2014, 161, H930. [Google Scholar] [CrossRef]
- Wang, L.; Ling, Y.; Han, L.; Zhou, J.; Sun, Z.; Li, N.B.; Luo, H.Q. Catalase Active Metal-Organic Framework Synthesized by Ligand Regulation for the Dual Detection of Glucose and Cysteine. Anal. Chim. Acta 2020, 1131, 118–125. [Google Scholar] [CrossRef]
- Liang, J.; Liang, K. Multi-Enzyme Cascade Reactions in Metal-Organic Frameworks. Chem. Rec. 2020, 20, 1100–1116. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ge, J.; Yang, C.; Hou, M.; Liu, Z. Facile Synthesis of Multiple Enzyme-Containing Metal–Organic Frameworks in a Biomolecule-Friendly Environment. Chem. Commun. 2015, 51, 13408–13411. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Vázquez-González, M.; Zoabi, A.; Abu-Reziq, R.; Willner, I. Biocatalytic Cascades Driven by Enzymes Encapsulated in Metal–Organic Framework Nanoparticles. Nat. Catal. 2018, 1, 689–695. [Google Scholar] [CrossRef]
- Fernando, D.; Mathesh, M.; Cai, J.; Yang, W. In situ Immobilization of Multi-Enzymes for Enhanced Substrate Channeling of Enzyme Cascade Reactions: A Nanoarchitectonics Approach by Directed Metal–Organic Frameworks. Langmuir 2023, 39, 7979–7985. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, N.; Huang, W.; Song, Y.; Jiang, Y. Enzymatic Cascade Reactions Mediated by Highly Efficient Biomimetic Quasi Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 22240–22253. [Google Scholar] [CrossRef] [PubMed]
- Man, T.; Xu, C.; Liu, X.-Y.; Li, D.; Tsung, C.-K.; Pei, H.; Wan, Y.; Li, L. Hierarchically Encapsulating Enzymes with Multi-Shelled Metal-Organic Frameworks for Tandem Biocatalytic Reactions. Nat. Commun. 2022, 13, 305. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Ma, X.; Xia, M.; Zhang, Y. Adsorption of Cholesterol Oxidase and Entrapment of Horseradish Peroxidase in Metal-Organic Frameworks for the Colorimetric Biosensing of Cholesterol. Talanta 2019, 200, 293–299. [Google Scholar] [CrossRef]
- Yimamumaimaiti, T.; Lu, X.; Zhang, J.-R.; Wang, L.; Zhu, J.-J. Efficient Blood-Toleration Enzymatic Biofuel Cell Via in situ Protection of an Enzyme Catalyst. ACS Appl. Mater. Interfaces 2020, 12, 41429–41436. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Jiang, H.; Zeng, H.; An, N.; Liu, B.; Sun, L.; Fan, Z. Self-Powered Enzyme-Linked Microneedle Patch for Scar-Prevention Healing of Diabetic Wounds. Sci. Adv. 2023, 9, eadh1415. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Huang, L.; Zhang, Z.; Dong, S. GOx@ZIF-8(NiPd) Nanoflower: An Artificial Enzyme System for Tandem Catalysis. Angew. Chem. Int. Ed. 2017, 56, 16082–16085. [Google Scholar] [CrossRef]
- Fu, L.; Liu, J.; Hu, Z.; Zhou, M. Recent Advances in the Construction of Biofuel Cells Based Self-Powered Electrochemical Biosensors: A Review. Electroanalysis 2018, 30, 2535–2550. [Google Scholar] [CrossRef]
- Zebda, A.; Alcaraz, J.-P.; Vadgama, P.; Shleev, S.; Minteer, S.D.; Boucher, F.; Cinquin, P.; Martin, D.K. Challenges for Successful Implantation of Biofuel Cells. Bioelectrochemistry 2018, 124, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xia, H.-Q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the Challenges of Enzymatic (Bio)Fuel Cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Morshed, J.; Tsujimura, S. Designing a Cross-Linked Redox Network for a Mediated Enzyme-Based Electrode. Chem. Commun. 2021, 57, 6999–7002. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Luo, Z.; Wang, R.; Pei, X.; Wu, S.; Chen, H.; Xie, T.; Sheldon, R.A.; Wang, A. Designing an Enzyme Assembly Line for Green Cascade Processes Using Bio-Orthogonal Chemistry. Green Chem. 2023, 25, 7547–7555. [Google Scholar] [CrossRef]
- Bauler, P.; Huber, G.; Leyh, T.; McCammon, J.A. Channeling by Proximity: The Catalytic Advantages of Active Site Colocalization Using Brownian Dynamics. J. Phys. Chem. Lett. 2010, 1, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Neto, S.A.; Minteer, S.D.; de Andrade, A.R. Developing Ethanol Bioanodes Using a Hydrophobically Modified Linear Polyethylenimine Hydrogel for Immobilizing an Enzyme Cascade. J. Electroanal. Chem. 2018, 812, 153–158. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in Bio-Catalysts Design: A Useful Crosslinker and a Versatile Tool in Enzyme Immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Vasylieva, N.; Maucler, C.; Meiller, A.; Viscogliosi, H.; Lieutaud, T.; Barbier, D.; Marinesco, S. Immobilization Method to Preserve Enzyme Specificity in Biosensors: Consequences for Brain Glutamate Detection. Anal. Chem. 2013, 85, 2507–2515. [Google Scholar] [CrossRef]
- Siritanaratkul, B. Design Principles for a Nanoconfined Enzyme Cascade Electrode Via Reaction–Diffusion Modelling. Phys. Chem. Chem. Phys. 2023, 25, 9357–9363. [Google Scholar] [CrossRef]
- Wheeldon, I.; Minteer, S.D.; Banta, S.; Barton, S.C.; Atanassov, P.; Sigman, M. Substrate Channelling as an Approach to Cascade Reactions. Nat. Chem. 2016, 8, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Toda, R.; Tatara, R.; Horiba, T.; Komaba, S. Multi-Enzyme-Modified Bioanode Utilising Starch as a Fuel. ChemElectroChem 2021, 8, 4199–4206. [Google Scholar] [CrossRef]
- Moehlenbrock, M.J.; Toby, T.K.; Waheed, A.; Minteer, S.D. Metabolon Catalyzed Pyruvate/Air Biofuel Cell. J. Am. Chem. Soc. 2010, 132, 6288–6289. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.; Kojima, K.; Sode, K. Review of Glucose Oxidases and Glucose Dehydrogenases: A Bird’s Eye View of Glucose Sensing Enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.J.; Svoboda, V.; Lau, C.; Martin, G.; Minteer, S.D. Enzyme Catalysed Biofuel Cells. Energy Environ. Sci. 2008, 1, 320–337. [Google Scholar] [CrossRef]
- Minteer, S.D.; Liaw, B.Y.; Cooney, M.J. Enzyme-Based Biofuel Cells. Curr. Opin. Biotechnol. 2007, 18, 228–234. [Google Scholar] [CrossRef]
- Ruff, A. Redox Polymers in Bioelectrochemistry: Common Playgrounds and Novel Concepts. Curr. Opin. Electrochem. 2017, 5, 66–73. [Google Scholar] [CrossRef]
- Rafighi, P.; Karlsson, E.N.; Ara, K.Z.; Pankratova, G.; Bollella, P.; Peterbauer, C.K.; Gorton, L. A Novel Membraneless Β-Glucan/O2 Enzymatic Fuel Cell Based on Β-Glucosidase (Rmbgl3b)/Pyranose Dehydrogenase (Ampdh) Co-Immobilized onto Buckypaper Electrode. Bioelectrochemistry 2022, 148, 108254. [Google Scholar] [CrossRef]
- Matsumoto, R.; Kakuta, M.; Sugiyama, T.; Goto, Y.; Sakai, H.; Tokita, Y.; Hatazawa, T.; Tsujimura, S.; Shirai, O.; Kano, K. A Liposome-Based Energy Conversion System for Accelerating the Multi-Enzyme Reactions. Phys. Chem. Chem. Phys. 2010, 12, 13904–13906. [Google Scholar] [CrossRef]
- Sakai, H.; Tokita, Y.; Tsujimura, S.; Shirai, O.; Kano, K. Re-Construction of Pentose Phosphate Pathway Coupled with a Bioelectrocatalytic NADPH Oxidation System for Bioanodes of Biofuel Cells. Electrochemistry 2013, 81, 981–984. [Google Scholar] [CrossRef][Green Version]
- Gracia, R.; Mecerreyes, D. Polymers with Redox Properties: Materials for Batteries, Biosensors and More. Polym. Chem. 2013, 4, 2206–2214. [Google Scholar] [CrossRef]
- Heller, A. Miniature Biofuel Cells. Phys. Chem. Chem. Phys. 2004, 6, 209–216. [Google Scholar] [CrossRef]
- Campbell, A.S.; Murata, H.; Carmali, S.; Matyjaszewski, K.; Islam, M.F.; Russell, A.J. Polymer-Based Protein Engineering Grown Ferrocene-Containing Redox Polymers Improve Current Generation in an Enzymatic Biofuel Cell. Biosens. Bioelectron. 2016, 86, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Degani, Y.; Heller, A. Electrical Communication between Redox Centers of Glucose Oxidase and Electrodes Via Electrostatically and Covalently Bound Redox Polymers. J. Am. Chem. Soc. 1989, 111, 2357–2358. [Google Scholar] [CrossRef]
- Yuan, M.; Minteer, S.D. Redox Polymers in Electrochemical Systems: From Methods of Mediation to Energy Storage. Curr. Opin. Electrochem. 2019, 15, 1–6. [Google Scholar] [CrossRef]
- Hickey, D.P.; Giroud, F.; Schmidtke, D.W.; Glatzhofer, D.T.; Minteer, S.D. Enzyme Cascade for Catalyzing Sucrose Oxidation in a Biofuel Cell. ACS Catal. 2013, 3, 2729–2737. [Google Scholar] [CrossRef]
- Lau, C.; Moehlenbrock, M.J.; Arechederra, R.L.; Falase, A.; Garcia, K.; Rincon, R.; Minteer, S.D.; Banta, S.; Gupta, G.; Babanova, S.; et al. Paper Based Biofuel Cells: Incorporating Enzymatic Cascades for Ethanol and Methanol Oxidation. Int. J. Hydrogen Energy 2015, 40, 14661–14666. [Google Scholar] [CrossRef]
- Nieh, C.-H.; Tsujimura, S.; Shirai, O.; Kano, K. Amperometric Biosensor Based on Reductive H2O2 Detection Using Pentacyanoferrate-Bound Polymer for Creatinine Determination. Anal. Chim. Acta 2013, 767, 128–133. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Omayu, A.; Yagi, S.; Nakamura, K.; Motonaka, J. Evaluation of Osmium(II) Complexes as Electron Transfer Mediators Accessible for Amperometric Glucose Sensors. Anal. Sci. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Zafar, M.N.; Wang, X.; Sygmund, C.; Ludwig, R.; Leech, D.; Gorton, L. Electron-Transfer Studies with a New Flavin Adenine Dinucleotide Dependent Glucose Dehydrogenase and Osmium Polymers of Different Redox Potentials. Anal. Chem. 2011, 84, 334–341. [Google Scholar] [CrossRef]
- Kopiec, G.; Starzec, K.; Kochana, J.; Kinnunen-Skidmore, T.P.; Schuhmann, W.; Campbell, W.H.; Ruff, A.; Plumeré, N. Bioelectrocatalytic and Electrochemical Cascade for Phosphate Sensing with up to 6 Electrons Per Analyte Molecule. Biosens. Bioelectron. 2018, 117, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Mano, N.; Heller, A. Long Tethers Binding Redox Centers to Polymer Backbones Enhance Electron Transport in Enzyme “Wiring” Hydrogels. J. Am. Chem. Soc. 2003, 125, 4951–4957. [Google Scholar] [CrossRef]
- Ruff, A.; Pinyou, P.; Nolten, M.; Conzuelo, F.; Schuhmann, W. A Self-Powered Ethanol Biosensor. ChemElectroChem 2017, 4, 890–897. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Enzyme-Based Biosensors: Tackling Electron Transfer Issues. Sensors 2020, 20, 3517. [Google Scholar] [CrossRef] [PubMed]


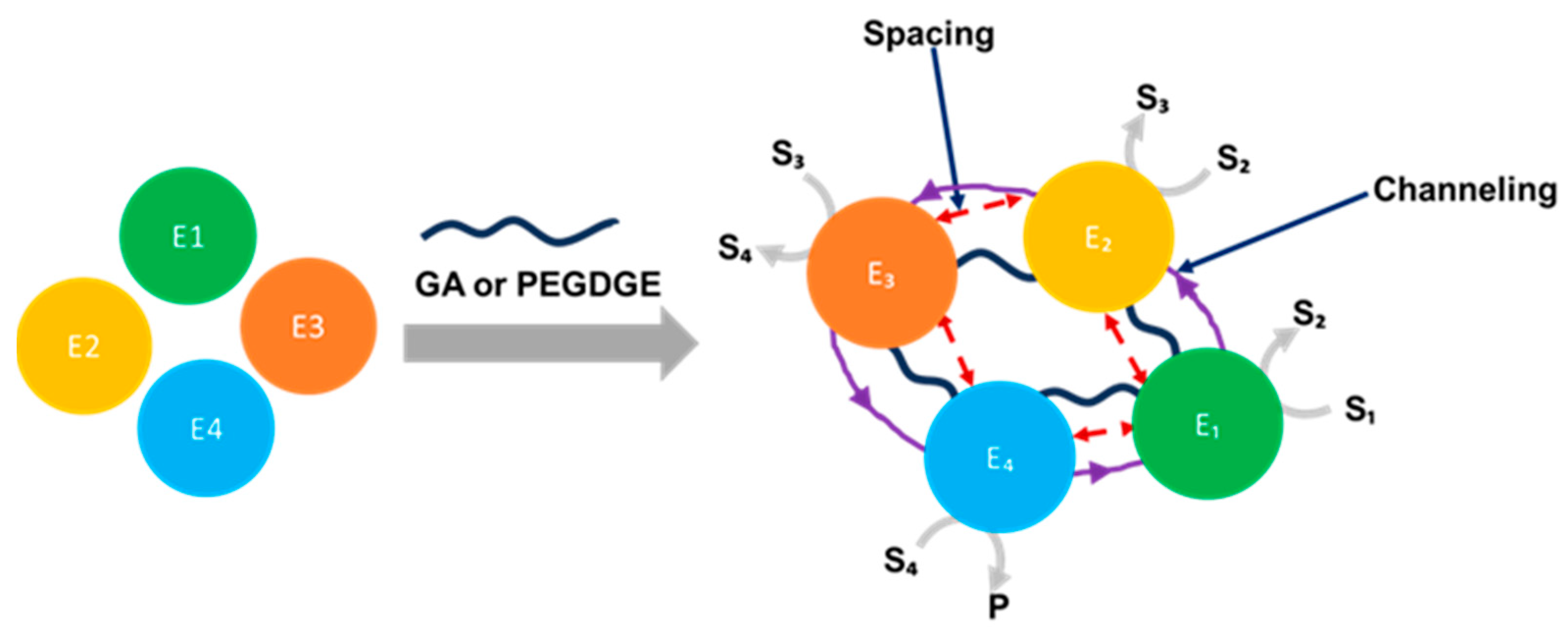
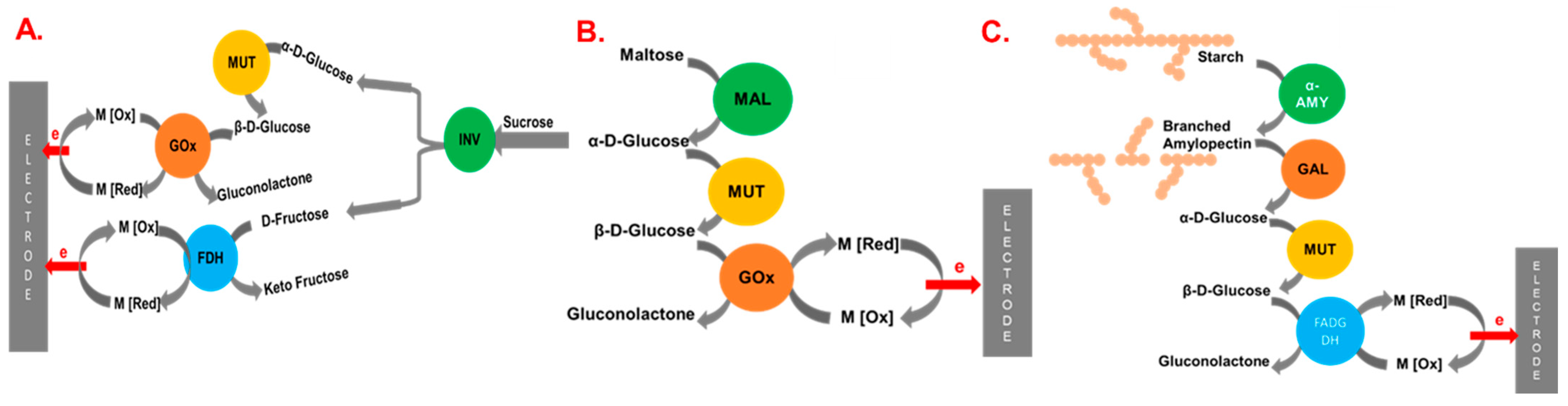
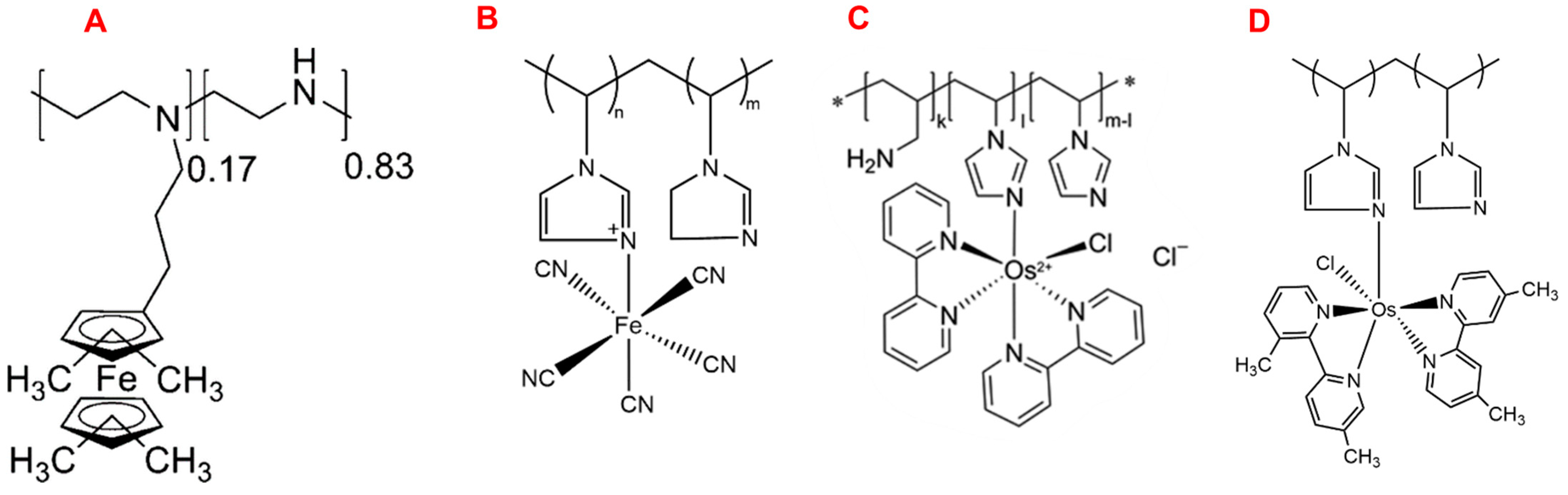
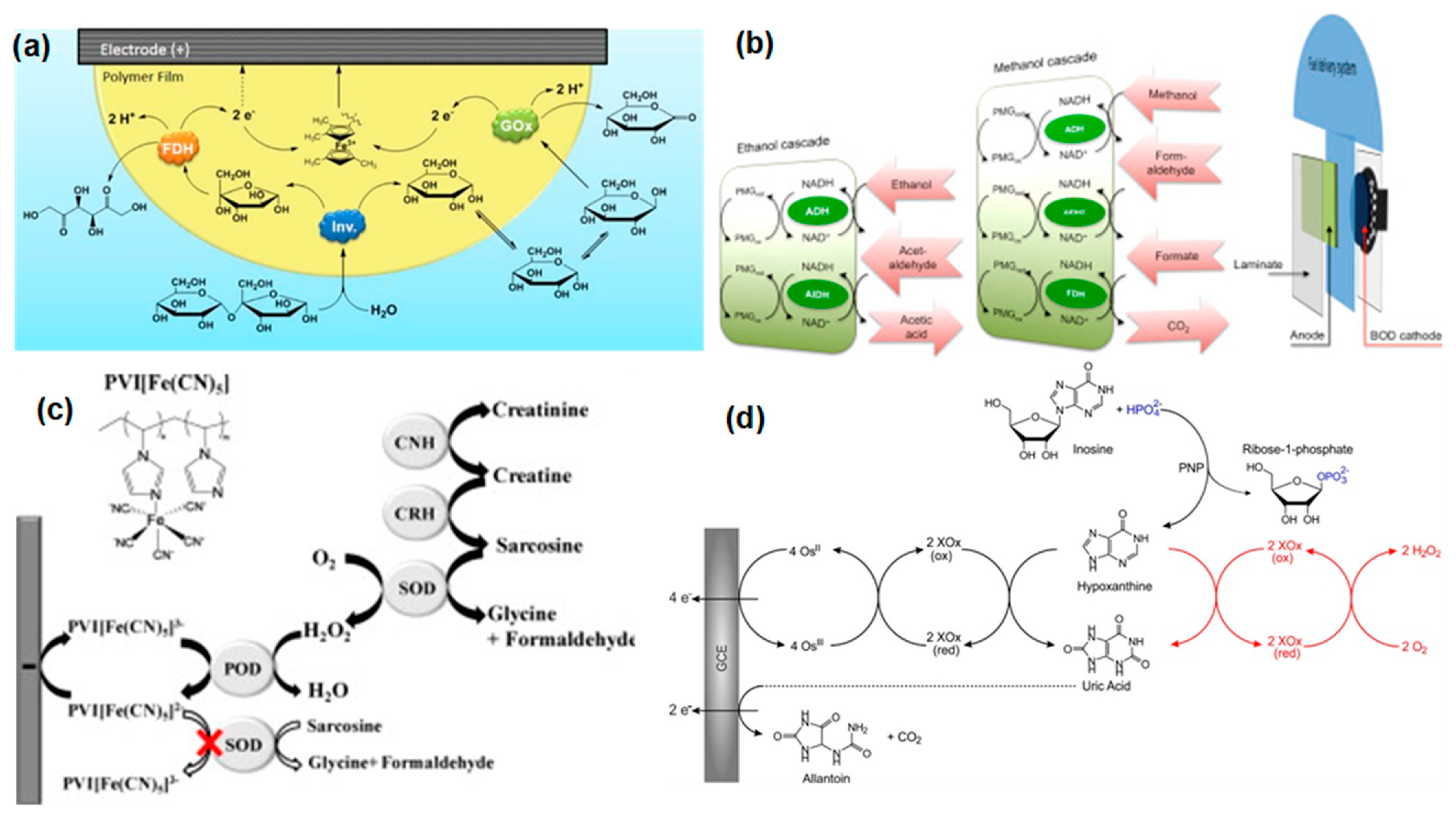
| Cascade System | Substrate | Immobilization Matrix | Power (μW/cm2) | Concentration (mM) | Ref. |
|---|---|---|---|---|---|
| LOx/POx | lactate | MgOC | 1750 | 200 | [25] |
| GA/GOD | starch | MWCNTs | 8.15 | 0.5% (w/w) | [31] |
| INV/MUT/GOD/FAD | sucrose | MWCNTs-modified carbon felt (CF) | 2900 | 500 | [36] |
| INV/GDH | sucrose | MWCNTs | 405 ± 6 | 200 | [32] |
| ADH/ALDH/ForDH | methanol | carbon nanodots | 68.7 ± 0.4 | 100 | [37] |
| MAL/MUT/GOx | maltose | MWCNT-modified CF | 2300 | 50 | [38] |
| Cascade System | Fuel or Substrate | DNA Scaffold Structure | Power (μW cm−2) | LOD/Detection Range | Fuel Concentration (mM) | Ref. |
|---|---|---|---|---|---|---|
| INV/GOx | sucrose | DNA template | 22 | − | 50 | [78] |
| ALD/DLD | methanol | DNA template | 24 | − | 120 | [75] |
| GOx/HRP | glucose | RCA DNA flower | − | 3 µM | [77] | |
| GOx/HRP | thrombin | tetrahedron (TDN)-lattice | − | 0.32 pM/0.001–10 nM | [70] | |
| GOx/HRP | DNA | Tetrahedron (TDN) | 3 fM/0.01 pM–10 nM | [79] |
| Immobilization Techniques | Description | Advantages | Disadvantages |
|---|---|---|---|
| One-step | The enzymes were immobilized at once. | Simple synthesis process Short distance of each enzyme will improve the cascade reaction efficiency | Cannot be used to immobilize unmatched enzyme |
| Multi-step | The first enzyme was immobilized, followed by the immobilization of the second enzyme, the third enzyme, and soon in a compartment structure. | Can immobilize various types of enzymes, even unmatched enzymes or enzyme that can inhibit each other’s activity Prevent the toxicity of the enzyme caused by another enzyme’s byproduct reaction | Longer synthesis steps Limiting analyte diffusion |
| Aspects | SWCNT-GOx/HRP | SWCNT-MAF-7-GOx/HRP |
|---|---|---|
| Current density (mA cm−2) | 0.3 | 1.68 |
| Temperature stability (remaining current density after heating at 65 °C) | 13% | 53% |
| Chemical stability (remaining current density in organic solvents) | Significant current density loss when exposed to organic solvents | 77% in THF 60% in methanol 60% in DMF 53% in pyridine |
| Pmax in blood (μW cm−2) | 14 | 119 |
| OCV in blood (V) | 0.22 | 0.34 |
| Operation stability (hours) | 2 | 14 |
| Multi-Enzyme as Anode | Fuel | Crosslinker | Current Output (mA cm−2) | Power (mW cm−2) | OCV (V) | Fuel Conc. | Ref. |
|---|---|---|---|---|---|---|---|
| INV, MUT, GOx, and FDH | Sucrose | GA | 12 | 2.9 | 0.69 | 50 mM | [36] |
| MAL, MUT, and GOx | Maltose | GA | 6.5 | 2.3 | 0.69 | 50 mM | [38] |
| AA, GluA, MUT, and FAD-GDH | Starch | PEGDGE | 5.8 | − | − | 0.8% (w/v) | [102] |
| β-glucosidase and PDH | β-glucan | GA | − | 0.031 | − | 0.5 % (w/v) | [108] |
| Metabolon | Pyruvate | GA, DMS | 0.1 | 0.03 | − | 100 mM | [103] |
| Multi-Enzyme as Anode | Fuel | Redox Polymer | Current Output (mA cm−2) | Power (mW cm−2) | OCV (V) | Fuel Conc. | Ref. |
|---|---|---|---|---|---|---|---|
| β-glucosidase, PDH | β-glucan | Os complex, glutaraldehyde | − | 0.031 | − | 0.5 % (w/v) | [108] |
| INV, FDH, GOx | Sucrose | Fc-LPEI, EGDGE | 0.36 | 0.06 | − | 100 mM | [116] |
| ADH, ALDH | Ethanol | Fc-LPEI, EGDGE | 0.23 | 0.02 | − | 30 mM | [97] |
| ADH, AldDH, S-acetyl-CoA synthetase, citrate synthase, aconitase, and isocitric dehydrogenase | Ethanol | Fc-LPEI, EGDGE | 0.12 | 0.01 | − | 30 mM | |
| ADH, AldDH | Ethanol | Poly-MG, LPEI-EGDGE | 0.12 | 0.04 | 0.53 | 100 mM | [117] |
| ADH, AldDH, ForDH | Methanol | 0.08 | 0.01 | 0.38 | 100 mM | ||
| PNP, XOx | Phosphate | Os complex, PEGDGE | − | − | − | − | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalyana Sundaram, S.d.; Hossain, M.M.; Rezki, M.; Ariga, K.; Tsujimura, S. Enzyme Cascade Electrode Reactions with Nanomaterials and Their Applicability towards Biosensor and Biofuel Cells. Biosensors 2023, 13, 1018. https://doi.org/10.3390/bios13121018
Kalyana Sundaram Sd, Hossain MM, Rezki M, Ariga K, Tsujimura S. Enzyme Cascade Electrode Reactions with Nanomaterials and Their Applicability towards Biosensor and Biofuel Cells. Biosensors. 2023; 13(12):1018. https://doi.org/10.3390/bios13121018
Chicago/Turabian StyleKalyana Sundaram, Shalini devi, Md. Motaher Hossain, Muhammad Rezki, Kotoko Ariga, and Seiya Tsujimura. 2023. "Enzyme Cascade Electrode Reactions with Nanomaterials and Their Applicability towards Biosensor and Biofuel Cells" Biosensors 13, no. 12: 1018. https://doi.org/10.3390/bios13121018
APA StyleKalyana Sundaram, S. d., Hossain, M. M., Rezki, M., Ariga, K., & Tsujimura, S. (2023). Enzyme Cascade Electrode Reactions with Nanomaterials and Their Applicability towards Biosensor and Biofuel Cells. Biosensors, 13(12), 1018. https://doi.org/10.3390/bios13121018