Recent Research Progress in Fluorescent Probes for Detection of Amyloid-β In Vivo
Abstract
:1. Introduction
2. Fluorescent Probes In Vivo for Amyloid-β Detection
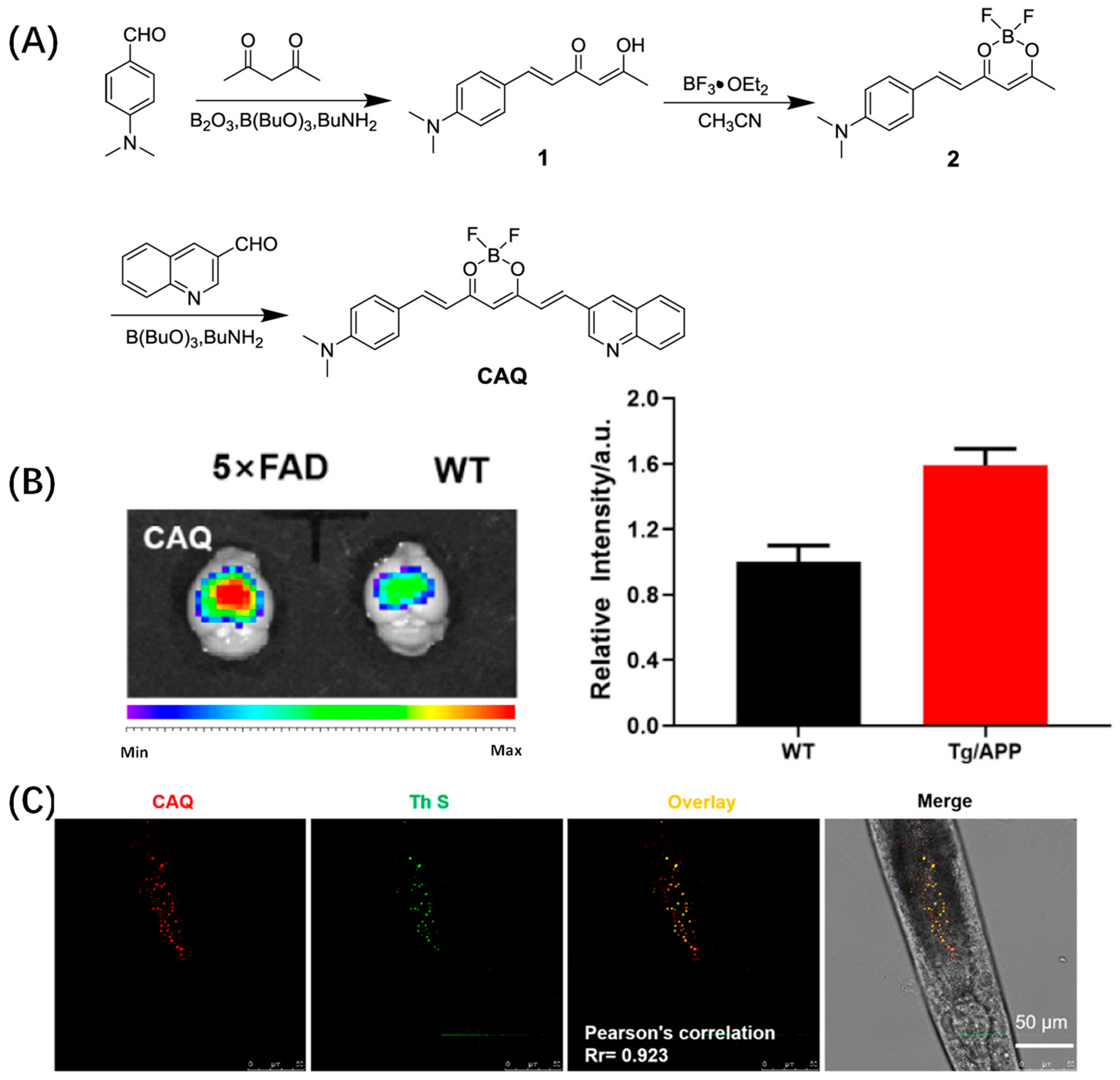
2.1. Curcumin-Based Probes for Aβ
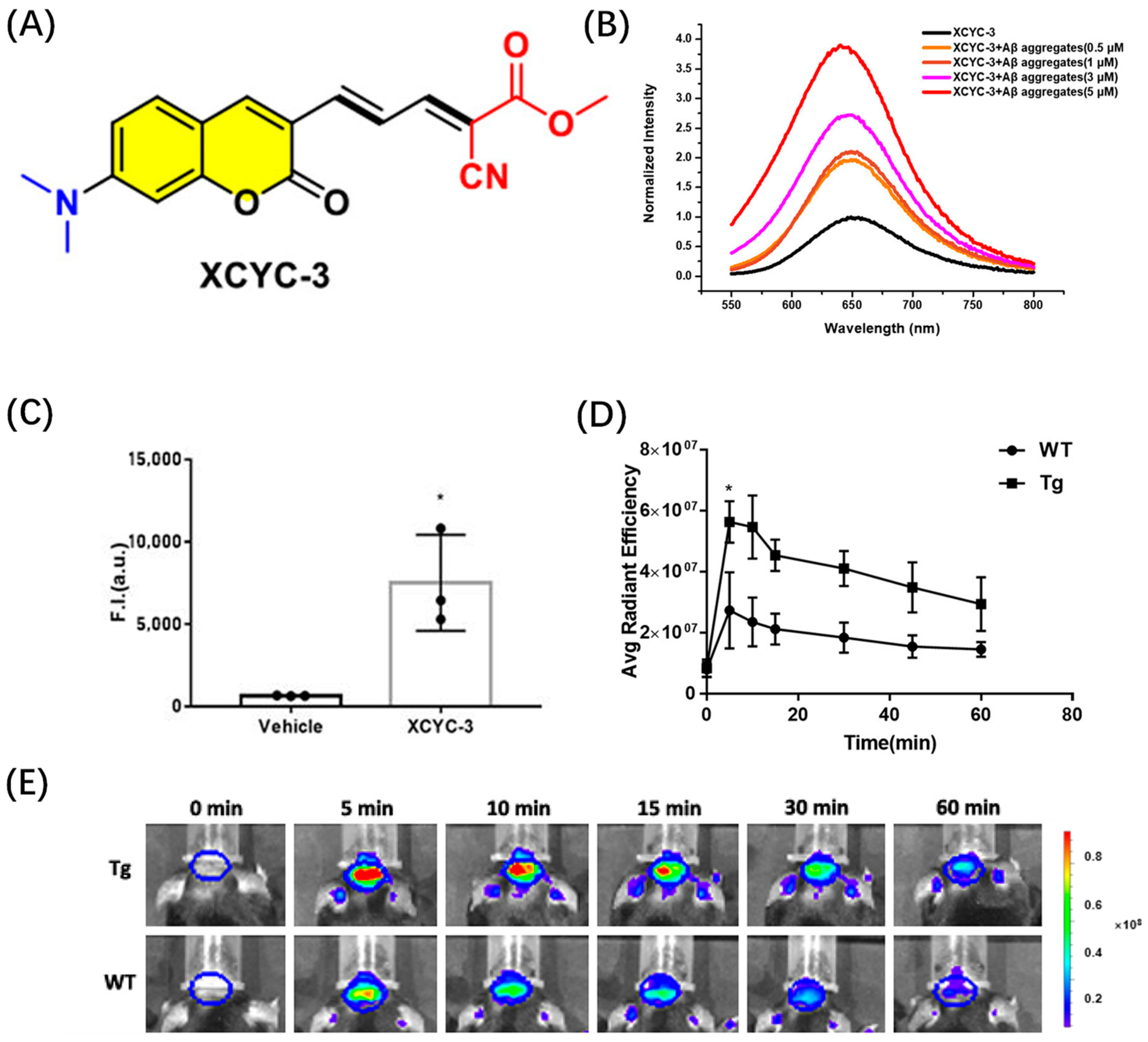
2.2. Coumarin-Based Probes for Aβ
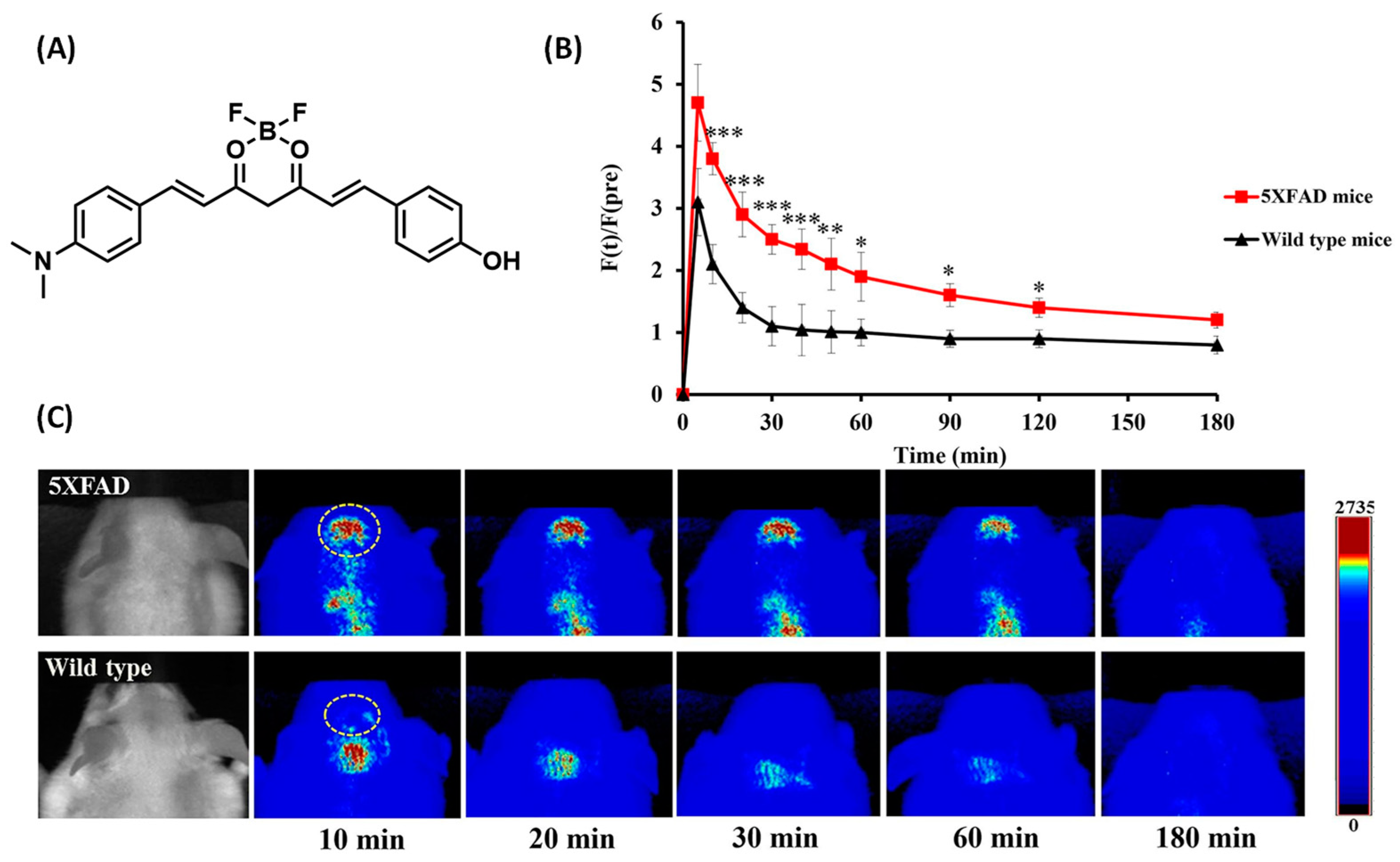
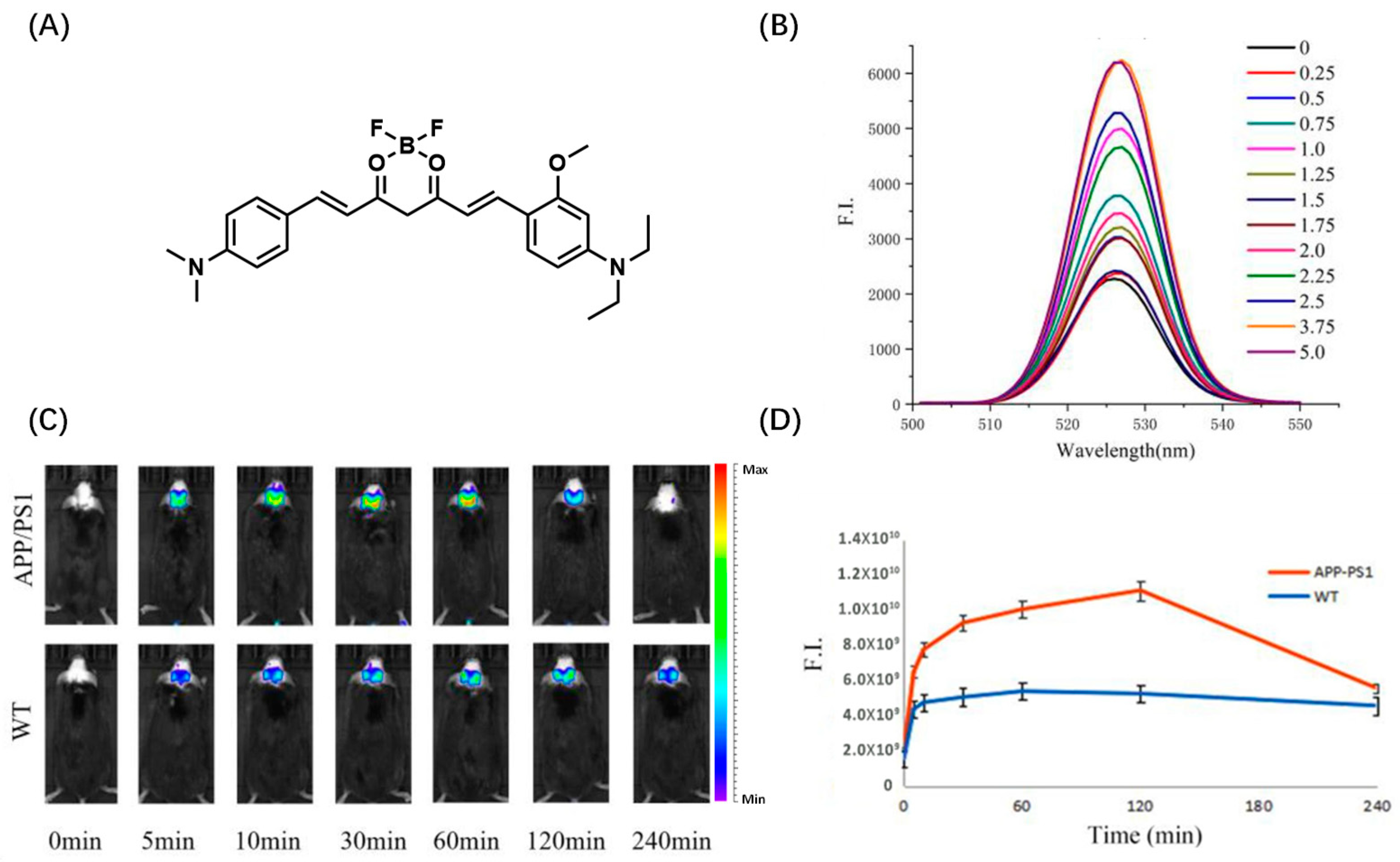
2.3. BODIPY-Based Probes for Aβ
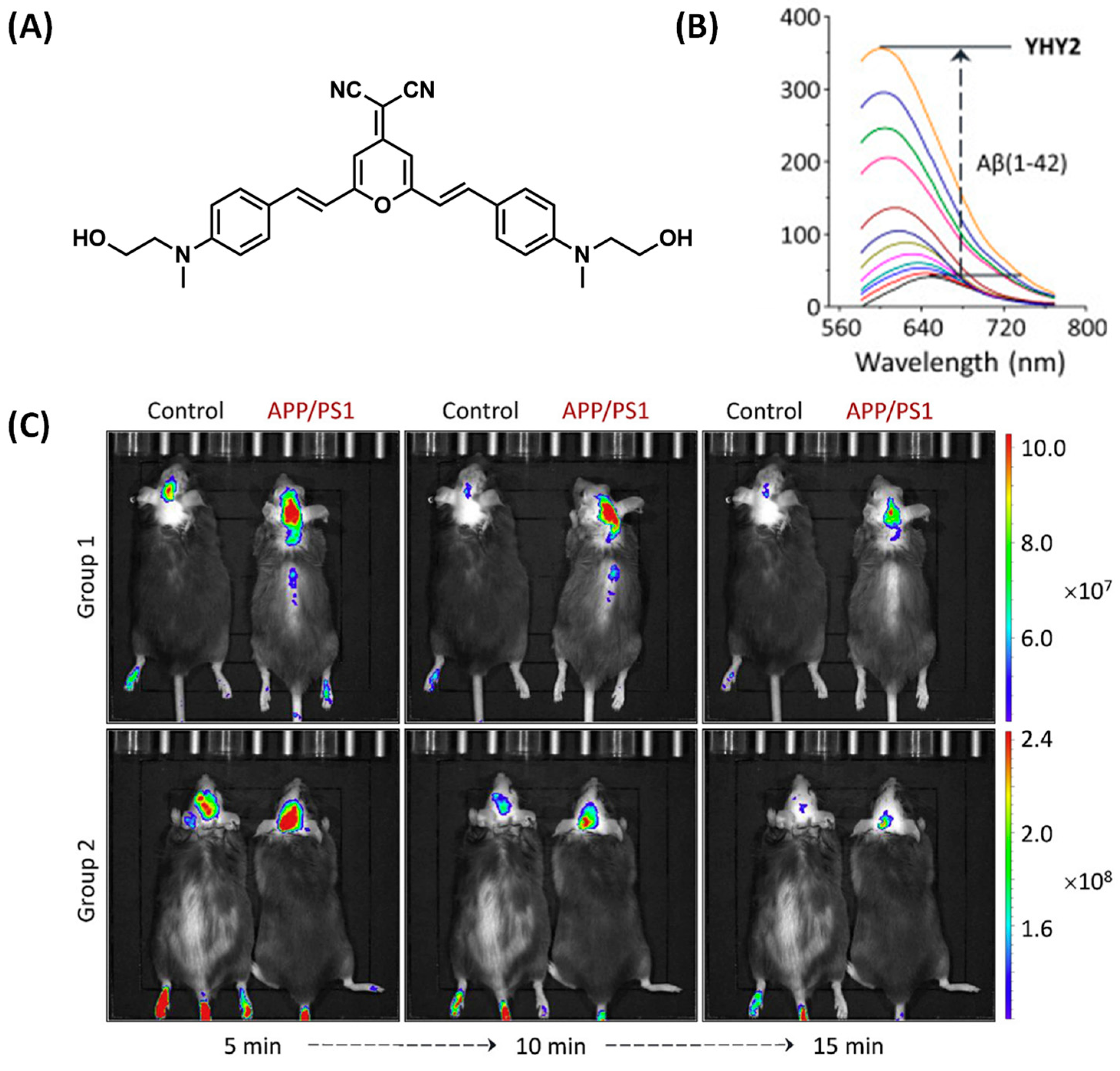
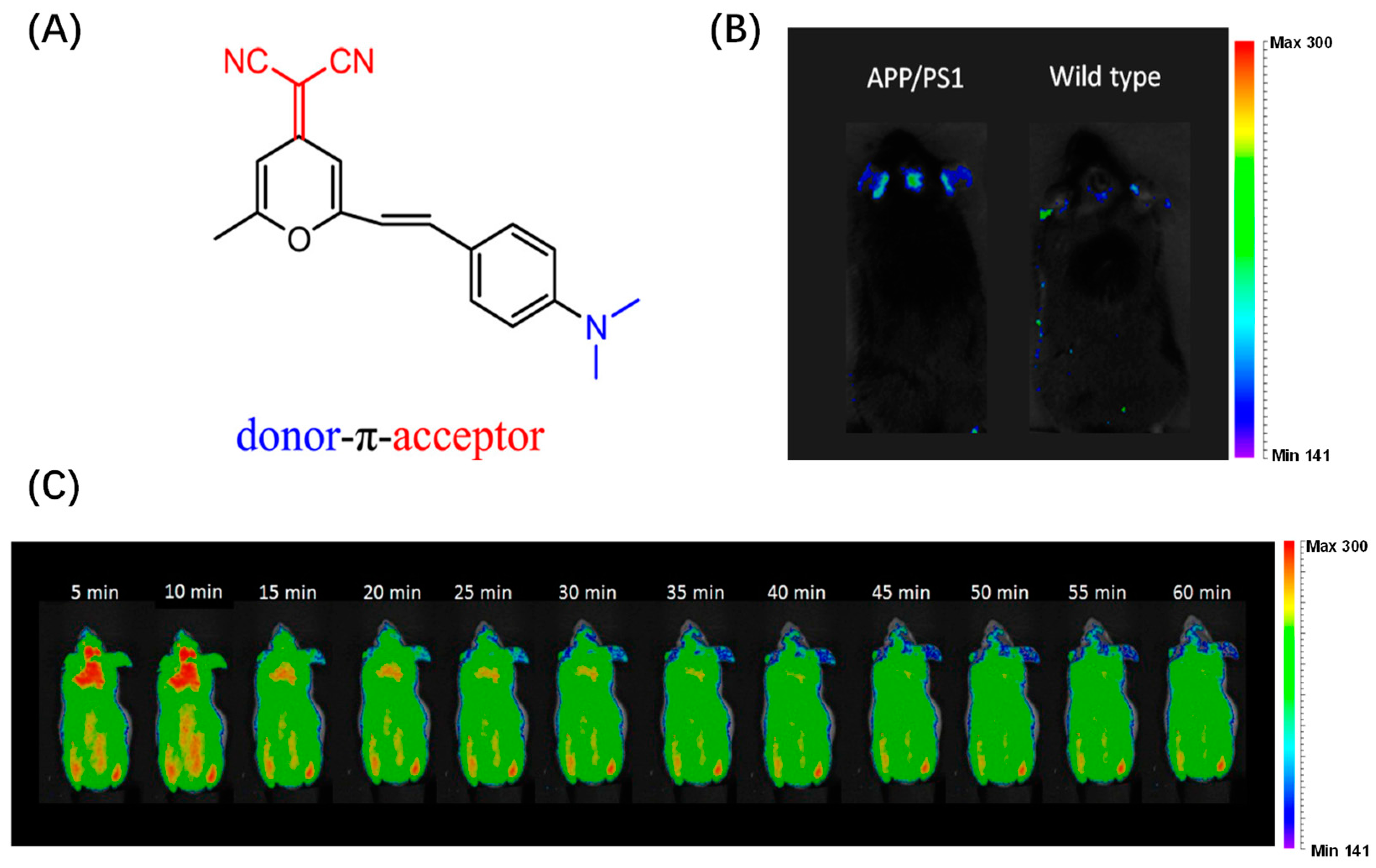
2.4. DCM-Based Probes for Aβ
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haghighijoo, Z.; Akrami, S.; Saeedi, M.; Zonouzi, A.; Iraji, A.; Larijani, B.; Fakherzadeh, H.; Sharifi, F.; Arzaghi, S.M.; Mahdavi, M.; et al. N-Cyclohexylimidazo[1,2-a]pyridine derivatives as multi-target-directed ligands for treatment of Alzheimer’s disease. Bioorg. Chem. 2020, 103, 104146. [Google Scholar] [CrossRef] [PubMed]
- Kubankova, M.; Lopez-Duarte, I.; Bull, J.A.; Vadukul, D.M.; Serpell, L.C.; de Saint Victor, M.; Stride, E.; Kuimova, M.K. Probing supramolecular protein assembly using covalently attached fluorescent molecular rotors. Biomaterials 2017, 139, 195–201. [Google Scholar] [CrossRef]
- Lassi, M.; Fabbiani, C.; Mazzeo, S.; Burali, R.; Vergani, A.A.; Giacomucci, G.; Moschini, V.; Morinelli, C.; Emiliani, F.; Scarpino, M.; et al. Degradation of EEG microstates patterns in subjective cognitive decline and mild cognitive impairment: Early biomarkers along the Alzheimer’s Disease continuum? NeuroImage Clin. 2023, 38, 103407. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ishikawa, M.; Kimura, H.; Hayashi, S.; Matsumura, K.; Watanabe, H.; Shimizu, Y.; Cheng, Y.; Cui, M.; Kawashima, H.; et al. Development of dual functional SPECT/fluorescent probes for imaging cerebral beta-amyloid plaques. Bioorg. Med. Chem. Lett. 2010, 20, 3885–3888. [Google Scholar] [CrossRef]
- Si, G.; Zhou, S.; Xu, G.; Wang, J.; Wu, B.; Zhou, S. A curcumin-based NIR fluorescence probe for detection of amyloid-beta (Aβ) plaques in Alzheimer’s disease. Dye. Pigment. 2019, 163, 509–515. [Google Scholar] [CrossRef]
- Espay, A.J. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 385, 666–667. [Google Scholar]
- Manus, J.-M. Leqembi: Une nouvelle étape dans la protection anti-amyloïde des neurones? Rev. Francoph. Des. Lab. 2023, 2023, 12. [Google Scholar] [CrossRef]
- Diner, I.; Dooyema, J.; Gearing, M.; Walker, L.C.; Seyfried, N.T. Generation of Clickable Pittsburgh Compound B for the Detection and Capture of beta-Amyloid in Alzheimer’s Disease Brain. Bioconjug. Chem. 2017, 28, 2627–2637. [Google Scholar] [CrossRef]
- Pacheco-Quinto, J.; Clausen, D.; Perez-Gonzalez, R.; Peng, H.; Meszaros, A.; Eckman, C.B.; Levy, E.; Eckman, E.A. Intracellular metalloprotease activity controls intraneuronal Abeta aggregation and limits secretion of Abeta via exosomes. FASEB J. 2019, 33, 3758–3771. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.E.; Cali, M.P.; Pazin, W.M.; Carlos-Lima, E.; Salles Trevisan, M.T.; Venancio, T.; Arcisio-Miranda, M.; Ito, A.S.; Carlos, R.M. Luminescent Ru(II) Phenanthroline Complexes as a Probe for Real-Time Imaging of Abeta Self-Aggregation and Therapeutic Applications in Alzheimer’s Disease. J. Med. Chem. 2016, 59, 9215–9227. [Google Scholar] [CrossRef]
- Jia, L.; Wang, W.; Yan, Y.; Hu, R.; Sang, J.; Zhao, W.; Wang, Y.; Wei, W.; Cui, W.; Yang, G.; et al. General Aggregation-Induced Emission Probes for Amyloid Inhibitors with Dual Inhibition Capacity against Amyloid beta-Protein and alpha-Synuclein. ACS Appl. Mater. Interfaces 2020, 12, 31182–31194. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Ono, M.; Ariyoshi, T.; Katayanagi, R.; Saji, H. Novel Benzothiazole Derivatives as Fluorescent Probes for Detection of beta-Amyloid and alpha-Synuclein Aggregates. ACS Chem. Neurosci. 2017, 8, 1656–1662. [Google Scholar] [CrossRef]
- Zhou, K.; Yuan, C.; Dai, B.; Wang, K.; Chen, Y.; Ma, D.; Dai, J.; Liang, Y.; Tan, H.; Cui, M. Environment-Sensitive Near-Infrared Probe for Fluorescent Discrimination of Abeta and Tau Fibrils in AD Brain. J. Med. Chem. 2019, 62, 6694–6704. [Google Scholar] [CrossRef] [PubMed]
- Bishay, J.; Beckett, T.; Lai, A.Y.; Hill, M.E.; McMahon, D.; Simpson, C.; Hynynen, K.; McLaurin, J. Venular amyloid and its relationship with cerebral amyloid angiopathy in TGF344AD rats. Alzheimers Dement. 2020, 16, e042458. [Google Scholar] [CrossRef]
- Bittar, A.; Bhatt, N.; Kayed, R. Advances and considerations in AD tau-targeted immunotherapy. Neurobiol. Dis. 2020, 134, 104707. [Google Scholar] [CrossRef]
- d’Oleire Uquillas, F.; Jacobs, H.I.L.; Hanseeuw, B.; Marshall, G.A.; Properzi, M.; Schultz, A.P.; LaPoint, M.R.; Johnson, K.A.; Sperling, R.A.; Vannini, P. Interactive versus additive relationships between regional cortical thinning and amyloid burden in predicting clinical decline in mild AD and MCI individuals. NeuroImage Clin. 2018, 17, 388–396. [Google Scholar] [CrossRef]
- Roberts, B.R.; Lind, M.; Wagen, A.Z.; Rembach, A.; Frugier, T.; Li, Q.X.; Ryan, T.M.; McLean, C.A.; Doecke, J.D.; Rowe, C.C.; et al. Biochemically-defined pools of amyloid-beta in sporadic Alzheimer’s disease: Correlation with amyloid PET. Brain 2017, 140, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, N.; Hendrix, S.B.; Wilke, S.; Nilan, D. In a pre-pivotal diagnostic study, β-amyloid plaque detection in the lens of the eye in MCI and mild AD patients was used to classify subjects as normal, MCI, and mild AD using Sapphire II. Alzheimers Dement. 2020, 16, e046437. [Google Scholar] [CrossRef]
- Walton, C.C.; Begelman, D.; Nguyen, W.; Andersen, J.K. Senescence as an Amyloid Cascade: The Amyloid Senescence Hypothesis. Front. Cell Neurosci. 2020, 14, 129. [Google Scholar] [CrossRef]
- Grover, S.; Pham, T.; Jones, A.; Sinobas-Pereira, C.; Villoch Diaz Maurino, M.; Garrad, E.C.; Makoni, N.J.; Parks, A.; Domalewski, R.J.; Riggio, G.; et al. A new class of monoclonal Aβ antibodies selectively targets and triggers deposition of Aβ protofibrils. J. Neurochem. 2023, 165, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, K.; Zhou, K.; Guo, W.; Dai, B.; Liang, Y.; Dai, J.; Cui, M. Novel D-A-D based near-infrared probes for the detection of beta-amyloid and Tau fibrils in Alzheimer’s disease. Chem. Commun. 2018, 54, 8717–8720. [Google Scholar] [CrossRef]
- Javed, M.; Ahmad, M.I.; Javed, H.; Naseem, S. D-ribose and pathogenesis of Alzheimer’s disease. Mol. Biol. Rep. 2020, 47, 2289–2299. [Google Scholar] [CrossRef]
- Lodeiro, M.; Puerta, E.; Ismail, M.A.; Rodriguez-Rodriguez, P.; Ronnback, A.; Codita, A.; Parrado-Fernandez, C.; Maioli, S.; Gil-Bea, F.; Merino-Serrais, P.; et al. Aggregation of the Inflammatory S100A8 Precedes Abeta Plaque Formation in Transgenic APP Mice: Positive Feedback for S100A8 and Abeta Productions. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 319–328. [Google Scholar]
- Starling, S. Alzheimer disease: Blood-derived Abeta induces AD pathology. Nat. Rev. Neurol. 2018, 14, 2. [Google Scholar]
- Hu, Z.; Zeng, L.; Huang, Z.; Zhang, J.; Li, T. The study of Golgi apparatus in Alzheimer’s disease. Neurochem. Res. 2007, 32, 1265–1277. [Google Scholar] [CrossRef]
- Quartey, M.O.; Nyarko, J.N.K.; Maley, J.M.; Barnes, J.R.; Bolanos, M.A.C.; Heistad, R.M.; Knudsen, K.J.; Pennington, P.R.; Buttigieg, J.; De Carvalho, C.E.; et al. The Abeta(1-38) peptide is a negative regulator of the Abeta(1-42) peptide implicated in Alzheimer disease progression. Sci. Rep. 2021, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Scheidt, H.A.; Adler, J.; Krueger, M.; Huster, D. Fibrils of Truncated Pyroglutamyl-Modified Abeta Peptide Exhibit a Similar Structure as Wildtype Mature Abeta Fibrils. Sci. Rep. 2016, 6, 33531. [Google Scholar] [CrossRef] [PubMed]
- Soloperto, A.; Quaglio, D.; Baiocco, P.; Romeo, I.; Mori, M.; Ardini, M.; Presutti, C.; Sannino, I.; Ghirga, S.; Iazzetti, A.; et al. Rational design and synthesis of a novel BODIPY-based probe for selective imaging of tau tangles in human iPSC-derived cortical neurons. Sci. Rep. 2022, 12, 5257. [Google Scholar] [CrossRef]
- Verwilst, P.; Kim, H.R.; Seo, J.; Sohn, N.W.; Cha, S.Y.; Kim, Y.; Maeng, S.; Shin, J.W.; Kwak, J.H.; Kang, C.; et al. Rational Design of in Vivo Tau Tangle-Selective Near-Infrared Fluorophores: Expanding the BODIPY Universe. J. Am. Chem. Soc. 2017, 139, 13393–13403. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Sekhon, H.; Launay, C.P.; Rolland, Y.; Schott, A.-M.; Allali, G. Motoric cognitive risk syndrome and incident dementia: Results from a population-based prospective and observational cohort study. Eur. J. Neurol. 2020, 27, 468–474. [Google Scholar] [CrossRef]
- Patel, D.; Chung, J.; Mez, J.; Zhang, X.; Haines, J.L.; Pericak-Vance, M.A.; Schellenberg, G.; Lunetta, K.L.; Farrer, L.A. O5-04-02: Rare Coding Mutations Associated with Alzheimer Disease and Other Dementias. Alzheimers Dement. 2018, 14, P1649–P1651. [Google Scholar] [CrossRef]
- Yao, P.L.; Zhuo, S.; Mei, H.; Chen, X.F.; Li, N.; Zhu, T.F.; Chen, S.T.; Wang, J.M.; Hou, R.X.; Le, Y.Y. Androgen alleviates neurotoxicity of beta-amyloid peptide (Abeta) by promoting microglial clearance of Abeta and inhibiting microglial inflammatory response to Abeta. CNS Neurosci. Ther. 2017, 23, 855–865. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Wang, X.; Chen, W.; Bian, W.; Choi, M.M.F. Silver-doped graphite carbon nitride nanosheets as fluorescent probe for the detection of curcumin. Luminescence 2018, 33, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Shi, D.; Li, X.; Zhu, J.; Mao, F.; Li, X.; Xia, C.; Jiang, B.; Guo, Y.; Li, J. Curcumin-based polarity fluorescent probes: Design strategy and biological applications. Dye. Pigment. 2020, 177, 108320. [Google Scholar] [CrossRef]
- Sato, T.; Hotsumi, M.; Makabe, K.; Konno, H. Design, synthesis and evaluation of curcumin-based fluorescent probes to detect Abeta fibrils. Bioorg. Med. Chem. Lett. 2018, 28, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, R.; Fu, H.; Yang, J.; Kumar, M.; Lu, J.; Xu, Y.; Liang, S.H.; Cui, M.; Ran, C. Half-curcumin analogues as PET imaging probes for amyloid beta species. Chem. Commun. 2019, 55, 3630–3633. [Google Scholar] [CrossRef]
- Park, Y.D.; Kinger, M.; Min, C.; Lee, S.Y.; Byun, Y.; Park, J.W.; Jeon, J. Synthesis and evaluation of curcumin-based near-infrared fluorescent probes for the in vivo optical imaging of amyloid-beta plaques. Bioorg. Chem. 2021, 115, 105167. [Google Scholar] [CrossRef]
- Wu, J.; Shao, C.; Ye, X.; Di, X.; Li, D.; Zhao, H.; Zhang, B.; Chen, G.; Liu, H.K.; Qian, Y. InVivo Brain Imaging of Amyloid-beta Aggregates in Alzheimer’s Disease with a Near-Infrared Fluorescent Probe. ACS Sens. 2021, 6, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiang, F.; Yao, L.; Zhang, C.; Jia, X.; Chen, A.; Liu, Y. Synthesis and evaluation of curcumin-based near-infrared fluorescent probes for detection of amyloid beta peptide in Alzheimer mouse models. Bioorg. Med. Chem. 2023, 92, 117410. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Wen, X.; Wang, Y.; Sun, Y.; An, R.; Zhou, Y.; Ye, D.; Liu, H. Engineering of donor-acceptor-donor curcumin analogues as near-infrared fluorescent probes for in vivo imaging of amyloid-beta species. Theranostics 2022, 12, 3178–3195. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, M.; Chutia, K.; Dutta, D.; Gogoi, P. Overview of coumarin-fused-coumarins: Synthesis, photophysical properties and their applications. Org. Biomol. Chem. 2021, 20, 55–72. [Google Scholar] [CrossRef]
- Kupeli Akkol, E.; Genc, Y.; Karpuz, B.; Sobarzo-Sanchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Lippe, D.S.; Elghawy, O.; Zucker, A.M.; Yanagawa, E.S.K.; Mathews, E.; Ahmed, Y.G.; D’Elia, P.N.; Bimson, S.; Walvoord, R.R. Synthesis of 7-Aminocoumarins from 7-Hydroxycoumarins via Amide Smiles Rearrangement. ACS Omega 2022, 7, 35269–35279. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Zhang, J.; Liu, Z.; Fu, Y.; Zhang, D.; Zheng, M.; Zhang, H.; Xu, M.H. Design of a Coumarin-Based Fluorescent Probe for Efficient In Vivo Imaging of Amyloid-beta Plaques. ACS Chem. Neurosci. 2023, 14, 829–838. [Google Scholar] [CrossRef]
- Dzyuba, S.V. BODIPY Dyes as Probes and Sensors to Study Amyloid-beta-Related Processes. Biosensors 2020, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Bacalum, M.; Wang, L.; Boodts, S.; Yuan, P.; Leen, V.; Smisdom, N.; Fron, E.; Knippenberg, S.; Fabre, G.; Trouillas, P.; et al. A Blue-Light-Emitting BODIPY Probe for Lipid Membranes. Langmuir 2016, 32, 3495–3505. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Gu, J.; Lin, W.; Wei, Y.; Lin, Y.; Liu, L.; Ding, H.; Pan, C.; Xie, Z.; Wu, T. A BODIPY biosensor to detect and drive self-assembly of diphenylalanine. Chem. Commun. 2019, 55, 8564–8566. [Google Scholar] [CrossRef]
- Collot, M.; Boutant, E.; Lehmann, M.; Klymchenko, A.S. BODIPY with Tuned Amphiphilicity as a Fluorogenic Plasma Membrane Probe. Bioconjug. Chem. 2019, 30, 192–199. [Google Scholar] [CrossRef]
- Ma, L.; Geng, Y.; Zhang, G.; Hu, Z.; James, T.D.; Wang, X.; Wang, Z. Near-Infrared Bodipy-Based Molecular Rotors for beta-Amyloid Imaging In Vivo. Adv. Healthc. Mater. 2023, 12, e2300733. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, G.; Hu, Z.; Zhu, C.; Chen, Y.; James, T.D.; Ma, L.; Wang, Z. A BODIPY-based probe for amyloid-β imaging in vivo. Org. Chem. Front. 2023, 10, 1903–1909. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, J.; Peng, C.; Xiang, H.; Chen, J.; Peng, C.; Zhu, W.; Huang, R.; Zhang, H.; Hu, Y. Fluorescent Imaging of beta-Amyloid Using BODIPY Based Near-Infrared Off-On Fluorescent Probe. Bioconjug. Chem. 2018, 29, 3459–3466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Chen, Q.; Su, J.; Shi, W.J.; Zhang, L.; Xia, C.; Yan, J. Rotor-Tuning Boron Dipyrromethenes for Dual-Functional Imaging of Abeta Oligomers and Viscosity. ACS Appl. Bio Mater. 2022, 5, 3049–3056. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.T.; Cai, Y.; Zhang, Z.Y.; Wang, C.Y.; Tang, Y.H.; Zhu, M.Q.; Wang, Y.L. Progress of Dicyanomethylene-4H-Pyran Derivatives in Biological Sensing Based on ICT Effect. Front. Chem. 2022, 10, 903253. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-Y.; Zhang, J.-J.; Zang, Y.; Zhang, H.-Y.; Li, J.; Chen, G.-R.; He, X.-P. D-A-D fluorogenic probe for the rapid imaging of amyloid β plaques in vivo. Dye. Pigment. 2017, 136, 224–228. [Google Scholar] [CrossRef]
- Fu, W.; Yan, C.; Guo, Z.; Zhang, J.; Zhang, H.; Tian, H.; Zhu, W.H. Rational Design of Near-Infrared Aggregation-Induced-Emission-Active Probes: In Situ Mapping of Amyloid-beta Plaques with Ultrasensitivity and High-Fidelity. J. Am. Chem. Soc. 2019, 141, 3171–3177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Zhou, L.F.; Li, Y.K.; Chen, S.B.; Yan, J.W.; Zhang, L. In vivo near-infrared fluorescence imaging of amyloid-beta plaques with a dicyanoisophorone-based probe. Anal. Chim. Acta 2017, 961, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhu, B.; Deng, Y.; Zhang, Z. In vivo detection of cerebral amyloid fibrils with smart dicynomethylene-4H-pyran-based fluorescence probe. Anal. Chem. 2015, 87, 4781–4787. [Google Scholar] [CrossRef] [PubMed]


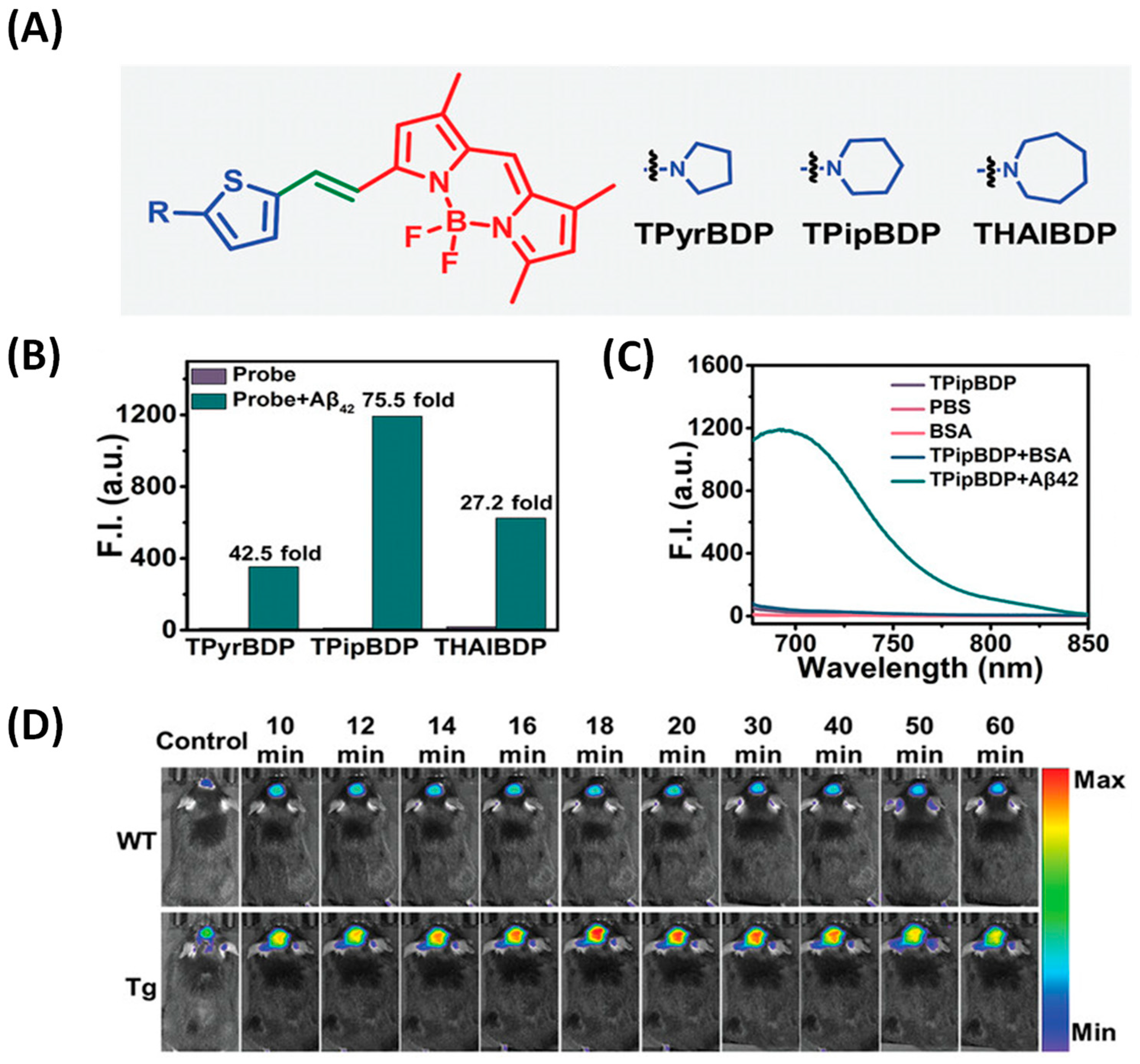
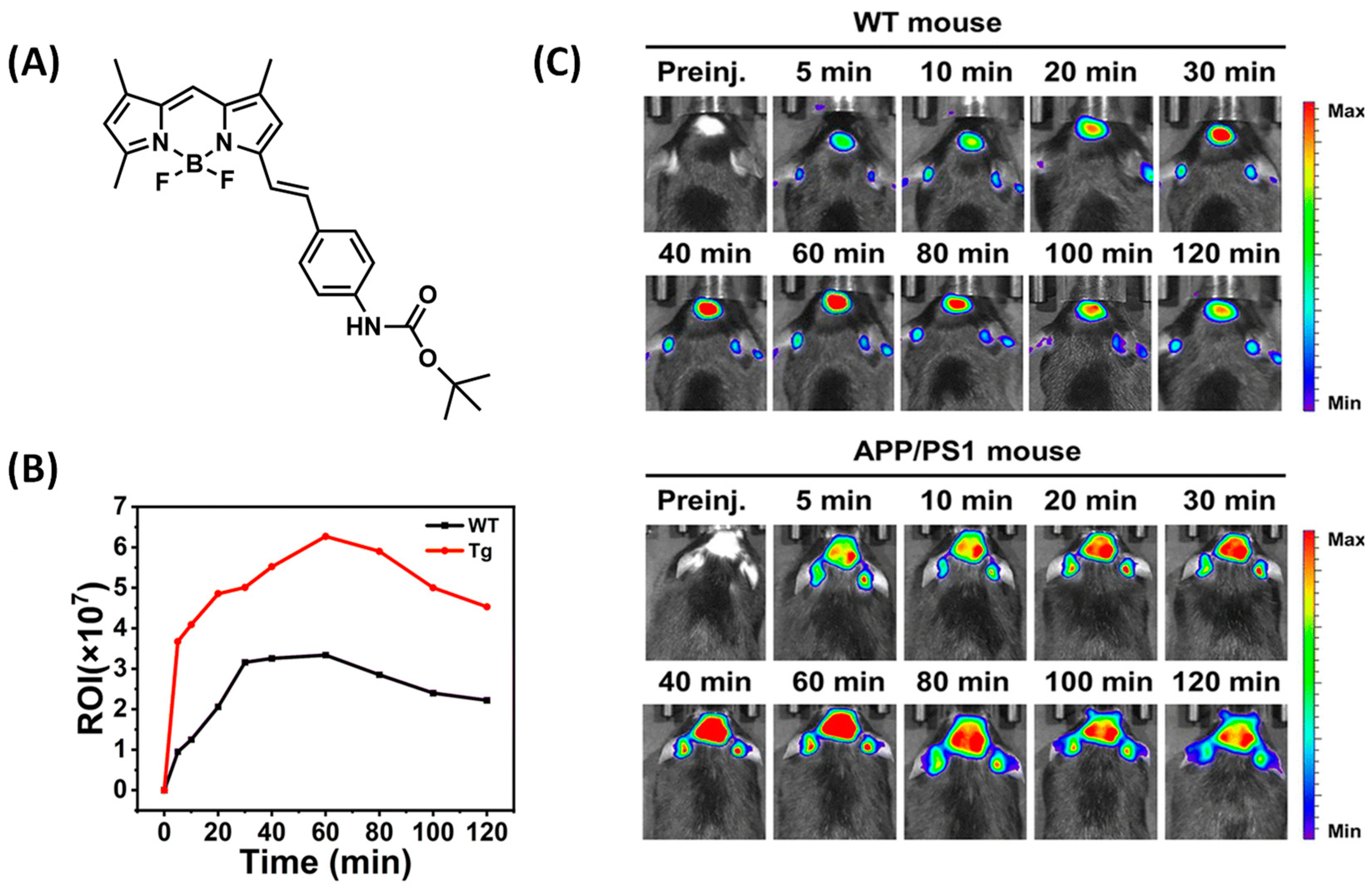
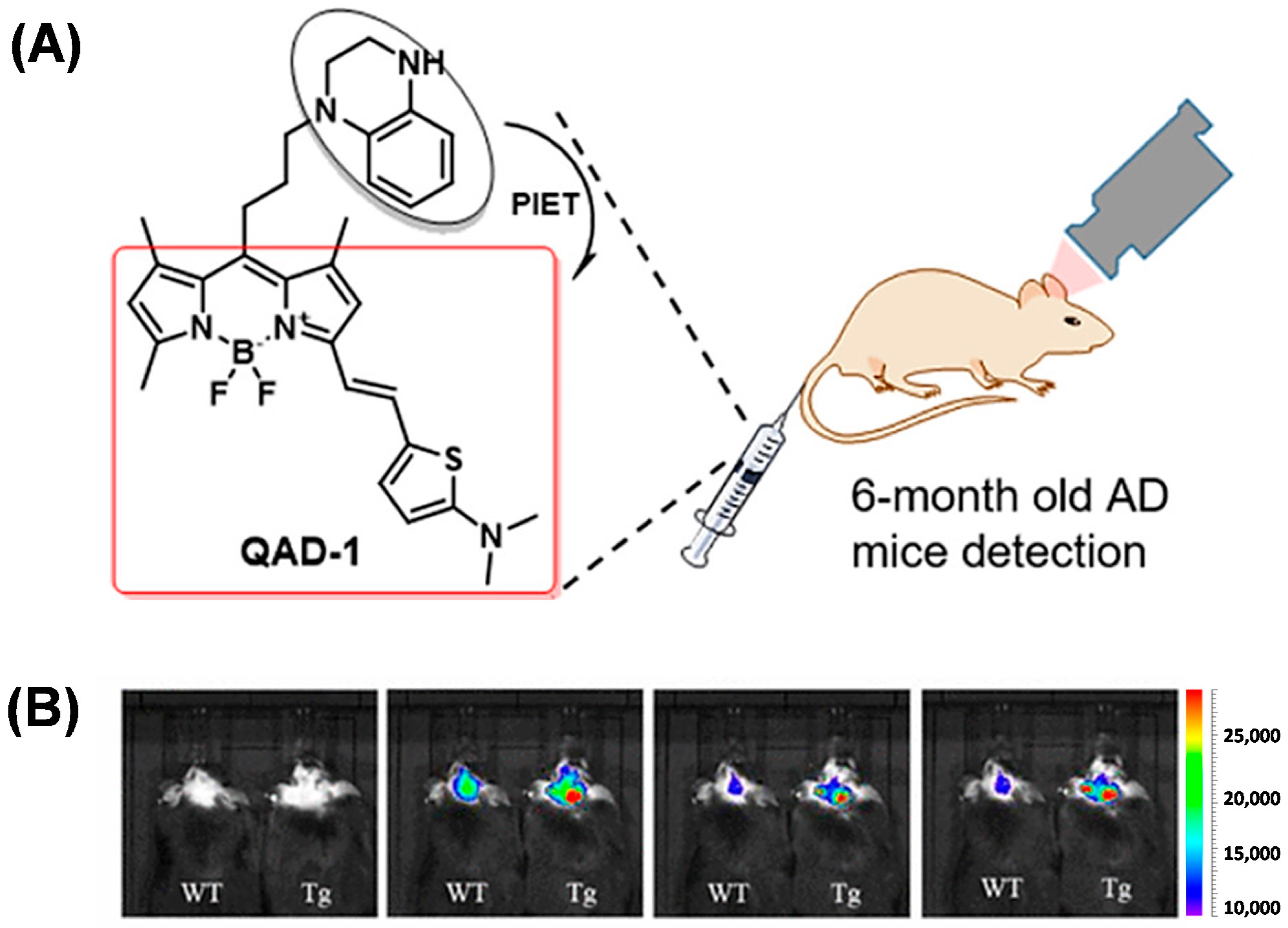
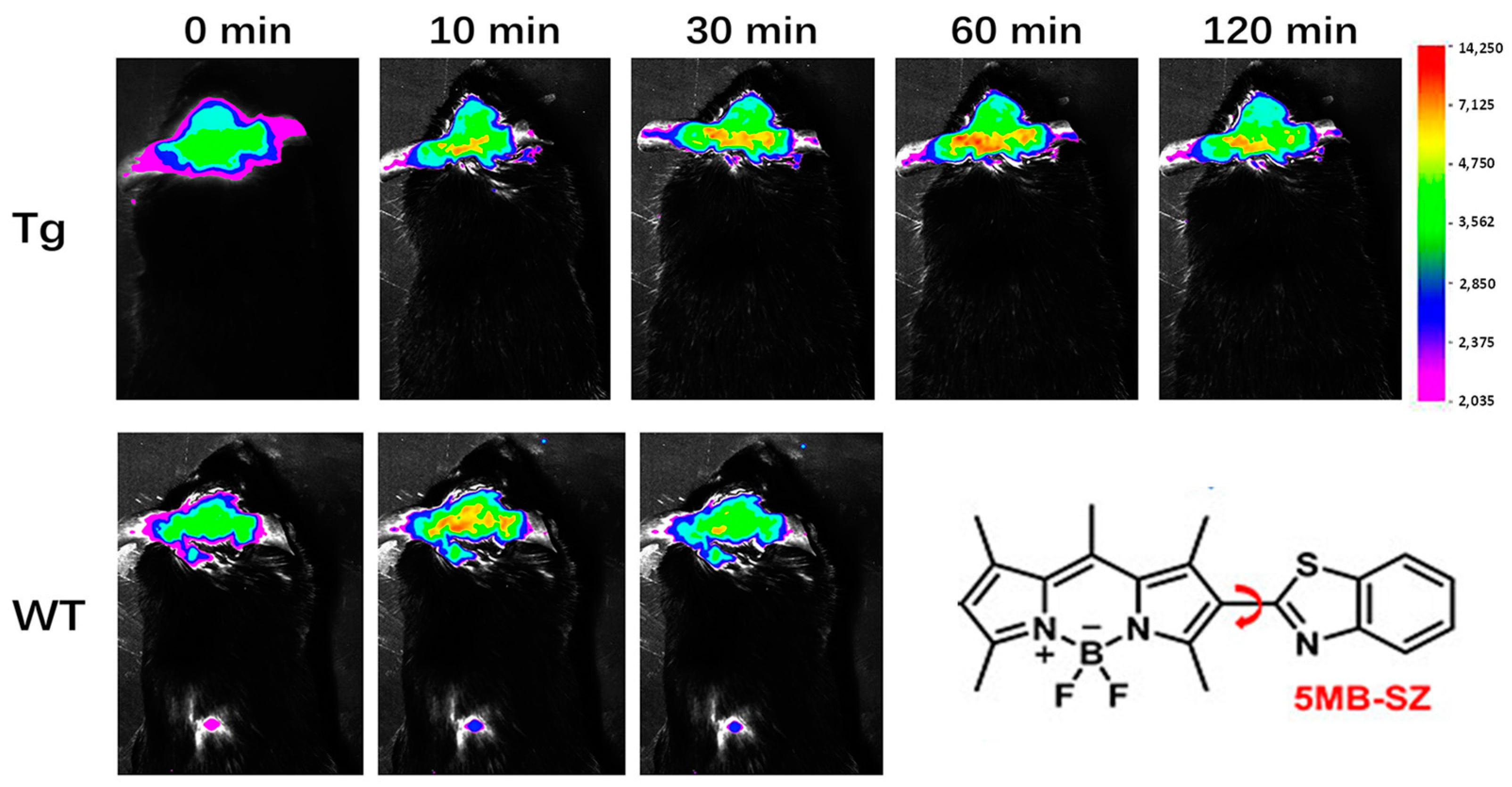


| Compounds | λex (nm) | λem (nm) with Aβ | (FAβ/F0) | Kd (nM) | |
|---|---|---|---|---|---|
| Curcumin | 8b | 560 | 667 | 21.4 | 91.2 ± 3.28 |
| CAQ | 565 | 635 | 10 | 78.89 | |
| 3b | 635 | 667 | / | 2.12 ± 0.77 | |
| probe 9 | 620 | 697 | 10 | 14.57 ± 1.27 | |
| Coumarin | XCYX-3 | 502 | 632 | / | 71.11 |
| BODIPY | TPipBDP | / | 692 | 75.5 | 28.30 ± 5.94 |
| BocBDP | / | / | 5 | 67.8 ± 3.18 | |
| QAD-1 | 635 | 700 | / | 27 | |
| 5MB-SZ | / | 550 | 43.64 | / | |
| DCM | YHY2 | / | 596 | 7.7 | 23.5 |
| QM-FN-SO3 | / | / | 50 | 170 | |
| DCIP-1 | / | 635 | / | 674.3 | |
| PAD-1 | / | / | 7 | 58.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-Y.; Li, Z.-J.; Tang, Y.-H.; Xu, L.; Zhang, D.-T.; Qin, T.-Y.; Wang, Y.-L. Recent Research Progress in Fluorescent Probes for Detection of Amyloid-β In Vivo. Biosensors 2023, 13, 990. https://doi.org/10.3390/bios13110990
Zhang Z-Y, Li Z-J, Tang Y-H, Xu L, Zhang D-T, Qin T-Y, Wang Y-L. Recent Research Progress in Fluorescent Probes for Detection of Amyloid-β In Vivo. Biosensors. 2023; 13(11):990. https://doi.org/10.3390/bios13110990
Chicago/Turabian StyleZhang, Zhen-Yu, Ze-Jun Li, Ying-Hao Tang, Liang Xu, De-Teng Zhang, Tian-Yi Qin, and Ya-Long Wang. 2023. "Recent Research Progress in Fluorescent Probes for Detection of Amyloid-β In Vivo" Biosensors 13, no. 11: 990. https://doi.org/10.3390/bios13110990
APA StyleZhang, Z.-Y., Li, Z.-J., Tang, Y.-H., Xu, L., Zhang, D.-T., Qin, T.-Y., & Wang, Y.-L. (2023). Recent Research Progress in Fluorescent Probes for Detection of Amyloid-β In Vivo. Biosensors, 13(11), 990. https://doi.org/10.3390/bios13110990





