Palladium Hydroxide (Pearlman’s Catalyst) Doped MXene (Ti3C2Tx) Composite Modified Electrode for Selective Detection of Nicotine in Human Sweat
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Characterizations
2.3. Preparation of MAX Phase (Ti3AlC2) and MXene (Ti3C2Tx)
2.4. Preparation of MXene/PHC Composite
2.5. Fabrication of MXene/PHC Sensor
2.6. Electrochemical Measurements
2.7. Real Sample Collection and Extraction
3. Results and Discussion
3.1. Material Characterizations
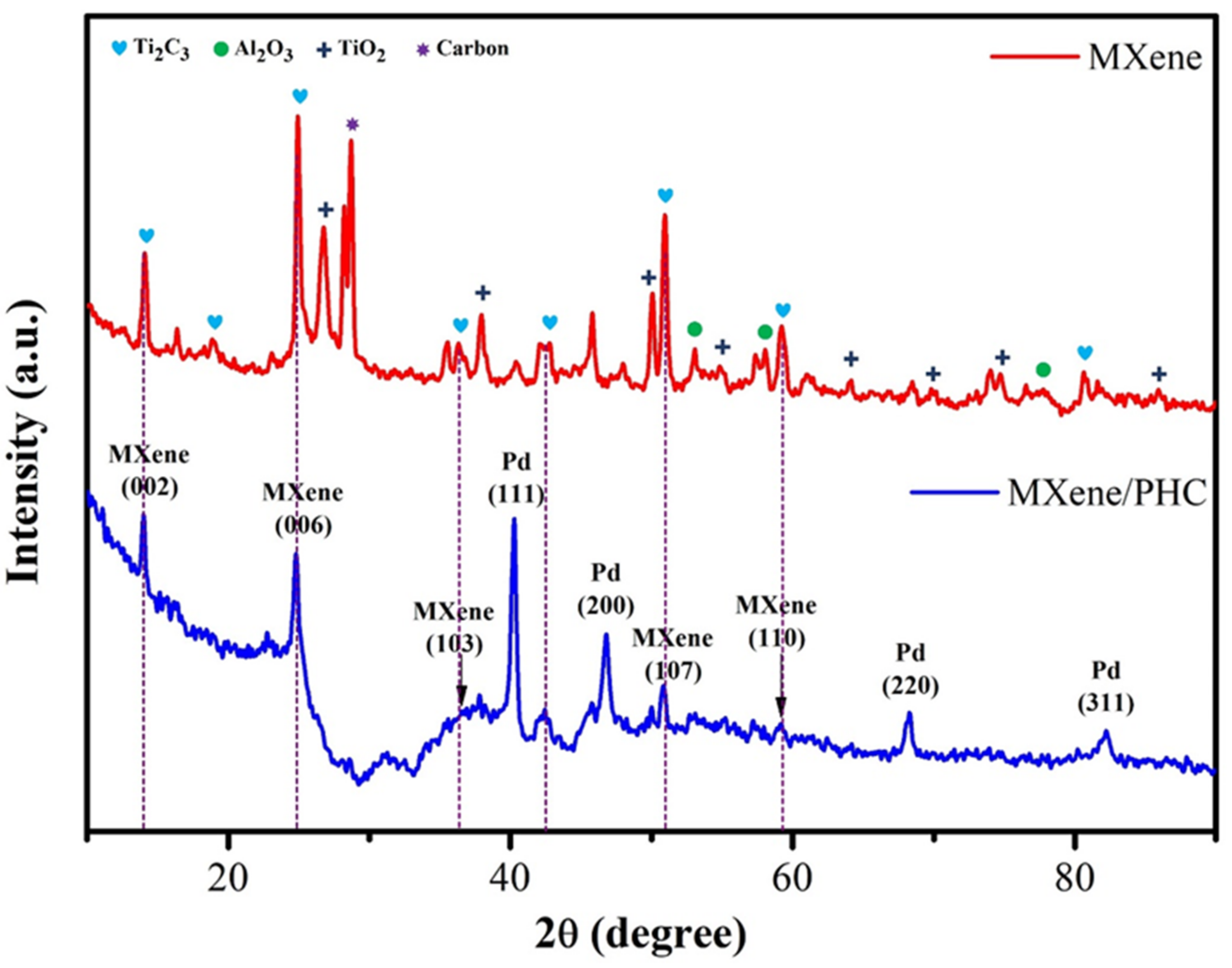
3.1.1. Raman and XRD Analysis
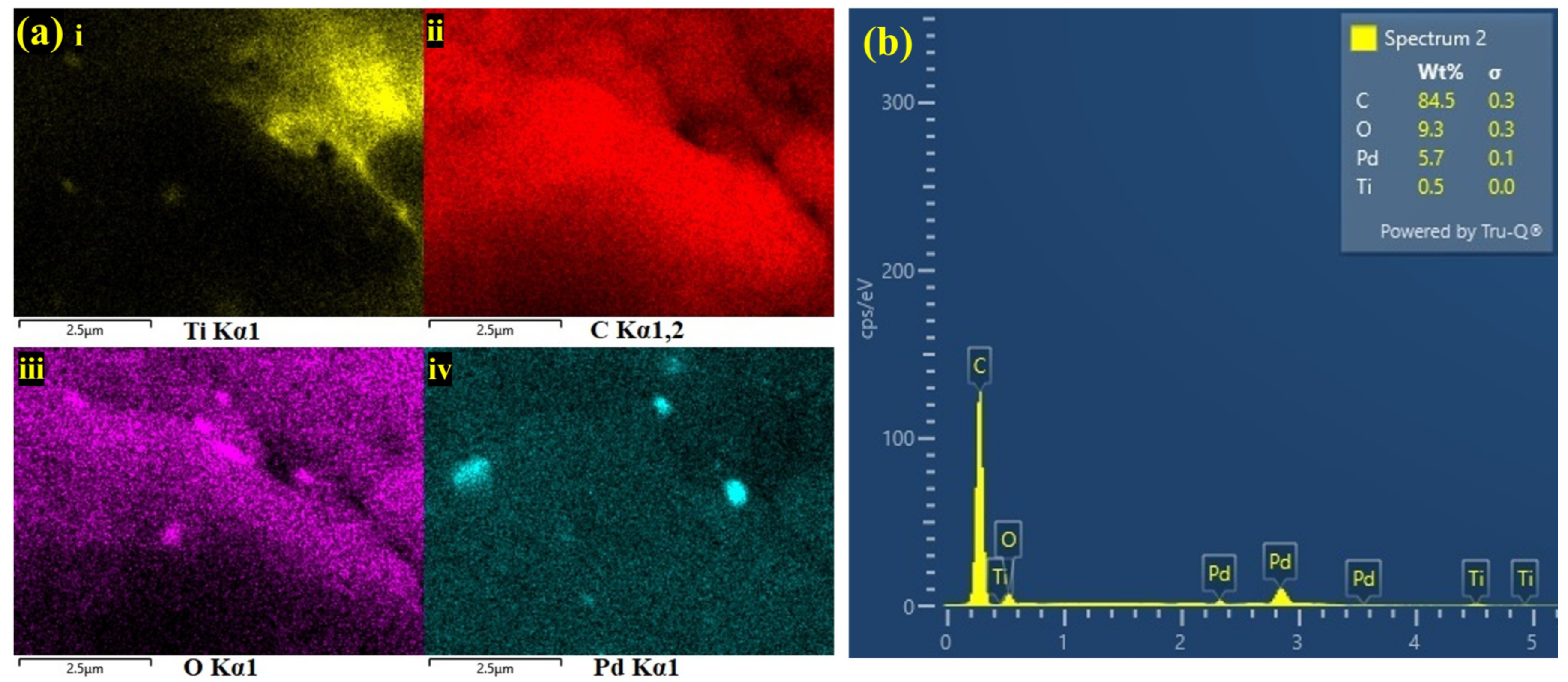
3.1.2. FE-SEM, EDS and E-Mapping Analysis
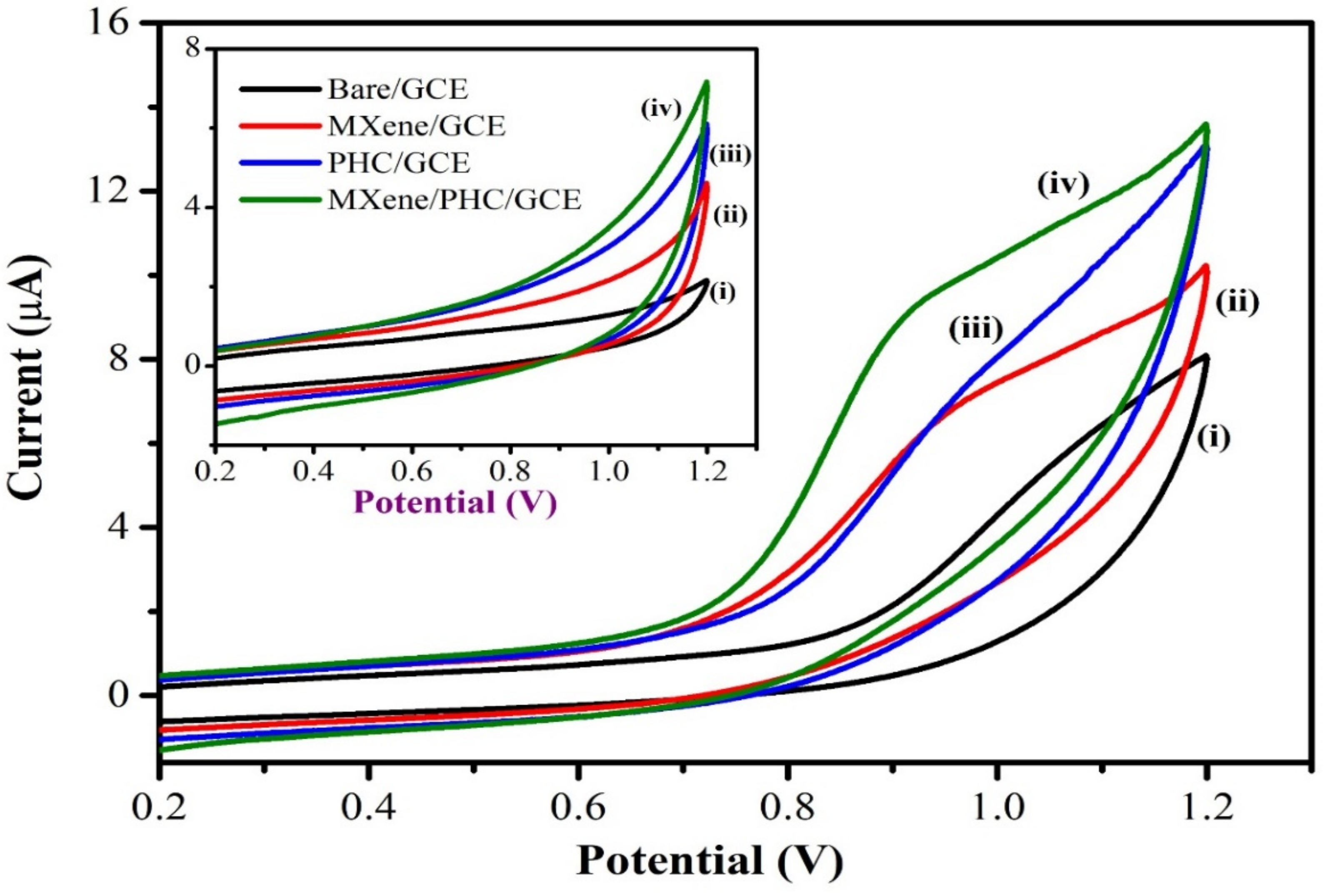
3.2. Electrochemical Properties of MXene/PHC Composite
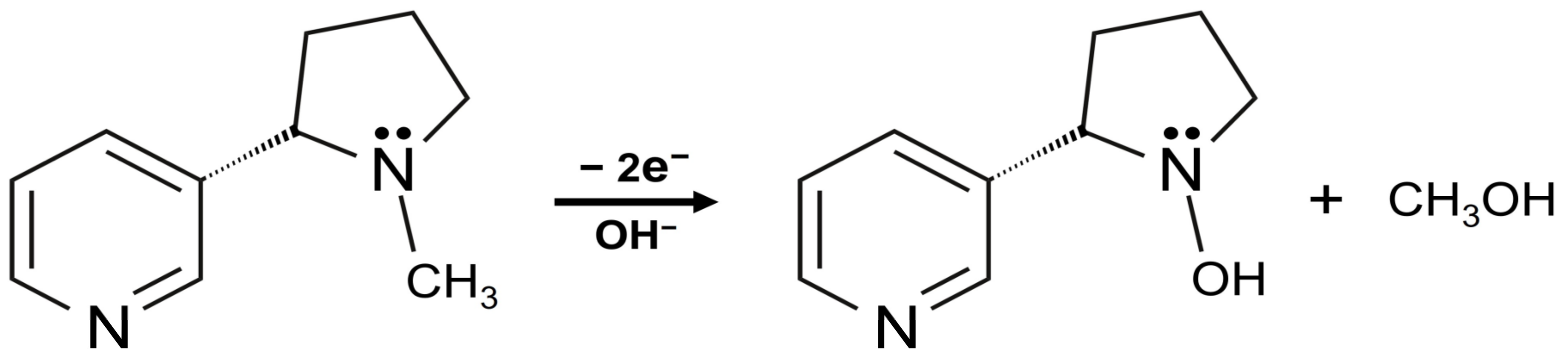
3.2.1. Electrochemical Oxidation of NIC
3.2.2. Detecting NIC Concentration
3.2.3. Amperometry and Interference Studies
3.2.4. Repeatability and Stability Studies
3.2.5. Real-World Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nouri, F.; Nourollahi-Fard, S.R.; Foroodi, H.R.; Sharifi, H. In Vitro Anthelmintic Effect of Tobacco (Nicotiana Tabacum) Extract on Parasitic Nematode, Marshallagia Marshalli. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2016, 40, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular Toxicity of Nicotine: Implications for Electronic Cigarette Use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. How Tobacco Smoke Causes Disease. In The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2010. [Google Scholar]
- Goldstein, A.L.; Faulkner, B.; Wekerle, C. The Relationship among Internal Resilience, Smoking, Alcohol Use, and Depression Symptoms in Emerging Adults Transitioning out of Child Welfare. Child Abuse Negl. 2013, 37, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Kumar, A.; Kaur, J.; Kumari, S.; Sharma, A.K.; Sinha, D.N.; Singh, H.; Mehrotra, R. In-Silico Study of Toxicokinetics and Disease Association of Chemicals Present in Smokeless Tobacco Products. Regul. Toxicol. Pharmacol. 2018, 95, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Shi, L.; Zhu, X.; Lan, M.; Zhang, Q.; Hugh Fan, Z. Electrochemical Sensing of Nicotine Using Screen-Printed Carbon Electrodes Modified with Nitrogen-Doped Graphene Sheets. J. Electroanal. Chem. 2017, 784, 77–84. [Google Scholar] [CrossRef]
- Karthika, A.; Karuppasamy, P.; Selvarajan, S.; Suganthi, A.; Rajarajan, M. Electrochemical Sensing of Nicotine Using CuWO4 Decorated Reduced Graphene Oxide Immobilized Glassy Carbon Electrode. Ultrason. Sonochem. 2019, 55, 196–206. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, S.-M.; Chang, M.-I.; Cho, Y.-J.; Lee, H.-J.; Chae, Y.-S.; Rhee, G.-S. Development of a Method for Analyzing the Nicotine Content in Synthetic Flavoring Substances as Unauthorized E-Cigarette Liquid by Using HPLC. Korean J. Food Sci. Technol. 2013, 45, 693–699. [Google Scholar] [CrossRef][Green Version]
- Vindatiche, I.; Roche, D.; Callais, F.; Lequang, N.T.; Labrousse, F. Analytical Improvements in Barlow Reaction Coupled to HPLC Detection of Nicotine and ITS Metabolites. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 1423–1437. [Google Scholar] [CrossRef]
- Ramírez, N.; Özel, M.Z.; Lewis, A.C.; Marcé, R.M.; Borrull, F.; Hamilton, J.F. Determination of Nicotine and N-Nitrosamines in House Dust by Pressurized Liquid Extraction and Comprehensive Gas Chromatography—Nitrogen Chemiluminiscence Detection. J. Chromatogr. A 2012, 1219, 180–187. [Google Scholar] [CrossRef]
- Baidoo, E.E.K.; Clench, M.R.; Smith, R.F.; Tetler, L.W. Determination of Nicotine and Its Metabolites in Urine by Solid-Phase Extraction and Sample Stacking Capillary Electrophoresis-Mass Spectrometry. J. Chromatogr. B 2003, 796, 303–313. [Google Scholar] [CrossRef]
- Vieira-Brock, P.L.; Miller, E.I.; Nielsen, S.M.; Fleckenstein, A.E.; Wilkins, D.G. Simultaneous Quantification of Nicotine and Metabolites in Rat Brain by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2011, 879, 3465–3474. [Google Scholar] [CrossRef]
- Langone, J.J.; Gjika, H.B.; Van Vunakis, H. Nicotine and Its Metabolites. Radioimmunoassays for Nicotine and Cotinine. Biochemistry 1973, 12, 5025–5030. [Google Scholar] [CrossRef]
- Hossain, A.M.; Salehuddin, S.M. Analytical Determination of Nicotine in Tobacco Leaves by Gas Chromatography–Mass Spectrometry. Arab. J. Chem. 2013, 6, 275–278. [Google Scholar] [CrossRef]
- Shrivas, K.; Patel, D.K. Liquid-Phase Microextraction Combined with Gas Chromatography Mass Spectrometry for Rapid Determination of Nicotine in One-Drop of Nightshades Vegetables and Commercial Food Products. Food Chem. 2010, 122, 314–318. [Google Scholar] [CrossRef]
- Aragón, M.; Marcé, R.M.; Borrull, F. Determination of N-Nitrosamines and Nicotine in Air Particulate Matter Samples by Pressurised Liquid Extraction and Gas Chromatography-Ion Trap Tandem Mass Spectrometry. Talanta 2013, 115, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Vollmer, T.; Martin, C.; Hendig, D.; Knabbe, C. Fast and Sample Cleanup-Free Measurement of Nicotine and Cotinine by Stable Isotope Dilution Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2012, 67–68, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Rodsud, S.; Limbut, W. A Simple Electrochemical Sensor Based on Graphene Nanoplatelets Modified Glassy Carbon Electrode (GrNPs/GCE) for Highly Sensitive Detection of Yohimbine (YOH). J. Electrochem. Soc. 2019, 166, B771–B779. [Google Scholar] [CrossRef]
- Martins, E.C.; Santana, E.R.; Spinelli, A. Nitrogen and Sulfur Co-Doped Graphene Quantum Dot-Modified Electrode for Monitoring of Multivitamins in Energy Drinks. Talanta 2023, 252, 123836. [Google Scholar] [CrossRef]
- Inam, O.; Demir, E.; Uslu, B. Voltammetric Pathways for the Analysis of Ophthalmic Drugs: A Review. Curr. Pharm. Anal. 2020, 16, 367–391. [Google Scholar] [CrossRef]
- Švorc, Ľ.; Stanković, D.M.; Kalcher, K. Boron-Doped Diamond Electrochemical Sensor for Sensitive Determination of Nicotine in Tobacco Products and Anti-Smoking Pharmaceuticals. Diam. Relat. Mater. 2014, 42, 1–7. [Google Scholar] [CrossRef]
- Nagarajan, R.D.; Murugan, P.; Sundramoorthy, A.K. Selective Electrochemical Sensing of NADH and NAD+ Using Graphene/Tungstate Nanocomposite Modified Electrode. ChemistrySelect 2020, 5, 14643–14651. [Google Scholar] [CrossRef]
- Kumar, S.A.; Chen, S.-L.; Chen, S.-M. Electrochemical Sensing of H[Sub 2]O[Sub 2] at Flavin Adenine Dinucleotide/Chitosan/CNT Nanocomposite Modified Electrode. Electrochem. Solid-State Lett. 2010, 13, K83. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Li, Y.; Shen, Y.; Huang, Y. Mesoporous Pd@ Pt Core–Shell Nanoparticles Supported on Multi-Walled Carbon Nanotubes as a Sensing Platform: Application in Simultaneous Electrochemical Detection of Anticancer Drugs Doxorubicin and Dasatinib. Anal. Methods 2019, 11, 443–453. [Google Scholar] [CrossRef]
- Huang, X.-J.; Choi, Y.-K. Chemical Sensors Based on Nanostructured Materials. Sens. Actuators B Chem. 2007, 122, 659–671. [Google Scholar] [CrossRef]
- Rajendran, J.; Kannan, T.S.; Dhanasekaran, L.S.; Murugan, P.; Atchudan, R.; ALOthman, Z.A.; Ouladsmane, M.; Sundramoorthy, A.K. Preparation of 2D Graphene/MXene Nanocomposite for the Electrochemical Determination of Hazardous Bisphenol A in Plastic Products. Chemosphere 2022, 287, 132106. [Google Scholar] [CrossRef]
- Rajendran, J.; Reshetilov, A.N.; Sundramoorthy, A.K. An Electrochemically Exfoliated Graphene/Poly(3,4-Ethylenedioxythiophene) Nanocomposite-Based Electrochemical Sensor for the Detection of Nicotine. Mater. Adv. 2021, 2, 3336–3345. [Google Scholar] [CrossRef]
- Jerome, R.; Sundramoorthy, A.K. Preparation of Hexagonal Boron Nitride Doped Graphene Film Modified Sensor for Selective Electrochemical Detection of Nicotine in Tobacco Sample. Anal. Chim. Acta 2020, 1132, 110–120. [Google Scholar] [CrossRef]
- Murugan, N.; Kumar, T.H.V.; Devi, N.R.; Sundramoorthy, A.K. A Flower-Structured MoS2-Decorated f-MWCNTs/ZnO Hybrid Nanocomposite-Modified Sensor for the Selective Electrochemical Detection of Vitamin C. New J. Chem. 2019, 43, 15105–15114. [Google Scholar] [CrossRef]
- Zribi, R.; Neri, G. Mo-Based Layered Nanostructures for the Electrochemical Sensing of Biomolecules. Sensors 2020, 20, 5404. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Catania, F.; Marras, E.; Giorcelli, M.; Jagdale, P.; Lavagna, L.; Tagliaferro, A.; Bartoli, M. A Review on Recent Advancements of Graphene and Graphene-Related Materials in Biological Applications. Appl. Sci. 2021, 11, 614. [Google Scholar] [CrossRef]
- Rubio, N.; Au, H.; Coulter, G.O.; Guetaz, L.; Gebel, G.; Mattevi, C.; Shaffer, M.S.P. Effect of Graphene Flake Size on Functionalisation: Quantifying Reaction Extent and Imaging Locus with Single Pt Atom Tags. Chem. Sci. 2021, 12, 14907–14919. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.G.; Shekhirev, M.; Wyatt, B.C.; Anasori, B.; Gogotsi, Y.; Seh, Z.W. Fundamentals of MXene Synthesis. Nat. Synth. 2022, 1, 601–614. [Google Scholar] [CrossRef]
- Rajendran, J.; Sundramoorthy, A.K.; Ganapathy, D.; Atchudan, R.; Habila, M.A.; Nallaswamy, D. 2D MXene/Graphene Nanocomposite Preparation and Its Electrochemical Performance towards the Identification of Nicotine Level in Human Saliva. J. Hazard. Mater. 2022, 440, 129705. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in Energy Conversion and Storage Systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Huang, W.-X.; Li, Z.-P.; Li, D.-D.; Hu, Z.-H.; Wu, C.; Lv, K.-L.; Li, Q. Ti3C2 MXene: Recent Progress in Its Fundamentals, Synthesis, and Applications. Rare Met. 2022, 41, 3268–3300. [Google Scholar] [CrossRef]
- Wang, Q.; Han, N.; Shen, Z.; Li, X.; Chen, Z.; Cao, Y.; Si, W.; Wang, F.; Ni, B.-J.; Thakur, V.K. MXene-Based Electrochemical (Bio) Sensors for Sustainable Applications: Roadmap for Future Advanced Materials. Nano Mater. Sci. 2022; in press. [Google Scholar] [CrossRef]
- Khazaei, M.; Mishra, A.; Venkataramanan, N.S.; Singh, A.K.; Yunoki, S. Recent Advances in MXenes: From Fundamentals to Applications. Curr. Opin. Solid State Mater. Sci. 2019, 23, 164–178. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, H.; Hu, D.; Li, L.-L.; Jin, J.; Ai, M.-J.; Wei, J.; Song, K. Recent Advances in Electrochemical Sensors Based on Palladium Nanoparticles. Chin. J. Anal. Chem. 2022, 50, 100144. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Z.; Huang, Z.; Oyama, M.; Jiang, Y.; Chen, X. Ultrafine Palladium Nanoparticles Grown on Graphene Nanosheets for Enhanced Electrochemical Sensing of Hydrogen Peroxide. Electrochim. Acta 2013, 97, 398–403. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-Titanium Carbide (MXene) Based Selective Electrochemical Sensor for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Challagulla, S.; Tarafder, K.; Ganesan, R.; Roy, S. Structure Sensitive Photocatalytic Reduction of Nitroarenes over TiO2. Sci. Rep. 2017, 7, 8783. [Google Scholar] [CrossRef]
- Hu, T.; Wang, J.; Zhang, H.; Li, Z.; Hu, M.; Wang, X. Vibrational Properties of Ti3C2 and Ti3C2T2 (T = O, F, OH) Monosheets by First-Principles Calculations: A Comparative Study. Phys. Chem. Chem. Phys. 2015, 17, 9997–10003. [Google Scholar] [CrossRef]
- Zhang, L.; Su, W.; Huang, Y.; Li, H.; Fu, L.; Song, K.; Huang, X.; Yu, J.; Lin, C.-T. In Situ High-Pressure X-ray Diffraction and Raman Spectroscopy Study of Ti3C2Tx MXene. Nanoscale Res. Lett. 2018, 13, 343. [Google Scholar] [CrossRef]
- Armano, A.; Agnello, S. Two-Dimensional Carbon: A Review of Synthesis Methods, and Electronic, Optical, and Vibrational Properties of Single-Layer Graphene. C J. Carbon Res. 2019, 5, 67. [Google Scholar] [CrossRef]
- Al-Sherbini, A.-S.; Bakr, M.; Ghoneim, I.; Saad, M. Exfoliation of Graphene Sheets via High Energy Wet Milling of Graphite in 2-Ethylhexanol and Kerosene. J. Adv. Res. 2017, 8, 209–215. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, R.; Jia, J.; Wu, C.; Zhou, A.; Xu, J.; Zhang, X. Enhanced Supercapacitive Performance of Delaminated Two-Dimensional Titanium Carbide/Carbon Nanotube Composites in Alkaline Electrolyte. J. Power Sources 2015, 284, 38–43. [Google Scholar] [CrossRef]
- Raj, S.M.M.; Sundramoorthy, A.K.; Atchudan, R.; Ganapathy, D.; Khosla, A. Review—Recent Trends on the Synthesis and Different Characterization Tools for MXenes and Their Emerging Applications. J. Electrochem. Soc. 2022, 169, 77501. [Google Scholar] [CrossRef]
- Applestone, D.; Manthiram, A. Cu6Sn5–TiC–C Nanocomposite Alloy Anodes with High Volumetric Capacity for Lithium Ion Batteries. RSC Adv. 2012, 2, 5411–5417. [Google Scholar] [CrossRef]
- Qi, B.; Di, L.-B.; Xu, W.; Zhang, X. Dry Plasma Reduction to Prepare a High Performance Pd/C Catalyst at Atmospheric Pressure for CO Oxidation. J. Mater. Chem. A 2014, 2, 11885. [Google Scholar] [CrossRef]
- Molaei, R.; Farhadi, K.; Forough, M.; Hajizadeh, S. Green Biological Fabrication and Characterization of Highly Monodisperse Palladium Nanoparticles Using Pistacia Atlantica Fruit Broth. J. Nanostruct. 2018, 8, 47–54. [Google Scholar] [CrossRef]
- Mahmood, M.; Rasheed, A.; Ayman, I.; Rasheed, T.; Munir, S.; Ajmal, S.; Agboola, P.; Warsi, M.; Shahid, M. Synthesis of Ultrathin MnO 2 Nanowire-Intercalated 2D-MXenes for High-Performance Hybrid Supercapacitors. Energy Fuels 2021, 35, 3469–3478. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Xu, A.; Weng, Y.; Zhao, R. Permeability and Equivalent Circuit Model of Ionically Conductive Mortar Using Electrochemical Workstation. Materials 2020, 13, 1179. [Google Scholar] [CrossRef] [PubMed]
- Mehmeti, E.; Kilic, T.; Laur, C.; Carrara, S. Electrochemical Determination of Nicotine in Smokers’ Sweat. Microchem. J. 2020, 158, 105155. [Google Scholar] [CrossRef]
- Suffredini, H.B.; Santos, M.C.; De Souza, D.; Codognoto, L.; Homem-de-Mello, P.; Honório, K.M.; da Silva, A.B.F.; Machado, S.A.S.; Avaca, L.A. Electrochemical Behavior of Nicotine Studied by Voltammetric Techniques at Boron-Doped Diamond Electrodes. Anal. Lett. 2005, 38, 1587–1599. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Papaefthimiou, S.; Yianoulis, P. Dependence of the Estimated Diffusion Coefficient of LixWO3 Films on the Scan Rate of Cyclic Voltammetry Experiments. Solid State Ion. 2007, 178, 259–263. [Google Scholar] [CrossRef]
- Opitz, M.; Yue, J.; Wallauer, J.; Smarsly, B.; Roling, B. Mechanisms of Charge Storage in Nanoparticulate TiO2 and Li4Ti5O12 Anodes: New Insights from Scan Rate-Dependent Cyclic Voltammetry. Electrochim. Acta 2015, 168, 125–132. [Google Scholar] [CrossRef]
- Abd-Elsabour, M.; Alsoghier, H.M.; Alhamzani, A.G.; Abou-Krisha, M.M.; Yousef, T.A.; Assaf, H.F. A Novel Electrochemical Sensor for Detection of Nicotine in Tobacco Products Based on Graphene Oxide Nanosheets Conjugated with (1,2-Naphthoquinone-4-Sulphonic Acid) Modified Glassy Carbon Electrode. Nanomaterials 2022, 12, 2354. [Google Scholar] [CrossRef]
- Fekry, A.M.; Azab, S.M.; Shehata, M.; Ameer, M.A. A Novel Electrochemical Nicotine Sensor Based on Cerium Nanoparticles with Anionic Surfactant. RSC Adv. 2015, 5, 51662–51671. [Google Scholar] [CrossRef]
- Cavalheiro, É.T.G.; Brajter-Toth, A. Amperometric Determination of Xanthine and Hypoxanthine at Carbon Electrodes. Effect of Surface Activity and the Instrumental Parameters on the Sensitivity and the Limit of Detection. J. Pharm. Biomed. Anal. 1999, 19, 217–230. [Google Scholar] [CrossRef]
- Wu, C.-T.; Chen, P.-Y.; Chen, J.-G.; Suryanarayanan, V.; Ho, K.-C. Detection of Nicotine Based on Molecularly Imprinted TiO2-Modified Electrodes. Anal. Chim. Acta 2009, 633, 119–126. [Google Scholar] [CrossRef]
- Wang, S.-J.; Liaw, H.-W.; Tsai, Y.-C. Low Potential Detection of Nicotine at Multiwalled Carbon Nanotube–Alumina-Coated Silica Nanocomposite. Electrochem. Commun. 2009, 11, 733–735. [Google Scholar] [CrossRef]
- Xiong, H.; Zhao, Y.; Liu, P.; Zhang, X.; Wang, S. Electrochemical Properties and the Determination of Nicotine at a Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode. Microchim. Acta 2010, 168, 31–36. [Google Scholar] [CrossRef]
- Sims, M.J.; Rees, N.V.; Dickinson, E.J.F.; Compton, R.G. Effects of Thin-Layer Diffusion in the Electrochemical Detection of Nicotine on Basal Plane Pyrolytic Graphite (BPPG) Electrodes Modified with Layers of Multi-Walled Carbon Nanotubes (MWCNT-BPPG). Sens. Actuators B Chem. 2010, 144, 153–158. [Google Scholar] [CrossRef]
- Shehata, M.; Azab, S.M.; Fekry, A.M.; Ameer, M.A. Nano-TiO2 Modified Carbon Paste Sensor for Electrochemical Nicotine Detection Using Anionic Surfactant. Biosens. Bioelectron. 2016, 79, 589–592. [Google Scholar] [CrossRef]
- Lo, T.W.B.; Aldous, L.; Compton, R.G. The Use of Nano-Carbon as an Alternative to Multi-Walled Carbon Nanotubes in Modified Electrodes for Adsorptive Stripping Voltammetry. Sens. Actuators B Chem. 2012, 162, 361–368. [Google Scholar] [CrossRef]
- Highton, L.; Kadara, R.O.; Jenkinson, N.; Logan Riehl, B.; Banks, C.E. Metallic Free Carbon Nanotube Cluster Modified Screen Printed Electrodes for the Sensing of Nicotine in Artificial Saliva. Electroanalysis 2009, 21, 2387–2389. [Google Scholar] [CrossRef]
- Geto, A.; Amare, M.; Tessema, M.; Admassie, S. Voltammetric Determination of Nicotine at Poly(4-Amino-3-Hydroxynaphthalene Sulfonic Acid)-Modified Glassy Carbon Electrode. Electroanalysis 2012, 24, 659–665. [Google Scholar] [CrossRef]
- Levent, A.; Yardim, Y.; Senturk, Z. Voltammetric Behavior of Nicotine at Pencil Graphite Electrode and Its Enhancement Determination in the Presence of Anionic Surfactant. Electrochim. Acta 2009, 55, 190–195. [Google Scholar] [CrossRef]
- Kassa, H.; Geto, A.; Admassie, S. Voltammetric Determination of Nicotine in Cigarette Tobacco at Electrochemically Activated Glassy Carbon Electrode. Bull. Chem. Soc. Ethiop. 2013, 27, 321–328. [Google Scholar] [CrossRef]
- Papadoyannis, I.N.; Samanidou, V.F.; Stefanidou, P.G. Clinical Assay of Nicotine and Its Metabolite, Cotinine, in Body Fluids by HPLC Following Solid Phase Extraction. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 2315–2335. [Google Scholar] [CrossRef]




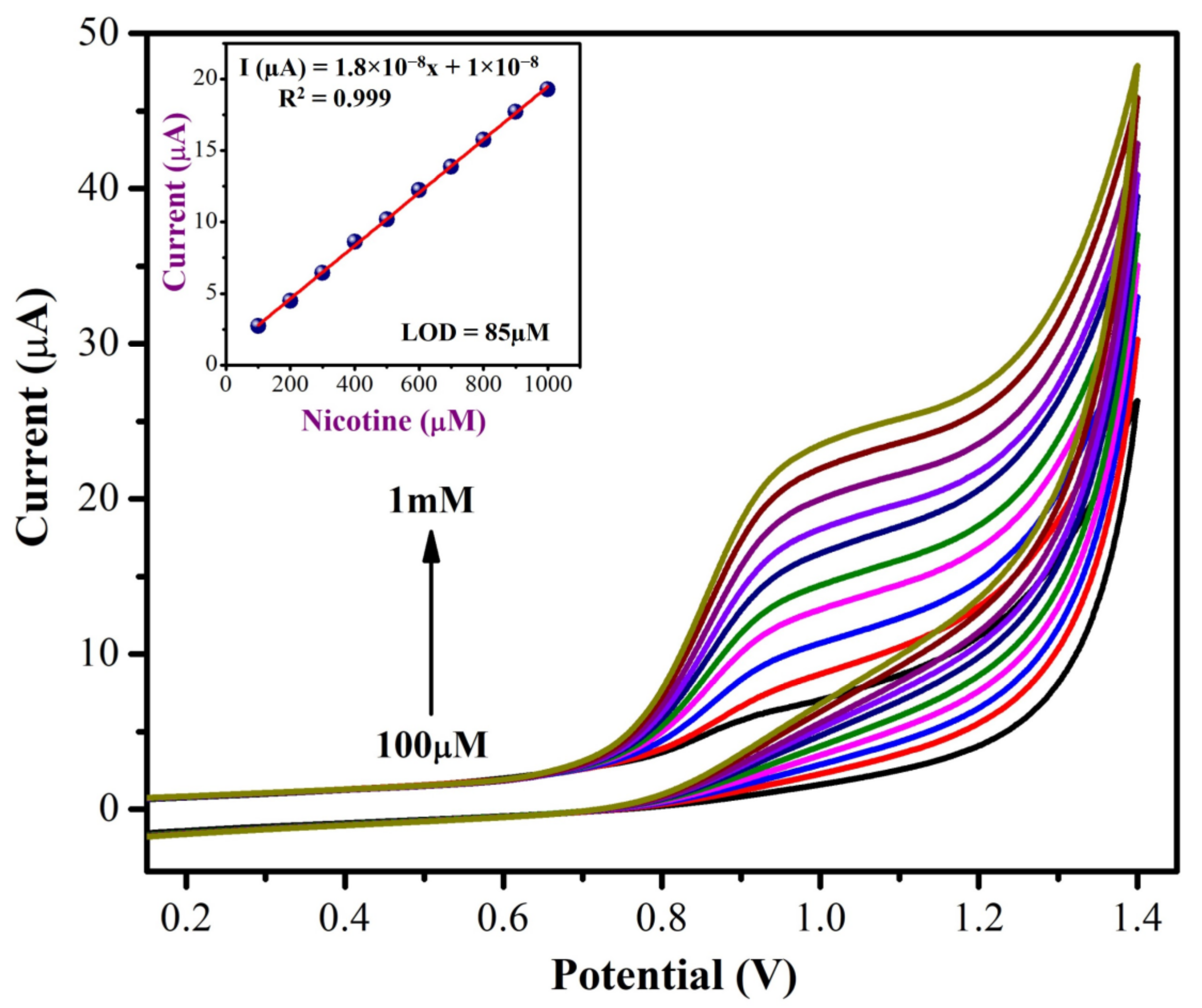



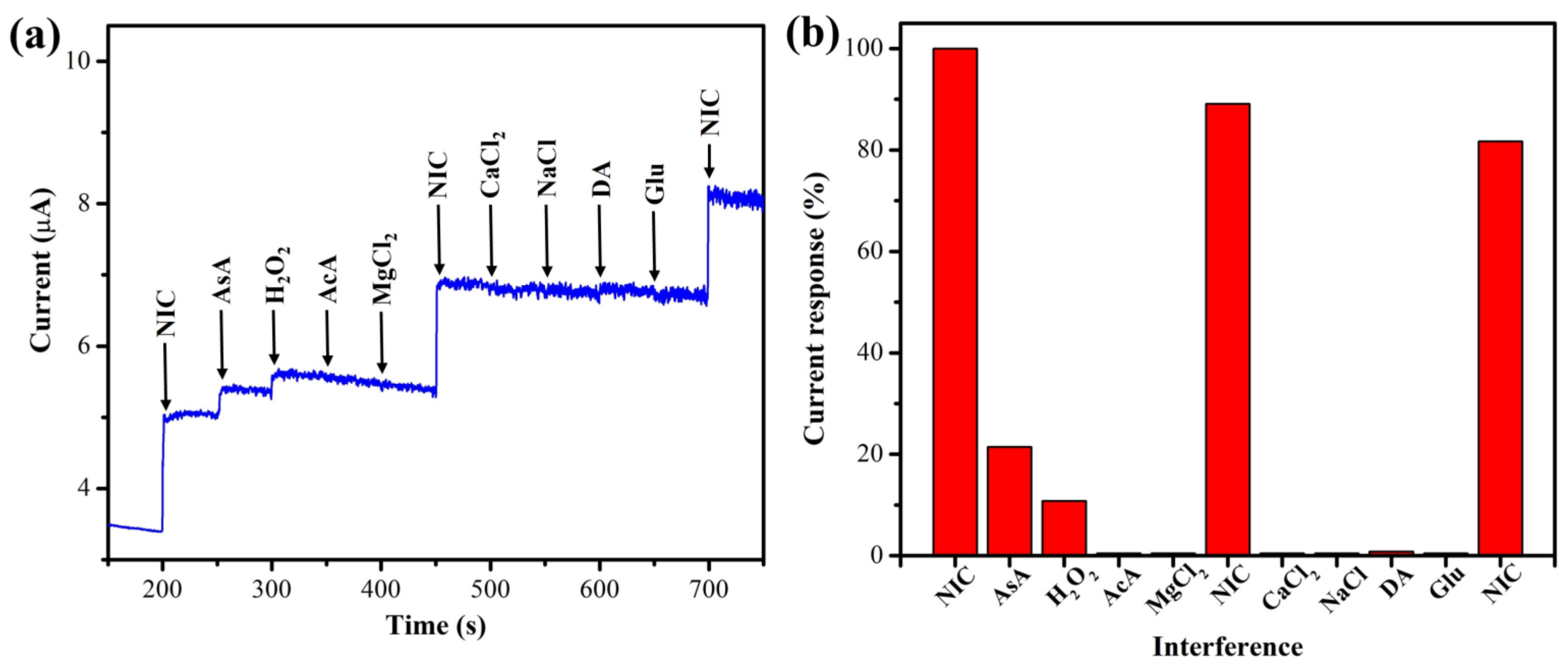
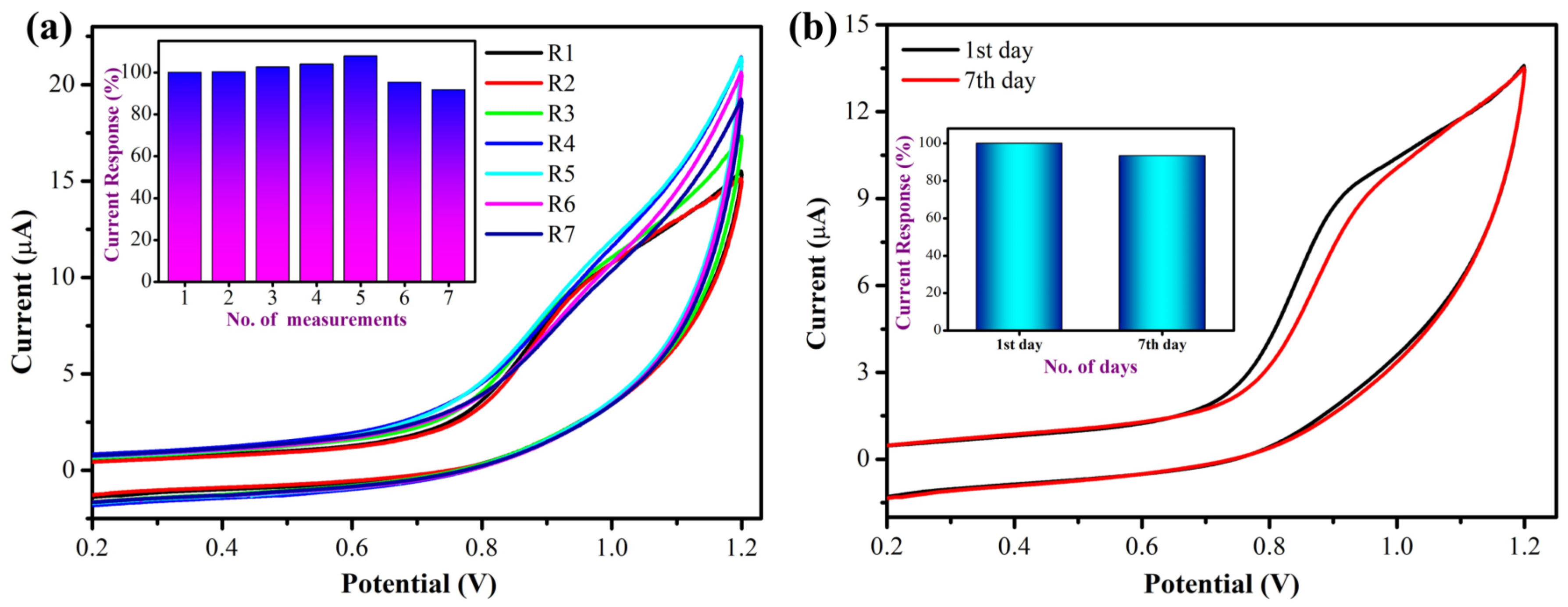
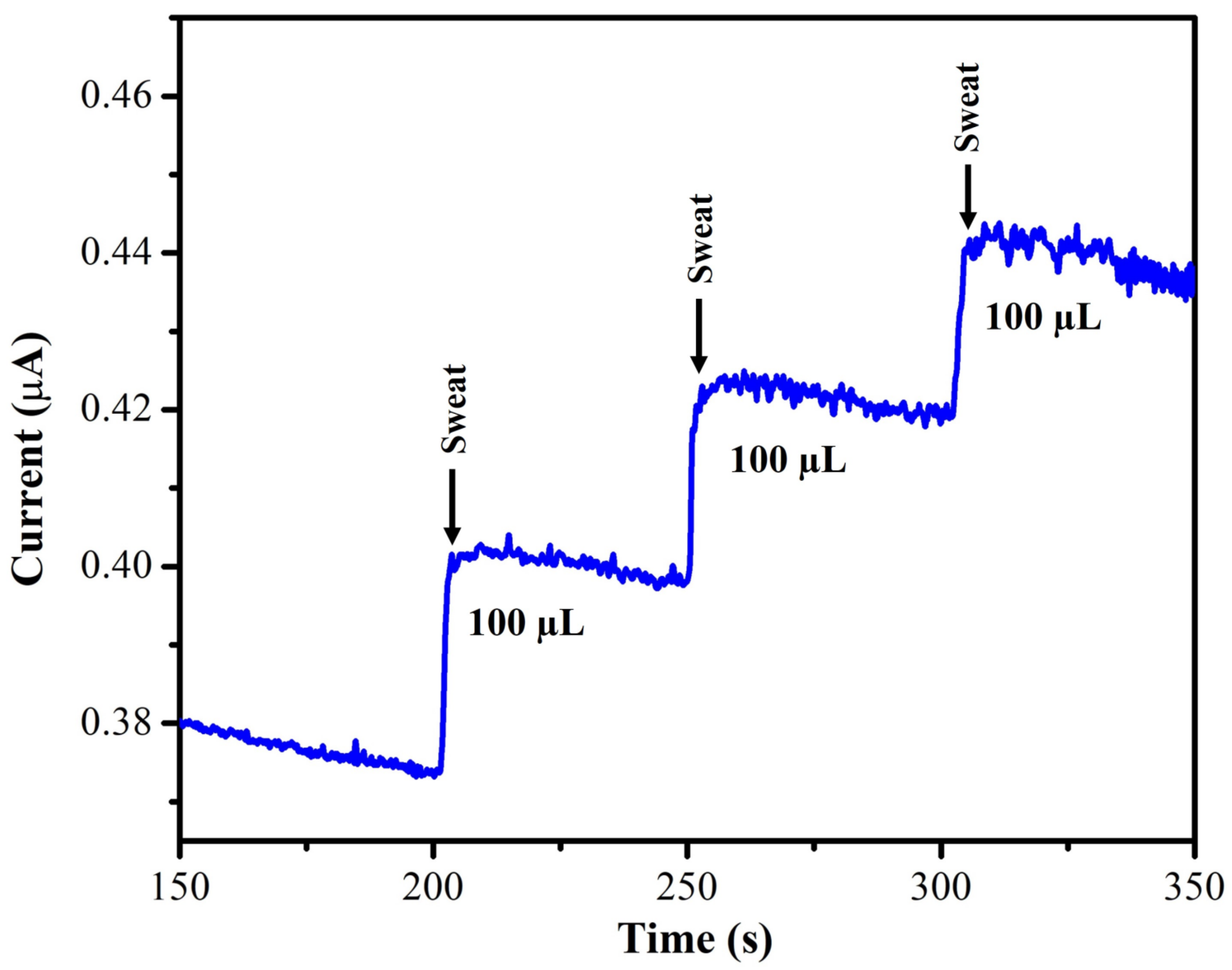

| Electrode | Catalyst | Electrolyte | Epa (V) | Technique | Linear Range (µM) | LOD (µM) | Test Sample | Reference |
|---|---|---|---|---|---|---|---|---|
| SPCE | N-GrS | PB, pH = 7.4 | 0.85 | CV | 0–200 | 0.047 | Cigarettes/Urine | [6] |
| GCE | CuWO4/rGO/Nf | PB, pH = 7 | 1.1 | CV | 0.1–0.9 | 0.035 | Cigarettes/Urine | [7] |
| BDD | - | BRB, pH = 8 | 1.35 | DPV | 0.5–202.5 | 0.3 | Tobacco products/Pharmaceuticals | [22] |
| GCE | HBN/Gr | PB, pH = 7.4 | 0.97 | AMP | 1–1000 | 0.42 | Tobacco samples | [29] |
| SPCE | - | PB, pH = 7.5 | 0.9 | DPV | 1–375 | 0.6 | Sweat | [57] |
| CPE | Ce-NPs | BRB, pH = 2 | 1 | CV | 4–500 | 0.0943 | Cigarettes | [62] |
| GCE | TiO2/PEDOT | PB, pH = 7.4 | 0.88 | AMP | 0–5000 | 4.9 | - | [64] |
| GCE | MWCNT/ACS | PB, pH = 8 | 0.65 | AMP | 5–1395 | 1.42 | - | [65] |
| GCE | MWCNT | Na2C2O4, pH = 4.5 | 1.4 | DPV | 31–1900 | 9.3 | Cigarettes | [66] |
| BPPGE | MWCNT | PB, pH = 8 | 0.65 | CV | 0–1000 | 1.5 | - | [67] |
| CPE | TiO2 | BRB, pH = 5 | 0.87 | CV/DPV | 2–540 | 0.0134 | Cigarettes/Urine | [68] |
| GCE | MWCNT/NCB | PB, pH = 7.4 | 0.72 | CV | 10–50 | 5 | - | [69] |
| SPE | CNC | PB, pH = 7 | 0.75 | CV | 10–1000 | 2 | Artificial Saliva | [70] |
| GCE | P-AHNSA | PB, pH = 7.5 | 0.88 | SWV | 1–200 | 0.866 | Cigarettes | [71] |
| PGE | SDS (surfactant) | PB, pH = 7 | 0.84 | SWV | 7.6–107.5 | 2 | Cigarettes | [72] |
| A-GCE | – | PB, pH = 7 | 0.83 | SWV | 1–200 | 0.7 | Cigarettes | [73] |
| GCE | MXene/PHC | PB, pH = 7.4 | 1 | CV/AMP | 0.25–37.5 | 0.027 | Human sweat | Current work |
| S.No. | Samples | Added (µM) | Found * (µM) | Recovery (%) |
|---|---|---|---|---|
| 1. | Human Sweat | - | 0.91 | - |
| 2. | Std. NIC | 1.0 | 1.88 | 97.0 |
| 3. | Std. NIC | 2.0 | 2.89 | 99.0 |
| 4. | Std. NIC | 4.0 | 5.05 | 103.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magesh, V.; Sundramoorthy, A.K.; Ganapathy, D.; Atchudan, R.; Arya, S.; Alshgari, R.A.; Aljuwayid, A.M. Palladium Hydroxide (Pearlman’s Catalyst) Doped MXene (Ti3C2Tx) Composite Modified Electrode for Selective Detection of Nicotine in Human Sweat. Biosensors 2023, 13, 54. https://doi.org/10.3390/bios13010054
Magesh V, Sundramoorthy AK, Ganapathy D, Atchudan R, Arya S, Alshgari RA, Aljuwayid AM. Palladium Hydroxide (Pearlman’s Catalyst) Doped MXene (Ti3C2Tx) Composite Modified Electrode for Selective Detection of Nicotine in Human Sweat. Biosensors. 2023; 13(1):54. https://doi.org/10.3390/bios13010054
Chicago/Turabian StyleMagesh, Vasanth, Ashok K. Sundramoorthy, Dhanraj Ganapathy, Raji Atchudan, Sandeep Arya, Razan A. Alshgari, and Ahmed Muteb Aljuwayid. 2023. "Palladium Hydroxide (Pearlman’s Catalyst) Doped MXene (Ti3C2Tx) Composite Modified Electrode for Selective Detection of Nicotine in Human Sweat" Biosensors 13, no. 1: 54. https://doi.org/10.3390/bios13010054
APA StyleMagesh, V., Sundramoorthy, A. K., Ganapathy, D., Atchudan, R., Arya, S., Alshgari, R. A., & Aljuwayid, A. M. (2023). Palladium Hydroxide (Pearlman’s Catalyst) Doped MXene (Ti3C2Tx) Composite Modified Electrode for Selective Detection of Nicotine in Human Sweat. Biosensors, 13(1), 54. https://doi.org/10.3390/bios13010054






