Ratiometric Fluorescence Immunoassay Based on Carbon Quantum Dots for Sensitive Detection of Malachite Green in Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of N-CQDs from Wolfberry
2.3. N-CQDs Characterization
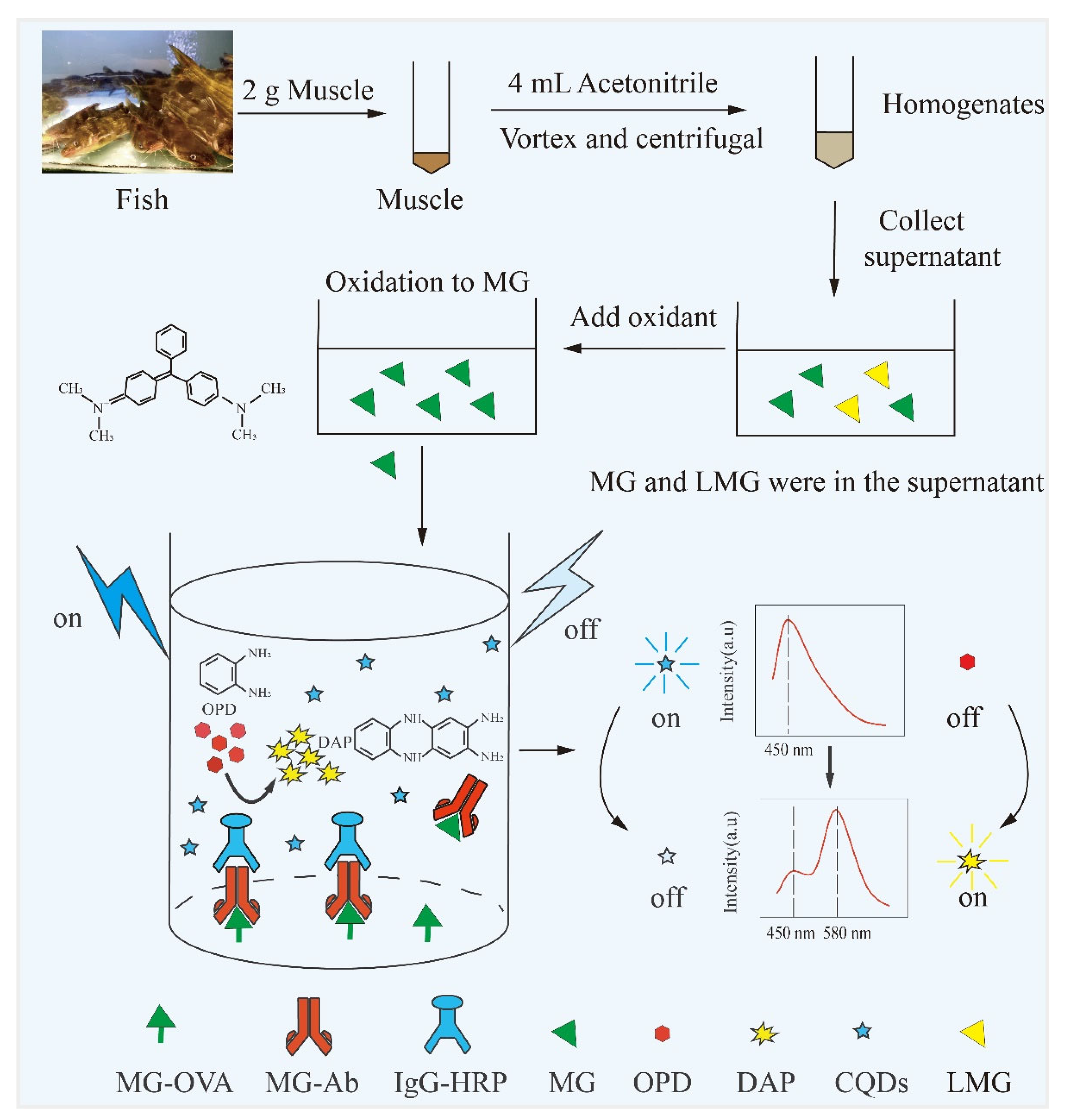
2.4. Development of Immunoassay
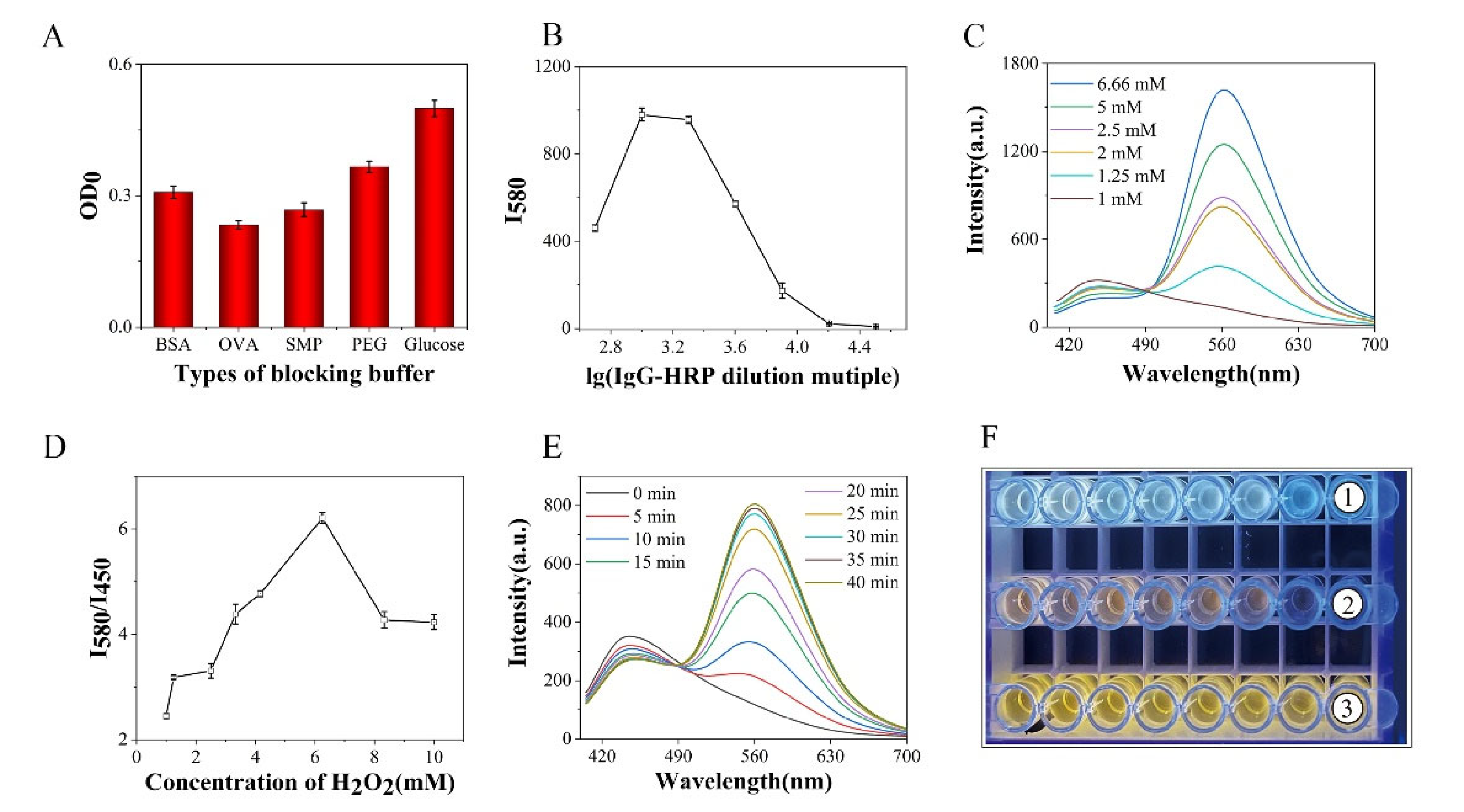
2.5. Optimization of Experimental Conditions
2.6. Antibody Specificity Analysis
2.7. Sample Preparation
3. Results and Discussion
3.1. Characterizations of N-CQDs
3.2. Evaluation of the Sensing Mechanism
3.3. Optimization of Experimental Conditions
3.4. Detection of MG
3.5. Specificity Test
3.6. Spiking and Real Sample Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, P.H.; Feng, J.X.; Wei, Y.P.; Xiao, J.Y.; Li, J.H.; He, Q.G. Fast and Ultrasensitive Trace Malachite Green Detection in Aquaculture and Fisheries by Using Hexadecylpyridinium Bromide Modified Electrochemical Sensor. J. Food Compos. Anal. 2021, 102, 104003. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Hong, C.Y.; Lin, Z.Z.; Huang, Z.Y. Rapid Detection of Malachite Green in Fish and Water Based on the Peroxidase-Like Activity of Fe3o4nps Enhanced with Aptamer. J. Food Compos. Anal. 2021, 104, 104162. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wu, R.Q.; Zhou, Y.H.; Lin, Q.L.; Fang, C.Q. A Novel Surface-Oxidized Rigid Carbon Foam with Hierarchical Macro-Nanoporous Structure for Efficient Removal of Malachite Green and Lead Ion. J. Mater. Sci. Technol. 2022, 103, 15–28. [Google Scholar] [CrossRef]
- Hu, Q.; Cui, Y.K.; Zhang, L.F.; Qian, M.J.; Xiao, L.X.; Yang, M.; Yang, Z.Q.; Rao, S.Q.; Gong, X.J.; Han, J. An Ultrasensitive Analytical Strategy for Malachite Green Determination in Fish Samples Based on Bright Orange-Emissive Carbon Dots. J. Food Compos. Anal. 2021, 102, 104032. [Google Scholar] [CrossRef]
- Gao, Z.X.; Li, Y.Y.; Ma, Y.L.; Ji, W.X.; Chen, T.; Ma Xi, X.; Xu, H. Functionalized Melamine Sponge Based on Β-Cyclodextrin-Graphene Oxide as Solid-Phase Extraction Material for Rapidly Pre-Enrichment of Malachite Green in Seafood. Microchem. J. 2019, 150, 104167. [Google Scholar] [CrossRef]
- Nebot, C.; Iglesias, A.; Barreiro, R.; Miranda, J.M.; Vázquez, B.; Franco, C.M.; Cepeda, A. A Simple and Rapid Method for the Identification and Quantification of Malachite Green and Its Metabolite in Hake by Hplc–Ms/Ms. Food Control 2013, 31, 102–107. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Munoz, G.; Vo Duy, S.; Do, D.T.; Bayen, S.; Sauvé, S. Analysis of Sulfonamides, Fluoroquinolones, Tetracyclines, Triphenylmethane Dyes and Other Veterinary Drug Residues in Cultured and Wild Seafood Sold in Montreal, Canada. J. Food Compos. Anal. 2020, 94, 103603. [Google Scholar] [CrossRef]
- Xu, D.P.; Zhang, Y.F.; Zhang, S.; Yang, W.; Chen, J. Ultrasensitive Sers Detection of Crystal Violet and Malachite Green Based on High Surface Roughness Copper Nanocorns Prepared Via Solid-State Ionics Method. Sensor. Actuat. A-phys. 2021, 331, 113042. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.; Zhang, Y.; Kang, Y.; Hu, H.L.; Iqbal, J.R.; Du, Y.P. Rapid Determination of Illegal Additives Chrysoidin and Malachite Green by Surface-Enhanced Raman Scattering with Silanized Support Based Substrate. Chin. Chem. Lett. 2018, 29, 981–984. [Google Scholar] [CrossRef]
- Dong, J.X.; Li, Z.F.; Wang, Y.; Jin, M.J.; Shen, Y.D.; Xu, Z.L.; Abd El-Aty, A.M.; Gee, S.J.; Hammock, B.D.; Sun, Y.M.; et al. Generation of Functional Single-Chain Fragment Variable from Hybridoma and Development of Chemiluminescence Enzyme Immunoassay for Determination of Total Malachite Green in Tilapia Fish. Food Chem. 2021, 337, 127780. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z.J.; Luo, J.F. Fluorescence Detection of Malachite Green in Fish Tissue Using Red Emissive Se,N,Cl-Doped Carbon Dots. Food Chem. 2021, 335, 127677. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.Y.; Na, L.H.; Li, Y.M.; Bai, W.F.; Zhang, J.P.; Jin, L. N,S-Gqds Mixed with Cdte Quantum Dots for Ratiometric Fluorescence Visual Detection and Quantitative Analysis of Malachite Green in Fish. Food Chem. 2022, 390, 133156. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.Y.; Li, Y.; Xu, S.; Li, J.G.; Li, M.; Jiang, L.Y.; Jie, M.S.; Bai, Y.H. A Portable Smartphone-Assisted Ratiometric Fluorescence Sensor for Intelligent and Visual Detection of Malachite Green. Food Chem. 2022, 371, 131164. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.B.; Zhang, Y.; Ren, X.H.; He, X.W.; Li, W.Y.; Zhang, Y.K. Dual-Reverse-Signal Ratiometric Fluorescence Method for Malachite Green Detection Based on Multi-Mechanism Synergistic Effect. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 276, 121196. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, D.; Zhang, G.Y.; Zhang, D.; Shi, X.B. Recent Improvements in Enzyme-Linked Immunosorbent Assays Based on Nanomaterials. Talanta 2021, 223, 121722. [Google Scholar] [CrossRef]
- Li, G.M.; Liu, C.; Zhang, X.C.; Luo, P.J.; Lin, G.M.; Jiang, W.X. Highly Photoluminescent Carbon Dots-Based Immunosensors for Ultrasensitive Detection of Aflatoxin M(1) Residues in Milk. Food Chem. 2021, 355, 129443. [Google Scholar] [CrossRef]
- Liu, Z.J.; Wang, X.Y.; Ren, X.X.; Li, W.B.; Sun, J.F.; Wang, X.W.; Huang, Y.Q.; Guo, Y.G.; Zeng, H.W. Novel Fluorescence Immunoassay for the Detection of Zearalenone Using Hrp-Mediated Fluorescence Quenching of Gold-Silver Bimetallic Nanoclusters. Food Chem. 2021, 355, 129633. [Google Scholar] [CrossRef]
- Shi, Y.H.; Sun, Y.H.; Qu, X.W.; Zhou, L.; Yue, T.L.; Yuan, Y.H. Preparation of Species-Specific Monoclonal Antibody and Development of Fluorescence Immunoassay Based on Fluorescence Resonance Energy Transfer of Carbon Dots for Accurate and Sensitive Detection of Alicyclobacillus Acidoterrestris in Apple Juice. Food Chem. 2021, 347, 129069. [Google Scholar] [CrossRef]
- Ge, Y.Q.; Zheng, X.L.; Ji, R.X.; Shen, S.L.; Cao, X.Q. A New Pyrido[1,2-a]Benzimidazole-Rhodamine Fret System as an Efficient Ratiometric Fluorescent Probe for Cu2+ in Living Cells. Anal. Chim. Acta. 2017, 965, 103–110. [Google Scholar] [CrossRef]
- Ju, Y.J.; Li, N.; Liu, S.G.; Han, L.; Xiao, N.; Luo, H.Q.; Li, N.B. Ratiometric Fluorescence Method for Malachite Green Detection Based on Dual-Emission Bsa-Protected Gold Nanoclusters. Sensor. Actuat. B-Chem. 2018, 275, 244–250. [Google Scholar] [CrossRef]
- Mehta, V.N.; Desai, M.L.; Basu, H.; Singhal, R.K.; Kailasa, S.K. Recent Developments on Fluorescent Hybrid Nanomaterials for Metal Ions Sensing and Bioimaging Applications: A Review. J. Mol. Liq. 2021, 333, 115950. [Google Scholar] [CrossRef]
- Atabaev, T.S. Doped Carbon Dots for Sensing and Bioimaging Applications: A Minireview. Nanomaterials 2018, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, J.S.; Zhang, P.; Niu, X.Q.; Zhao, W.; Zhu, Z.Y.; Ding, H.; Xiong, H.M. Red-Emissive Carbon Dots for Fingerprints Detection by Spray Method: Coffee Ring Effect and Unquenched Fluorescence in Drying Process. ACS Appl. Mater. Inter. 2017, 9, 18429–18433. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Shen, D.K.; Liu, Q.; Wu, C.F.; Gu, S. Sustainable Synthesis of Bright Green Fluorescent Carbon Quantum Dots from Lignin for Highly Sensitive Detection of Fe3+ Ions. Appl. Surf. Sci. 2021, 565, 150526. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Hua, Y.; Zhang, M.; Zhou, Z.; Yuan, J.; Wang, J.; Peng, W.; Zhang, L.; Xia, S.; et al. Multicolor Nitrogen-Doped Carbon Dots for Live Cell Imaging. J. Biomed. Nanotechnol. 2015, 11, 780–788. [Google Scholar] [CrossRef]
- Yang, H.; Long, Y.W.; Li, H.X.; Pan, S.; Liu, H.; Yang, J.D.; Hu, X.L. Carbon Dots Synthesized by Hydrothermal Process Via Sodium Citrate and Nh4hco3 for Sensitive Detection of Temperature and Sunset Yellow. J. Colloid Interface Sci. 2018, 516, 192–201. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, J.R.; Yang, G.X.; Tang, Y.Y.; Zhang, X.; Huang, X.Y.; Zhai, W.L.; Fodjo, E.K.; Kong, C. Green Preparation of Carbon Quantum Dots with Wolfberry as on-Off-on Nanosensors for the Detection of Fe(3+) and L-Ascorbic Acid. Food Chem. 2022, 376, 131898. [Google Scholar] [CrossRef]
- Latief, U.; Ul Islam, S.; Khan, Z.; Khan, M.S. A Facile Green Synthesis of Functionalized Carbon Quantum Dots as Fluorescent Probes for a Highly Selective and Sensitive Detection of Fe(3+) Ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 262, 120132. [Google Scholar] [CrossRef]
- Yang, X.P.; Wang, D.; Luo, N.; Feng, M.; Peng, X.H.; Liao, X.J. Green Synthesis of Fluorescent N,S-Carbon Dots from Bamboo Leaf and the Interaction with Nitrophenol Compounds. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 239, 118462. [Google Scholar] [CrossRef]
- Yuan, C.L.; Qin, X.; Xu, Y.J.; Shi, R.; Cheng, S.Q.; Wang, Y.L. Dual-Signal Uric Acid Sensing Based on Carbon Quantum Dots and O-Phenylenediamine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 254, 119678. [Google Scholar] [CrossRef]
- Nicell, J.A.; Wright, H. A Model of Peroxidase Activity with Inhibition by Hydrogen Peroxide. Enzym. Microb. Technol. 1997, 21, 302–310. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China, Announcement of the Ministry of Agriculture of the People’s Republic of China No. 250. Available online: http://www.moa.gov.cn/gk/tzgg_1/gg/202001/t20200106_6334375.htm/ (accessed on 6 January 2020).
- European Commission. Commission Decision of 17 June 2008 amending Decision 2002/994/EC Concerning Certain Protective Measures with Regard to the Products of Animal Origin Imported from China. Available online: http://data.europa.eu/eli/dec/2008/463/oj (accessed on 17 June 2008).
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed; Document No. SANTE/11312/2021; European Union: Mestreech, The Netherlands, 2022. [Google Scholar]
- Schneider, M.J.; Andersen, W.C. Determination of Triphenylmethane Dyes and Their Metabolites in Salmon, Catfish, and Shrimp by Lc-Ms/Ms Using Aoac First Action Method 2012.25: Collaborative Study. J. Aoac. Int. 2015, 98, 658–670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, R.; Singh, R.K. Detection of Malachite Green in Water Using Edge Excited Label Free Fluorescent Probe Ncqds. J. Fluoresc. 2020, 30, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lin, Z.Z.; Zhong, H.P.; Peng, A.H.; Chen, X.M.; Huang, Z.Y. Rapid Detection of Malachite Green in Fish Based on Cdte Quantum Dots Coated with Molecularly Imprinted Silica. Food Chem. 2017, 229, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, A.H.; Lin, Z.Z.; Zhong, H.P.; Chen, X.M.; Huang, Z.Y. Biomimetic Elisa Detection of Malachite Green Based on Molecularly Imprinted Polymer Film. Food Chem. 2017, 229, 403–408. [Google Scholar] [CrossRef]
- Ran, H.; Lin, Z.Z.; Yao, Q.H.; Hong, C.Y.; Huang, Z.Y. Ratiometric Fluorescence Probe of Mips@Cdte Qds for Trace Malachite Green Detection in Fish. Anal. Bioanal. Chem. 2019, 411, 537–544. [Google Scholar] [CrossRef]


| Interferents | Structural Formula | CR (%) |
|---|---|---|
| MG |  | 100 |
| LMG | 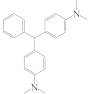 | 1.14 |
| CV | 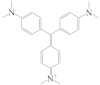 | 121 |
| LCV |  | 2.34 |
| MB | 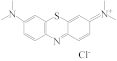 | 2.40 |
| AZB | 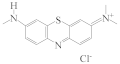 | 9.97 |
| AZC |  | 0.43 |
| Sample | Added Amount (ng g−1) | Found (ng g−1, n = 3) | Recovery (%) | RSD(%) |
|---|---|---|---|---|
| 1 | 3.2 | 2.72 ± 0.05 | 85.00 | 1.71 |
| 2 | 1.6 | 1.31 ± 0.04 | 81.88 | 2.84 |
| 3 | 0.25 | 0.27 ± 0.01 | 108.00 | 2.75 |
| Sample | Species | Detected Value by LC-MS/MS (ng·g−1) | Test Result (ng g−1) (n = 5) | Relative Discrepancy (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | Yellow catfish | 4.79 | 4.43 ± 0.03 | 7.5 | 0.6 |
| 2 | Yellow catfish | 2.99 | 3.39 ± 0.14 | 13.3 | 4.2 |
| 3 | Flat fish | 3 | 2.75 ± 0.07 | 7.0 | 2.5 |
| 4 | Grass carp | Not Found | Not Found | -- | -- |
| 5 | Crucian carp | Not Found | Not Found | -- | -- |
| 6 | Turbot | Not Found | Not Found | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Zhang, J.; Gu, L.; Tang, Y.; Zhang, X.; Huang, X.; Shen, X.; Zhai, W.; Fodjo, E.K.; Kong, C. Ratiometric Fluorescence Immunoassay Based on Carbon Quantum Dots for Sensitive Detection of Malachite Green in Fish. Biosensors 2023, 13, 38. https://doi.org/10.3390/bios13010038
Yang G, Zhang J, Gu L, Tang Y, Zhang X, Huang X, Shen X, Zhai W, Fodjo EK, Kong C. Ratiometric Fluorescence Immunoassay Based on Carbon Quantum Dots for Sensitive Detection of Malachite Green in Fish. Biosensors. 2023; 13(1):38. https://doi.org/10.3390/bios13010038
Chicago/Turabian StyleYang, Guangxin, Jingru Zhang, Lin Gu, Yunyu Tang, Xuan Zhang, Xuanyun Huang, Xiaosheng Shen, Wenlei Zhai, Essy Kouadio Fodjo, and Cong Kong. 2023. "Ratiometric Fluorescence Immunoassay Based on Carbon Quantum Dots for Sensitive Detection of Malachite Green in Fish" Biosensors 13, no. 1: 38. https://doi.org/10.3390/bios13010038
APA StyleYang, G., Zhang, J., Gu, L., Tang, Y., Zhang, X., Huang, X., Shen, X., Zhai, W., Fodjo, E. K., & Kong, C. (2023). Ratiometric Fluorescence Immunoassay Based on Carbon Quantum Dots for Sensitive Detection of Malachite Green in Fish. Biosensors, 13(1), 38. https://doi.org/10.3390/bios13010038






