A Novel Optical Fiber Terahertz Biosensor Based on Anti-Resonance for the Rapid and Nondestructive Detection of Tumor Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
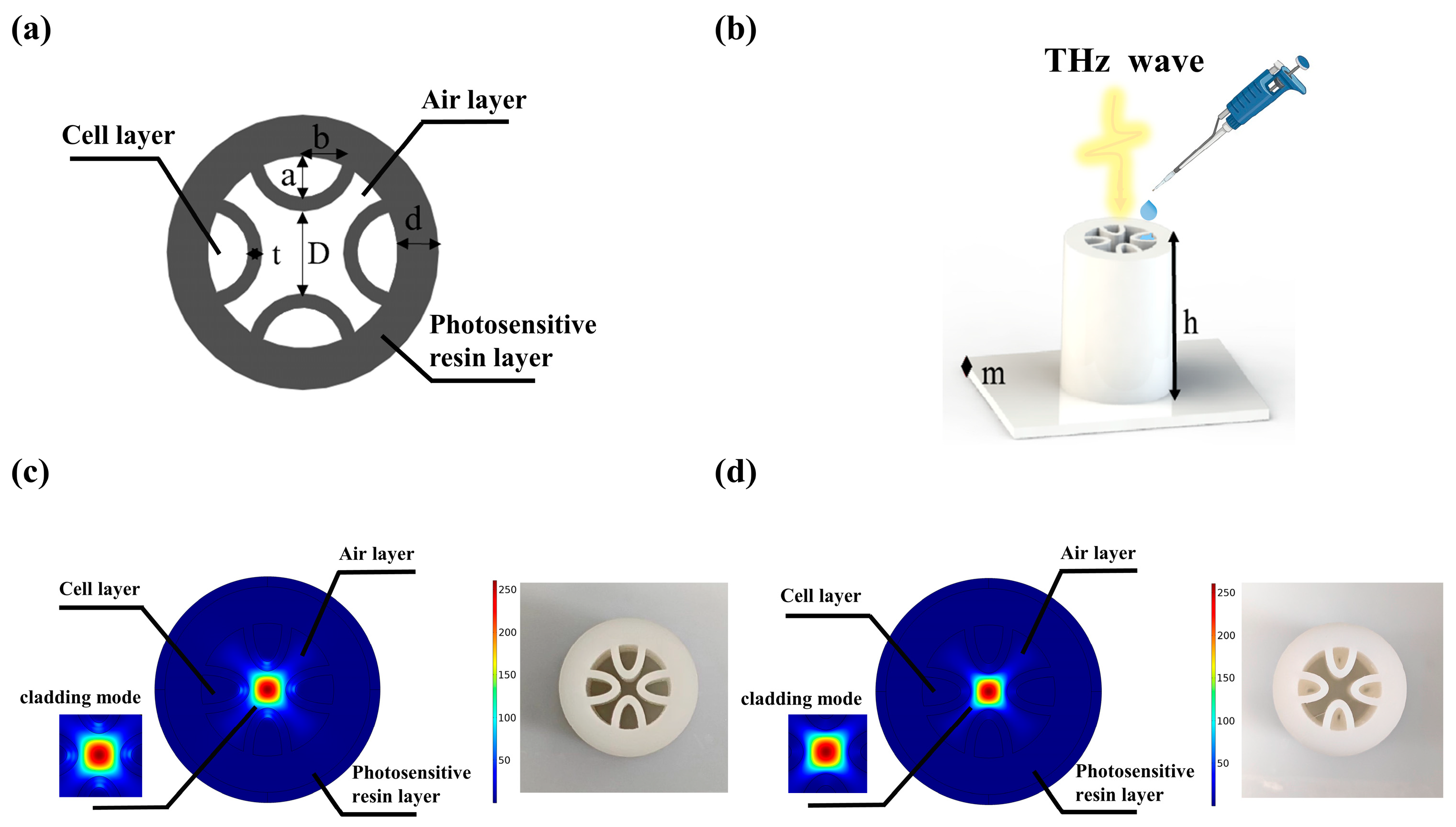
2.2. Fabrication of THz AR-HCFs
2.3. Cell Culture
2.4. Cell Suspension Preparation and Measurement
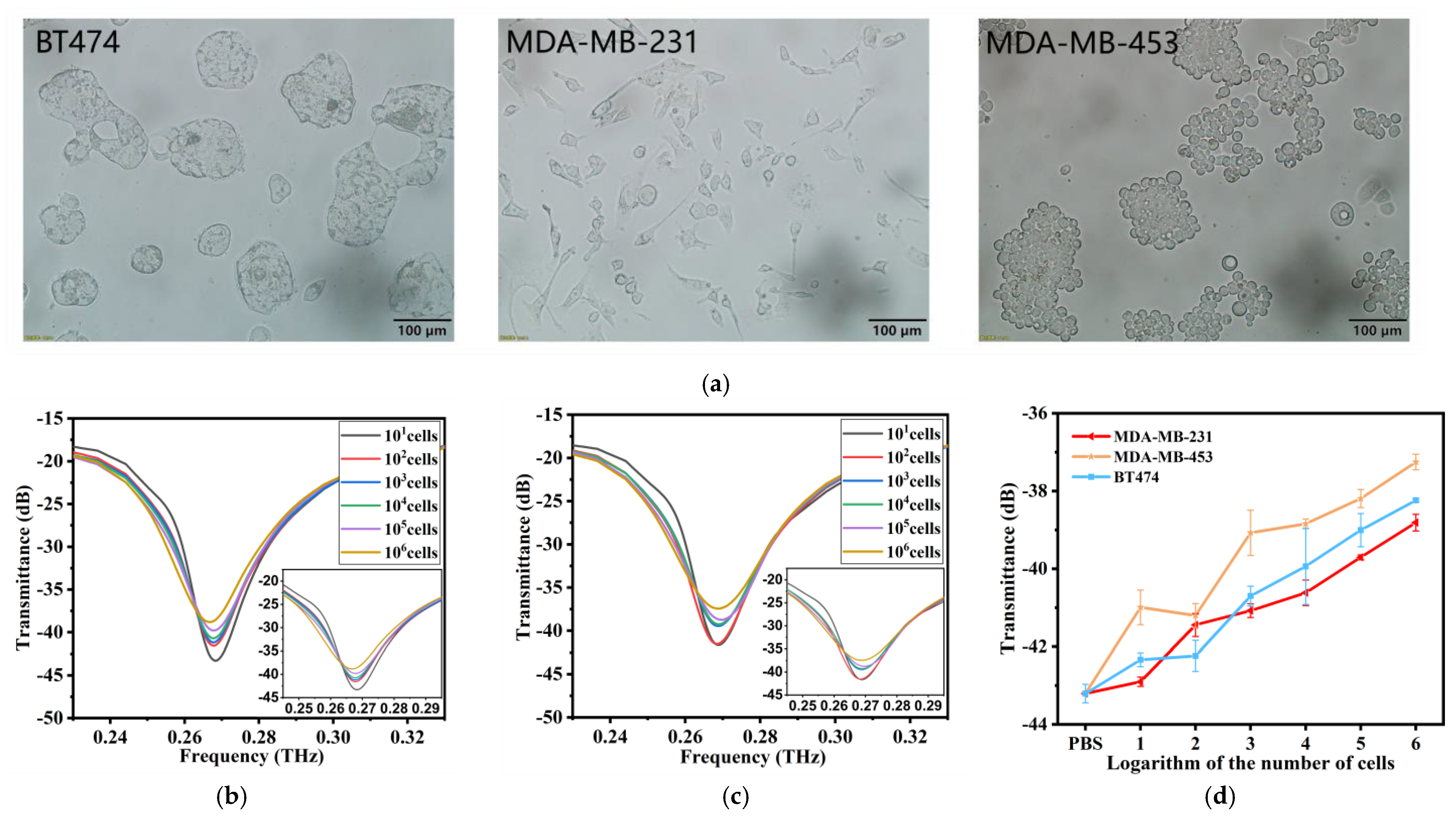
2.5. Morphology of the Cells
2.6. Simulation
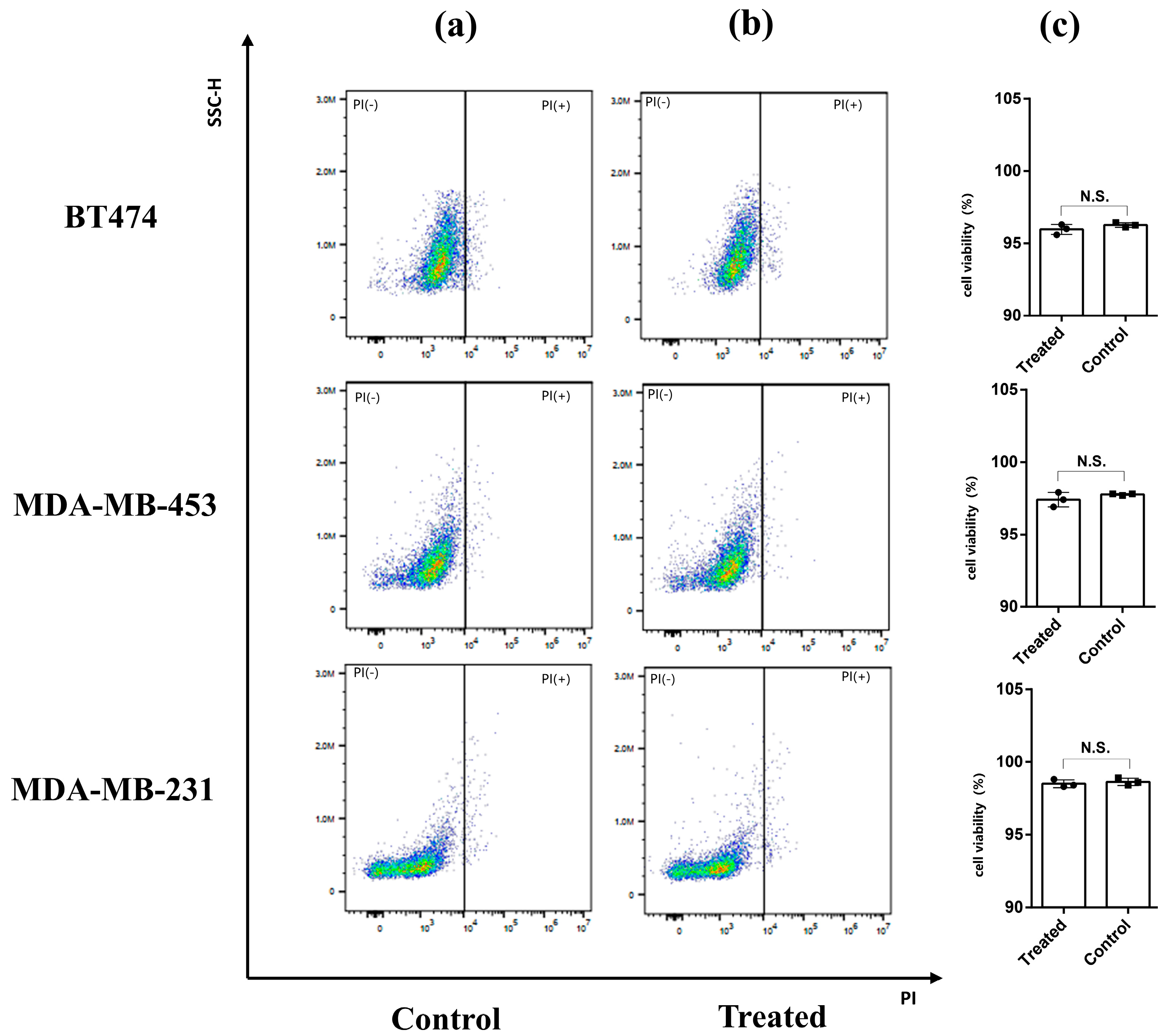
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
3.1. Theory of THz AR-HCFs
3.2. Design and Analysis of the THz AR-HCF
3.3. Sensitivity of the THz AR-HCF Biosensor
3.4. Specificity of the THz AR-HCF Biosensor
3.5. Cell Activity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milojkovic Kerklaan, B.; Pluim, D.; Bol, M.; Hofland, I.; Westerga, J.; van Tinteren, H.; Beijnen, J.H.; Boogerd, W.; Schellens, J.H.M.; Brandsma, D. EpCAM-based flow cytometry in cerebrospinal fluid greatly improves diagnostic accuracy of leptomeningeal metastases from epithelial tumors. Neuro-Oncology 2016, 18, 855–862. [Google Scholar] [CrossRef]
- Rawal, S.; Yang, Y.P.; Cote, R.; Agarwal, A. Identification and Quantitation of Circulating Tumor Cells. Annu. Rev. Anal. Chem. 2017, 10, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Murlidhar, V.; Reddy, R.M.; Fouladdel, S.; Zhao, L.L.; Ishikawa, M.K.; Grabauskiene, S.; Zhang, Z.; Lin, J.; Chang, A.C.; Carrott, P.; et al. Poor Prognosis Indicated by Venous Circulating Tumor Cell Clusters in Early-Stage Lung Cancers. Cancer Res. 2017, 77, 5194–5206. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yu, S.; Wei, J.; Ding, L.; Yang, X.; Wu, Y. New horizons in the identification of circulating tumor cells (CTCs): An emerging paradigm shift in cytosensors. Biosens. Bioelectron. 2022, 203, 114043. [Google Scholar] [CrossRef] [PubMed]
- Alfihed, S.; Holzman, J.F.; Foulds, I.G. Developments in the integration and application of terahertz spectroscopy with microfluidics. Biosens. Bioelectron. 2020, 165, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical Applications of Terahertz Spectroscopy and Imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhu, L.G.; Meng, K.; Huang, W.; Shi, Q. THz medical imaging: From in vitro to in vivo. Trends Biotechnol. 2022, 40, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Peng, Y.; Shi, C.J.; Wang, L.P.; Chen, X.H.; Wu, W.W.; Wu, X.; Zhu, Y.M.; Zhang, J.C.; Cheng, G.L.; et al. Qualitative and quantitative recognition of chiral drugs based on terahertz spectroscopy. Analyst 2021, 146, 3888–3898. [Google Scholar] [CrossRef]
- Liu, H.B.; Plopper, G.; Earley, S.; Chen, Y.; Ferguson, B.; Zhang, X.C. Sensing minute changes in biological cell monolayers with THz differential time-domain spectroscopy. Biosens. Bioelectron. 2007, 22, 1075–1080. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, M.; Xia, L.; Yu, W.; Peng, J.; Zhang, Y.; de la Chapelle, M.L.; Yang, K.; Cui, H.-L.; Fu, W. Cell viability and hydration assay based on metamaterial-enhanced terahertz spectroscopy. RSC Adv. 2017, 7, 53963–53969. [Google Scholar] [CrossRef]
- Reid, C.B.; Reese, G.; Gibson, A.P.; Wallace, V.P. Terahertz time-domain spectroscopy of human blood. IEEE J. Biomed. Health Inform. 2013, 17, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.; Huang, P.; Ge, W.; Hou, D.; Zhang, G. Inspecting human colon adenocarcinoma cell lines by using terahertz time-domain reflection spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.; Zhang, G.; Fan, S.; Ge, W.; Hu, W.; Huang, P.; Hou, D.; Zheng, S. Characterization and discrimination of human colorectal cancer cells using terahertz spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 256, 119713. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, L.; Peng, R.Y. Research progress in the effects of terahertz waves on biomacromolecules. Mil. Med. Res. 2021, 8, 28. [Google Scholar] [CrossRef]
- Islam, M.S.; Cordeiro, C.M.B.; Franco, M.A.R.; Sultana, J.; Cruz, A.L.S.; Abbott, D. Terahertz optical fibers [Invited]. Opt. Express 2020, 28, 16089–16117. [Google Scholar] [CrossRef]
- Mei, S.; Kong, D.P.; Wang, L.L.; Ma, T.; Zhu, Y.F.; Zhang, X.D.; He, Z.Q.; Huang, X.S.; Zhang, Y.N. Suspended graded-index porous core POF for ultra-flat near-zero dispersion terahertz transmission. Opt. Fiber Technol. 2019, 52, 7. [Google Scholar] [CrossRef]
- Gandhi, M.S.A.; Zhao, Y.; Fu, H.Y.; Li, Q. A Highly Versatile Porous Core Photonic Quasicrystal Fiber Based Refractive Index Terahertz Sensor. Sensors 2022, 22, 3469. [Google Scholar] [CrossRef]
- Chen, H.; Lee, W.J.; Huang, H.Y.; Chiu, C.M.; Tsai, Y.F.; Tseng, T.F.; Lu, J.T.; Lai, W.L.; Sun, C.K. Performance of THz fiber-scanning near-field microscopy to diagnose breast tumors. Opt. Express 2011, 19, 19523–19531. [Google Scholar] [CrossRef]
- Hou, M.; Wang, N.; Ma, J.; Chen, Y.; Chen, X. Intensity Modulated Gas RI Sensor Based on Inornate Antiresonant Hollow-Core Fiber with Ultrahigh Sensitivity. IEEE Access 2021, 9, 45270–45276. [Google Scholar] [CrossRef]
- Wu, H.; Ma, Z.; Wei, C.; Jiang, M.; Hong, X.; Li, Y.; Chen, D.; Huang, X. Three-Dimensional Microporous Hollow Fiber Membrane Microfluidic Device Integrated with Selective Separation and Capillary Self-Driven for Point-of-Care Testing. Anal. Chem. 2020, 92, 6358–6365. [Google Scholar] [CrossRef]
- Sultana, J.; Islam, M.S.; Ahmed, K.; Dinovitser, A.; Ng, B.W.; Abbott, D. Terahertz detection of alcohol using a photonic crystal fiber sensor. Appl. Opt. 2018, 57, 2426–2433. [Google Scholar] [CrossRef]
- Hossain, M.B.; Podder, E. Design and investigation of PCF-based blood components sensor in terahertz regime. Appl. Phys. A 2019, 125, 861. [Google Scholar] [CrossRef]
- Islam, M.R.; Iftekher, A.N.M.; Mou, F.A.; Rahman, M.M.; Bhuiyan, M.I.H. Design of a Topas-based ultrahigh-sensitive PCF biosensor for blood component detection. Appl. Phys. A 2021, 127, 109. [Google Scholar] [CrossRef]
- Habib, A.; Rashed, A.N.Z.; El-Hageen, H.M.; Alatwi, A.M. Extremely Sensitive Photonic Crystal Fiber–Based Cancer Cell Detector in the Terahertz Regime. Plasmonics 2021, 16, 1297–1306. [Google Scholar] [CrossRef]
- Yadav, S.; Lohia, P.; Dwivedi, D.K. Eminently sensitive mono-rectangular photonic crystal fiber-based sensor for cancer cell detection in THz regime. J. Opt. 2023. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Miao, T.; Mu, Q.Y.; Zhou, L.; Meng, C.; Xue, J.; Yao, Y.M. A Novel High-Sensitivity Terahertz Microstructure Fiber Biosensor for Detecting Cancer Cells. Photonics 2022, 9, 639. [Google Scholar] [CrossRef]
- Xiao, H.; Li, H.S.; Wu, B.L.; Dong, Y.; Xiao, S.Y.; Jian, S.S. Low-loss polarization-maintaining hollow-core anti-resonant terahertz fiber. J. Opt. 2019, 21, 7. [Google Scholar] [CrossRef]
- Lu, W.; Argyros, A. Terahertz spectroscopy and imaging with flexible tube-lattice fiber probe. J. Light. Technol. 2014, 32, 4019–4025. [Google Scholar]
- Van Putten, L.; Gorecki, J.; Fokoua, E.N.; Apostolopoulos, V.; Poletti, F. 3D-printed polymer antiresonant waveguides for short-reach terahertz applications. Appl. Opt. 2018, 57, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Lou, S.; Wang, X.; Zhao, T.; Zhang, W. High-birefringence hollow-core anti-resonant THz fiber. Opt. Quant. Electron. 2018, 50, 162. [Google Scholar] [CrossRef]
- Atakaramians, S.; Afshar, S.; Fischer, B.M.; Abbott, D.; Monro, T.M. Porous fibers: A novel approach to low loss THz waveguides. Opt. Express 2008, 16, 8845–8854. [Google Scholar] [CrossRef]
- Vincetti, L.; Setti, V.; Zoboli, M. Terahertz Tube Lattice Fibers with Octagonal Symmetry. IEEE Photonics Technol. Lett. 2010, 22, 972–974. [Google Scholar] [CrossRef]
- Sultana, J.; Islam, M.S.; Cordeiro CM, B.; Habib, M.S.; Dinovitser, A.; Kaushik, M.; Ng, B.W.H.; Ebendorff-Heidepriem, H.; Abbott, D. Hollow Core Inhibited Coupled Antiresonant Terahertz Fiber: A Numerical and Experimental Study. IEEE Trans. Terahertz Sci. Technol. 2021, 11, 245–260. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Pappas, D. Isolation of leukemia and breast cancer cells from liquid biopsies and clinical samples at low concentration in a 3D printed cell separation device via transferrin-receptor affinity. Talanta 2023, 253, 124107. [Google Scholar] [CrossRef]
- Li, J.; Wuethrich, A.; Zhang, Z.; Wang, J.; Lin, L.L.; Behren, A.; Wang, Y.; Trau, M. SERS Multiplex Profiling of Melanoma Circulating Tumor Cells for Predicting the Response to Immune Checkpoint Blockade Therapy. Anal. Chem. 2022, 94, 14573–14582. [Google Scholar] [CrossRef]
- Wang, W.; Liu, S.; Li, C.; Wang, Y.; Yan, C. Dual-target recognition sandwich assay based on core-shell magnetic mesoporous silica nanoparticles for sensitive detection of breast cancer cells. Talanta 2018, 182, 306–313. [Google Scholar] [CrossRef]
- Wu, X.; Xia, Y.; Huang, Y.; Li, J.; Ruan, H.; Chen, T.; Luo, L.; Shen, Z.; Wu, A. Improved SERS-Active Nanoparticles with Various Shapes for CTC Detection without Enrichment Process with Supersensitivity and High Specificity. ACS Appl. Mater. Interfaces 2016, 8, 19928–19938. [Google Scholar] [CrossRef]
- Yin, X.; Chen, B.; He, M.; Hu, B. A Multifunctional Platform for the Capture, Release, And Enumeration of Circulating Tumor Cells Based on Aptamer Binding, Nicking Endonuclease-Assisted Amplification, and Inductively Coupled Plasma Mass Spectrometry Detection. Anal. Chem. 2020, 92, 10308–10315. [Google Scholar] [CrossRef]
- Luo, J.; Liang, D.; Zhao, D.; Yang, M. Photoelectrochemical detection of circulating tumor cells based on aptamer conjugated Cu2O as signal probe. Biosens. Bioelectron. 2020, 151, 111976. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Piskin, A.K.; Yavuz, H.; Denizli, A. Quartz crystal microbalance biosensor for label-free MDA MB 231 cancer cell detection via notch-4 receptor. Talanta 2019, 204, 840–845. [Google Scholar] [CrossRef]


| Classification | Target Cell Line | Limits of Detection (Cells/mL) | Linear Ranges (Cells/mL) | Ref. |
|---|---|---|---|---|
| Fluorescence | MCF-7 | 100 | 100–1 × 105 | [36] |
| Surface-enhanced Raman scattering | HeLa | 1 | 3–100 | [37] |
| ICP-MS | MCF-7 | 87 | 250–10,000 | [38] |
| Electrochemical | MCF-7 | 1 | 3–3000 | [39] |
| Quartz crystal microbalances | MDA-MB-231 | 12 | 50–300 | [40] |
| THz AR-HCF | BT 474, MDA-MB-453, MDA-MB-231 | 100 | 100–1 × 107 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Luo, Y.; Huang, G.; Lamy de la Chapelle, M.; Tian, H.; Xie, F.; Jin, W.; Shi, J.; Yang, X.; Fu, W. A Novel Optical Fiber Terahertz Biosensor Based on Anti-Resonance for the Rapid and Nondestructive Detection of Tumor Cells. Biosensors 2023, 13, 947. https://doi.org/10.3390/bios13100947
He Z, Luo Y, Huang G, Lamy de la Chapelle M, Tian H, Xie F, Jin W, Shi J, Yang X, Fu W. A Novel Optical Fiber Terahertz Biosensor Based on Anti-Resonance for the Rapid and Nondestructive Detection of Tumor Cells. Biosensors. 2023; 13(10):947. https://doi.org/10.3390/bios13100947
Chicago/Turabian StyleHe, Zhe, Yueping Luo, Guorong Huang, Marc Lamy de la Chapelle, Huiyan Tian, Fengxin Xie, Weidong Jin, Jia Shi, Xiang Yang, and Weiling Fu. 2023. "A Novel Optical Fiber Terahertz Biosensor Based on Anti-Resonance for the Rapid and Nondestructive Detection of Tumor Cells" Biosensors 13, no. 10: 947. https://doi.org/10.3390/bios13100947
APA StyleHe, Z., Luo, Y., Huang, G., Lamy de la Chapelle, M., Tian, H., Xie, F., Jin, W., Shi, J., Yang, X., & Fu, W. (2023). A Novel Optical Fiber Terahertz Biosensor Based on Anti-Resonance for the Rapid and Nondestructive Detection of Tumor Cells. Biosensors, 13(10), 947. https://doi.org/10.3390/bios13100947








