Controlling the Nucleation and Growth of Salt from Bodily Fluid for Enhanced Biosensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. SERS Substrate Fabrication
- Formation of Polystyrene Ball Layer: A single layer of self-assembled polystyrene (PS) balls (500 nm) was generated using Langmuir–Blodgett patterning.
- Transfer and Deposition: The layer was transferred to a 4″ (001) silicon wafer with 50 nm SiO2 deposited on top, followed by a deposition of 50 nm Cr.
- PS Ball Removal: PS balls were removed using chloroform, and the SiO2 was exposed using reactive-ion etching.
- Silicon Etching: The silicon was etched using KOH to create inverted nanopyramids with 57.5°-angle sidewalls, exploiting the different etching rates along the [001] and [111] silicon directions.
- Gold Film Deposition: A 200 nm film of gold was deposited on the pitted surface using electron beam deposition and bonded to a carrier wafer using epoxy before lifting off.
2.2. sEV Isolation Procedure
- Preliminary Centrifugation: Cell culture supernatants were first centrifuged at 300 g (4 °C, 10 min) and 2000 g (4 °C, 15 min) to remove cells and apoptotic bodies.
- Further Centrifugation: Supernatants were centrifuged at 12,000 g (4 °C, 45 min) to remove cell debris, followed by filtration using 0.22 μm-pore filters.
- Ultracentrifugation: Supernatants were ultracentrifuged at 110,000 g (4 °C, 70 min), and the pellets were resuspended in prechilled PBS. The process was repeated, and the final pellets were resuspended in 50–100 μL of PBS for NTA measurement.
2.3. Droplet Drying Method with and without Plasmon Resonance Hotspots on a Plasmonic Substrate
2.4. Raman Spectroscopy
- Sample Preparation: 5 μL of each sEV sample solution was deposited on the SERS substrate and dried before Raman testing.
- Spectrometer: Measurements were performed using a Reinshaw inVia Raman spectrometer at room temperature, with a laser excitation wavelength of 785 nm and a power of 5 mW.
- Calibration: The system was calibrated using the 520 cm−1 peak of silicon.
- Rough Mapping: Initial scouting for sEV locations was performed at a step width of 2 μm, with an exposure time of 0.2 s to prevent sample overheating.
- Fine Mapping: After spotting an sEV, fine mapping was performed at a step width of 0.1 μm to collect characteristic spectra from the sEV sample, maintaining the exposure time of 0.2 s to avoid overheating.
2.5. Polystyrene (PS) Beads
2.6. Phosphate Buffered Saline (PBS)
2.7. Biological-Simulated Sample Solution
3. Results and Discussion
3.1. Challenges Limiting Throughput in SERS Analysis
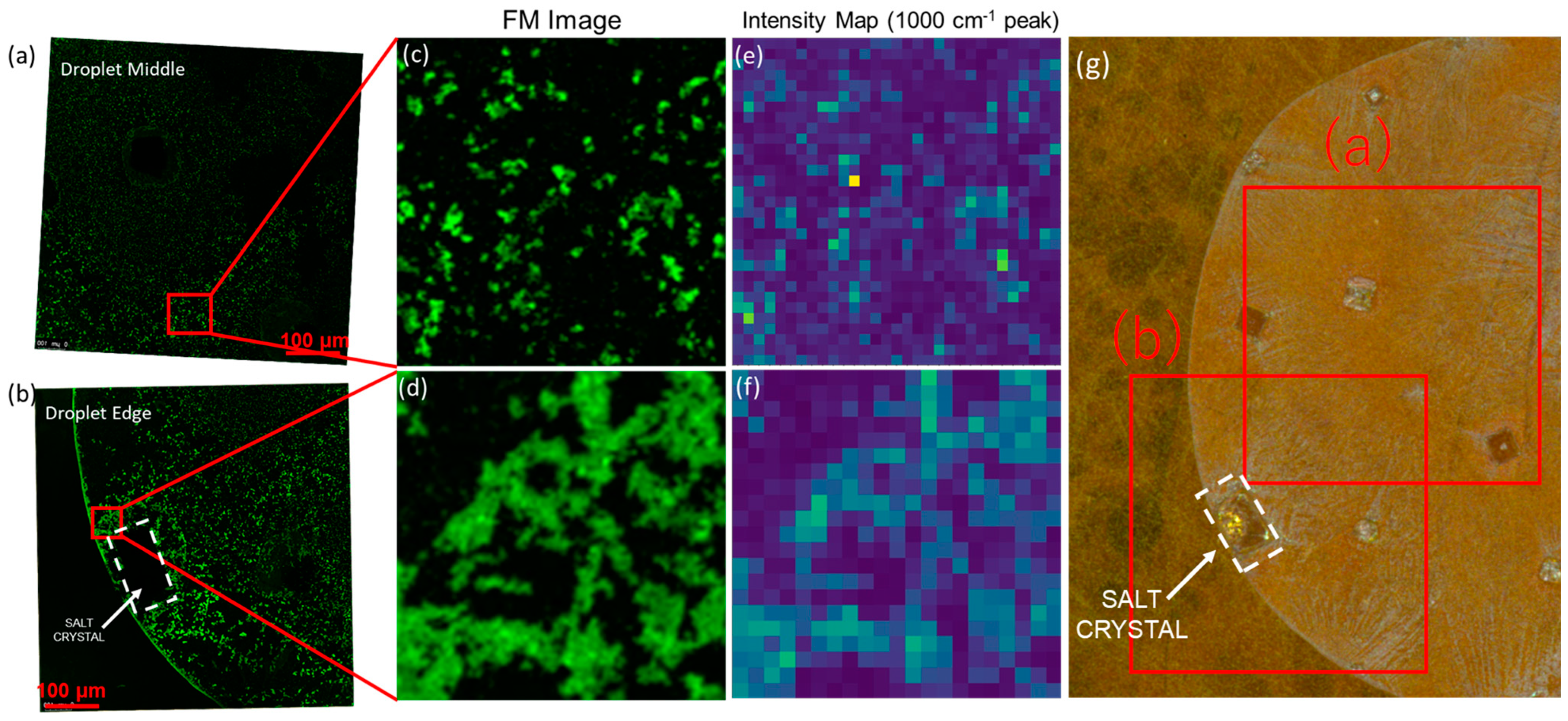
3.2. SERS Spatial-Overlap Study
3.3. Controlled Nucleation: Plasmonic-Induced Precipitation on SERS Substrate
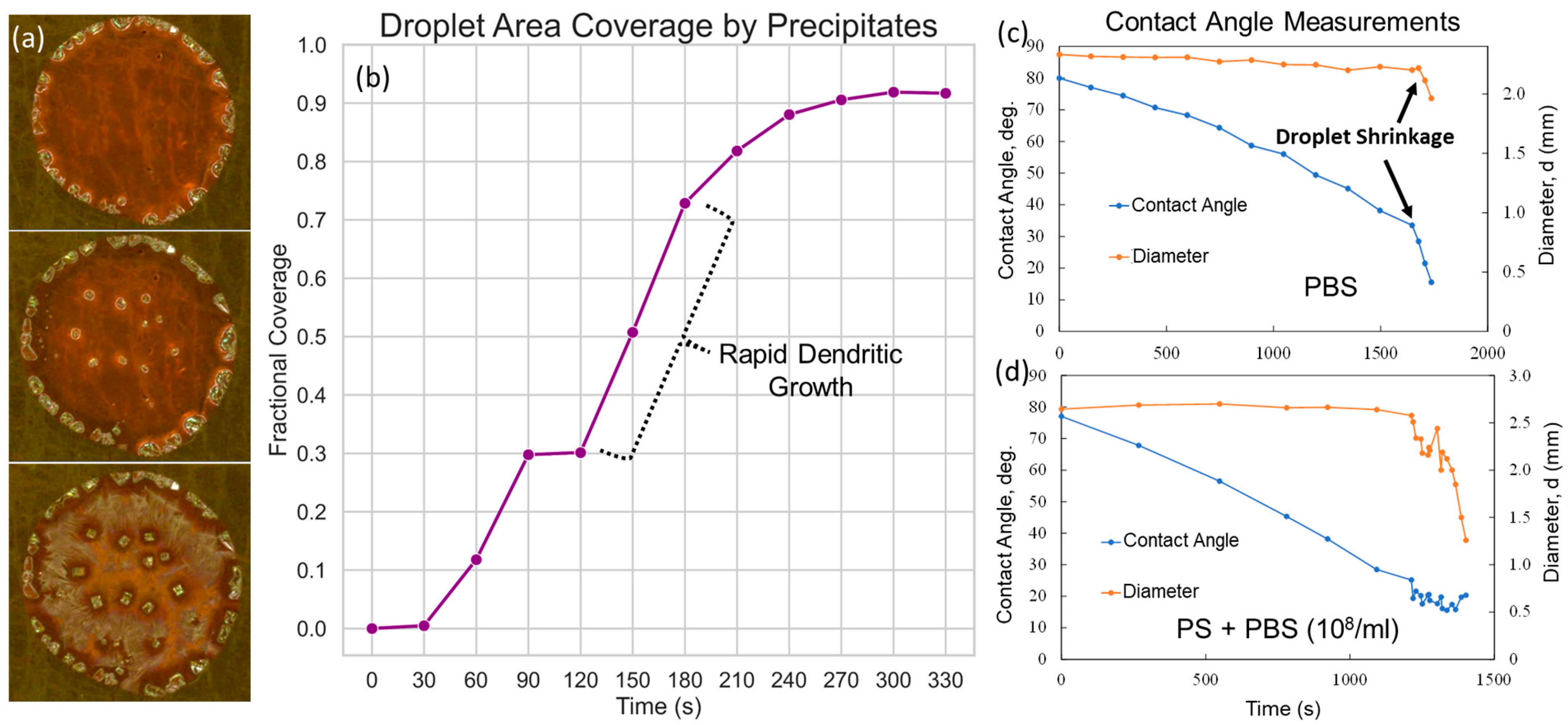
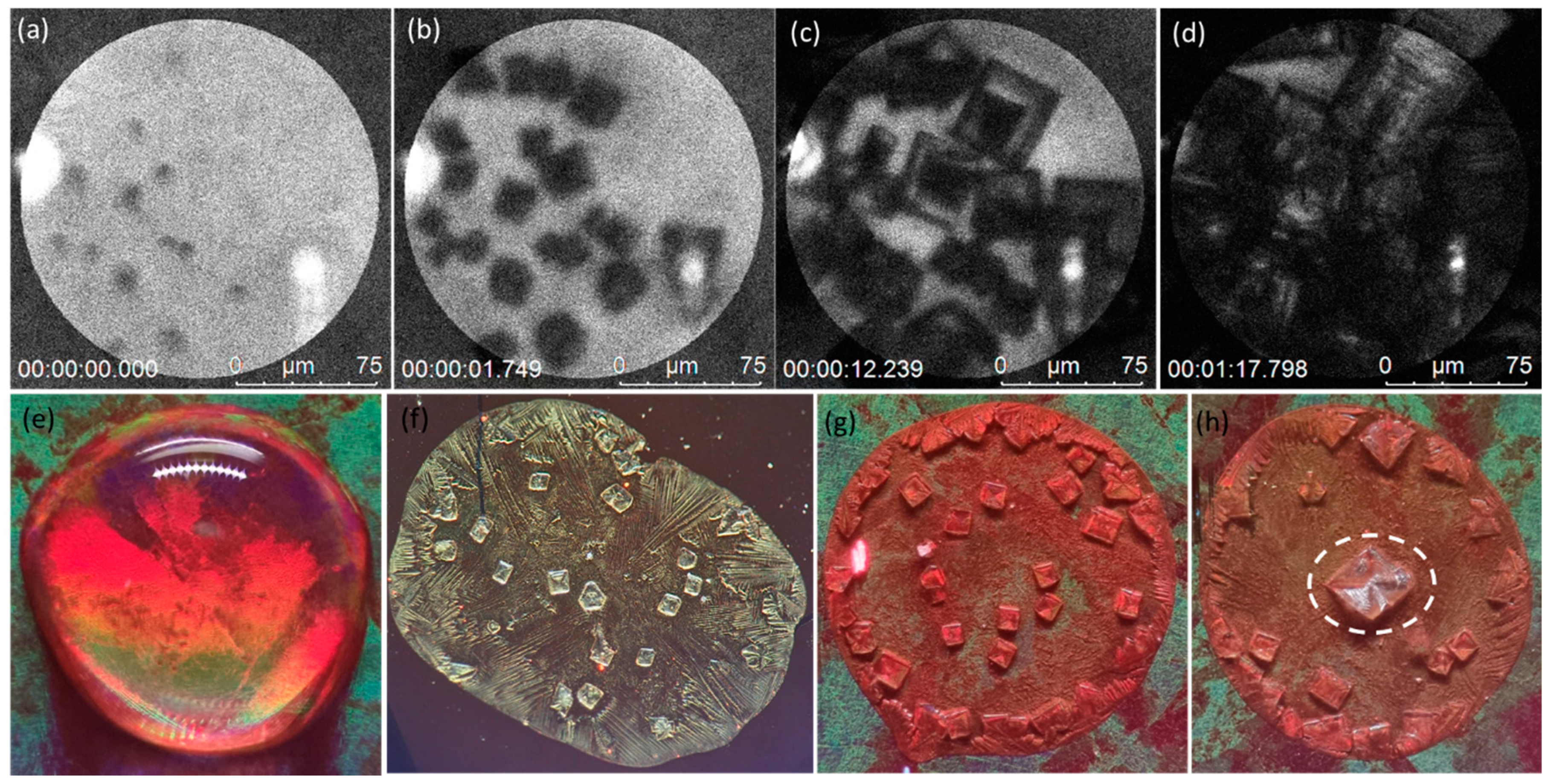
3.3.1. Coffee Ring Formation Mechanism
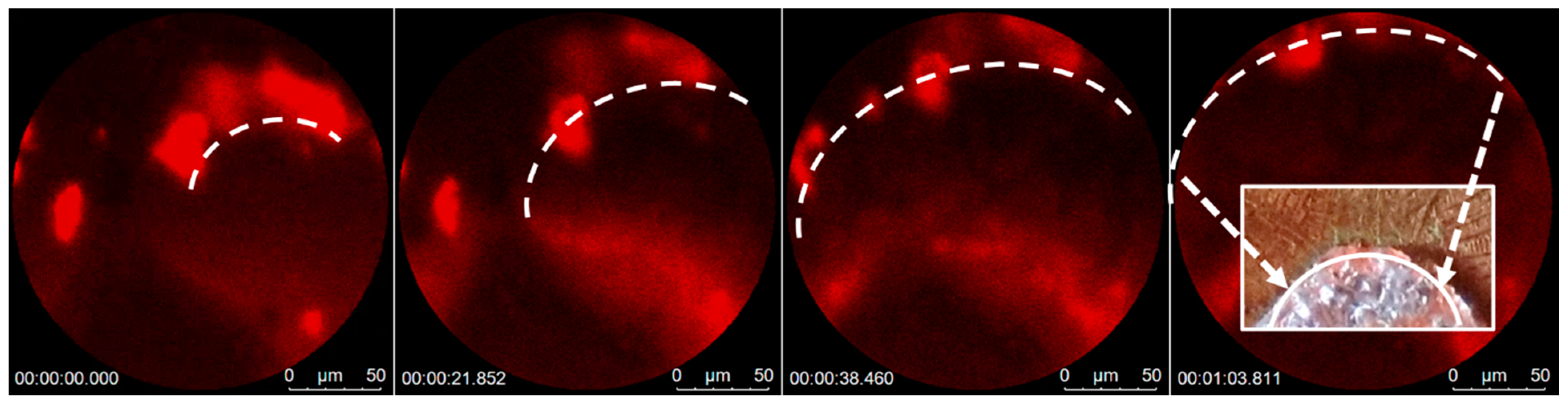
3.3.2. Plasmonic-Induced Precipitation
- 1.
- Laser Contribution on Plasmonic Substrate:
- 2.
- LSPR Contribution:
3.4. Segregation Mechanism of the Growth Process
3.5. Reduced Crystallization Area Coverage and Increased Throughput
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, M.; Zhou, Q.; Xie, H.; Liu, C.; Zheng, M.; Zhang, S.; Zhou, S.; Zhao, J. Role of CD36 in Central Nervous System Diseases. Neural Regen. Res. 2024, 19, 512–518. [Google Scholar] [CrossRef]
- Hornung, S.; Dutta, S.; Bitan, G. CNS-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Tukova, A.; Wang, Y. Emerging SERS Biosensors for the Analysis of Cells and Extracellular Vesicles. Nanoscale 2022, 14, 15242–15268. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef]
- Han, X.X.; Rodriguez, R.S.; Haynes, C.L.; Ozaki, Y.; Zhao, B. Surface-Enhanced Raman Spectroscopy. Nat. Rev. Methods Prim. 2022, 1, 87. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Wang, Z.; Liu, J.; Huang, S.; Min, B.H.; An, J.Y.; Kim, K.M.; Kim, S.; Chen, Y.; et al. Gold Nanopyramid Arrays for Non-Invasive Surface-Enhanced Raman Spectroscopy-Based Gastric Cancer Detection via SEVs. ACS Appl. Nano Mater. 2022, 2022, 12506–12517. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid Biopsy: Current Technology and Clinical Applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a New Frontier of Cancer Liquid Biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.B.; Hou, L.K.; Yu, F.; Zhang, J.; Wu, W.; Tang, X.M.; Sun, F.; Lu, H.M.; Deng, J.; et al. Liquid Biopsy in Lung Cancer: Significance in Diagnostics, Prediction, and Treatment Monitoring. Mol. Cancer 2022, 21, 25. [Google Scholar] [CrossRef]
- Li, X.; Keshavarz, M.; Kassanos, P.; Kidy, Z.; Roddan, A.; Yeatman, E.; Thompson, A.J. SERS Detection of Breast Cancer-Derived Exosomes Using a Nanostructured Pt-Black Template. Adv. Sens. Res. 2023, 2, 2200039. [Google Scholar] [CrossRef]
- Rojalin, T.; Koster, H.J.; Liu, J.; Mizenko, R.R.; Tran, D.; Wachsmann-Hogiu, S.; Carney, R.P. Hybrid Nanoplasmonic Porous Biomaterial Scaffold for Liquid Biopsy Diagnostics Using Extracellular Vesicles. ACS Sens. 2020, 5, 2820–2833. [Google Scholar] [CrossRef]
- Boriachek, K.; Islam, M.N.; Möller, A.; Salomon, C.; Nguyen, N.T.; Hossain, M.S.A.; Yamauchi, Y.; Shiddiky, M.J.A. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small 2018, 14, 1702153. [Google Scholar] [CrossRef]
- Yamanaka, K.; Vestergaard, M.C.; Tamiya, E. Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application. Sensors 2016, 16, 1761. [Google Scholar] [CrossRef]
- Dak, P.; Ebrahimi, A.; Swaminathan, V.; Duarte-Guevara, C.; Bashir, R.; Alam, M.A. Droplet-Based Biosensing for Lab-on-a-Chip, Open Microfluidics Platforms. Biosensors 2016, 6, 14. [Google Scholar] [CrossRef]
- Barea, J.S.; Lee, J.; Kang, D.K. Recent Advances in Droplet-Based Microfluidic Technologies for Biochemistry and Molecular Biology. Micromachines 2019, 10, 412. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; Do, H.D.K.; Nam, N.N.; Dan, T.T.; Trinh, K.T.L.; Lee, N.Y. Droplet-Based Microfluidics: Applications in Pharmaceuticals. Pharmaceuticals 2023, 16, 937. [Google Scholar] [CrossRef]
- Chen, G.; Mohamed, G.J. Complex Protein Patterns Formation via Salt-Induced Self-Assembly and Droplet Evaporation. Eur. Phys. J. E 2010, 33, 19–26. [Google Scholar] [CrossRef]
- Dugyala, V.R.; Basavaraj, M.G. Control over Coffee-Ring Formation in Evaporating Liquid Drops Containing Ellipsoids. Langmuir 2014, 30, 8680–8686. [Google Scholar] [CrossRef]
- Jeong, H.; Han, C.; Cho, S.; Gianchandani, Y.; Park, J. Analysis of Extracellular Vesicles Using Coffee Ring. ACS Appl. Mater. Interfaces 2018, 10, 22877–22882. [Google Scholar] [CrossRef]
- Wang, P.; Xia, M.; Liang, O.; Sun, K.; Cipriano, A.F.; Schroeder, T.; Liu, H.; Xie, Y.H. Label-Free SERS Selective Detection of Dopamine and Serotonin Using Graphene-Au Nanopyramid Heterostructure. Anal. Chem. 2015, 87, 10255–10261. [Google Scholar] [CrossRef]
- Yan, Z.; Dutta, S.; Liu, Z.; Yu, X.; Mesgarzadeh, N.; Ji, F.; Bitan, G.; Xie, Y.H. A Label-Free Platform for Identification of Exosomes from Different Sources. ACS Sens. 2019, 4, 488–497. [Google Scholar] [CrossRef]
- Wang, P.; Liang, O.; Zhang, W.; Schroeder, T.; Xie, Y.H. Ultra-Sensitive Graphene-Plasmonic Hybrid Platform for Label-Free Detection. Adv. Mater. 2013, 25, 4918–4924. [Google Scholar] [CrossRef]
- Yu, X.; Srivastava, S.; Huang, S.; Hayden, E.Y.; Teplow, D.B.; Xie, Y.H. The Feasibility of Early Alzheimer’s Disease Diagnosis Using a Neural Network Hybrid Platform. Biosensors 2022, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Jonak, S.T.; Liu, Z.; Liu, J.; Li, T.; D’Souza, B.V.; Schiaffino, J.; Ph, S.; Xie, Y.-H. Analyzing Bronchoalveolar Fluid Derived Small Extracellular Vesicles Using Single-Vesicle SERS for Non-Small Cell Lung Cancer Detection. Sens. Diagn. 2023, 2, 90–99. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Contact Line Deposits in an Evaporating Drop. Phys. Rev. E 2000, 62, 756. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary Flow as the Cause of Ring Stains from Dried Liquid Drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- van der Pol, E.; Sturk, A.; van Leeuwen, T.; Nieuwland, R.; Coumans, F.; Mobarrez, F.; Arkesteijn, G.; Wauben, M.; Siljander, P.R.M.; Sánchez-López, V.; et al. Standardization of Extracellular Vesicle Measurements by Flow Cytometry through Vesicle Diameter Approximation. J. Thromb. Haemost. 2018, 16, 1236–1245. [Google Scholar] [CrossRef]
- Yang, K.S.; Lin, H.Y.; Curley, C.; Welch, M.W.; Wolpin, B.M.; Lee, H.; Weissleder, R.; Im, H.; Castro, C.M. Bead-Based Extracellular Vesicle Analysis Using Flow Cytometry. Adv. Biosyst. 2020, 4, e2000203. [Google Scholar] [CrossRef]
- Suárez, H.; Gámez-Valero, A.; Reyes, R.; López-Martín, S.; Rodríguez, M.J.; Carrascosa, J.L.; Cabañas, C.; Borràs, F.E.; Yáñez-Mó, M. A Bead-Assisted Flow Cytometry Method for the Semi-Quantitative Analysis of Extracellular Vesicles. Sci. Rep. 2017, 7, 11271. [Google Scholar] [CrossRef]
- Kuiper, M.; van de Nes, A.; Nieuwland, R.; Varga, Z.; van der Pol, E. Reliable Measurements of Extracellular Vesicles by Clinical Flow Cytometry. Am. J. Reprod. Immunol. 2021, 85, e13350. [Google Scholar] [CrossRef]
- Palm, A. Raman Spectrum of Polystyrene. J. Phys. Colloid Chem. 1951, 55, 1320–1324. [Google Scholar] [CrossRef]
- Sefiane, K. Patterns from Drying Drops. Adv. Colloid Interface Sci. 2014, 206, 372–381. [Google Scholar] [CrossRef]
- Kaya, D.; Belyi, V.A.; Muthukumar, M. Pattern Formation in Drying Droplets of Polyelectrolyte and Salt. J. Chem. Phys. 2010, 133, 114905. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Analysis of the Effects of Marangoni Stresses on the Microflow in an Evaporating Sessile Droplet. Langmuir 2005, 21, 3972–3980. [Google Scholar] [CrossRef]
- Rivera, N.; Kaminer, I. Light–Matter Interactions with Photonic Quasiparticles. Nat. Rev. Phys. 2020, 2, 538–561. [Google Scholar] [CrossRef]
- Maher, R.C. Raman Spectroscopy for Nanomaterials Characterization. SERS Hot Spots; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–260. [Google Scholar] [CrossRef]
- Rojalin, T.; Phong, B.; Koster, H.; Carney, R.P. Nanoplasmonic Approaches for Sensitive Detection and Molecular Characterization of Extracellular Vesicles. Front. Chem. 2019, 7, 279. [Google Scholar] [CrossRef]
- Ward, M.R.; Alexander, A.J. Nonphotochemical Laser-Induced Nucleation of Potassium Halides: Effects of Wavelength and Temperature. Cryst. Growth Des. 2012, 12, 4554–4561. [Google Scholar] [CrossRef]
- Karthika, S.; Radhakrishnan, T.K.; Kalaichelvi, P. A Review of Classical and Nonclassical Nucleation Theories. Cryst. Growth Des. 2016, 16, 6663–6681. [Google Scholar] [CrossRef]
- Cheng, T.; Fang, D.; Yang, Y. A Temperature-Dependent Surface Free Energy Model for Solid Single Crystals. Appl. Surf. Sci. 2017, 393, 364–368. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E.; Easterling, K.E. Phase Transformations in Metals and Alloys (Revised Reprint); CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Duyne, R.P. Van Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; Ratcliffe, G.; Wormald, M.; De Paula, J. Physical Chemistry for the Life Sciences; Oxford University Press: Oxford, UK, 2023; p. 528. [Google Scholar]
- Fang, Z.; Zhen, Y.R.; Neumann, O.; Polman, A.; García De Abajo, F.J.; Nordlander, P.; Halas, N.J. Evolution of Light-Induced Vapor Generation at a Liquid-Immersed Metallic Nanoparticle. Nano Lett. 2013, 13, 1736–1742. [Google Scholar] [CrossRef]
- Lukianova-Hleb, E.; Hu, Y.; Latterini, L.; Tarpani, L.; Lee, S.; Drezek, R.A.; Hafner, J.H.; Lapotko, D.O. Plasmonic Nanobubbles as Transient Vapor Nanobubbles Generated around Plasmonic Nanoparticles. ACS Nano 2010, 4, 2109–2123. [Google Scholar] [CrossRef]
- Adleman, J.R.; Boyd, D.A.; Goodwin, D.G.; Psaltis, D. Heterogenous Catalysis Mediated by Plasmon Heating. Nano Lett. 2009, 9, 4417–4423. [Google Scholar] [CrossRef]

| PS Concentration, Particles/mL | Solution |
|---|---|
| DI | |
| DI or PBS | |
| DI or PBS | |
| DI or PBS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, S.; Terai, Y.; Liu, J.; Capellini, G.; Xie, Y.-H. Controlling the Nucleation and Growth of Salt from Bodily Fluid for Enhanced Biosensing Applications. Biosensors 2023, 13, 1016. https://doi.org/10.3390/bios13121016
Srivastava S, Terai Y, Liu J, Capellini G, Xie Y-H. Controlling the Nucleation and Growth of Salt from Bodily Fluid for Enhanced Biosensing Applications. Biosensors. 2023; 13(12):1016. https://doi.org/10.3390/bios13121016
Chicago/Turabian StyleSrivastava, Siddharth, Yusuke Terai, Jun Liu, Giovanni Capellini, and Ya-Hong Xie. 2023. "Controlling the Nucleation and Growth of Salt from Bodily Fluid for Enhanced Biosensing Applications" Biosensors 13, no. 12: 1016. https://doi.org/10.3390/bios13121016
APA StyleSrivastava, S., Terai, Y., Liu, J., Capellini, G., & Xie, Y.-H. (2023). Controlling the Nucleation and Growth of Salt from Bodily Fluid for Enhanced Biosensing Applications. Biosensors, 13(12), 1016. https://doi.org/10.3390/bios13121016





