Abstract
Cancer is one of the major killers across the globe. According to the WHO, more than 10 million people succumbed to cancer in the year 2020 alone. The early detection of cancer is key to reducing the mortality rate. In low- and medium-income countries, the screening facilities are limited due to a scarcity of resources and equipment. Paper-based microfluidics provide a platform for a low-cost, biodegradable micro-total analysis system (µTAS) that can be used for the detection of critical biomarkers for cancer screening. This work aims to review and provide a perspective on various available paper-based methods for cancer screening. The work includes an overview of paper-based sensors, the analytes that can be detected and the detection, and readout methods used.
1. Introduction
Cancer is a major cause of death worldwide [1,2]. It is estimated to be the cause of every 1 in 6 deaths [3,4]. According to the World Health Organisation (WHO), in the year 2020 more than 10 million people lost their life to cancer [5]. Worldwide, an estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths occurred in 2020 [6]. Although the causes of cancer may vary depending on the type, it has been observed that the incidence rate of disease is on the rise [7,8].Worldwide, by 2040, 28.7 million new cases of cancer are projected to occur, a 47% rise compared to 19.3 million in 2020. An increase in the global cancer burden in the next fifty years will come from low- and middle-income countries (400% in low-income countries, 168% in middle-income countries, and 53% in high-income countries) [9,10]. Though there are different treatment strategies that have been developed and the disease is no longer ’incurable’ [11,12,13,14], the success rate of the treatment depends on the stage of disease progression [15,16,17]. An individual undergoing treatment in the early stages of cancer has a many times higher chance of survival than in the later stages [18,19]. Estimates suggest that approximately 30–50% of cancer deaths can be prevented by early detection and treatment [20,21,22].
Although the incidence rate of cancer is higher in wealthy nations compared to low- and middle-income countries, low- and middle-income countries have a lower survival rate, partly due to the late presentation of the disease [23]. The barriers of cancer care in developing countries are due to late-stage presentation, quality of care, affordability, and a lack of access to advanced clinical resources. Late-stage presentation puts tremendous burden on clinicians [10]. The premature death and loss of productive life in the working population results in a significant economic impact on these countries.
In low- and middle-income countries, access to health care facilities is not readily available [24,25,26,27,28]. The number of physicians in low- to middle-income countries can be as low as 0.1 to 2 per 1000 people. In many cases, they are heavily burdened, leading to long waiting times [29]. In many low- and middle-income countries, cancer screening is not covered under insurance, thus discouraging patients from undergoing cancer screening.
To address the issue of cancer screening in these countries, various strategies have been developed [30]. These screening devices need to be easy to manufacture, low-cost, portable, and should not require any special training. Paper-based sensors are gaining significance in this field as they possess all the features of an ideal screening device [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50].
Paper was invented by the Egyptians in the fourth century BC. It is one material that has existed continuously since the beginning of Egyptian civilization. Paper-based products are the most sold products in the world. For example, the Bible is the most sold book in the world and is printed on paper. The question then becomes: can we print cancer screening devices on paper to enable their use as low-cost sensors for cancer screening? Paper-based sensors for cancer screening are akin to an at-home pregnancy test kit. These kits will indicate whether a specific cancer biomarker is present in the person’s body. In many cases, the result will be qualitative, i.e., yes/no type. However, novel sensors have been developed that provide a quantitative output [51].
In case the test returns a positive result, the patient can consult medical professionals so that exhaustive testing can be performed and the stage of the disease identified.
The most common sensing principle used in paper-based sensors for cancer screening is ELISA, wherein the analyte is labelled and detected using a sandwich assay [52]. The results are then readout using a plate reader to specify analyte concentration against a standard curve [53,54,55,56,57]. Various other methods are also being explored for the development of sensors.
This work reviews the various paper-based sensors that have been developed for cancer screening. In the next sections, a brief introduction about paper-based sensors, the analytes used for cancer screening, and the detection and read-out techniques used are discussed.
2. Paper-based Sensors: Low-Cost Screening Devices for the Developing World
Advancements in microfluidics have led to their widespread usage in sensing applications. Microfluidics, as the name suggests, uses small fluidic channels where liquids, proteins and cells can be manipulated through flow control [58]. The channels are designed in such a way that various operations such as separation of phases, biological cells, the size-based separation of particles, to name a few, can be performed. However, fabrication of microfluidic devices may not be possible in low-income countries due to the lack of availability of materials. Even poly dimethyl siloxance (PDMS)-based soft lithography techniques require a master that can be expensive to make in low-income countries and set-ups such as mask makers and lithography may not be readily available. Paper is a low-cost alternative for sensors in the fight against cancer that may not need lithographic processing. Droplet-based paper devices could be highly useful for biomarker detection. Even microfluidic devices using screen-printing techniques can be printed on paper.
Paper-based sensors are devices that are fabricated on a paper substrate. These devices are printed on a cellulose-based paper substrate using readily available printers, making them easily accessible.
A typical paper-based sensor is based on exploiting the capability of paper to wick liquid, leading to capillary action in the paper. A typical paper-based sensor is developed on an hydrophilic paper substrate with wicking capabilities. Using surface treatment methods, a hydrophobic barrier is created in order to guide the flow of liquid through the specific path or channels.
The most commonly used paper in the fabrication of paper-based sensors is filter paper. Due to its pores, it has sufficient wicking capabilities, providing a moderate flow rate. Whatmann-branded filter paper manufactured by General Electric Health Care is the most widely used filter paper due to its uniform pore size and distribution. In applications where filter paper is not suitable, nitro-cellulose paper is used as a substrate. The main advantage of nitrocellulose substrate is its easy and efficient binding of proteins [59].
For example, nitrocellulose film is used for protein immobilization and filter paper is used for its water absorption. In one particular study for detection of bladder cancer, a glass-cellulose film was used for sampling, a nitro-cellulose film was used for protein immobilization, and filter paper was used for sample transfer due to its adsorption capabilities [60].
Table 1 highlights the different types of paper substrates that have been developed for paper-based sensing applications.

Table 1.
Paper substrates of interest for paper-based microfluidics.
For patterning of the microfluidic channels on paper substrate, various printing techniques such as wax-printing, ink-jet printing, screen printing, lithography, plasma processing, and manual pattern drawing have been explored. In a typical printing-based fabrication process, a CAD model of the microfluidic channels is printed using wax/ink-jet–printer on the substrate. Since the model is printed on only one side of the substrate, the substrate is heated to cause the reflow of the hydrophobic ink/wax barrier over the complete cross-section of the substrate. These barriers act as microfluidic channels guiding the flow of the fluid (Figure 1).
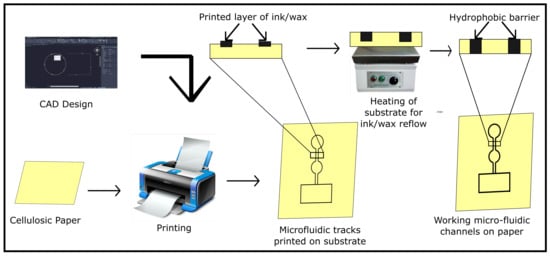
Figure 1.
Workflow of the fabrication process for printing microfluidic channels on a paper substrate.
Although paper-based microfluidic channels can be used for most of applications as conventional microfluidic chips, there may be concerns regarding the utility of paper substrate for use in microfluidic applications requiring multiple layers of fluidic channels for phase separation applications. For such applications Japanese paper, folding techniques such as origami and Kirigami are being explored [87].
The low cost of substrate, the ability to print continuously, the ability to make arrays of devices, and minimal capital requirements for the fabrication setup make paper-based sensors an interesting candidate for use in mass screening process. Paper being a naturally derived substance is biodegradable in nature. This will also reduce the environmental impact due mass production and usage of screening kits.
3. Design and Working of a Typical Paper-Based Sensor
The paper-based sensor used for screening must be fast, accurate, reliable, and must have a low limit of detection. A low limit of detection will allow for successful detection even during the early onset of cancer. A typical sensing setup comprises the following elements:
- Analyte: It can be simply be defined as the chemical substance to be measured. In the case of cancer screening, cancer specific biomarkers, tumour markers, antigen, and proteins are essential analytes. More about the different types of analytes for cancer screening in Section 4
- Labeling: In most of the biosensors, labeling plays an important role. For the detection of the analyte, labels that attach to the molecule are used. The selection of label depends on the detection method used.
- Recognition: The recognition element is used to convert the biological information into signals. The most common detection method used in cancer screeing is enzyme-linked immunoassay (ELISA). In Section 5, various recognition methods that have been used for paper-based cancer screening are discussed.
- Readout: The readout method is used to obtain the outcome of the test. Some common readout methods are electrochemical, optical, and colorimetric. Depending on the detection technique used, the results obtained can either be qualitative (yes/no) type or quantitative (numerical values).
The paper-based microfluidic platform is divided into different zones, with each zone having a specific functionality, as shown in Figure 2. In the sampling zone, the sample is placed on the paper substrate. While passing through the microfluidic channels, labeling elements get attached to the analyte. In the detection zone, the analyte is detected and a signal is generated for the readout. In the case of colorimetry-based devices, there is a special zone termed as ’control zone’. In case a test yields a positive or negative result, there is a color change observed in the control zone.
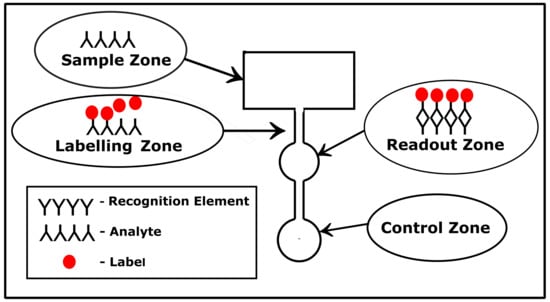
Figure 2.
Typical layout of a paper-based sensor with the different zones. Each zone performs a specific function.
4. Analytes for Cancer Screening
For cancer detection, biomarkers play an important role. According to the National Cancer Institute (NCI), biomarkers are defined as “a biological molecule found in blood, other body fluids, or tissues that is a sign of a normal or abnormal process, or of a condition or disease” [88]. Though biomarkers may be generated due to various factors such as somatic mutations, transcriptional changes, or post-translational modifications, they an important differentiators for an affected individual compared to a healthy individual [88]. A handful of biomarkers have been approved for cancer detection. For example, high levels of carcino embryogenic antigen may mean the presence of cancer. Similarly, CA 125 is a protein that is detected in blood for ovarian cancer. Lysophosphatidic acid, leptin, osteopotin, and insulin-like growth factor receptor 2 are used as a biomarker for ovarian carcinoma. Early prostate cancer antigen 2 is used as a novel biomarker for prostate cancer [89]. Biomarkers are not only useful for the detection of screening of cancer, they are also used in monitoring the effectiveness on any treatment or therapy. Various biomarkers are present in blood, saliva, urine, stools, etc., making it possible to obtain samples for analysis in a non-invasive or minimally invasive manner. There are various biomarkers that are used for cancer screening, including antigens, micro-RNAs, proteins, antibodies, and tumor cells. For example, the antibody–antigen interaction creates a signal that is measured qualitatively or quantitatively on a paper substrate.
5. Recognition Element
Recognition elements are responsible for the recognition of target analytes (ex: receptors) and their conversion into a signal, which can be qualitative, semi-quantitative, or quantitative [90]. An ideal recognition element has a highly specific binding affinity towards the analyte of interest [90]. Recognition elements can either be natural or artificial bio-molecules that are synthetically obtained [90,91,92,93,94]. When the target analyte molecule attaches to the recognition element, it undergoes a biochemical reaction producing a signal [90]. In some cases, for the detection of analyte, labeling agents such as nanoparticles are attached to the analyte molecule [90].
5.1. Antibodies
Antibodies are a type of natural bio-receptor that can be derived from living organisms [90]. Monoclonal antibodies are widely preferred in cancer screening applications due to their high specificity to the target antigen [95,96]. Through the development of hybridoma technology, it is possible to obtain a reliable and uniform supply of monoclonal antibodies [96]. This has also led to better reliability and accuracy of sensors using monoclonal antibodies. Covalent binding of antibodies to cellulose paper discs has been developed for colorimetric immunoassays. The antibodies were coated on the amine-functionalized cellulose paper discs. Through a glutaraldehyde cross-linking agent, the antibodies showed enhanced binding activity to the target when compared to the periodate oxidation method [97]. Other methods for antibody immobilization on a paper with shelf-life up to 12 months have been described [98,99]. Polyclonal antibodies have multiple binding sites, each specific to a particular antigen. Although they are cheaper to produce compared to monoclonal antibodies, they are not suitable for sensing applications due to the multiple binding sites [100].
For the detection of analytes, the principle of enzyme linked immunosorbent assay (ELISA) is applied in sensor design. Nanoparticles such as gold are used as antibody carriers and signal enhancers for ELISA. In the range between 0 and 60 U/mL, the ELISA assay adopting gold nanoparticles as an optical signal enhancer resulted in higher sensitivity and shorter assay time when compared to classical ELISA procedures. This was used to detect breast cancer biomarkers [101].
A sensor for the detection of prostate-specific antigens (PSA) used multiwalled carbon nanotubes (MWCNT) activated with anti-PSA antibody for the detection [102]. Due to the site-selective interaction between the antigen and the antibody there was a change in the resistance. This change in resistance could be measured using a benchtop multi-meter [102]. This method was found to be cheaper and faster than the ELISA method used in cancer diagnosis [102]. Similarly, single-wall carbon nanotube based biosensors have been used in the identification of cancer antibody–antigen interactions in blood samples using electrical conductance measurements. Following the measurements, a classification algorithm was implemented to differentiate between cancer and controls with 90% accuracy [103,104,105].
In a colorimetric sensor for the detection of pancreatic cancer biomarker (PEAK1), gold nanoparticles that were used as a labeling element acted as a color dye catalyst to produce colorimetric signals [106]. A photothermal-effect-based sensor used a graphene oxide (GO)-gold–anti-EpCAM antibody composite as the recognition element for the detection of MCF-7 cancer cells specific antigen [107]. After laser irradiation at the test zone, the temperature contrast was recorded for the detection of cell numbers (Figure 3) [107].
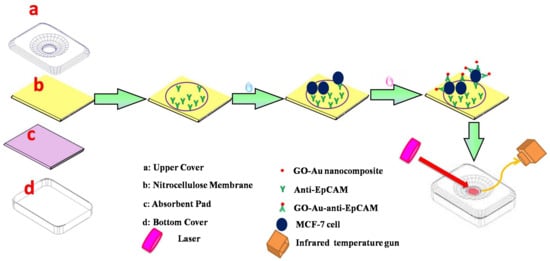
Figure 3.
Working of photothermal effect based sensor for MCF-7 cancer cell detection. (Reproduced with permission from [107] Copyright 2016, Elsevier).
5.2. Aptamers
Aptamers are single-stranded nucleic acids that are folded into a specific architecture [108,109]. Due to their specific binding of the target proteins, they are used for sensing applications [108,110]. Their size and chemical stability make them widely preferred for the detection of proteins and small molecules [110,111]. Their low cost makes them a preferred alternative to antibodies in sensing applications [111]. For example, carbon-nanotube-based RNA apatamer sensors were developed for detecting IL6 in blood samples. Apatamer sensors based on field effect transistor arrays suggested a shift in drain current versus gate voltage for 1 pg and 1 ng of IL-6 exposure. The concentration of 1 pg falls below the diagnostic gray zone for cancer (2.3 pg–4 ng/mL), which is an indicator of early-stage cancer [112].
For the design of aptamer-based sensors, various strategies such as sandwich, target-induced structure switching, or competitive replacement modes have been used for biosensor design [111]. Electrochemical sensing is the most preferred sensing method with aptamer as the recognition element; however, other methods such as optical sensing have also been explored [110].
In a fluorescence-based paper-based sensor designed for the detection of multiple types of cancer cells, graphene oxide-coated with mesoporus-silica-labelled high-specificity aptamers was used as a labeling element [113]. Using the excitation wavelength of 350 nm, a color change was produced that could be observed through naked eye [113].
6. Sensing and Readout Methods
For achieving higher utility of paper-based sensors, the readout method used should be cost-effective and portable. The method provides fast and accurate results without a requirement for extensive handling by experts.
In most of the cases, qualitative readout methods should suffice. However, with focus on providing health professionals with important data at the point-of-care, qualitative readout methods are also gaining significance. Although there are various readout methods for sensing applications, electrochemical and optical are the most widely used readout methods for paper-based sensing applications. With advancements in smartphone and machine learning technologies, there have been works that use smartphones for signal interpretation and readout.
6.1. Modified Electrodes
For electrochemical sensing, the potential difference between the electrodes is proportional to the concentration of the analyte. In paper-based sensors, the working electrode is modified such that the binding of the analyte produces an electrical signal through a change in resistance, current, capacitance, or impedance.
Various nanocomposites have been used for the fabrication of modified electrodes. These nanocomposites perform a dual function: recognition and amplification. Amino functional graphene (NH2-G)/thionine (Thi)/gold nanoparticles (AuNPs) nanocomposites are coated with recognition elements such as üimmobilized anti-CEA [114] and anti-NSE [115] for the detection of specific analytes. The sensor could provide fast results with a low limit of detection of 10 pg/mL [114]. In a more recent work, an aptasensor with two working electrodes capable of the simultaneous detection of CEA and NSE has been developed. Along with the NH2-G/Thi/Au nanocomposite, Prussian blue (PB)- poly (3,4- ethylenedioxythiophene) (PEDOT)- AuNPs nanocomposite was used for the fabrication of the second electrode, which was coated with immobilized CEA and NSE aptamers [116]. The device worked on the principle of electrochemluminescnce and could achieve fast and accurate detection of CEA and NSE with a limit of detection of 2 pg/mL and 10 pg/mL, respectively (Figure 4) [116].
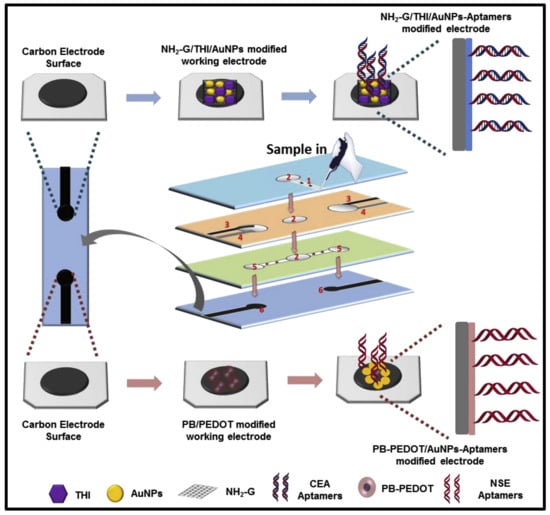
Figure 4.
Mechanics of modified electrode paper-based apta sensor. (Reproduced with permission from [116] Copyright 2019, Elsevier).
6.2. Electrochemical
In electrochemical sensing method, the analyte generates an electrical signal proportional to its concentration [90]. The signals may be generated through a biorecognition event, modified electrodes, or enzyme mediated electrodes [90,117]. For electrochemical detection, the sensor should have three electrode systems with reference, working, and counter electrodes. For measuring the signals, electrochemical devices such as electrochemical workstations or bench-top multimeters are used.
Various routes such as the use of labeling agents or modified electrodes may be used for generating electrical output from biological signals. In a sensor developed for the detection of cancer antigens, a marker for ovarian cancer, a reduced graphene oxide/gold nanoparticle/thionine nanocomposite was used as working electrode [118].
For the reliable detection of signals, signal amplification techniques are used. In paper-based sensors for the detection of CEA using horseradish peroxidase (HRP)–O-phenylenediamine–HO as a detection element, graphene was coated on the substrate for accelerating the electron transfer and amplifying the signals [119].
6.3. Optical
For optical sensing, signals are generated through a recognition process by the formation of an antigen–antibody complex [90]. The optical signals could be fluorosence, chemiluminescence, or color change [90]. Other than the signals that display a direct color change, a photo-detector is used for measuring the signals [90].
Surface-enhanced Raman scattering is a popularly used method for signal detection in paper-based sensors. Gold nanostar@Raman reporter@silica-sandwiched nanoparticles have been developed as surface-enhanced Raman scattering (SERS) probes for the paper-based lateral flow strip (PLFS) assay [120]. A sensor for the detection of CEA used a portable raman sensor for measurement (Figure 5) [120]. Using a paper-based lateral flow strip capable of plasma separation and using silica nanoparticles for labeling the sensor displayed a limit of detection of 1 ng/mL [120].
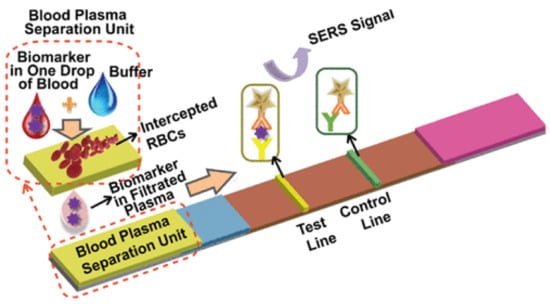
Figure 5.
Working of a paper-based sensor for CEA concentration detection using surface-enhanced Raman scattering (SERS). (Reproduced with permission from [120] Copyright 2021, American Chemical Society).
For naked eye detection, luminiscent reporters are used as labeling elements These can be nanoparticles [51], conjugated polyelectrolytes [121], or multi walled carbon nanotubes [102].
6.4. Smartphone/Machine-Learning-Based
Smartphones are devices that are readily available, even in low- and middle-income countries. Mobile health is becoming increasingly popular in developing countries [122]. It is widely explored as a tool for the efficient delivery of services, including in healthcare. Smartphones have been explored as a readout method for both optical and electrical signals [123,124,125]. For optical signals, a smartphone camera is used for data acquisition [126,127,128]. Using a custom application, the acquired image is compared with reference values and the result is calculated [126,127,128,129,130].
Smart phone-based imaging was used for calculating and displaying results in a multi-layered paper-based sensor for cancer screening [131]. The movable layers allowed one to control the flow of the solution. Using the special design and smartphone-based readout, it was possible to achieve a low detection limit of 0.015 ng/mL [131].
Smartphones are also used for coupling with an electrochemical sensing device for the readout of signals [132]. A screen-printed sensor with multi-walled carbon nanotubes (MwCNT)/thionine (Thi)/gold nanoparticles (AuNPs) electrodes is capable of detecting cancer antigen (CA125) with a limit of detection of 2mU/mL [132]. The sensor uses a electrochemical detector powered using a smartphone, and it transfers data to the smartphone, where it is readout using a custom app (Figure 6) [132].
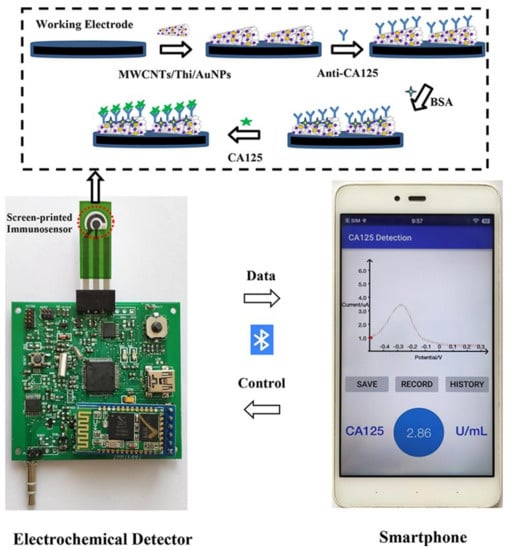
Figure 6.
Sensor for detection of cancer antigen (CA125). The screen-printed sensor used electrochemical deterrence for signal measurement and a smartphone with a custom application for readout (reproduced with permission from [132] Copyright 2022, Elsevier).
Table 2 summarizes recent works using paper-based sensors for cancer screening. It provides the breakdown of the sensor in terms of the biomarker(s) detected, recognition element, readout method used, and the types of cancer detected.

Table 2.
Paper-based sensors for cancer screening.
7. Limitations of Current Paper-based Methods
Despite the promising future, there are currently several limitations that hinder the large-scale acceptance of paper-based sensors. Ranging from fabrication methods, a requirement of measurement equipment, to regulatory requirements, many limitations need to be addressed before paper sensors are actually put into service. Sensitivity and accuracy are also concerns in paper-based devices.
However, paper-based sensors are excellent for qualitative and semi-quantitative screening. One can further improve the accuracy of paper-based testing through implementing paper-based testing with artificial-intelligence-based analysis. Machine learning and deep learning can even predict the sequence of DNA and RNA that can point to cancer mutations. They could also potentially detect cancer cells in blood droplets. The ability to differentiate between cancer versus normal cells in blood based on AI can be a very powerful approach in making paper-based testing a reality for mass screening [152].
With the exception of laser printing and screen printing, different fabrication methods used for the fabrication of paper-based microfluidic devices such as photo-lithography, e-beam lithography, reactive ion etching, and metal or oxide deposition require extensive capital investment and training, making them non-feasible for use in low- and middle-income countries.
For devices with qualitative measurement of signals, despite the low cost of single paper-based sensing device, expensive equipment such as electrochemical workstations, photo detectors, or electrochemiluminscence detectors are required for a results readout. This not only affects the portability of the device but also increases the overall cost of the screening, and it requires a trained technician to carry out the readout.
Nevertheless, paper-based sensors can make a significant impact in terms of testing blood for infectious diseases and even in the fight against cancer. The qualitative assessment of whether a person has cancer based on biomarkers in blood can reduce the cancer clinical burden in low- and middle-income countries. The paper-based testing method will definitely have an advantage here to reduce the clinical burden through the large-scale screening of populations. People with cancer undergoing chemotherapy may be prone to infectious diseases. Life-threatening infectious diseases can kill people in a few days. Here, POC systems based on paper and colorimetric detection methods for parasites, viruses, and other agents would be highly valuable. Before vaccination, people, including doctors, were dying of COVID-19 within a few days to two weeks. Low-cost qualitative paper-based sensors with instant results through color change are an absolute necessity to differentiate between population who has the virus from those who do not. This may be cheaper than the current PCR test that is used and is done in the laboratory. In the era of the pandemic, low-cost sensors for personal safety are very important. Qualitative paper-based sensors with instant read out will be highly useful in such pandemics as one cannot run tests in the laboratory frequently.
8. Conclusions
Paper-based sensors have immense potential to to act as low-cost tools in the mass screening of cancer. They can be used in cancer-screening camps, especially in low- and middle-income countries. Due to the health care resources being highly stressed in these countries, screening using paper-based sensors will act as a filter. The samples of the patients who test positive in the screening stages can then be treated as a priority. This will in a way help in resource allocation and management in these countries. It must be mentioned that the paper-based sensors with their current state of the art are insufficient for providing data to healthcare professionals in making important decisions. Further, testing will be required to ascertain the stage of the disease before any treatment plan is decided. Many times, the same biomarker is produced for multiple types of cancers. CEA is a common marker for multiple types of cancer such as lung, breast, ovaries, stomach, and intestine, to name a few. Thus, it is necessary to determine the type of cancer and the stage of disease progression before starting the treatment. Normal CEA levels are 2.5 ng/mL. A CEA level of 10 ng/mL would indicate the presence of cancer, and anything above 20 ng/mL would indicate the spread of cancer.
Patients with continuously decreasing levels of CEA do better after treatment than patients with increasing CEA levels. A regular paper-based CEA test with an instant read out can tell the doctors the potential for cancer progression. Their use will reduce the number of expensive tests (CT scans, PET-scans, etc.) a patient undergoes during follow-up visits and also help reduce the cost of treatment. In the era of telemedicine, it is becoming increasingly convenient to deliver healthcare at home. Cancer-screening tests can be conducted at home, and the results of the test can be emailed or texted to a doctor automatically using smart phones. So, paper-based testing is important here for home-based low-cost sensors.
Although paper-based sensors may not potentially be the knight in shining armor against humanity’s fight against cancer, they can potentially be the important foot soldier in the fight. Future integrated paper-based sensors where all the sensing and electronic circuitry are printed in paper can make an impact on low-cost testing. Paper-based cantilevers with optical sensors and electronics integrated in a hand-held chip could enable the detection of cancer biomarkers such as prostate-specific antigen (PSA) from blood samples. Thus, there are exciting opportunities for paper-based sensors in the fight against cancer. The places where paper-based sensors along with mobile phones and AI-based techniques could make an impact in the fight against cancer are (1) the low-cost qualitative screening of large populations; (2) reducing the clinical burden through proper resource allocation; (3) estimating cancer prognosis; (4) monitoring cancer treatment; and (5) detecting cancer recurrence qualitatively or semi-quantitatively. With the ability to miniaturize anything, from detectors to spectrometers, one can implement miniaturized low-cost detectors along with paper-based sensors for cancer detection. Such miniaturized sensors are already made by many companies and could be bought off the shelf and integrated with a paper-based sensor. Finally, paper-based methods with artificial intelligence techniques can enable low cost and further improve the sensitivity and accuracy of paper-based sensors.
Author Contributions
Conceptualization, A.D. and A.J.N.; writing—original draft preparation, A.D.; writing—review and editing, A.D., A.J.N. and B.P.; and supervision, A.J.N., B.P., and T.-H.O. All authors have read and agreed to the published version of the manuscript.
Funding
B.P. acknowledges funding from the United States-India Educational Foundation through the Fulbright-Nehru Academic and Research Professional Excellence Award, Grant Number: 2021/ APE-R/63. A.J.N. acknowledges the financial support from the Department of Biotechnology, Government of India, through Ramalingaswami Re-entry fellowship (D.O. No. BT/HRD/35/02/2006) and VIT SEED GRANT 2021-2022 (SG20210234).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
A.J.N. would like to acknowledge the financialsupport from the Department of Biotechnology, Government of India, through Ramalingaswami Re-entry fellowship (D.O. No. BT/HRD/35/02/2006) and VIT SEED GRANT 2021-2022 (SG20210234). B.P. acknowledges funding from the United States-India Educational Foundation through the Fulbright-Nehru Academic and Research Professional Excellence Award, Grant Number: 2021/APE-R/63.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PoCT | Point of Care Testing |
| CEA | Carcino Embryonic Antigen |
| MCF-7 | Michigan Cancer Foundation |
| AFP | Alpha-fetoprotein |
| MWCNT | Multi-walled carbon nano tubes |
| CA125 | Cancer antigen 125 |
| CA153 | Carbohydrate antigen 153 |
| PSA | Prostate-specific antigen |
| HRP | Horse radish peroxidase |
| MOF | Metal–Organic Framework |
| miR-141 | microRNA-141 |
| miR-21 | microRNA-21 |
| NSE | Neuron-specific enolase |
| PEAK1 | Pseudopodium-enriched atypical kinase one |
| EGFR | Epidermal Growth Factor Receptor |
| Cyt C | Cytochrome c |
| PCF | Photonic crystal fiber |
| DSN | Duplex-specific nuclease |
| CPE | Conjugated polyelectrolyte |
| VEGF-C | Vascular endothelial growth factor C |
| NMB | New methylene blue |
| NH2-SWCNTs | Amino-functional single-walled carbon nanotubes |
| AuNPs | Gold nanoparticles |
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.; Ezzati, M. Comparative Risk Assessment Collaborating Group. Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef]
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Cancer. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 10 July 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Damgacioglu, H.; Sonawane, K.; Zhu, Y.; Li, R.; Balasubramanian, B.A.; Lairson, D.R.; Giuliano, A.R.; Deshmukh, A.A. Oropharyngeal cancer incidence and mortality trends in all 50 states in the US, 2001–2017. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 155–165. [Google Scholar] [CrossRef]
- Sekeroglu, B.; Tuncal, K. Prediction of cancer incidence rates for the European continent using machine learning models. Health Inform. J. 2021, 27, 1460458220983878. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef]
- Pramesh, C.; Badwe, R.A.; Bhoo-Pathy, N.; Booth, C.M.; Chinnaswamy, G.; Dare, A.J.; de Andrade, V.P.; Hunter, D.J.; Gopal, S.; Gospodarowicz, M.; et al. Priorities for cancer research in low-and middle-income countries: A global perspective. Nat. Med. 2022, 28, 649–657. [Google Scholar] [CrossRef]
- Kretz, A.L.; Trauzold, A.; Hillenbrand, A.; Knippschild, U.; Henne-Bruns, D.; von Karstedt, S.; Lemke, J. TRAILblazing strategies for cancer treatment. Cancers 2019, 11, 456. [Google Scholar] [CrossRef] [Green Version]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J. Clin. Med. 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- L Arias, J. Drug targeting strategies in cancer treatment: An overview. Mini Rev. Med. Chem. 2011, 11, 1–17. [Google Scholar] [CrossRef]
- Isaeva, O.; Osipov, V. Different strategies for cancer treatment: Mathematical modelling. Comput. Math. Methods Med. 2009, 10, 253–272. [Google Scholar] [CrossRef]
- Tunali, I.; Gillies, R.J.; Schabath, M.B. Application of radiomics and artificial intelligence for lung cancer precision medicine. Cold Spring Harb. Perspect. Med. 2021, 11, a039537. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Kajani, A.; Haghjooy Javanmard, S.; Asadnia, M.; Razmjou, A. Recent advances in nanomaterials development for nanomedicine and cancer. ACS Appl. Bio Mater. 2021, 4, 5908–5925. [Google Scholar] [CrossRef]
- Kenner, B.; Chari, S.T.; Kelsen, D.; Klimstra, D.S.; Pandol, S.J.; Rosenthal, M.; Rustgi, A.K.; Taylor, J.A.; Yala, A.; Abul-Husn, N.; et al. Artificial intelligence and early detection of pancreatic cancer: 2020 summative review. Pancreas 2021, 50, 251. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, N. Cancer survival data emphasise importance of early diagnosis. BMJ 2019, 364, l408. [Google Scholar] [CrossRef] [PubMed]
- Why Is Early Diagnosis Important? Cancer Research UK. Available online: https://www.cancerresearchuk.org/about-cancer/cancer-symptoms/why-is-early-diagnosis-important (accessed on 22 June 2021).
- Brown, M.L.; Yabroff, K.R. 12, Economic impact of cancer in the United States. Cancer Epidemiol. Prev. 2006, 202, 202–214. [Google Scholar]
- IJzerman, M.J.; Berghuis, A.S.; de Bono, J.S.; Terstappen, L.W. Health economic impact of liquid biopsies in cancer management. Expert Rev. Pharmacoecon. Outcomes Res. 2018, 18, 593–599. [Google Scholar] [CrossRef]
- Janovsky, C.C.P.S.; Bittencourt, M.S.; Novais, M.A.P.D.; Maciel, R.; Biscolla, R.P.M.; Zucchi, P. Thyroid cancer burden and economic impact on the Brazilian public health system. Arch. Endocrinol. Metab. 2018, 62, 537–544. [Google Scholar] [CrossRef]
- Shah, S.C.; Kayamba, V.; Peek, R.M., Jr.; Heimburger, D. Cancer control in low-and middle-income countries: Is it time to consider screening? J. Glob. Oncol. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Rivera-Franco, M.M.; Leon-Rodriguez, E. Delays in breast cancer detection and treatment in developing countries. Breast Cancer Basic Clin. Res. 2018, 12, 1178223417752677. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.; Yu, J.; Awolude, O.A.; Chuang, L. Cervical cancer worldwide. Curr. Probl. Cancer 2018, 42, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, O.; Yip, C.H.; Brooks, A.; Cabanes, A.; Caleffi, M.; Dunstan Yataco, J.A.; Gyawali, B.; McCormack, V.; McLaughlin de Anderson, M.; Mehrotra, R.; et al. Breast cancer early detection: A phased approach to implementation. Cancer 2020, 126, 2379–2393. [Google Scholar] [CrossRef]
- Cortes, J.; Perez-García, J.M.; Llombart-Cussac, A.; Curigliano, G.; El Saghir, N.S.; Cardoso, F.; Barrios, C.H.; Wagle, S.; Roman, J.; Harbeck, N.; et al. Enhancing global access to cancer medicines. CA Cancer J. Clin. 2020, 70, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Moodley, J.; Cairncross, L.; Naiker, T.; Constant, D. From symptom discovery to treatment-women’s pathways to breast cancer care: A cross-sectional study. BMC Cancer 2018, 18, 312. [Google Scholar] [CrossRef]
- Darj, E.; Chalise, P.; Shakya, S. Barriers and facilitators to cervical cancer screening in Nepal: A qualitative study. Sex. Reprod. Healthc. 2019, 20, 20–26. [Google Scholar] [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Derda, R.; Tang, S.K.Y.; Laromaine, A.; Mosadegh, B.; Hong, E.; Mwangi, M.; Mammoto, A.; Ingber, D.E.; Whitesides, G.M. Multizone paper platform for 3D cell cultures. PLoS ONE 2011, 6, e18940. [Google Scholar] [CrossRef] [PubMed]
- Ellerbee, A.K.; Phillips, S.T.; Siegel, A.C.; Mirica, K.A.; Martinez, A.W.; Striehl, P.; Jain, N.; Prentiss, M.; Whitesides, G.M. Quantifying colorimetric assays in paper-based microfluidic devices by measuring the transmission of light through paper. Anal. Chem. 2009, 81, 8447–8452. [Google Scholar] [CrossRef] [PubMed]
- Glavan, A.C.; Martinez, R.V.; Maxwell, E.J.; Subramaniam, A.B.; Nunes, R.M.D.; Soh, S.; Whitesides, G.M. Rapid fabrication of pressure-driven open-channel microfluidic devices in omniphobic R(F) paper. Lab Chip 2013, 13, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Glavan, A.C.; Christodouleas, D.C.; Mosadegh, B.; Yu, H.D.; Smith, B.S.; Lessing, J.; Fernández-Abedul, M.T.; Whitesides, G.M. Folding analytical devices for electrochemical ELISA in hydrophobic R(H) paper. Anal. Chem. 2014, 86, 11999–12007. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Wiley, B.J.; Gupta, M.; Whitesides, G.M. FLASH: A rapid method for prototyping paper-based microfluidic devices. Lab Chip 2008, 8, 2146. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Nie, Z.; Cheng, C.M.; Carrilho, E.; Wiley, B.J.; Whitesides, G.M. Programmable diagnostic devices made from paper and tape. Lab Chip 2010, 10, 2499–2504. [Google Scholar] [CrossRef]
- Martinez, R.V.; Fish, C.R.; Chen, X.; Whitesides, G.M. Elastomeric Origami: Programmable Paper-Elastomer Composites as Pneumatic Actuators. Adv. Funct. Mater. 2012, 22, 1376–1384. [Google Scholar] [CrossRef]
- Nie, Z.; Deiss, F.; Liu, X.; Akbulut, O.; Whitesides, G.M. Integration of paper-based microfluidic devices with commercial electrochemical readers. Lab Chip 2010, 10, 3163–3169. [Google Scholar] [CrossRef]
- Nemiroski, A.; Christodouleas, D.C.; Hennek, J.W.; Kumar, A.A.; Maxwell, E.J.; Fernández-Abedul, M.T.; Whitesides, G.M. Universal mobile electrochemical detector designed for use in resource-limited applications. Proc. Natl. Acad. Sci. USA 2014, 111, 11984–11989. [Google Scholar] [CrossRef]
- Lan, W.J.; Maxwell, E.J.; Parolo, C.; Bwambok, D.K.; Subramaniam, A.B.; Whitesides, G.M. Paper-based electroanalytical devices with an integrated, stable reference electrode. Lab Chip 2013, 13, 4103–4108. [Google Scholar] [CrossRef]
- Lan, W.J.; Zou, X.U.; Hamedi, M.M.; Hu, J.; Parolo, C.; Maxwell, E.J.; Bühlmann, P.; Whitesides, G.M. Paper-based potentiometric ion sensing. Anal. Chem. 2014, 86, 9548–9553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, A.C.; Phillips, S.T.; Wiley, B.J.; Whitesides, G.M. Thin, lightweight, foldable thermochromic displays on paper. Lab Chip 2009, 9, 2775–2781. [Google Scholar] [CrossRef]
- Mosadegh, B.; Dabiri, B.E.; Lockett, M.R.; Derda, R.; Campbell, P.; Parker, K.K.; Whitesides, G.M. rThree-Dimensional Paper-Based Model for Cardiac Ischemia. Adv. Healthc. Mater. 2014, 3, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Lessing, J.; Glavan, A.C.; Walker, S.B.; Keplinger, C.; Lewis, J.A.; Whitesides, G.M. Inkjet printing of conductive inks with high lateral resolution on omniphobic “R(F) paper” for paper-based electronics and MEMS. Adv. Mater. 2014, 26, 4677–4682. [Google Scholar] [CrossRef]
- Liu, X.; Mwangi, M.; Li, X.; O’Brien, M.; Whitesides, G.M. Paper-based piezoresistive MEMS sensors. Lab Chip 2011, 11, 2189–2196. [Google Scholar] [CrossRef]
- Glavan, A.C.; Martinez, R.V.; Subramaniam, A.B.; Yoon, H.J.; Nunes, R.M.; Lange, H.; Thuo, M.M.; Whitesides, G.M. Omniphobic “RF paper” produced by silanization of paper with fluoroalkyltrichlorosilanes. Adv. Funct. Mater. 2014, 24, 60–70. [Google Scholar] [CrossRef]
- Thuo, M.M.; Martinez, R.V.; Lan, W.J.; Liu, X.; Barber, J.; Atkinson, M.B.; Bandarage, D.; Bloch, J.F.; Whitesides, G.M. Fabrication of low-cost paper-based microfluidic devices by embossing or cut-and-stack methods. Chem. Mater. 2014, 26, 4230–4237. [Google Scholar] [CrossRef]
- Mazzeo, A.D.; Kalb, W.B.; Chan, L.; Killian, M.G.; Bloch, J.F.; Mazzeo, B.A.; Whitesides, G.M. Paper-based, capacitive touch pads. Adv. Mater. 2012, 24, 2850–2856. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Lin, H.T.; Chen, T.H.; Chen, C.A.; Chang, H.T.; Chen, C.F. Signal amplified gold nanoparticles for cancer diagnosis on paper-based analytical devices. ACS Sens. 2018, 3, 174–182. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef]
- Ito, E.; Iha, K.; Yoshimura, T.; Nakaishi, K.; Watabe, S. Early diagnosis with ultrasensitive ELISA. Adv. Clin. Chem. 2021, 101, 121–133. [Google Scholar] [PubMed]
- Arya, S.K.; Estrela, P. Recent advances in enhancement strategies for electrochemical ELISA-based immunoassays for cancer biomarker detection. Sensors 2018, 18, 2010. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. Advantages, disadvantages and modifications of conventional ELISA. In Enzyme-Linked Immunosorbent Assay (ELISA); Springer: Berlin/Heidelberg, Germany, 2018; pp. 67–115. [Google Scholar]
- Qiu, J.; Keyser, B.; Lin, Z.T.; Wu, T. Autoantibodies as potential biomarkers in breast cancer. Biosensors 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Estrela, P. Electrochemical ELISA-based platform for bladder cancer protein biomarker detection in urine. Biosens. Bioelectron. 2018, 117, 620–627. [Google Scholar] [CrossRef]
- Al Mughairy, B.; Al-Lawati, H.A. Recent analytical advancements in microfluidics using chemiluminescence detection systems for food analysis. TrAC Trends Anal. Chem. 2020, 124, 115802. [Google Scholar] [CrossRef]
- Tonkinson, J.L.; Stillman, B.A. Nitrocellulose: A tried and true polymer finds utility as a post-genomic substrate. Front. Biosci.-Landmark 2002, 7, 1–12. [Google Scholar]
- Jiang, Q.; Han, T.; Ren, H.; Aziz, A.U.R.; Li, N.; Zhang, H.; Zhang, Z.; Liu, B. Bladder cancer hunting: A microfluidic paper-based analytical device. Electrophoresis 2020, 41, 1509–1516. [Google Scholar] [CrossRef]
- Evans, E.; Gabriel, E.F.M.; Coltro, W.K.T.; Garcia, C.D. Rational selection of substrates to improve color intensity and uniformity on microfluidic paper-based analytical devices. Analyst 2014, 139, 2127–2132. [Google Scholar] [CrossRef]
- Swahn, B. A new micromethod for the determination of total lipids in serum. Scand. J. Clin. Lab. Investig. 1952, 4, 247–248. [Google Scholar] [CrossRef]
- Chandler, R.; Gutierrez, C. The filter-paper method of suction measurement. Geotechnique 1986, 36, 265–268. [Google Scholar] [CrossRef]
- Chardon, W.; Menon, R.; Chien, S. Iron oxide impregnated filter paper (Pi test): A review of its development and methodological research. Nutr. Cycl. Agroecosystems 1996, 46, 41–51. [Google Scholar] [CrossRef]
- Fawcett, R.; Collis-George, N. A filter-paper method for determining the moisture characteristics of soil. Aust. J. Exp. Agric. 1967, 7, 162–167. [Google Scholar] [CrossRef]
- Partridge, S. Filter-paper partition chromatography of sugars: I. General description and application to the quantitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by RG Westall. Biochem. J. 1948, 42, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Li, M.; Yan, X.; Xie, M.; Liu, L.N.; Li, Z.; Xu, F. Comparison of paper-based nucleic acid extraction materials for point-of-care testing applications. Cellulose 2022, 29, 2479–2495. [Google Scholar] [CrossRef] [PubMed]
- Minamide, L.; Bamburg, J. A filter paper dye-binding assay for quantitative determination of protein without interference from reducing agents or detergents. Anal. Biochem. 1990, 190, 66–70. [Google Scholar] [CrossRef]
- Aryal, R.; Terman, P.; Voss, K.J. Comparison of two filter-based reflectance methods to measure the light absorption by atmospheric aerosols. J. Atmos. Ocean. Technol. 2014, 31, 923–929. [Google Scholar] [CrossRef]
- Rauf, S.; Ali, Y.; Hussain, S.; Ullah, F.; Hayat, A. Design of a novel filter paper-based construct for rapid analysis of acetone. PLoS ONE 2018, 13, e0199978. [Google Scholar] [CrossRef]
- Kurdekar, A.; Chunduri, L.; Bulagonda, E.P.; Haleyurgirisetty, M.K.; Kamisetti, V.; Hewlett, I.K. Comparative performance evaluation of carbon dot-based paper immunoassay on Whatman filter paper and nitrocellulose paper in the detection of HIV infection. Microfluid. Nanofluidics 2016, 20, 99. [Google Scholar] [CrossRef]
- Fierro-Mercado, P.M.; Hernández-Rivera, S.P. Highly sensitive filter paper substrate for SERS trace explosives detection. Int. J. Spectrosc. 2012, 2012, 716527. [Google Scholar] [CrossRef]
- Gan, W.; Zhuang, B.; Zhang, P.; Han, J.; Li, C.X.; Liu, P. A filter paper-based microdevice for low-cost, rapid, and automated DNA extraction and amplification from diverse sample types. Lab Chip 2014, 14, 3719–3728. [Google Scholar] [CrossRef]
- Kim, H.; Prezzi, M.; Salgado, R. Calibration of Whatman Grade 42 filter paper for soil suction measurement. Can. J. Soil Sci. 2016, 97, 93–98. [Google Scholar] [CrossRef]
- Mei, J.V.; Zobel, S.D.; Hall, E.M.; De Jesús, V.R.; Adam, B.W.; Hannon, W.H. Performance properties of filter paper devices for whole blood collection. Bioanalysis 2010, 2, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Rottinghaus, E.; Bile, E.; Modukanele, M.; Maruping, M.; Mine, M.; Nkengasong, J.; Yang, C. Comparison of Ahlstrom grade 226, Munktell TFN, and Whatman 903 filter papers for dried blood spot specimen collection and subsequent HIV-1 load and drug resistance genotyping analysis. J. Clin. Microbiol. 2013, 51, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Langer, E.K.; Johnson, K.J.; Shafer, M.M.; Gorski, P.; Overdier, J.; Musselman, J.; Ross, J.A. Characterization of the elemental composition of newborn blood spots using sector-field inductively coupled plasma-mass spectrometry. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Linton, C.J.; Miles, S.J.; Campbell, C.K.; Johnson, E.M. Ultra-rapid preparation of total genomic DNA from isolates of yeast and mould using Whatman FTA filter paper technology–a reusable DNA archiving system. Med. Mycol. 2006, 44, 389–398. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Santos, G.; Liu, N.; Tsao, M.S.; Kamel-Reid, S.; Chin, K.; Geddie, W.R. Detection of EGFR and KRAS mutations in fine-needle aspirates stored on Whatman FTA cards: Is this the tool for biobanking cytological samples in the molecular era? Cancer Cytopathol. 2010, 118, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Cards, F. FTA Cards for Preservation of Nucleic Acids for Molecular Assays. Arch. Pathol. Lab. Med. 2018, 142, 308–312. [Google Scholar]
- Davis, E.H.; Velez, J.O.; Russell, B.J.; Basile, A.J.; Brault, A.C.; Hughes, H.R. Evaluation of Whatman FTA cards for the preservation of yellow fever virus RNA for use in molecular diagnostics. PLoS Neglected Trop. Dis. 2022, 16, e0010487. [Google Scholar] [CrossRef]
- Kurien, B.T.; Scofield, R.H. Western blotting. Methods 2006, 38, 283–293. [Google Scholar] [CrossRef]
- Mansfield, M.A. The Use of Nitrocellulose Membranes in Lateral-Flow Assays. In Drugs of Abuse: Body Fluid Testing; Wong, R.C., Tse, H.Y., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 71–85. [Google Scholar] [CrossRef]
- Koga, H.; Nagashima, K.; Suematsu, K.; Takahashi, T.; Zhu, L.; Fukushima, D.; Huang, Y.; Nakagawa, R.; Liu, J.; Uetani, K.; et al. Nanocellulose Paper Semiconductor with a 3D Network Structure and Its Nano–Micro–Macro Trans-Scale Design. ACS Nano 2022, 16, 8630–8640. [Google Scholar] [CrossRef]
- Tang, R.; Liu, L.; Li, M.; Yao, X.; Yang, Y.; Zhang, S.; Li, F. Transparent microcrystalline cellulose/polyvinyl alcohol paper as a new platform for three-dimensional cell culture. Anal. Chem. 2020, 92, 14219–14227. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Soum, V.; Lee, S.N.; Choi, J.H.; Shin, J.H.; Shin, K.; Oh, B.K. Pumpless three-dimensional photo paper–based microfluidic analytical device for automatic detection of thioredoxin-1 using enzyme-linked immunosorbent assay. Anal. Bioanal. Chem. 2022, 414, 3219–3230. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Luo, J.; Xiong, Y.; Ming, T.; Liu, J.; Ma, Y.; Yan, S.; Yang, Y.; Yang, Z.; et al. Low sample volume origami-paper-based graphene-modified aptasensors for label-free electrochemical detection of cancer biomarker-EGFR. Microsyst. Nanoeng. 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Diamandis, E.P. Cancer biomarkers: Can we turn recent failures into success? J. Natl. Cancer Inst. 2010, 102, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.; Fussenegger, M. Emerging biomedical applications of synthetic biology. Nat. Rev. Genet. 2012, 13, 21–35. [Google Scholar] [CrossRef]
- Zourob, M.; Elwary, S.; Khademhosseini, A. Recognition Receptors in Biosensors; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Justino, C.I.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Santos, T.A.R. Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Whitcombe, M.J. Designing Receptors for The Next Generation of Biosensors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 12. [Google Scholar]
- Zhang, X.; Soori, G.; Dobleman, T.J.; Xiao, G.G. The application of monoclonal antibodies in cancer diagnosis. Expert Rev. Mol. Diagn. 2014, 14, 97–106. [Google Scholar] [CrossRef]
- Modjtahedi, H.; Ali, S.; Essapen, S. Therapeutic application of monoclonal antibodies in cancer: Advances and challenges. Br. Med. Bull. 2012, 104, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Van Gelder, V.; Amaladoss, A.; Patel, K.H. Covalent binding of antibodies to cellulose paper discs and their applications in naked-eye colorimetric immunoassays. J. Vis. Exp. 2016, e54111. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.A.; McLiesh, H.; Then, W.L.; Garnier, G. Activity and longevity of antibody in paper-based blood typing diagnostics. Front. Chem. 2018, 6, 193. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.H.; Liu, L.N.; Zhang, S.F.; He, X.C.; Li, X.J.; Xu, F.; Ni, Y.H.; Li, F. A review on advances in methods for modification of paper supports for use in point-of-care testing. Microchim. Acta 2019, 186, 521. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.D.; Gaudet, R.G. Antibodies in infectious diseases: Polyclonals, monoclonals and niche biotechnology. New Biotechnol. 2011, 28, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Airo, F.; Merkoçi, A. Enhanced gold nanoparticle based ELISA for a breast cancer biomarker. Anal. Chem. 2010, 82, 1151–1156. [Google Scholar] [CrossRef]
- Ji, S.; Lee, M.; Kim, D. Detection of early stage prostate cancer by using a simple carbon nanotube@ paper biosensor. Biosens. Bioelectron. 2018, 102, 345–350. [Google Scholar] [CrossRef]
- Shao, N.; Wickstrom, E.; Panchapakesan, B. Nanotube–antibody biosensor arrays for the detection of circulating breast cancer cells. Nanotechnology 2008, 19, 465101. [Google Scholar] [CrossRef]
- Loeian, M.S.; Aghaei, S.M.; Farhadi, F.; Rai, V.; Yang, H.W.; Johnson, M.D.; Aqil, F.; Mandadi, M.; Rai, S.N.; Panchapakesan, B. Liquid biopsy using the nanotube-CTC-chip: Capture of invasive CTCs with high purity using preferential adherence in breast cancer patients. Lab Chip 2019, 19, 1899–1915. [Google Scholar] [CrossRef]
- Khoshroo, A.; Fattahi, A.; Hosseinzadeh, L. Development of paper-based aptasensor for circulating tumor cells detection in the breast cancer. J. Electroanal. Chem. 2022, 910, 116182. [Google Scholar] [CrossRef]
- Prasad, K.S.; Abugalyon, Y.; Li, C.; Xu, F.; Li, X. A new method to amplify colorimetric signals of paper-based nanobiosensors for simple and sensitive pancreatic cancer biomarker detection. Analyst 2020, 145, 5113–5117. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Y.; Liu, J.; Bing, X.; Hua, J.; Zhang, H. A paper-based detection method of cancer cells using the photo-thermal effect of nanocomposite. J. Pharm. Biomed. Anal. 2016, 117, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, P.J.J.; Ding, J.; Liu, J. Aptamer-based biosensors for biomedical diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-based biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Han, K.; Liang, Z.; Zhou, N. Design strategies for aptamer-based biosensors. Sensors 2010, 10, 4541–4557. [Google Scholar] [CrossRef]
- Khosravi, F.; Loeian, S.M.; Panchapakesan, B. Ultrasensitive Label-Free Sensing of IL-6 Based on PASE Functionalized Carbon Nanotube Micro-Arrays with RNA-Aptamers as Molecular Recognition Elements. Biosensors 2017, 7, 17. [Google Scholar] [CrossRef]
- Liang, L.; Su, M.; Li, L.; Lan, F.; Yang, G.; Ge, S.; Yu, J.; Song, X. Aptamer-based fluorescent and visual biosensor for multiplexed monitoring of cancer cells in microfluidic paper-based analytical devices. Sens. Actuators B Chem. 2016, 229, 347–354. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Luo, J.; Liu, J.; Wang, L.; Fan, Y.; Yan, S.; Yang, Y.; Cai, X. A novel label-free microfluidic paper-based immunosensor for highly sensitive electrochemical detection of carcinoembryonic antigen. Biosens. Bioelectron. 2016, 83, 319–326. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Wang, Y.; Luo, J.; Xu, H.; Xu, S.; Cai, X. A wireless point-of-care testing system for the detection of neuron-specific enolase with microfluidic paper-based analytical devices. Biosens. Bioelectron. 2017, 95, 60–66. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
- Cass, A.E.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.; Turner, A.P. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. A paper-based electrochemical immunosensor with reduced graphene oxide/thionine/gold nanoparticles nanocomposites modification for the detection of cancer antigen 125. Biosens. Bioelectron. 2019, 135, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xue, P.; Kang, Y.; Hui, K.M. based microfluidic electrochemical immunodevice integrated with nanobioprobes onto graphene film for ultrasensitive multiplexed detection of cancer biomarkers. Anal. Chem. 2013, 85, 8661–8668. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Boryczka, J.; Kasani, S.; Wu, N. Enabling direct protein detection in a drop of whole blood with an “on-strip” plasma separation unit in a paper-based lateral flow strip. Anal. Chem. 2020, 93, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, U.H.; Alagappan, P.; Liedberg, B. Naked eye detection of lung cancer associated miRNA by paper-based biosensing platform. Anal. Chem. 2013, 85, 820–824. [Google Scholar] [CrossRef]
- Källander, K.; Tibenderana, J.K.; Akpogheneta, O.J.; Strachan, D.L. Zelee Hill, Augustinus HA ten Asbroek, Lesong Conteh, Betty R Kirkwood, and Sylvia R Meek. 2013. Mobile health (mHealth) approaches and lessons for increased performance and retention of community health workers in low-and middle-income countries: A review. J. Med. Internet Res. 2013, 15, e17. [Google Scholar] [CrossRef]
- Aydindogan, E.; Guler Celik, E.; Timur, S. Paper-Based analytical methods for smartphone sensing with functional nanoparticles: Bridges from smart surfaces to global health. Anal. Chem. 2018, 90, 12325–12333. [Google Scholar]
- Kassal, P.; Horak, E.; Sigurnjak, M.; Steinberg, M.D.; Steinberg, I.M. Wireless and mobile optical chemical sensors and biosensors. Rev. Anal. Chem. 2018, 37, 20170024. [Google Scholar] [CrossRef]
- Chandra Kishore, S.; Samikannu, K.; Atchudan, R.; Perumal, S.; Edison, T.N.J.I.; Alagan, M.; Sundramoorthy, A.K.; Lee, Y.R. Smartphone-Operated Wireless Chemical Sensors: A Review. Chemosensors 2022, 10, 55. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Khan, H.; Yang, L. A dual-readout paper-based sensor for on-site detection of penicillinase with a smartphone. Sens. Actuators B Chem. 2021, 335, 129707. [Google Scholar] [CrossRef]
- Shrivas, K.; Monisha; Patel, S.; Thakur, S.S.; Shankar, R. Food safety monitoring of the pesticide phenthoate using a smartphone-assisted paper-based sensor with bimetallic Cu@ Ag core–shell nanoparticles. Lab Chip 2020, 20, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Niu, X.; Li, X.; He, Y.; Song, H.; Peng, Y.; Pan, J.; Qiu, F.; Zhao, H.; Lan, M. A smartphone-integrated ready-to-use paper-based sensor with mesoporous carbon-dispersed Pd nanoparticles as a highly active peroxidase mimic for H2O2 detection. Sens. Actuators B Chem. 2018, 265, 412–420. [Google Scholar] [CrossRef]
- Shrivas, K.; Monisha; Kant, T.; Karbhal, I.; Kurrey, R.; Sahu, B.; Sinha, D.; Patra, G.K.; Deb, M.K.; Pervez, S. Smartphone coupled with paper-based chemical sensor for on-site determination of iron (III) in environmental and biological samples. Anal. Bioanal. Chem. 2020, 412, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Chatterjee, S.; Bandyopadhyay, S.; Kar, S.; Som, N.K.; Saha, S.; Chakraborty, S. Smartphone-enabled paper-based hemoglobin sensor for extreme point-of-care diagnostics. ACS Sens. 2021, 6, 1077–1085. [Google Scholar] [CrossRef]
- Wang, K.; Yang, J.; Xu, H.; Cao, B.; Qin, Q.; Liao, X.; Wo, Y.; Jin, Q.; Cui, D. Smartphone-imaged multilayered paper-based analytical device for colorimetric analysis of carcinoembryonic antigen. Anal. Bioanal. Chem. 2020, 412, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. Smartphone-based electrochemical system with multi-walled carbon nanotubes/thionine/gold nanoparticles modified screen-printed immunosensor for cancer antigen 125 detection. Microchem. J. 2022, 174, 107044. [Google Scholar] [CrossRef]
- Feng, Q.M.; Pan, J.B.; Zhang, H.R.; Xu, J.J.; Chen, H.Y. Disposable paper-based bipolar electrode for sensitive electrochemiluminescence detection of a cancer biomarker. Chem. Commun. 2014, 50, 10949–10951. [Google Scholar] [CrossRef]
- Tian, R.; Li, Y.; Bai, J. Hierarchical assembled nanomaterial paper-based analytical devices for simultaneously electrochemical detection of microRNAs. Anal. Chim. Acta 2019, 1058, 89–96. [Google Scholar] [CrossRef]
- Fakhri, N.; Abarghoei, S.; Dadmehr, M.; Hosseini, M.; Sabahi, H.; Ganjali, M.R. Paper-based colorimetric detection of miRNA-21 using Ag/Pt nanoclusters. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2020, 227, 117529. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, H.; Yu, X.; Wang, W. A microfluidic paper-based laser-induced fluorescence sensor based on duplex-specific nuclease amplification for selective and sensitive detection of miRNAs in cancer cells. Talanta 2020, 216, 120996. [Google Scholar] [CrossRef]
- Eid, M.; Rashed, A.N.Z.; Bulbul, A.A.M.; Podder, E. Mono-rectangular core photonic crystal fiber (MRC-PCF) for skin and blood cancer detection. Plasmonics 2021, 16, 717–727. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.J.; Ahn, Y.J.; Choi, S.; Lee, G.J. A simple and facile paper-based colorimetric assay for detection of free hydrogen sulfide in prostate cancer cells. Sens. Actuators B Chem. 2018, 256, 828–834. [Google Scholar] [CrossRef]
- Mazzu-Nascimento, T.; Morbioli, G.G.; Milan, L.A.; Donofrio, F.C.; Mestriner, C.A.; Carrilho, E. Development and statistical assessment of a paper-based immunoassay for detection of tumor markers. Anal. Chim. Acta 2017, 950, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Cao, X.; Gao, N.; Jin, Q.; Sanjay, S.T.; Henao-Pabon, G.; Li, X. A low-cost nanomaterial-based electrochemical immunosensor on paper for high-sensitivity early detection of pancreatic cancer. Sens. Actuators B Chem. 2020, 305, 127516. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, B.; Tian, C.; Yu, F.; Zhou, N.; Zhan, Y.; Chen, L. Rotational paper-based electrochemiluminescence immunodevices for sensitive and multiplexed detection of cancer biomarkers. Anal. Chim. Acta 2018, 1007, 33–39. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, S.; Xue, J.; Zhou, X.; Lu, W.; Li, G.; Cao, X. Highly sensitive detection of cytochrome c in the NSCLC serum using a hydrophobic paper-based–gold nanourchin substrate. Biomed. Opt. Express 2020, 11, 7062–7078. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Wang, Y.; Ge, S.; Liu, H.; Yan, M.; Yu, J. A paper-based electrochemiluminescence electrode as an aptamer-based cytosensor using PtNi@ carbon dots as nanolabels for detection of cancer cells and for in-situ screening of anticancer drugs. Microchim. Acta 2016, 183, 1873–1880. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Ming, T.; Luo, J.; Xing, Y.; Liu, J.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; et al. An origami paper-based nanoformulated immunosensor detects picograms of VEGF-C per milliliter of blood. Commun. Biol. 2021, 4, 121. [Google Scholar] [CrossRef]
- Sharma, B.; Parajuli, P.; Podila, R. Rapid detection of urokinase plasminogen activator using flexible paper-based graphene-gold platform. Biointerphases 2020, 15, 011004. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, R.; Huang, M.; Shen, J.; Shan, Y.; Xing, D. CRISPR/Cas13a powered portable electrochemiluminescence chip for ultrasensitive and specific MiRNA detection. Adv. Sci. 2020, 7, 1903661. [Google Scholar] [CrossRef]
- Mukama, O.; Wu, W.; Wu, J.; Lu, X.; Liu, Y.; Liu, Y.; Liu, J.; Zeng, L. A highly sensitive and specific lateral flow aptasensor for the detection of human osteopontin. Talanta 2020, 210, 120624. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Ge, L.; Yan, M.; Song, X.; Yu, J.; Liu, S. A disposable immunosensor device for point-of-care test of tumor marker based on copper-mediated amplification. Biosens. Bioelectron. 2013, 43, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Aydindogan, E.; Ceylan, A.E.; Timur, S. based colorimetric spot test utilizing smartphone sensing for detection of biomarkers. Talanta 2020, 208, 120446. [Google Scholar] [CrossRef] [PubMed]
- Bahavarnia, F.; Saadati, A.; Hassanpour, S.; Hasanzadeh, M.; Shadjou, N.; Hassanzadeh, A. Paper-based immunosensing of ovarian cancer tumor protein CA 125 using novel nano-ink: A new platform for efficient diagnosis of cancer and biomedical analysis using microfluidic paper-based analytical devices (μPAD). Int. J. Biol. Macromol. 2019, 138, 744–754. [Google Scholar] [CrossRef]
- Yen, Y.K.; Chao, C.H.; Yeh, Y.S. A graphene-PEDOT: PSS modified paper-based aptasensor for electrochemical impedance spectroscopy detection of tumor marker. Sensors 2020, 20, 1372. [Google Scholar] [CrossRef]
- Patel, D.; Shah, Y.; Thakkar, N.; Shah, K.; Shah, M. Implementation of artificial intelligence techniques for cancer detection. Augment. Hum. Res. 2020, 5, 6. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).