Obtaining a Monoclonal Antibody against a Novel Prometryn-Like Hapten and Characterization of Its Selectivity for Triazine Herbicides
Abstract
1. Introduction
2. Materials and Reagents
3. Methods
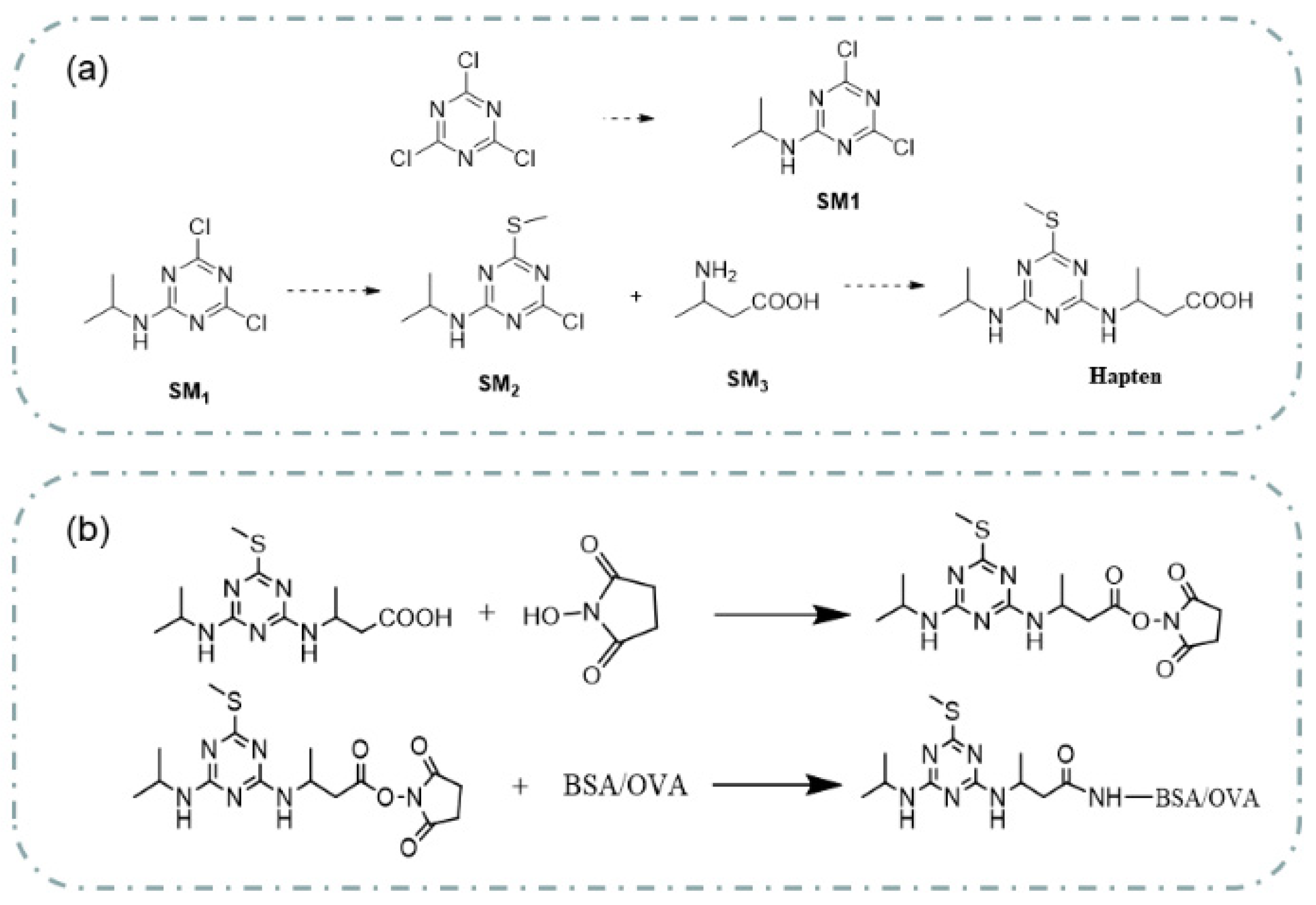
3.1. Synthesis of Prometryn Hapten
3.2. Immunogen and Coating Antigen Preparation
3.3. Production and Characteristics of Prometryn mAb
3.3.1. Immunization
3.3.2. Ic-ELISA Procedure
3.3.3. Production of mAb
4. Results and Discussion
4.1. The Results of Hapten, Immunogen, and Coating Antigen Identification
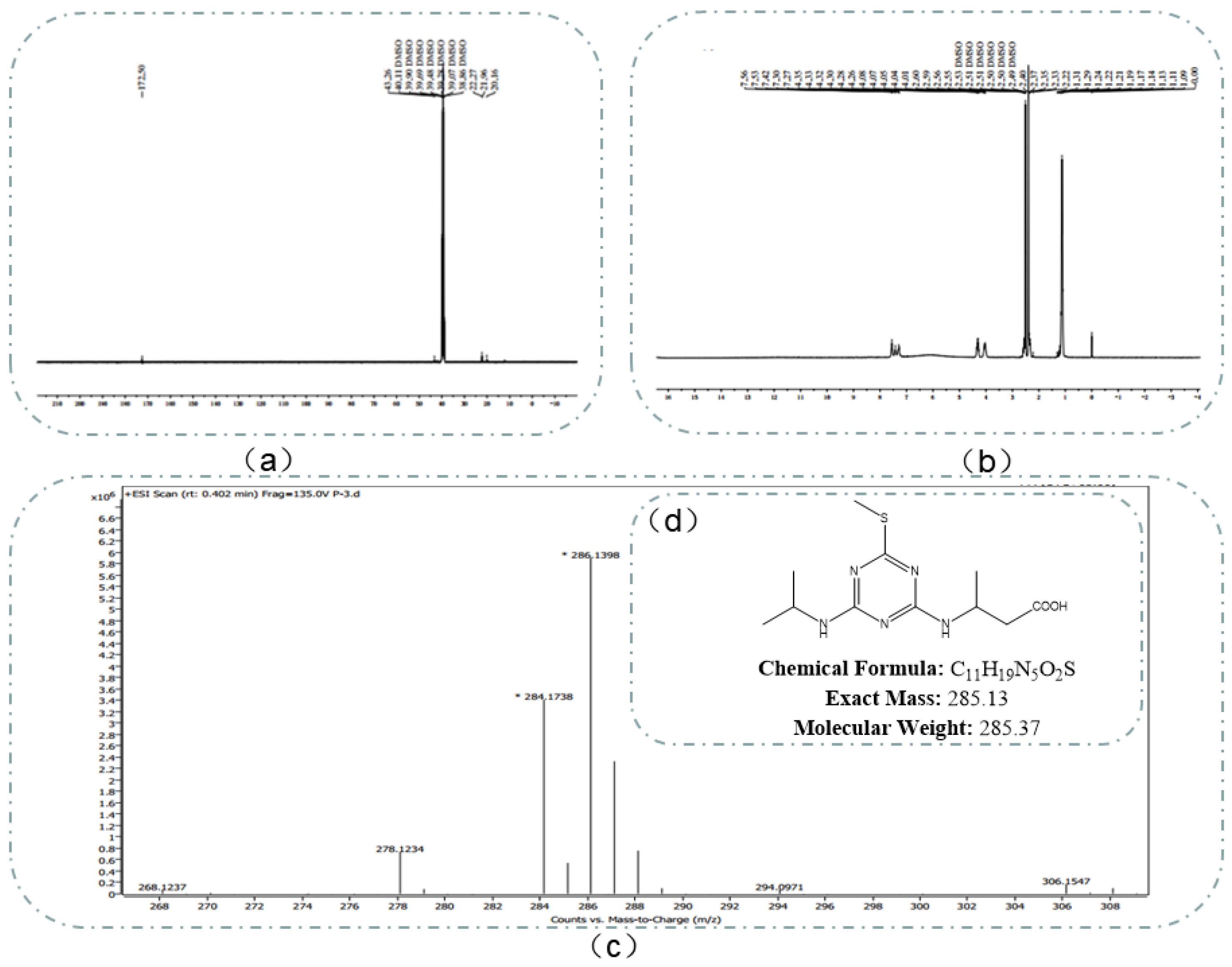
4.1.1. Identification of Hapten
4.1.2. Identification of the Prometryn Antigen and Immunogen
4.2. Characterization and Cross-Reactivity of the 7D4 mAb
4.2.1. Characterization of mAb
4.2.2. Cross-Reactivities of the 7D4 mAb
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Triazine Herbicides: 50 Years Revolutionizing Agriculture, 1st ed.; LeBaron, H.M., McFarland, J.E., Burnside, O., Eds.; Elsevier: Amsterdam, The Netherlands; Boston, CA, USA, 2008; ISBN 978-0-444-51167-6. [Google Scholar]
- Khan, S.U. Distribution and Characteristics of Bound Residues of Prometryn in an Organic Soil. J. Agric. Food Chem. 1982, 30, 175–179. [Google Scholar] [CrossRef]
- Khan, S.U.; Hamilton, H.A. Extractable and Bound (Nonextractable) Residues of Prometryn and Its Metabolites in an Organic Soil. J. Agric. Food Chem. 1980, 28, 126–132. [Google Scholar] [CrossRef]
- Guo, L.J.; Qu, J.R.; Miao, S.S.; Geng, H.R.; Yang, H. Development of a Molecularly Imprinted Polymer for Prometryne Clean-up in the Environment. J. Sep. Sci. 2013, 36, 3911–3917. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, E.N.; Vryzas, Z.; Kotopoulou, A.; Kintzikoglou, K.; Makris, K.C.; Papadopoulou-Mourkidou, E. A Pesticide Monitoring Survey in Rivers and Lakes of Northern Greece and Its Human and Ecotoxicological Risk Assessment. Ecotoxicol. Environ. Saf. 2015, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, M.P.; Sterling, T.M.; Khan, R.A.; Molin, W.T. Fate of Prometryn in Prometryn-Tolerant and -Susceptible Cotton Cultivars. Pestic. Biochem. Physiol. 1996, 56, 111–122. [Google Scholar] [CrossRef]
- Molin, W.T.; Khan, R.A. Differential Tolerance of Cotton (Gossypium sp.) Cultivars to the Herbicide Prometryn. Pestic. Biochem. Physiol. 1996, 56, 1–11. [Google Scholar] [CrossRef]
- Bester, K. Effects of Pesticides on Seagrass Beds. Helgol Mar Res. 2000, 54, 95–98. [Google Scholar] [CrossRef]
- Brvar, M.; Okrajšek, R.; Kosmina, P.; Starič, F.; Kapš, R.; Koželj, G.; Bunc, M. Metabolic Acidosis in Prometryn (Triazine Herbicide) Self-Poisoning. Clin. Toxicol. 2008, 46, 270–273. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Wang, L. Interaction of Prometryn to Human Serum Albumin: Insights from Spectroscopic and Molecular Docking Studies. Pestic. Biochem. Physiol. 2014, 108, 66–73. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Koutnik, D.; Machova, J. Effects of Prometryne on Early Life Stages of Common Carp (Cyprinus carpio L.). Pestic. Biochem. Physiol. 2015, 118, 58–63. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, F.; Jiao, J.; Liu, M.; Li, H. Effects of Bacterial-Feeding Nematodes and Prometryne-Degrading Bacteria on the Dissipation of Prometryne in Contaminated Soil. J. Soils Sediments. 2012, 12, 576–585. [Google Scholar] [CrossRef]
- Sun, S.; Li, Y.; Lv, P.; Punamiya, P.; Sarkar, D.; Dan, Y.; Ma, J.; Zheng, Y. Determination of Prometryn in Vetiver Grass and Water Using Gas Chromatography–Nitrogen Chemiluminescence Detection. J. Chromatogr Sci. 2016, 2, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, J.; Cheng, Y.; Li, D.; Hu, F.; Li, H. Determination of Prometryne in Water and Soil by HPLC–UV Using Cloud-Point Extraction. Talanta. 2009, 79, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, T.; Zheng, C. Analysis of prometryn residue in roots of Codonopsis lanceolata. Plant Prot. Sci. 2011, 36, 100–102. [Google Scholar]
- Chae, Y.-S.; Cho, Y.-J.; Jang, K.-J.; Kim, J.-Y.; Lee, S.-M.; Chang, M.-I. Establishment of an Analytical Method for Prometryn Residues in Clam Using GC-MS. Korean J. Food Sci. Technol. 2013, 45, 531–536. [Google Scholar] [CrossRef][Green Version]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Miranda-Cruz, E.; Domínguez-Álvarez, J.; Hernández-Méndez, J. Comparison of a Non-Aqueous Capillary Electrophoresis Method with High Performance Liquid Chromatography for the Determination of Herbicides and Metabolites in Water Samples. J. Chromatogr. A 2006, 1122, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Abd El-Aty, A.M.; Shim, J.-H.; Eun, J.-B.; Lei, X.; Zhao, J.; Zhang, X.; Cui, X.; She, Y.; Jin, F.; et al. Design and Characterization of a Novel Hapten and Preparation of Monoclonal Antibody for Detecting Atrazine. Foods 2022, 11, 1726. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Zhang, L.; Qin, J.; Dou, X.; Fu, Y.; Li, Q.; Zhao, X.; Yang, M. An Integrated Strategy for Rapid On-Site Screening and Determination of Prometryn Residues in Herbs. Anal. Bioanal Chem. 2020, 412, 621–633. [Google Scholar] [CrossRef]
- Guo, L.; Xu, X.; Zhao, J.; Hu, S.; Xu, L.; Kuang, H.; Xu, C. Multiple detection of 15 triazine herbicides by gold nanoparticle based-paper sensor. Nano Res. 2022, 15, 5483–5491. [Google Scholar] [CrossRef]
- Schlaeppi, J.M.; Foery, W.; Ramsteiner, K. Hydroxyatrazine and atrazine determination in soil and water by enzyme-linked immunosorbent assay using specific monoclonal antibodies. J. Agric. Food Chem. 1989, 37, 1532–1538. [Google Scholar] [CrossRef]
- Shim, W.B.; Yang, Z.Y.; Kim, J.Y.; Choi, J.G.; Je, J.H.; Kang, S.J.; Kolosova, A.Y.; Eremin, S.A.; Chung, D.H. Immunochromatography using colloidal gold− antibody probe for the detection of atrazine in water samples. J. Agric. Food Chem. 2006, 54, 9728–9734. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G. A new monoclonal antibody for the sensitive detection of atrazine with immunoassay in microtiter plate and dipstick format. J. Agric. Food. Chem. 1993, 41, 1006–1011. [Google Scholar]
- Sai, N.; Sun, W.; Wu, Y.; Sun, Z.; Yu, G.; Huang, G. A highly sensitive immunoassay for atrazine based on covalently linking the small molecule hapten to a urea–glutaraldehyde network on a polystyrene surface. Int. Immunopharmacol. 2016, 40, 480–486. [Google Scholar] [CrossRef] [PubMed]


| Triazine Compounds | Chemical Structure | IC50 (ng/mL) | Cross-Reactivity |
|---|---|---|---|
| Prometryn | 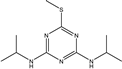 | 3.901 | 100% |
| Ametryn |  | 11.221 | 34.77% |
| Desmetryn |  | 21.566 | 18.09% |
| Terbumeton | 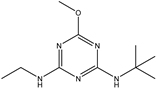 | 51.091 | 7.64% |
| Propazine | 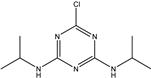 | 398.011 | 0.98% |
| Terbuthylazine |  | >1000 | <0.39% |
| Simazine | 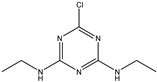 | >1000 | <0.39% |
| Simetryn | 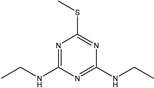 | >1000 | <0.39% |
| Atrazine | 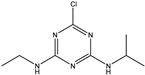 | >1000 | <0.39% |
| Prometon | 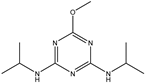 | >1000 | <0.39% |
| Terbutryn | 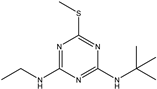 | >1000 | <0.39% |
| Hapten Name | Hapten Structure | Reference |
|---|---|---|
| H1: 4-((4-(isopropylamino)-6-(methylthio)-1,3,5-triazin-2-yl)amino)butanoic acid H2: 3-((4-amino-6-(cyclopropylamino)-1,3,5-triazin-2-yl)thio)propanoic acid | 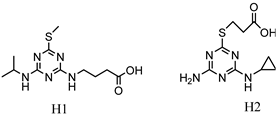 | [19] |
| 4-((4-(isopropylamino)-6-(methylthio)-1,3,5-triazin-2-yl)amino)butanoic acid | 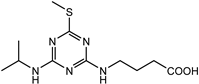 | [20,21] |
| 4-chloro-6- (isopropyl amino)-l,3,5-triazine-2-(6-ami- nohexanecarboxylic acid) | 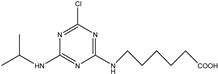 | [22,23] |
| 2-mercaptopropionic acid-4-ethylamino-6-isopropylamino-1,3,5-triazine | 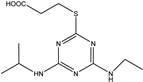 | [24] |
| 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine | 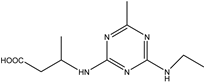 | [18] |
| 3-((4-(isopropylamino)-6-(methylthio)-1,3,5-triazin-2-yl)amino)butanoic acid |  | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Abd El-Aty, A.M.; Zhao, J.; Lei, X.; Zhang, X.; Zhao, Y.; Cui, X.; She, Y.; Jin, F.; Wang, J.; et al. Obtaining a Monoclonal Antibody against a Novel Prometryn-Like Hapten and Characterization of Its Selectivity for Triazine Herbicides. Biosensors 2023, 13, 22. https://doi.org/10.3390/bios13010022
Xu L, Abd El-Aty AM, Zhao J, Lei X, Zhang X, Zhao Y, Cui X, She Y, Jin F, Wang J, et al. Obtaining a Monoclonal Antibody against a Novel Prometryn-Like Hapten and Characterization of Its Selectivity for Triazine Herbicides. Biosensors. 2023; 13(1):22. https://doi.org/10.3390/bios13010022
Chicago/Turabian StyleXu, Lingyuan, A. M. Abd El-Aty, Jing Zhao, Xingmei Lei, Xiuyuan Zhang, Yun Zhao, Xueyan Cui, Yongxin She, Fen Jin, Jing Wang, and et al. 2023. "Obtaining a Monoclonal Antibody against a Novel Prometryn-Like Hapten and Characterization of Its Selectivity for Triazine Herbicides" Biosensors 13, no. 1: 22. https://doi.org/10.3390/bios13010022
APA StyleXu, L., Abd El-Aty, A. M., Zhao, J., Lei, X., Zhang, X., Zhao, Y., Cui, X., She, Y., Jin, F., Wang, J., Jin, M., & Hammock, B. D. (2023). Obtaining a Monoclonal Antibody against a Novel Prometryn-Like Hapten and Characterization of Its Selectivity for Triazine Herbicides. Biosensors, 13(1), 22. https://doi.org/10.3390/bios13010022








