Recent Advances in Stretchable and Wearable Capacitive Electrophysiological Sensors for Long-Term Health Monitoring
Abstract
:1. Introduction
2. Sensing Strategies
2.1. Piezoresistive
2.2. Piezoelectric
2.3. Iontronic
2.4. Capacitive
3. Functional Materials for Wearable Sensors
3.1. Substrate Materials
3.2. Active Element/Electrode Materials
3.2.1. Carbon Materials
3.2.2. Metallic Materials
3.3. Hydrogels/Ion Gels in Wearable Electronics
3.3.1. Hydrogels
3.3.2. Iongels
4. Fabrication of Wearable Sensors
5. Wearable Capacitive Electrophysiological Sensors
5.1. Electrode-Skin Model
5.2. Design
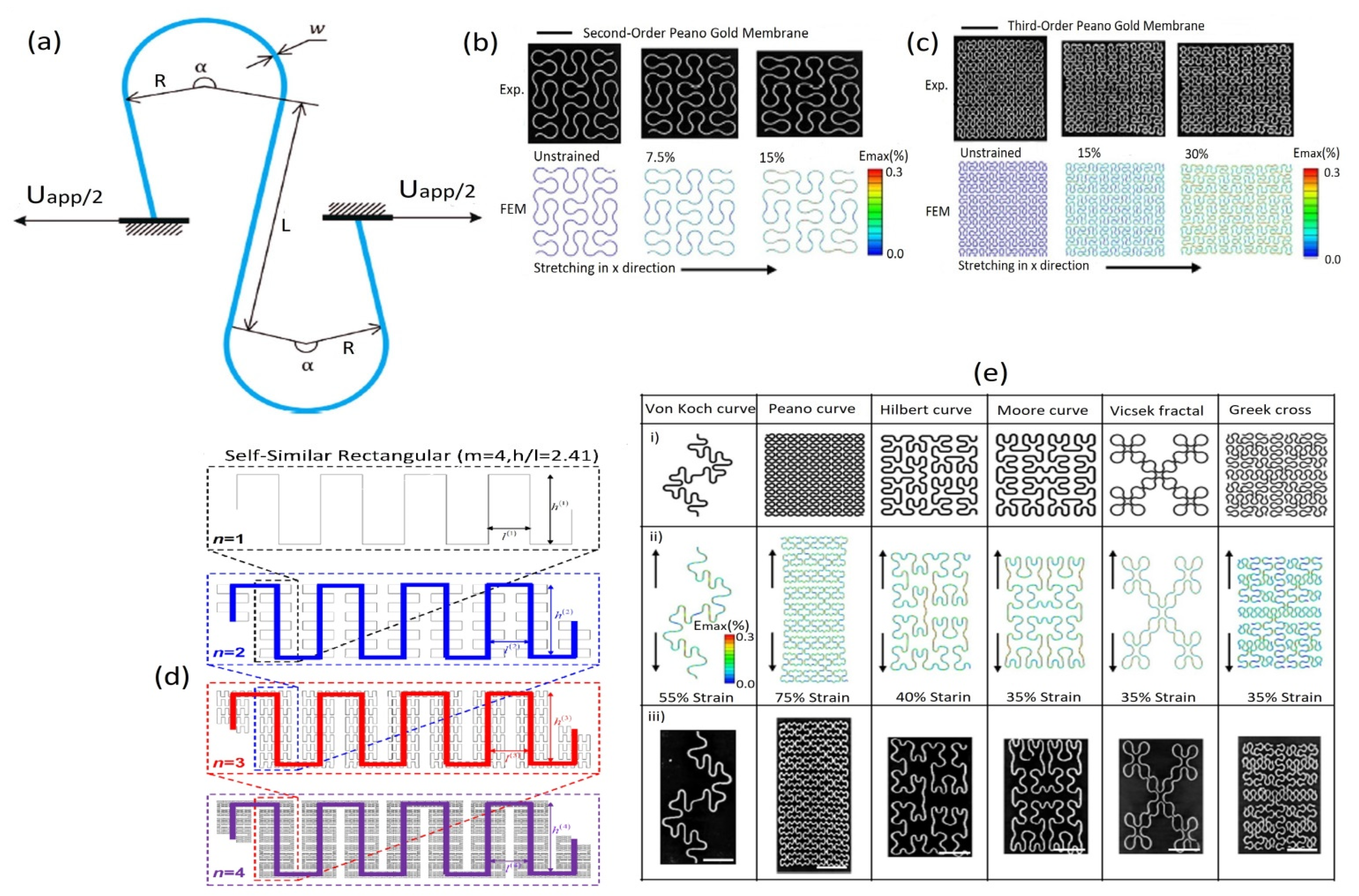
5.2.1. Out-of-Plane Design
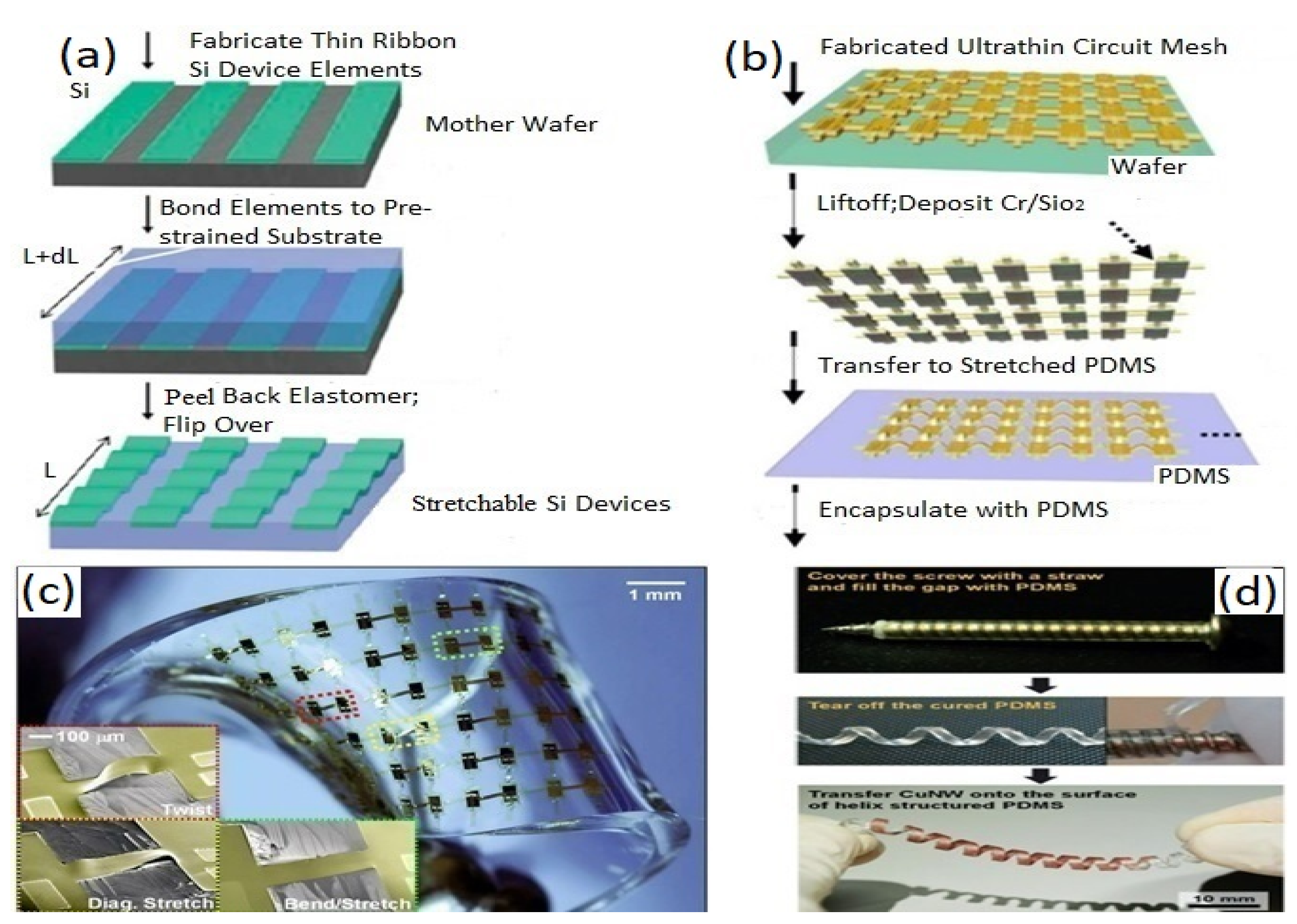
5.2.2. In-Plane Design
5.3. Fabrication and Implementation
6. Body-to-Electrode Signal Transduction and Measurements
7. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Yang, B.; Hua, Z.; Zhang, Z.; Guo, P.; Hao, D.; Gao, Y.; Huang, J. Recent advancements in flexible and wearable sensors for biomedical and healthcare applications. J. Phys. D Appl. Phys. 2021, 55, 134001. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Yang, T.; Li, X.; Zang, X.; Zhu, M.; Wang, K.; Wu, D.; Zhu, H. Wearable and highly sensitive graphene strain sensors for human motion monitoring. Adv. Funct. Mater. 2014, 24, 4666–4670. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, P.; Chou, J.B.; Xu, R.; Zhao, R.; Hart, A.J.; Kim, S.-G. Extremely Elastic Wearable Carbon Nanotube Fiber Strain Sensor for Monitoring of Human Motion. ACS Nano 2015, 9, 5929–5936. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Hyun, W.J.; Mun, S.C.; Park, Y.T.; Park, O.O. Highly stretchable and wearable graphene strain sensors with controllable sensitivity for human motion monitoring. ACS Appl. Mater. Interfaces 2015, 7, 6317–6324. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-molded flexible, ultrasensitive, and highly stable electronic skin for monitoring human physiological signals. Adv. Mater. 2014, 26, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Mishra, K.; Lau, K.; Aguirre-Pablo, A.A.; Hussain, M.M. Recyclable Nonfunctionalized Paper-Based Ultralow-Cost Wearable Health Monitoring System. Adv. Mater. Technol. 2017, 2, 1600228. [Google Scholar] [CrossRef]
- Tian, L.; Li, Y.; Webb, R.C.; Krishnan, S.; Bian, Z.; Song, J.; Ning, X.; Crawford, K.; Kurniawan, J.; Bonifas, A.; et al. Flexible and Stretchable 3ω Sensors for Thermal Characterization of Human Skin. Adv. Funct. Mater. 2017, 27, 1701282. [Google Scholar] [CrossRef]
- Webb, R.C.; Pielak, R.M.; Bastien, P.; Ayers, J.; Niittynen, J.; Kurniawan, J.; Manco, M.; Lin, A.; Cho, N.H.; Malyrchuk, V.; et al. Thermal Transport Characteristics of Human Skin Measured In Vivo Using Ultrathin Conformal Arrays of Thermal Sensors and Actuators. PLoS ONE 2015, 10, e0118131. [Google Scholar] [CrossRef]
- Su, M.; Li, F.; Chen, S.; Huang, Z.; Qin, M.; Li, W.; Zhang, X.; Song, Y. Nanoparticle Based Curve Arrays for Multirecognition Flexible Electronics. Adv. Mater. 2016, 28, 1369–1374. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Lao, J.; Zhang, R.; Zhang, Y.; Zhu, M.; Li, M.; Zang, X.; Wang, K.; Yu, W.; et al. Ultra-sensitive graphene strain sensor for sound signal acquisition and recognition. Nano Res. 2015, 8, 1627–1636. [Google Scholar] [CrossRef]
- Yi, L.; Jiao, W.; Wu, K.; Qian, L.; Yu, X.; Xia, Q.; Mao, K.; Yuan, S.; Wang, S.; Jiang, Y. Nanoparticle monolayer-based flexible strain gauge with ultrafast dynamic response for acoustic vibration detection. Nano Res. 2015, 8, 2978–2987. [Google Scholar] [CrossRef]
- Tao, L.-Q.; Tian, H.; Liu, Y.; Ju, Z.-Y.; Pang, Y.; Chen, Y.-Q.; Wang, D.-Y.; Tian, X.-G.; Yan, J.-C.; Deng, N.-Q.; et al. An intelligent artificial throat with sound-sensing ability based on laser induced graphene. Nat. Commun. 2017, 8, 14579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Yi, N.; Zhu, J.; Cheng, Z.; Yin, X.; Zhang, X.; Zhu, H.; Cheng, H. Novel gas sensing platform based on a stretchable laser-induced graphene pattern with self-heating capabilities. J. Mater. Chem. A 2020, 8, 6487–6500. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Cheng, H.Y. Flexible and stretchable metal oxide gas sensors for healthcare. Sci. China Technol. Sci. 2019, 62, 209–223. [Google Scholar] [CrossRef]
- Imani, S.; Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Xue, Y.; Xia, W.; Ray, T.R.; Reeder, J.; Bandodkar, A.; Kang, D.; Xu, S.; Huang, Y.; Rogers, J. Soft, skin-mounted microfluidic systems for measuring secretory fluidic pressures generated at the surface of the skin by eccrine sweat glands. Lab. Chip. 2017, 17, 2572–2580. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Kim, M.K.; Cheng, H.; Yeo, W.-H.; Huang, X.; Liu, Y.; Zhang, Y.; Huang, Y.; Rogers, J.A. Capacitive epidermal electronics for electrically safe, long-term electrophysiological measurements. Adv. Healthc. Mater. 2014, 3, 642–648. [Google Scholar] [CrossRef]
- Yeo, W.-H.; Kim, Y.-S.; Lee, J.; Ameen, A.; Shi, L.; Li, M.; Wang, S.; Ma, R.; Jin, S.H.; Kang, J.; et al. Multifunctional Epidermal Electronics Printed Directly Onto the Skin. Adv. Mater. 2013, 25, 2773–2778. [Google Scholar] [CrossRef]
- Norton, J.J.S.; Lee, D.S.; Lee, J.W.; Lee, W.; Kwon, O.; Won, P.; Jung, S.-Y.; Cheng, H.; Jeong, J.-W.; Akce, A.; et al. Soft, curved electrode systems capable of integration on the auricle as a persistent brain-computer interface. Proc. Natl. Acad. Sci. USA 2015, 112, 3920–3925. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Cheng, X.; Xiong, T.; Wang, X. Stretchable bio-potential electrode with self-similar serpentine structure for continuous, long-term, stable ECG recordings. Biomed. Microdev. 2019, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Shahandashti, P.F.; Pourkheyrollah, H.; Jahanshahi, A.; Ghafoorifard, H. Highly conformable stretchable dry electrodes based on inexpensive flex substrate for long-term biopotential (EMG/ECG) monitoring. Sens. Actuat. A Phys. 2019, 295, 678–686. [Google Scholar] [CrossRef]
- Koo, J.H.; Jeong, S.; Shim, H.J.; Son, D.; Kim, J.; Kim, D.C.; Choi, S.; Hong, J.-I.; Kim, D.-H. Wearable Electrocardiogram Monitor Using Carbon Nanotube Electronics and Color-Tunable Organic Light-Emitting Diodes. ACS Nano 2017, 11, 10032–10041. [Google Scholar] [CrossRef] [PubMed]
- Searle, A.; Kirkup, L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 2000, 21, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.M.; Jung, T.P.; Cauwenberghs, G. Dry-contact and noncontact biopotential electrodes: Methodological review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.-W.; Yeo, W.-H.; Akhtar, A.; Norton, J.J.S.; Kwack, Y.-J.; Li, S.; Jung, S.-Y.; Su, Y.; Lee, W.; Xia, J.; et al. Materials and optimized designs for human-machine interfaces via epidermal electronics. Adv. Mater. 2013, 25, 6839–6846. [Google Scholar] [CrossRef]
- Mishra, S.; Norton, J.J.S.; Lee, Y.; Lee, D.S.; Agee, N.; Chen, Y.; Chun, Y.; Yeo, W.-H. Soft, conformal bioelectronics for a wireless human-wheelchair interface. Biosens. Bioelectron. 2017, 91, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Akhtar, A.; Liu, Y.; Chen, H.; Yeo, W.-H.; Park, S., II; Boyce, B.; Kim, H.; Yu, J.; Lai, H.-Y.; et al. An Epidermal Stimulation and Sensing Platform for Sensorimotor Prosthetic Control, Management of Lower Back Exertion, and Electrical Muscle Activation. Adv. Mater. 2016, 28, 4462–4471. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.G.; Kim, K.K.; Park, K.S. ECG recording on a bed during sleep without direct skin-contact. IEEE Trans. Biomed. Eng. 2007, 54, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Leleux, P.; Johnson, C.; Strakosas, X.; Rivnay, J.; Hervé, T.; Owens, R.M.; Malliaras, G.G. Ionic Liquid Gel-Assisted Electrodes for Long-Term Cutaneous Recordings. Adv. Healthc. Mater. 2014, 3, 1377–1380. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Yang, J.; Xu, C.; Zhang, Q.; Peng, Z. Ionic Gels and Their Applications in Stretchable Electronics. Macromol. Rapid Commun. 2018, 39, 1800246. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Lonjaret, T.; Sardon, S.; Marcilla, R.; Hervé, T.; Malliaras, G.G.; Ismailova, E.; Mecerreyes, D. Cholinium-based ion gels as solid electrolytes for long-term cutaneous electrophysiology. J. Mater. Chem. C 2018, 3, 8942–8948. [Google Scholar] [CrossRef] [Green Version]
- Shay, T.; Velev, O.D.; Dickey, M.D. Soft electrodes combining hydrogel and liquid metal. Soft Matter 2018, 14, 296–3303. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, Q.; He, P.; Liu, K.; Ni, Y.; Ouyang, X.; Chen, L.; Huang, L.; Wang, H.; Tan, Y. Mussel-Inspired Nanocomposite Hydrogel-Based Electrodes with Reusable and Injectable Properties for Human Electrophysiological Signals Detection. ACS Sustain. Chem. Eng. 2019, 7, 7918–7925. [Google Scholar] [CrossRef]
- Feng, B.; Wei, H.; Shi, B.; Zhao, D.; Ye, S.; Wu, G.; Wang, R.; Zuo, G.; Wu, Z.; Chen, Z.; et al. Sleeping Heart Monitoring Using Hydrogel-Textile Capacitive ECG Electrodes. IEEE Sens. J. 2022, 22, 9255–9267. [Google Scholar] [CrossRef]
- Nagamine, K.; Chihara, S.; Kai, H.; Kaji, H.; Nishizawa, M. Totally shape-conformable electrode/hydrogel composite for on-skin electrophysiological measurements. Sens. Actuator. B Chem. 2016, 237, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Chang, C.L.; Chien, T.F.; Luo, C.H. Flexible PDMS electrode for one-point wearable wireless bio-potential acquisition. Sens. Actuat. A Phys. 2013, 203, 20–28. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, C.-L.; Chang, C.-W.; Lai, S.-C.; Chien, T.-F.; Huang, H.-Y.; Chiou, J.-C.; Luo, C.-H. A Low-Power Bio-Potential Acquisition System with Flexible PDMS Dry Electrodes for Portable Ubiquitous Healthcare Applications. Sensors 2013, 13, 3077–3091. [Google Scholar] [CrossRef]
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D printed dry electrode for ECG/EEG recording. Sens. Actuat. A Phys. 2012, 174, 96–102. [Google Scholar] [CrossRef]
- O’Mahony, C.; Pini, F.; Blake, A.; Webster, C.; O’Brien, J.; McCarthy, K.G. Microneedle-based electrodes with integrated through-silicon via for biopotential recording. Sens. Actuat. A Phys. 2012, 186, 130–136. [Google Scholar] [CrossRef]
- Jung, H.-C.; Moon, J.-H.; Baek, D.-H.; Lee, J.-H.; Choi, Y.-Y.; Hong, J.-S.; Lee, S.-H. CNT/PDMS composite flexible dry electrodesfor long-term ECG monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Kim, D.H.; McCormick, M.; Coleman, T.; Rogers, J. A stretchable electrode array for non-invasive, skin-mounted measurement of electrocardiography (ECG), electromyography (EMG) and electroencephalography (EEG). In Proceedings of the Annual International Conference of IEEE Engineering in Medicine and Biology Society (EMBC), Buenos Aires, Argentina, 31 August–4 September 2010; Volume 2010, pp. 6405–6408. [Google Scholar]
- Oh, T.I.; Yoon, S.; Kim, T.E.; Wi, H.; Kim, K.J.; Woo, E.J.; Sadleir, R.J. Nanofiber web textile dry electrodes for long-term biopotential recording. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 204–211. [Google Scholar] [PubMed]
- Lee, S.M.; Byeon, H.J.; Lee, J.H.; Baek, D.H.; Lee, K.H.; Hong, J.S.; Lee, S.-H. Self-adhesive epidermal carbon nanotube electronics for tether-free long-term continuous recording of biosignals. Sci. Rep. 2014, 4, 6074. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.C.; Huang, H.; Zhu, Y. Wearable silver nanowire dry electrodes for electrophysiological sensing. RSC Adv. 2015, 5, 11627–11632. [Google Scholar] [CrossRef]
- Jung, H.; Kwon, D.; Lee, S.A.; Kim, Y.J.; Ahn, J.W. Carbon Nanofiber-Based Wearable Patches for Bio-Potential Monitoring. J. Med. Biol. Eng. 2019, 39, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Haick, H.; Guo, S.; Wang, C.; Lee, S.; Yokota, T.; Someya, T. Skin bioelectronics towards long-term, continuous health monitoring. Chem. Soc. Rev. 2022, 51, 3759–3793. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yang, L.; Gravina, R.; Fortino, G. ANFIS fusion algorithm for eye movement recognition via soft multi-functional electronic skin. Inf. Fusion 2021, 71, 99–108. [Google Scholar] [CrossRef]
- Leleux, P.; Badier, J.-M.; Rivnay, J.; Bénar, C.; Hervé, T.; Chauvel, P.; Malliaras, G.G. Conducting Polymer Electrodes for Electroencephalograph. Adv. Healthc. Mater. 2014, 3, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.R.; Duarte, L.; Rodrigues, D.; Martins, A.C.; Machado, A.V.; Vaz, F.; Fiedler, P.; Haueisen, J.; Nóbrega, J.M.; Fonseca, C. Development of a quasi-dry electrode for EEG recording. Sens. Actuat. A Phys. 2013, 199, 310–317. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Y.-C.; Nicolini, L.; Pasupathy, P.; Sacks, J.; Su, B.; Yang, R.; Sanchez, D.; Chang, Y.-F.; Wang, P.; et al. Cut-and-Paste’ Manufacture of Multiparametric Epidermal Sensor Systems. Adv. Mater. 2015, 27, 6423–6430. [Google Scholar] [CrossRef]
- Wang, X.; Dong, L.; Zhang, H.; Yu, R.; Pan, C.; Wang, Z.L. Recent Progress in Electronic Skin. Adv. Sci. 2015, 2, 1500169. [Google Scholar] [CrossRef]
- Lacour, S.P.; Benmerah, S.; Tarte, E.; Gerald, J.F.; Serra, J.; McMahon, S.; Fawcett, J.; Graudejus, O.; Yu, Z.; Morrison, B. Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces. Med. Biol. Eng. Comput. 2010, 48, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Delivopoulos, E.; Chew, D.J.; Minev, I.R.; Fawcett, J.W.; Lacour, S.P. Concurrent recordings of bladder afferents from multiple nerves using a microfabricated PDMS microchannel electrode array. Lab Chip 2012, 12, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- Nittala, A.S.; Steimle, J. Next Steps in Epidermal Computing: Opportunities and Challenges for Soft On-Skin Devices. CHI Conf. Hum. Factors Comput. Syst. 2022, 2022, 1–22. [Google Scholar]
- Hammock, M.L.; Chortos, A.; Tee, B.C.-K.; Tok, J.B.-H.; Bao, Z. 25th Anniversary Article: The Evolution of Electronic Skin (E-Skin): A Brief History, Design Considerations, and Recent Progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, S.H.; Ha, K.H.; Lee, S.; Lu, N.; Kim, D.H. Wearable and Implantable Soft Bioelectronics: Device Designs and Material Strategies. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 359–391. [Google Scholar] [CrossRef]
- Fujita, T.; Shiono, S.; Kanda, K.; Maenaka, K.; Hamada, H.; Higuchi, K. Flexible sensor for human monitoring system by using P (VDF/TrFE) thin film. In Proceedings of the International Conference on Emerging Trends in Engineering and Technology (ICETET), Himeji, Japan, 5–7 November 2012. [Google Scholar]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwödiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458–463. [Google Scholar] [CrossRef]
- Ha, D.; De Vries, W.N.; John, S.W.M.; Irazoqui, P.P.; Chappell, W.J. Polymer-based miniature flexible capacitive pressure sensor for intraocular pressure (IOP) monitoring inside a mouse eye. Biomed. Microdevices 2012, 14, 207–215. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, Q.; Ding, Y.; Lin, Z.; Wang, C.; Li, Y.; Xu, F.; Li, J.; Yuan, Y.; He, X.; et al. Lightweight, Superelastic, and Mechanically Flexible Graphene/Polyimide Nanocomposite Foam for Strain Sensor Application. ACS Nano 2015, 9, 8933–8941. [Google Scholar] [CrossRef]
- Wagner, S.; Bauer, S. Materials for stretchable electronics. MRS Bull. 2012, 37, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Zhu, J.; Yeom, B.; Prima, M.D.; Su, X.; Kim, J.-G.; Yoo, S.J.; Uher, C.; Kotov, N.A. Stretchable nanoparticle conductors with self-organized conductive pathways. Nature 2013, 500, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.; Jaisutti, R.J.; Lee, H.; Lee, W.; Heo, J.-S.; Lee, J.-Y.; Park, S.K.; Kim, Y.-H. Highly Sensitive Textile Strain Sensors and Wireless User-Interface Devices Using All-Polymeric Conducting Fibers. ACS Appl. Mater. Interfaces 2017, 9, 10190–10197. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Honda, W.; Harada, S.; Arie, T.; Akita, S. Toward flexible and wearable human-interactive health-monitoring devices. Adv. Healthc. Mater. 2015, 4, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Matsuhisa, N.; Liu, Z.; Qi, D.; Cai, P.; Jiang, Y.; Wan, C.; Cui, Y.; Leow, W.R.; Liu, Z.; et al. Plasticizing Silk Protein for On-Skin Stretchable Electrodes. Adv. Mater. 2018, 30, e1800129. [Google Scholar] [CrossRef]
- Carlson, A.; Bowen, A.M.; Huang, Y.; Nuzzo, R.G.; Rogers, J.A. Transfer printing techniques for materials assembly and micro/nanodevice fabrication. Adv. Mater. 2012, 24, 5284–5318. [Google Scholar] [CrossRef] [PubMed]
- Windmiller, J.R.; Bandodkar, A.J.; Valdés-Ramírez, G.; Parkhomovsky, S.; Martinez, A.G.; Wang, J. Electrochemical sensing based on printable temporary transfer tattoos. Chem. Commun. 2012, 48, 6794–6796. [Google Scholar] [CrossRef]
- Takei, K. Printed Multifunctional Flexible Healthcare Patch. In Proceedings of the 2018 International Flexible Electronics Technology Conference (IFETC 2018), Ottawa, ON, Canada, 7–9 August 2018. [Google Scholar]
- Ma, L.Y.; Soin, N. Recent Progress in Printed Physical Sensing Electronics for Wearable Health-Monitoring Devices: A Review. IEEE Sens. J. 2022, 22, 3844–3859. [Google Scholar] [CrossRef]
- Hussin, H.; Soin, N.; Hatta, S.F.W.M.; Rezali, F.A.M.; Wahab, Y.A. Review—Recent Progress in the Diversity of Inkjet-Printed Flexible Sensor Structures in Biomedical Engineering Applications. J. Electrochem. Soc. 2021, 168, 077508. [Google Scholar] [CrossRef]
- Kim, D.H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, S.; Islam, A.; et al. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, C.; Sun, X.; Huang, W. Current development of materials science and engineering towards epidermal sensors. Prog. Mater. Sci. 2022, 128, 100962. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Kafy, A.; Zhai, L.; Ko, H.U.; Mun, S.; Kim, J. Transparent and flexible cellulose nanocrystal/reduced graphene oxide film for proximity sensing. Small 2015, 11, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, X.; Song, W. Physical and Chemical Sensors on the Basis of Laser-Induced Graphene: Mechanisms, Applications, and Perspectives. ACS Nano 2021, 15, 18708–18741. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Mitra, D.; Peterson, K.; Maharbiz, M.M. A highly elastic, capacitive strain gauge based on percolating nanotube networks. Nano Lett. 2012, 12, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Jost, K.; Stenger, D.; Perez, C.R.; McDonough, J.K.; Lian, K.; Gogotsia, Y.; Dion, G. Knitted and screen printed carbon-fiber supercapacitors for applications in wearable electronics. Energy Environ. Sci. 2013, 6, 2698–2705. [Google Scholar] [CrossRef]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 2014, 5, 3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Jiu, J.; Nogi, M.; Sugahara, T.; Nagao, S.; Koga, H.; He, P.; Suganumab, K. A highly sensitive and flexible pressure sensor with electrodes and elastomeric interlayer containing silver nanowires. Nanoscale 2015, 7, 2926–2932. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Chen, K.; Chuang, S.; Yu, K.M.; Kiriya, D.; Javey, A. Highly uniform and stable n-type carbon nanotube transistors by using positively charged silicon nitride thin films. Nano Lett. 2015, 15, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.H.; Takei, K.; Wang, C.; Ju, Y.; Kim, J.; Yu, Z.; Takahashi, T.; Cho, G.; Javey, A. Fully printed, high performance carbon nanotube thin-film transistors on flexible substrates. Nano Lett. 2013, 13, 3864–3869. [Google Scholar] [CrossRef]
- Park, B.S.; Ha, T.J. Carbon-based pressure sensors with wavy configuration. IEEE Electron Device Lett. 2017, 38, 979–982. [Google Scholar] [CrossRef]
- Kim, T.; Seong, N.; Ha, J.; Kim, H.; Ha, T.J.; Hong, Y. The rapid and dense assembly of solution-processed single-wall carbon nanotube semiconducting films: Via an acid-based additive in the aqueous dispersion. J. Mater. Chem. C 2016, 4, 5461–5468. [Google Scholar] [CrossRef]
- Kang, B.C.; Ha, T.J. Wearable carbon nanotube based dry-electrodes for electrophysiological sensors. Jpn. J. Appl. Phys. 2018, 57, 05GD02. [Google Scholar] [CrossRef]
- Maiti, R.; Gerhardt, L.-C.; Lee, Z.S.; Byers, R.A.; Woods, D.; Sanz-Herrera, J.A.; Franklin, S.E.; Roger Lewis, R.; Matcher, S.J.; Carré, M.J. In vivo measurement of skin surface strain and sub-surface layer deformation induced by natural tissue stretching. J. Mech. Behav. Biomed. Mater. 2016, 62, 556–569. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, S.; Li, X.; Fan, J.A.; Xu, S.; Song, Y.M.; Choi, K.-J.; Yeo, W.-H.; Lee, W.; Nazaar, S.N.; et al. Experimental and Theoretical Studies of Serpentine Microstructures Bonded To Prestrained Elastomers for Stretchable Electronics. Adv. Funct. Mater. 2014, 24, 2028–2037. [Google Scholar] [CrossRef]
- Jang, K.-I.; Li, K.; Chung, H.U.; Xu, S.; Jung, H.N.; Yang, Y.; Kwak, J.W.; Jung, H.H.; Song, J.; Yang, C.; et al. Self-assembled three dimensional network designs for soft electronics. Nat. Commun. 2017, 8, 15894. [Google Scholar] [CrossRef]
- Huang, Y.A.; Donga, W.; Huang, T.; Wang, Y.; Xiao, L.; Su, Y.; Yin, Z. Self-similar design for stretchable wireless LC strain sensors. Sens. Actuat. A Phys. 2015, 224, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.A.; Yeo, W.-H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S.-Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; et al. Fractal design concepts for stretchable electronics. Nat. Commun. 2014, 5, 3266. [Google Scholar] [CrossRef] [Green Version]
- Jang, K.-I.; Chung, H.U.; Xu, S.; Lee, C.H.; Luan, H.; Jeong, J.; Cheng, H.; Kim, G.-T.; Han, S.Y.; Lee, J.W.; et al. Soft network composite materials with deterministic and bio-inspired designs. Nat. Commun. 2015, 6, 6566. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Pharr, M.; Ma, Y.; Ning, R.; Yan, Z.; Xu, R.; Feng, X.; Huang, Y.; Rogers, J.A. Experimental and Theoretical Studies of Serpentine Interconnects on Ultrathin Elastomers for Stretchable Electronics. Adv. Funct. Mater. 2017, 27, 1702589. [Google Scholar] [CrossRef]
- Lee, C.H.; Ma, Y.; Jang, K.-I.; Banks, A.; Pan, T.; Feng, X.; Kim, J.S.; Kang, D.; Raj, M.S.; McGrane, B.L.; et al. Soft Core/Shell Packages for Stretchable Electronics. Adv. Funct. Mater. 2015, 25, 3698–3704. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Chen, K.; Shin, W.-J.; Lu, C.-J.; Kong, G.-W.; Patnaik, D.; Lee, S.-H.; Cortes, J.F.; Rogers, J.A. Stretchable, Wireless Sensors and Functional Substrates for Epidermal Characterization of Sweat. Small 2014, 10, 3083–3090. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Cheng, H.; Shin, W.-J.; Fan, J.A.; Liu, Z.; Lu, C.-J.; Kong, G.-W.; Chen, K.; Patnaik, D.; et al. Materials and Designs for Wireless Epidermal Sensors of Hydration and Strain. Adv. Funct. Mater. 2014, 24, 3846–3854. [Google Scholar] [CrossRef]
- Huang, X.; Yeo, W.H.; Liu, Y.; Rogers, J.A. Epidermal differential impedance sensor for conformal skin hydration monitoring. Biointerphases 2012, 7, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, K.I.; Han, S.Y.; Xu, S.; Mathewson, K.E.; Zhang, Y.; Jeong, J.-W.; Kim, G.-T.; Webb, R.C.; Lee, J.W.; Dawidczyk, T.J.; et al. Rugged and breathable forms of stretchable electronics with adherent composite substrates for transcutaneous monitoring. Nat. Commun. 2014, 5, 4779. [Google Scholar] [CrossRef]
- Jeong, Y.R.; Park, H.; Jin, S.W.; Hong, S.Y.; Lee, S.-S.; Ha, J.S. Highly Stretchable and Sensitive Strain Sensors Using Fragmentized Graphene Foam. Adv. Funct. Mater. 2015, 25, 4228–4236. [Google Scholar] [CrossRef]
- Luo, N.; Dai, W.; Li, C.; Zhou, Z.; Lu, L.; Poon, C.C.Y.; Chen, S.-C.; Zhang, Y.; Zhao, N. Flexible Piezoresistive Sensor Patch Enabling Ultralow Power Cuffless Blood Pressure Measurement. Adv. Funct. Mater. 2016, 26, 1178–1187. [Google Scholar] [CrossRef]
- Zhu, S.E.; Krishna Ghatkesar, M.; Zhang, C.; Janssen, G.C.A.M. Graphene based piezoresistive pressure sensor. Appl. Phys. Lett. 2013, 102, 161904. [Google Scholar] [CrossRef] [Green Version]
- Park, K., II; Son, J.H.; Hwang, G.-H.; Jeong, C.K.; Ryu, J.; Koo, M.; Choi, I.; Lee, S.H.; Byun, M.; Wang, Z.L.; et al. Highly-efficient, flexible piezoelectric PZT thin film nanogenerator on plastic substrates. Adv. Mater. 2014, 26, 2514–2520. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.-S.; Huang, Y.A.; Damadoran, A.R.; Xia, J.; Martin, L.W.; et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar]
- Mandal, D.; Yoon, S.; Kim, K.J. Origin of piezoelectricity in an electrospun poly(vinylidene fluoride-trifluoroethylene) nanofiber web-based nanogenerator and nano-pressure sensor. Macromol. Rapid Commun. 2011, 32, 831–837. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y.; Zheng, H.; Ramakrishna, S. Piezoelectric materials for flexible and wearable electronics: A review. Mater. Des. 2021, 211, 110164. [Google Scholar] [CrossRef]
- Nie, B.; Li, R.; Brandt, J.D.; Pan, T. Iontronic microdroplet array for flexible ultrasensitive tactile sensing. Lab Chip 2014, 14, 1107–1116. [Google Scholar] [CrossRef]
- Nie, B.; Li, R.; Cao, J.; Brandt, J.D.; Pan, T. Flexible Transparent Iontronic Film for Interfacial Capacitive Pressure Sensing. Adv. Mater. 2015, 27, 6055–6062. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Luo, H.; Qin, L.; Wang, X.; Xiong, Z.; Ding, H.; Gu, Y.; Liu, Z.; Zhang, T. Flexible Capacitive Tactile Sensor Based on Micropatterned Dielectric Layer. Small 2016, 12, 5042–5048. [Google Scholar] [CrossRef]
- Sheng, L.; Teo, S.; Liu, J. Liquid-Metal-Painted Stretchable Capacitor Sensors for Wearable Healthcare Electronics. J. Med. Biol. Eng. 2016, 36, 265–272. [Google Scholar] [CrossRef]
- Li, R.Z.; Hu, A.; Zhang, T.; Oakes, K.D. Direct writing on paper of foldable capacitive touch pads with silver nanowire inks. ACS Appl. Mater. Interfaces 2014, 6, 21721–21729. [Google Scholar] [CrossRef]
- Mazzeo, A.D.; Kalb, W.B.; Chan, L.; Killian, M.G.; Bloch, J.-F.; Mazzeo, B.A.; Whitesides, G.M. Paper-based, capacitive touch pads. Adv. Mater. 2012, 24, 2850–2856. [Google Scholar] [CrossRef]
- Torres, F.; Graças, M.D.; Melo, M.; Tosti, A. Management of contact dermatitis due to nickel allergy: An update. Clin. Cosmet. Investig. Dermatol. 2009, 2, 39–48. [Google Scholar]
- Lee, S.M.; Kim, J.H.; Byeon, H.J.; Choi, Y.Y.; Park, K.S.; Lee, S.H. A capacitive, biocompatible and adhesive electrode for long-term and cap-free monitoring of EEG signals. J. Neural Eng. 2013, 10, 036006. [Google Scholar] [CrossRef]
- Ueno, A.; Yamaguchi, T.; Iida, T.; Fukuoka, Y.; Uchikawa, Y.; Noshiro, M. Feasibility of capacitive sensing of surface electromyographic potential through cloth. Sens. Mater. 2012, 24, 335–346. [Google Scholar]
- Mannsfeld, S.C.B.; Tee, B.C.-K.; Stoltenberg, R.M.; Chen, C.V.H.-H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.-K.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, C.; Wang, Y.; Xiao, L.; Ye, D.; Huang, Y.A. Stretchable sEMG electrodes conformally laminated on skin for continuous electrophysiological monitoring. In Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2017; Volume 10464, pp. 77–86. [Google Scholar]
- Yan, Z.; Pan, T.; Xue, M.; Chen, C.; Cui, Y.; Yao, G.; Huang, L.; Liao, F.; Jing, W.; Zhang, H.; et al. Thermal Release Transfer Printing for Stretchable Conformal Bioelectronics. Adv. Sci. 2017, 4, 1700251. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.; Kovacs, P.; Laguna, P.; Meier, J.; Huemer, M. Ecg beat representation and delineation by means of variable projection. IEEE Trans. Biomed. Eng. 2021, 68, 2997–3008. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Shekar, D.D.; Hiremath, A.C.; Chemmangat, K. Detection of arrhythmia from electrocardiogram signals using a novel gaussian assisted signal smoothing and pattern recognition. Biomed. Signal Process. Control 2022, 73, 103469. [Google Scholar] [CrossRef]
- Lecun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Chen, C.; Hua, Z.; Zhang, R.; Liu, G.; Wen, W. Automated arrhythmia classification based on a combination network of CNN and LSTM. Biomed. Signal Process. Control 2020, 57, 101819. [Google Scholar] [CrossRef]
- Ullah, H.; Bu, Y.; Pan, T.; Gao, M.; Islam, S.; Lin, Y.; Lai, D. Cardiac Arrhythmia Recognition Using Transfer Learning with a Pre-trained DenseNet. In Proceedings of the 2nd International Conference on Pattern Recognition and Machine Learning (PRML), Chengdu, China, 16–18 July 2021. [Google Scholar]
- Ullah, H.; Heyat, M.B.B.; AlSalman, H.; Khan, H.M.; Akhtar, F.; Gumaei, A.; Mehdi, A.; Muaad, A.Y.; Islam, M.S.; Ali, A.; et al. An Effective and Lightweight Deep Electrocardiography Arrhythmia Recognition Model Using Novel Special and Native Structural Regularization Techniques on Cardiac Signal. J. Healthc. Eng. 2022, 2022, 3408501. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab A Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Chortos, A.; Yu, G.; Wang, Y.; Isaacson, S.; Allen, R.; Shi, Y.; Dauskardt, R.; Bao, Z. An ultra-sensitive resistive pressure sensor based on hollow-sphere microstructure induced elasticity in conducting polymer film. Nat. Commun. 2014, 5, 3002. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Hong, G. Wearable Electromechanical Sensors and Its Applications. In Wearable Devices—The Big Wave of Innovation, 1st ed.; Nasiri, N., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Choong, C.-L.; Shim, M.-B.; Lee, B.-S.; Jeon, S.; Ko, D.-S.; Kang, T.-H.; Bae, J.; Lee, S.H.; Byun, K.-E.; Im, J.; et al. Highly Stretchable Resistive Pressure Sensors Using a Conductive Elastomeric Composite on a Micropyramid Array. Adv. Mater. 2014, 26, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wei, Y.; Wei, S.; Lin, Y.; Liu, L. Ultrasensitive Cracking-Assisted Strain Sensors Based on Silver Nanowires/Graphene Hybrid Particles. ACS Appl. Mater. Interfaces 2016, 8, 25563–25570. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, X.; Jiang, X.; Lin, S.; Lao, J.; Shi, J.; Zhen, Z.; Li, Z.; Zhu, H. Structural engineering of gold thin films with channel cracks for ultrasensitive strain sensing. Mater. Horiz. 2016, 3, 248–255. [Google Scholar] [CrossRef]
- Song, H.; Zhang, J.; Chen, D.; Wang, K.; Niu, S.; Han, Z.; Ren, L. Superfast and high-sensitivity printable strain sensors with bioinspired micron-scale cracks. Nanoscale 2017, 9, 1166–1173. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, Z.; Liang, Q.; Liao, Q.; Zhang, Y. Flexible, cuttable, and self-waterproof bending strain sensors using microcracked gold nanofilms@paper substrate. ACS Appl. Mater. Interfaces 2017, 9, 4151–4158. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire-elastomer nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-D.; Ke, K.; Jia, J.; Pu, J.-H.; Zhao, X.; Bao, R.-Y.; Liu, Z.-Y.; Bai, L.; Zhang, K.; Yang, M.-B.; et al. Recent Advances in Multiresponsive Flexible Sensors towards E-skin: A Delicate Design for Versatile Sensing. Small 2022, 18, 2103734. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lei, K.F.; Tsai, S.W.; Tsang, N.M. Development of graphene-based sensors on paper substrate for the measurement of pH value of analyte. Biochip. J. 2016, 10, 182–188. [Google Scholar] [CrossRef]
- Kong, H.; Song, Z.; Li, W.; Bao, Y.; Qu, D.; Ma, Y.; Liu, Z.; Wang, W.; Wang, Z.; Han, D.; et al. Skin-Inspired Hair-Epidermis-Dermis Hierarchical Structures for Electronic Skin Sensors with High Sensitivity over a Wide Linear Range. ACS Nano 2021, 15, 16218–16227. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Qin, Y.; Xu, C.; Wei, Y.; Yang, R.; Wang, Z.L. Self-powered nanowire devices. Nat. Nanotechnol. 2010, 5, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, H.; Xie, G.; Jiang, Y.; Chen, C.; Su, Y.; Wang, Y.; Tai, H. Flexible piezoelectric pressure sensor based on polydopamine-modified BaTiO3/PVDF composite film for human motion monitoring. Sens. Actuat. A Phys. 2020, 301, 111789. [Google Scholar] [CrossRef]
- Lin, L.; Xie, Y.; Wang, S.; Wu, W.; Niu, S.; Wen, X.; Wang, Z.L. Triboelectric active sensor array for self-powered static and dynamic pressure detection and tactile imaging. ACS Nano 2013, 7, 8266–8274. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Shi, Y.; Joe, P.; Ghaffari, R.; Balooch, G.; Usgaonkar, K.; Gur, O.; Tran, P.L.; Crosby, J.R.; Meyer, M.; et al. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater. 2015, 14, 728–736. [Google Scholar] [CrossRef]
- Park, D.Y.; Joe, D.J.; Kim, D.H.; Park, H.; Han, J.H.; Jeong, C.K.; Park, H.; Park, J.G.; Joung, B.; Lee, K.J. Self-Powered Real-Time Arterial Pulse Monitoring Using Ultrathin Epidermal Piezoelectric Sensors. Adv. Mater. 2017, 29, 1702308. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Liu, Z.; Li, W.; Ruan, T.; Chen, X.; Liu, J.; Yang, B.; Zhang, W. Piezoelectric Dynamics of Arterial Pulse for Wearable Continuous Blood Pressure Monitoring. Adv. Mater. 2022, 34, 2110291. [Google Scholar] [CrossRef]
- Su, Y.; Chen, C.; Pan, H.; Yang, Y.; Chen, G.; Zhao, X.; Li, W.; Gong, Q.; Xie, G.; Zhou, Y.; et al. Muscle Fibers Inspired High-Performance Piezoelectric Textiles for Wearable Physiological Monitoring. Adv. Funct. Mater. 2021, 31, 2010962. [Google Scholar] [CrossRef]
- Lin, M.F.; Xiong, J.; Wang, J.; Parida, K.; Lee, P.S. Core-shell nanofiber mats for tactile pressure sensor and nanogenerator applications. Nano Energy 2018, 44, 248–255. [Google Scholar] [CrossRef]
- Nie, B.; Li, R.; Brandt, J.D.; Pan, T. Microfluidic tactile sensors for three-dimensional contact force measurements. Lab Chip 2014, 14, 4344–4353. [Google Scholar] [CrossRef]
- Nie, B.; Xing, S.; Brandt, J.D.; Pan, T. Droplet-based interfacial capacitive sensing. Lab Chip 2012, 12, 1110–1118. [Google Scholar] [CrossRef]
- Li, R.; Nie, B.; Digiglio, P.; Pan, T. Microflotronics: A flexible, transparent, pressure-sensitive microfluidic film. Adv. Funct. Mater. 2014, 24, 6195–6203. [Google Scholar] [CrossRef]
- Li, R.; Nie, B.; Zhai, C.; Cao, J.; Pan, J.; Chi, Y.-W.; Pan, T. Telemedical Wearable Sensing Platform for Management of Chronic Venous Disorder. Ann. Biomed. Eng. 2016, 44, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhu, Y. Wearable multifunctional sensors using printed stretchable conductors made of silver nanowires. Nanoscale 2014, 6, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.D.P.; Posner, J.D.; Santos, V.J. Flexible microfluidic normal force sensor skin for tactile feedback. Sens. Actuat. A Phys. 2012, 179, 62–69. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, H.; Seo, J.; Shin, S.; Koo, J.H.; Pang, C.P.; Son, S.; Kim, J.H.; Jang, Y.H.; Kim, D.E.; et al. Conductive fiber-based ultrasensitive textile pressure sensor for wearable electronics. Adv. Mater. 2015, 27, 2433–2439. [Google Scholar] [CrossRef]
- Tee, B.C.K.; Chortos, A.; Dunn, R.R.; Schwartz, G.; Eason, E.; Bao, Z. Tunable flexible pressure sensors using microstructured elastomer geometries for intuitive electronics. Adv. Funct. Mater. 2014, 24, 5427–5434. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Vosgueritchian, M.; Cheon, S.; Kim, H.; Koo, J.H.; Kim, T.R.; Lee, S.; Schwartz, G.; Chang, H.; et al. Stretchable energy-harvesting tactile electronic skin capable of differentiating multiple mechanical stimuli modes. Adv. Mater. 2014, 26, 7324–7332. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef]
- Tien, N.T.; Jeon, S.; Kim, D.-I.; Trung, T.Q.; Jang, M.; Hwang, B.-U.; Byun, K.-E.; Bae, J.; Lee, E.; Tok, J.B.-H.; et al. A flexible bimodal sensor array for simultaneous sensing of pressure and temperature. Adv. Mater. 2014, 26, 796–804. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Zhang, K.; Deng, H.; Fu, Q. Recent progress in flexible capacitive sensors: Structures and properties. Nano Mater. Sci. 2022. [Google Scholar] [CrossRef]
- Chortos, A.; Liu, J.; Bao, Z. Pursuing prosthetic electronic skin. Nat. Mater. 2016, 15, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, S.; Park, H.W.; Park, D.H.; Jeong, Y.; Kim, D.H. Highly Sensitive and Multimodal All-Carbon Skin Sensors Capable of Simultaneously Detecting Tactile and Biological Stimuli. Adv. Mater. 2015, 27, 4178–4185. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z. Ionic skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Koo, J.H.; Nguyen, A.; Caves, J.M.; Kim, M.-G.; Chortos, A.; Kim, K.; Wang, P.J.; Tok, J.B.-H.; Bao, Z. Highly Skin-Conformal Microhairy Sensor for Pulse Signal Amplification. Adv. Mater. 2015, 27, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hua, Q.; Yu, R.; Zhang, Y.; Pan, C. Flexible, Stretchable and Wearable Multifunctional Sensor Array as Artificial Electronic Skin for Static and Dynamic Strain Mapping. Adv. Electron. Mater. 2015, 1, 1500142. [Google Scholar] [CrossRef]
- An, B.W.; Heo, S.; Ji, S.; Bien, F.; Park, J.U. Transparent and flexible fingerprint sensor array with multiplexed detection of tactile pressure and skin temperature. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Pyo, S.; Choi, J.; Kim, J. Flexible, Transparent, Sensitive, and Crosstalk-Free Capacitive Tactile Sensor Array Based on Graphene Electrodes and Air Dielectric. Adv. Electron. Mater. 2018, 4, 1700427. [Google Scholar] [CrossRef]
- Wan, Y.; Qiu, Z.; Hong, Y.; Wang, Y.; Zhang, J.; Liu, Q.; Wu, Z.; Guo, C.F. A Highly Sensitive Flexible Capacitive Tactile Sensor with Sparse and High-Aspect-Ratio Microstructures. Adv. Electron. Mater. 2018, 4, 1700586. [Google Scholar] [CrossRef]
- Maiolino, P.; Maggiali, M.; Cannata, G.; Metta, G.; Natale, L. A flexible and robust large scale capacitive tactile system for robots. IEEE Sens. J. 2013, 13, 3910–3917. [Google Scholar] [CrossRef] [Green Version]
- Viry, L.; Levi, A.; Totaro, M.; Mondini, A.; Mattoli, V.; Mazzolai, B.; Beccai, L. Flexible three-axial force sensor for soft and highly sensitive artificial touch. Adv. Mater. 2014, 26, 2659–2664. [Google Scholar] [CrossRef] [Green Version]
- Amjadi, M.; Park, I. Sensitive and stable strain sensors based on the wavy structured electrodes. In Proceedings of the 14th IEEE Conference on Nanotechnology, Toronto, ON, Canada, 18–21 August 2014. [Google Scholar]
- Kanaparthi, S.; Sekhar, V.R.; Badhulika, S. Flexible, eco-friendly and highly sensitive paper antenna based electromechanical sensor for wireless human motion detection and structural health monitoring. Extrem. Mech. Lett. 2016, 9, 324–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, C.; Kim, W.S. Design optimized membrane-based flexible paper accelerometer with silver nano ink. Appl. Phys. Lett. 2013, 103, 073304. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, W.S. Highly Sensitive Flexible Printed Accelerometer System for Monitoring Vital Signs. Soft Robot. 2014, 1, 132–135. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Badhulik, S. Low cost, flexible and biodegradable touch sensor fabricated by solvent-free processing of graphite on cellulose paper. Sens. Actuat. B Chem. 2017, 242, 857–864. [Google Scholar] [CrossRef]
- Motha, L.; Kim, J.; Kim, W.S. Instrumented rubber insole for plantar pressure sensing. Org. Electron. 2015, 23, 82–86. [Google Scholar] [CrossRef]
- Kim, J.; Wang, Z.; Kim, W.S. Stretchable RFID for wireless strain sensing with silver nano ink. IEEE Sens. J. 2014, 14, 4395–4401. [Google Scholar] [CrossRef]
- Hassan, M.; Abbas, G.; Li, N.; Afzal, A.; Haider, Z.; Ahmed, S.; Xu, X.; Pan, C.; Peng, Z. Significance of Flexible Substrates for Wearable and Implantable Devices: Recent Advances and Perspectives. Adv. Mater. Technol. 2022, 7, 2100773. [Google Scholar] [CrossRef]
- Lee, C.; Jug, L.; Meng, E. High strain biocompatible polydimethylsiloxane-based conductive graphene and multiwalled carbon nanotube nanocomposite strain sensors. Appl. Phys. Lett. 2013, 102, 183511. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.A.; Kim, H.S.; Lee, S.S.; Park, M. Single-walled carbon nanotube/silicone rubber composites for compliant electrodes. Carbon 2012, 50, 444–449. [Google Scholar] [CrossRef]
- Choi, K.M.; Rogers, J.A. A photocurable poly(dimethylsiloxane) chemistry designed for soft lithographic molding and printing in the nanometer regime. J. Am. Chem. Soc. 2003, 125, 4060–4061. [Google Scholar] [CrossRef]
- Kokkinis, D.; Schaffner, M.; Studart, A.R. Multimaterial magnetically assisted 3D printing of composite materials. Nat. Commun. 2015, 6, 8643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, L.Q.; Wang, D.-Y.; Tian, H.; Ju, Z.; Liu, Y.; Chen, Y.-Q.; Xie, Q.-Y.; Zhao, H.; Yang, Y.; Ren, T. Tunable and wearable high performance strain sensors based on laser patterned graphene flakes. In Proceedings of the International Electron Devices Meeting, San Francisco, CA, USA, 3–7 December 2016. [Google Scholar]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.-C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Nyein, H.Y.Y.; Shahpar, Z.; Fahad, H.M.; Chen, K.C.; Emaminejad, S.; Gao, Y.; Tai, L.-C.; Ota, H.; Wu, E. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar] [CrossRef] [Green Version]
- Harito, C.; Listya Utari, L.; Putra, B.R.; Yuliarto, B.; Purwanto, S.; Zaidi, S.Z.J.Z.; Bavykin, D.V.; Marken, F.; Walsh, F.C. The development of wearable polymer-based sensors: Perspectives. J. Electrochem. Soc. 2020, 167, 037566. [Google Scholar] [CrossRef]
- Webb, R.C.; Bonifas, A.P.; Behnaz, A.; Zhang, Y.; Yu, K.J.; Cheng, H.; Shi, M.; Bian, Z.; Liu, Z.; Kim, Y.-S.; et al. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013, 12, 938–944. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, K.; Tian, G.; Jiang, B.; Huang, Y. Stretchable Electronics Based on PDMS Substrates. Adv. Mater. 2021, 33, 2003155. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Z.; Liu, Y.; Jiang, Y.; Leow, W.R.; Pal, M.; Pan, S.; Yang, H.; Wang, Y.; Zhang, X.; et al. Highly Stretchable, Compliant, Polymeric Microelectrode Arrays for In Vivo Electrophysiological Interfacing. Adv. Mater. 2017, 29, 1702800. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Kim, Y.J.; Yoon, T.L.; Park, S.A.; Cho, I.H.; Kim, E.J.; Kim, I.A.; Shin, J.-W. The characteristics of a hydroxyapatite-chitosan-PMMA bone cement. Biomaterials 2004, 25, 5715–5723. [Google Scholar] [CrossRef]
- Yang, W.; Gong, Y.; Li, W. A Review: Electrode and Packaging Materials for Neurophysiology Recording Implants. Front. Bioeng. Biotechnol. 2021, 8, 1515. [Google Scholar] [CrossRef]
- Weltman, A.; Yoo, J.; Meng, E. Flexible, Penetrating Brain Probes Enabled by Advances in Polymer Microfabrication. Micromachines 2016, 7, 180. [Google Scholar] [CrossRef] [Green Version]
- Kanyanta, V.; Ivankovic, A. Mechanical characterisation of polyurethane elastomer for biomedical applications. J. Mech. Behav. Biomed. Mater. 2010, 3, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; McCall, J.G.; Shin, G.; Zhang, Y.; Al-Hasani, R.; Kim, M.; Li, S.; Sim, J.Y.; Jang, K.-I.; Shi, Y.; et al. Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics. Cell 2015, 162, 662–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurra, N.; Kulkarni, G.U. Pencil-on-paper: Electronic devices. Lab Chip 2013, 13, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liao, Q.; Yan, X.; Liang, Q.; Si, H.; Li, M.; Wu, H.; Cao, S.; Zhang, Y. Flexible and highly sensitive strain sensors fabricated by pencil drawn for wearable monitor. Adv. Funct. Mater. 2015, 25, 2395–2401. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef]
- Lee, K.; Park, J.; Lee, M.-S.; Kim, J.; Hyun, B.G.; Kang, D.J.; Na, K.; Lee, C.Y.; Bien, F.; Park, J.-U. In-situ synthesis of carbon nanotube-graphite electronic devices and their integrations onto surfaces of live plants and insects. Nano Lett. 2014, 14, 2647–2654. [Google Scholar] [CrossRef]
- Roch, A.; Greifzu, M.; Talens, R.E.; Stepien, L.; Roch, T.; Hege, J.; Nong, N.V.; Schmiel, T.; Dani, I.; Leyens, C.; et al. Ambient effects on the electrical conductivity of carbon nanotubes. Carbon 2015, 95, 347–353. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, K.; Kim, S.-Y.; Lee, H.; Park, J.; Choi, K.-H.; Kim, H.-K.; Kim, D.-G.; Lee, D.-Y.; Nam, S.W.; et al. High-performance, transparent, and stretchable electrodes using graphene-metal nanowire hybrid structures. Nano Lett. 2013, 13, 2814–2821. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M.-S.; Jeon, S.; Kim, M.; Kim, S.; Kim, K.; Bien, F.; Hong, S.Y.; Park, J.-U. Highly transparent and stretchable field-effect transistor sensors using graphene-nanowire hybrid nanostructures. Adv. Mater. 2015, 27, 3292–3297. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, B.U.; Kim, D.; Kim, B.Y.; Lee, N.E. Stretchable, Transparent, Ultrasensitive, and Patchable Strain Sensor for Human-Machine Interfaces Comprising a Nanohybrid of Carbon Nanotubes and Conductive Elastomers. ACS Nano 2015, 9, 6252–6261. [Google Scholar] [CrossRef]
- Lu, C.C.; Lin, Y.C.; Yeh, C.H.; Huang, J.C.; Chiu, P.W. High mobility flexible graphene field-effect transistors with self-healing gate dielectrics. ACS Nano 2012, 6, 4469–4474. [Google Scholar] [CrossRef] [PubMed]
- Park, J.U.; Nam, S.; Lee, M.S.; Lieber, C.M. Synthesis of monolithic graphene-graphite integrated electronics. Nat. Mater. 2012, 11, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Sire, S.; Ardiaca, F.; Lepilliet, S.; Seo, J.-W.T.; Hersam, M.C.; Dambrine, G.D.; Happy, H.; Derycke, V. Flexible gigahertz transistors derived from solution-based single-layer graphene. Nano Lett. 2012, 12, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Xu, F.; Lu, W.; Zhu, Y. Controlled 3D buckling of silicon nanowires for stretchable electronics. ACS Nano 2011, 5, 672–678. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, Y. Highly conductive and stretchable silver nanowire conductors. Adv. Mater. 2012, 24, 5117–5122. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.; Ji, S.; Shin, S.-H.; Kim, S.-Y.; Kim, Y.-C.; Kim, J.-Y.; Park, J.-U. Fully-integrated, bezel-less transistor arrays using reversibly foldable interconnects and stretchable origami substrates. Nanoscale 2016, 8, 9504–9510. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, Y. Patterning of Metal Nanowire Networks: Methods and Applications. ACS Appl. Mater. Interfaces 2021, 13, 60736–60762. [Google Scholar] [CrossRef]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef] [Green Version]
- Sekitani, T.; Someya, T. Stretchable, large-area organic electronics. Adv. Mater. 2010, 22, 2228–2246. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wu, Z. A Microfluidic, Reversibly Stretchable, Large-Area Wireless Strain Sensor. Adv. Funct. Mater. 2011, 21, 2282–2290. [Google Scholar] [CrossRef]
- Liu, X.; Miao, J.; Fan, Q.; Zhang, W.; Zuo, X.; Tian, M.; Zhu, S.; Zhang, X.; Qu, L. Recent Progress on Smart Fiber and Textile Based Wearable Strain Sensors: Materials, Fabrications and Applications. Adv. Fiber Mater. 2022, 4, 361–389. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, F.; Huang, S.; Wang, H.; Yang, C.; Hang, T.; Tao, J.; Xia, W.; Xie, X. Progress of flexible strain sensors for physiological signal monitoring. Biosens. Bioelectron. 2022, 211, 114298. [Google Scholar] [CrossRef]
- Kim, H.J.; Son, C.; Ziaie, B. A multiaxial stretchable interconnect using liquid-alloy-filled elastomeric microchannels. Appl. Phys. Lett. 2008, 92, 011904. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.R.; Hayes, G.J.; Zhang, S.; Dickey, M.D.; Lazzi, G. A pressure responsive fluidic microstrip open stub resonator using a liquid metal alloy. IEEE Microw. Wirel. Compon. Lett. 2012, 22, 577–579. [Google Scholar] [CrossRef]
- Mazlouman, S.J.; Jiang, X.J.; Mahanfar, A.; Menon, C.; Vaughan, R.G. A reconfigurable patch antenna using liquid metal embedded in a silicone substrate. IEEE Trans. Antennas Propag. 2011, 59, 4406–4412. [Google Scholar] [CrossRef]
- Zhu, S.; So, J.-H.; Mays, R.; Desai, S.; Barnes, W.R.; Pourdeyhimi, B.; Dickey, M.D. Ultrastretchable Fibers with Metallic Conductivity Using a Liquid Metal Alloy Core. Adv. Funct. Mater. 2013, 23, 2308–2314. [Google Scholar] [CrossRef]
- Kim, D.H.; Song, J.; Choi, W.M.; Kim, H.-S.; Kim, R.-H.; Liu, Z.; Huang, Y.Y.; Hwang, K.C.; Zhang, Y.-W.; Rogers, J.A. Materials and noncoplanar mesh designs for integrated circuits with linear elastic responses to extreme mechanical deformations. Proc. Natl. Acad. Sci. USA 2008, 105, 18675–18680. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lu, B.; Chen, Y.; Feng, X. Breathable and Stretchable Temperature Sensors Inspired by Skin. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnaushenko, D.D.; Karnaushenko, D.; Makarov, D.; Schmidt, O.G. Compact helical antenna for smart implant applications. NPG Asia Mater. 2015, 7, e188. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, H.O.; Tao, A.R.; Schwartz, A.; Gracias, D.H.; Whitesides, G.M. Fabrication of a cylindrical display by patterned assembly. Science 2002, 296, 323–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Lee, D.-K.; Kim, B.-S.; Jeon, H.; Lee, N.-E.; Whang, D.; Lee, H.-J.; Kim, Y.J.; Ahn, J.-H. Stretchable, Transparent Zinc Oxide Thin Film Transistors. Adv. Funct. Mater. 2010, 20, 3577–3582. [Google Scholar] [CrossRef]
- Kim, R.-H.; Tao, H.; Kim, T.-I.; Zhang, Y.; Kim, S.; Panilaitis, B.; Yang, M.; Kim, D.-H.; Jung, Y.H.; Kim, B.H.; et al. Materials and Designs for Wirelessly Powered Implantable Light-Emitting Systems. Small 2012, 8, 2812–2818. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kumar, V.; Adesida, I.; Rogers, J.A. Buckled and Wavy Ribbons of GaAs for High-Performance Electronics on Elastomeric Substrates. Adv. Mater. 2006, 18, 2857–2862. [Google Scholar] [CrossRef]
- Wallentin, J.; Anttu, N.; Asoli, D.; Huffman, M.; Aberg, I.; Magnusson, M.H.; Siefer, G.; Fuss-Kailuweit, P.; Dimroth, F.; Witzigmann, B.; et al. InP nanowire array solar cells achieving 13.8% efficiency by exceeding the ray optics limit. Science 2013, 339, 1057–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwinner, M.C.; Pietro, R.D.; Vaynzof, Y.; Greenberg, K.J.; Ho, P.K.H.; Friend, R.F.; Sirringhaus, H. Doping of Organic Semiconductors Using Molybdenum Trioxide: A Quantitative Time-Dependent Electrical and Spectroscopic Study. Adv. Funct. Mater. 2011, 21, 1432–1441. [Google Scholar] [CrossRef]
- Mikhnenko, O.V.; Blom, P.W.M.; Nguyen, T.Q. Exciton diffusion in organic semiconductors. Energy Environ. Sci. 2015, 8, 1867–1888. [Google Scholar] [CrossRef]
- Sun, Y.; Choi, W.M.; Jiang, H.; Huang, Y.Y.; Rogers, J.A. Controlled buckling of semiconductor nanoribbons for stretchable electronics. Nat. Nanotechnol. 2006, 1, 201–207. [Google Scholar] [CrossRef]
- Kim, D.; Rogers, J.A. Stretchable Electronics: Materials Strategies and Devices. Adv. Mater. 2008, 20, 4887–4892. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Zhang, H.; Xie, M.; Duan, X. PEDOT:PSS: From conductive polymers to sensors. Nanotechnol. Precis. Eng. 2021, 4, 045004. [Google Scholar] [CrossRef]
- Jia, H.; Wang, J.; Zhang, X.; Wang, Y. Pen-writing polypyrrole arrays on paper for versatile cheap sensors. ACS Macro Lett. 2014, 3, 86–90. [Google Scholar] [CrossRef]
- Park, M.; Im, J.; Shin, M.; Min, Y.; Park, J.; Cho, H.; Park, S.; Shim, M.-B.; Jeon, S.; Chung, D.-Y.; et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotechnol. 2012, 7, 803–809. [Google Scholar] [CrossRef]
- Shang, S.; Zeng, W.; Tao, X.M. High stretchable MWNTs/polyurethane conductive nanocomposites. J. Mater. Chem. 2011, 21, 7274–7280. [Google Scholar] [CrossRef]
- Rahimzadeh, Z.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. An overview on the synthesis and recent applications of conducting poly(3,4-ethylenedioxythiophene) (PEDOT) in industry and biomedicine. J. Mater. Sci. 2020, 55, 7575–7611. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Sevil, B.; Zuhal, K. Synthesis and characterization of polypyrrole nanoparticles and their nanocomposites with poly(propylene). Macromol. Symp. 2010, 295, 59–64. [Google Scholar] [CrossRef]
- Wang, X.S.; Feng, X.Q. Effects of thickness on mechanical properties of conducting polythiophene films. J. Mater. Sci. Lett. 2002, 21, 715–717. [Google Scholar] [CrossRef]
- Park, D.-W.; Schendel, A.A.; Mikael, S.; Brodnick, S.K.; Richner, T.J.; Ness, J.P.; Hayat, M.R.; Atry, F.; Frye, S.T.; Pashaie, R.; et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, H.; Shim, B.S. Graphene: An emerging material for biological tissue engineering. Carbon Lett. 2013, 14, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Rusinek, C.A.; Thompson, C.H.; Setien, M.; Guo, Y.; Rechenberg, R.; Gong, Y.; Weber, A.J.; Becker, M.F.; Purcell, E.; et al. Flexible, diamond-based microelectrodes fabricated using the diamond growth side for neural sensing. Microsyst. Nanoeng. 2020, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jiang, S.; Grena, B.J.B.; Kimbrough, I.F.; Thompson, E.G.; Fink, Y.; Sontheimer, H.; Yoshinobu, T.; Jia, X. Polymer Composite with Carbon Nanofibers Aligned during Thermal Drawing as a Microelectrode for Chronic Neural Interfaces. ACS Nano 2017, 11, 6574–6585. [Google Scholar] [CrossRef]
- Lawrence, J.G.; Berhan, L.M.; Nadarajah, A. Elastic properties and morphology of individual carbon nanofibers. ACS Nano 2008, 2, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Vomero, M.; Castagnola, E.; Ciarpella, F.; Maggiolini, E.; Goshi, N.; Zucchini, E.; Carli, S.; Fadiga, L.; Kassegne, S.; Ricci, D. Highly Stable Glassy Carbon Interfaces for Long-Term Neural Stimulation and Low-Noise Recording of Brain Activity. Sci. Rep. 2017, 7, 40332. [Google Scholar] [CrossRef] [Green Version]
- Vomero, M.; Niekerk, P.V.; Nguyen, V.; Gong, N.; Hirabayashi, M.; Cinopri, A.; Logan, K.; Moghadasi, A.; Varma, P.; Kassegne, S. A novel pattern transfer technique for mounting glassy carbon microelectrodes on polymeric flexible substrates. J. Micromechanics Microengineering 2016, 26, 25018. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pana, X.; Lin, C.; Lin, D.; Ni, Y.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Biocompatible, self-wrinkled, antifreezing and stretchable hydrogel-based wearable sensor with PEDOT:sulfonated lignin as conductive materials. Chem. Eng. J. 2019, 370, 1039–1047. [Google Scholar] [CrossRef]
- Gilshteyn, E.P.; Lin, S.; Kondrashov, V.A.; Kopylova, D.S.; Tsapenko, A.P.; Anisimov, A.S.; Hart, A.J.; Zhao, X.; Nasibulin, A.G. A One-Step Method of Hydrogel Modification by Single-Walled Carbon Nanotubes for Highly Stretchable and Transparent Electronics. ACS Appl. Mater. Interfaces 2018, 10, 28069–28075. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Lee, W.; Yokota, T.; Fukuda, K.; Someya, T. Ultrathin Organic Electrochemical Transistor with Nonvolatile and Thin Gel Electrolyte for Long-Term Electrophysiological Monitoring. Adv. Funct. Mater. 2019, 29, 1906982. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, S.; Yin, R.; Li, L.; Lou, Z.; Shen, G. Recent advanced applications of ion-gel in ionic-gated transistor. npj Flex. Electron. 2021, 5, 13. [Google Scholar] [CrossRef]
- Luque, G.C.; Picchio, M.L.; Martins, A.P.S.; Dominguez-Alfaro, A.; Ramos, N.; Agua, I.D.; Marchiori, B.; Mecerreyes, D.; Minari, R.J.; Tomé, L.C. 3D Printable and Biocompatible Iongels for Body Sensor Applications. Adv. Electron. Mater. 2021, 7, 2100178. [Google Scholar] [CrossRef]
- Aguzin, A.; Luque, G.C.; Ronco, L.I.; Agua, I.D.; Guzmán-González, G.; Marchiori, B.; Gugliotta, A.; Tomé, L.C.; Gugliotta, L.M.; Mecerreyes, D.; et al. Gelatin and Tannic Acid Based Iongels for Muscle Activity Recording and Stimulation Electrodes. ACS Biomater. Sci. Eng. 2022, 8, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Zhao, C.X.; Wong, J.I.; Sun, X.W.; Yang, H.Y. Printed all-solid flexible microsupercapacitors: Towards the general route for high energy storage devices. Nanotechnology 2014, 25, 094010. [Google Scholar] [CrossRef]
- Kanoun, O.; Müller, C.; Benchirouf, A.; Sanli, A.; Dinh, T.N.; Al-Hamry, A.; Bu, L.; Gerlach, C.; Bouhamed, A. Flexible Carbon Nanotube Films for High Performance Strain Sensors. Sensors 2014, 14, 10042–10071. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Huang, X. Mechanisms and materials of flexible and stretchable skin sensors. Micromachines 2017, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Zhang, R.; Yang, T.; Lin, S.; Chen, Q.; Zhen, Z.; Xie, D.; Zhu, H. Foldable and electrically stable graphene film resistors prepared by vacuum filtration for flexible electronics. Surf. Coat. Technol. 2016, 299, 22–28. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, Z.; Liao, Q.; Liang, Q.; Ou, Y.; Xu, M.; Li, M.; Zhang, G.; Zhang, Y. Flexible and printable paper-based strain sensors for wearable and large-area green electronics. Nanoscale 2016, 8, 13025–13032. [Google Scholar] [CrossRef] [Green Version]
- Amjadi, M.; Yoon, Y.J.; Park, I. Ultra-stretchable and skin-mountable strain sensors using carbon nanotubes-Ecoflex nanocomposites. Nanotechnology 2015, 26, 375501. [Google Scholar] [CrossRef] [PubMed]
- Savagatrup, S.; Chan, E.; Renteria-Garcia, S.M.; Printz, A.D.; Zaretski, A.V.; O’Connor, T.F.; Rodriquez, D.; Valle, E.; Lipomi, D.J. Plasticization of PEDOT: PSS by common additives for mechanically robust organic solar cells and wearable sensors. Adv. Funct. Mater. 2015, 25, 427–436. [Google Scholar] [CrossRef]
- Zheng, Y.; He, Z.Z.; Yang, J.; Liu, J. Personal electronics printing via tapping mode composite liquid metal ink delivery and adhesion mechanism. Sci. Rep. 2014, 4, 4588. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, Z.; Gao, Y.; Liu, J. Direct desktop printed-circuits-on-paper flexible electronics. Sci. Rep. 2013, 3, srep01786. [Google Scholar] [CrossRef]
- Lee, H.; Cook, B.S.; Murali, K.P.; Raj, M.; Tentzeris, M.M. Inkjet Printed High-Q RF Inductors on Paper Substrate with Ferromagnetic Nanomaterial. IEEE Microw. Wirel. Compon. Lett. 2016, 26, 419–421. [Google Scholar] [CrossRef]
- Cook, B.S.; Cooper, J.R.; Tentzeris, M.M. Multi-layer RF capacitors on flexible substrates utilizing inkjet printed dielectric polymers. IEEE Microw. Wirel. Compon. Lett. 2013, 23, 353–355. [Google Scholar] [CrossRef]
- Riggs, B.C.; Elupula, R.; Grayson, S.M.; Chrisey, D.B. Photonic curing of aromatic thiol-ene click dielectric capacitors via inkjet printing. J. Mater. Chem. A 2014, 2, 17380–17386. [Google Scholar] [CrossRef]
- Graddage, N.; Chu, T.Y.; Ding, H.; Py, C.; Dadvand, A.; Tao, Y. Inkjet printed thin and uniform dielectrics for capacitors and organic thin film transistors enabled by the coffee ring effect. Org. Electron. 2016, 29, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Sou, A.; Gili, E.; Sirringhaus, H. Inkjet-printed resistors with a wide resistance range for printed read-only memory applications. Org. Electron. 2013, 14, 699–702. [Google Scholar] [CrossRef]
- Kang, B.J.; Lee, C.K.; Oh, J.H. All-inkjet-printed electrical components and circuit fabrication on a plastic substrate. Microelectron. Eng. 2012, 97, 251–254. [Google Scholar] [CrossRef]
- Verma, A.; Zink, D.M.; Fléchon, C.; Carballo, J.L.; Flügge, H.; Navarro, J.M.; Baumann, T.; Volz, D. Efficient, inkjet-printed TADF-OLEDs with an ultra-soluble NHetPHOS complex. Appl. Phys. A Mater. Sci. Process. 2016, 122, 191. [Google Scholar] [CrossRef]
- Haverinen, H.M.; Myllylä, R.A.; Jabbour, G.E.; Jabbour, G.E. Inkjet Printed RGB Quantum Dot-Hybrid LED. J. Disp. Technol. 2010, 6, 87–89. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dötz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 457, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Park, Y. Inkjet Etching of Polymers and Its Applications in Organic Electronic Devices. Polymers 2017, 9, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leigh, S.J.; Bradley, R.J.; Purssell, C.P.; Billson, D.R.; Hutchins, D.A. A Simple, Low-Cost Conductive Composite Material for 3D Printing of Electronic Sensors. PLoS ONE 2012, 7, e49365. [Google Scholar] [CrossRef]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Mengüç, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D Printing of Strain Sensors within Highly Stretchable Elastomers. Adv. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-Z.; Qiu, K.; Meng, F.; Park, S.H.; McAlpine, M.C. 3D Printed Stretchable Tactile Sensors. Adv. Mater. 2017, 29, 1701218. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, H.; Ouyang, C.; Wee, W.H.; Cui, X.; Lu, T.J.; Pingguan-Murphy, B.; Li, F.; Xu, F. Recent Advances in Pen-Based Writing Electronics and their Emerging Applications. Adv. Funct. Mater. 2016, 26, 165–180. [Google Scholar] [CrossRef]
- Gong, S.; Lai, D.T.H.; Wang, Y.; Yap, L.W.; Si, K.J.; Shi, Q.; Jason, N.N.; Sridhar, T.; Uddin, H.; Cheng, W. Tattoolike Polyaniline Microparticle-Doped Gold Nanowire Patches as Highly Durable Wearable Sensors. ACS Appl. Mater. Interfaces 2015, 7, 19700–19708. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Liu, J. Direct Writing of Flexible Electronics through Room Temperature Liquid Metal Ink. PLoS ONE 2012, 7, e45485. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Liu, J. Directly Writing Resistor, Inductor and Capacitor to Composite Functional Circuits: A Super-Simple Way for Alternative Electronics. PLoS ONE 2013, 8, e69761. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Liu, J. Biomedical Implementation of Liquid Metal Ink as Drawable ECG Electrode and Skin Circuit. PLoS ONE 2013, 8, e58771. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ahn, J.-H.; Choi, W.M.; Kim, H.-S.; Kim, T.-H.; Song, J.; Huang, Y.Y.; Liu, Z.; Lu, C.; Rogers, J.A. Stretchable and foldable silicon integrated circuits. Science 2008, 320, 507–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, A.M.V.; Kim, N.H.; Gu, Y.; Bandodkar, A.J.; You, J.-M.; Kumar, R.; Kurniawan, J.F.; Xu, S.; Wang, J. Merging of Thin- and Thick-Film Fabrication Technologies: Toward Soft Stretchable ‘Island-Bridge’ Devices. Adv. Mater. Technol. 2017, 2, 1600284. [Google Scholar] [CrossRef]
- Luo, S.; Hoang, P.T.; Liu, T. Direct laser writing for creating porous graphitic structures and their use for flexible and highly sensitive sensor and sensor arrays. Carbon 2016, 96, 522–531. [Google Scholar] [CrossRef]
- Zheng, C.; Hu, A.; Kihm, K.D.; Ma, Q.; Li, R.; Chen, T.; Duley, W.W. Femtosecond Laser Fabrication of Cavity Microball Lens (CMBL) inside a PMMA Substrate for Super-Wide Angle Imaging. Small 2015, 11, 3007–3016. [Google Scholar] [CrossRef]
- Dorin, B.; Parkinson, P.; Scully, P. Direct laser write process for 3D conductive carbon circuits in polyimide. J. Mater. Chem. C 2017, 5, 4923–4930. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, R.; Ochoa, M.; Ziaie, B. Direct Laser Writing of Porous-Carbon/Silver Nanocomposite for Flexible Electronics. ACS Appl. Mater. Interfaces 2016, 8, 16907–16913. [Google Scholar] [CrossRef]
- Strong, V.; Dubin, S.; El-Kady, M.F.; Lech, A.; Wang, Y.; Weiller, B.H.; Kaner, R.B. Patterning and electronic tuning of laser scribed graphene for flexible all-carbon devices. ACS Nano 2012, 6, 1395–1403. [Google Scholar] [CrossRef]
- Cao, Q.; Kim, H.-S.; Pimparkar, N.; Kulkarni, J.P.; Wang, C.; Shim, M.; Roy, K.; Alam, M.A.; Rogers, J.A. Medium-scale carbon nanotube thin-film integrated circuits on flexible plastic substrates. Nature 2008, 454, 495–500. [Google Scholar] [CrossRef]
- Park, S., II; Xiong, Y.; Kim, R.-H.; Elvikis, P.; Meitl, M.; Kim, D.-H.; Wu, J.; Yoon, J.; Yu, C.-J.; Liu, Z.; et al. Printed assemblies of inorganic light-emitting diodes for deformable and semitransparent displays. Science 2009, 325, 977–981. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.H.; Kim, H.S.; Lee, K.J.; Jeon, S.; Kang, S.J.; Sun, Y.; Nuzzo, R.G.; Rogers, J.A. Heterogeneous three-dimensional electronics by use of printed semiconductor nanomaterials. Science 2006, 314, 1754–1757. [Google Scholar] [CrossRef] [Green Version]
- Webster, J.G. Medical Instrumentation: Application and Design, 4th ed.; Wiley: Hoboken, NJ, USA, 2009; p. 205. [Google Scholar]
- Griss, P.; Tolvanen-Laakso, H.K.; Meriläinen, P.; Stemme, G. Characterization of micromachined spiked biopotential electrodes. IEEE Trans. Biomed. Eng. 2002, 49, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-D.; Wang, I.-J.; Chen, S.-F.; Chang, J.-Y.; Lin, C.-T. Design, Fabrication and Experimental Validation of a Novel Dry-Contact Sensor for Measuring Electroencephalography Signals without Skin Preparation. Sensors 2011, 11, 5819–5834. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhu, Y. Nanomaterial-Enabled Dry Electrodes for Electrophysiological Sensing: A Review. JOM 2016, 68, 1145–1155. [Google Scholar] [CrossRef]
- Cömert, A.; Honkala, M.; Hyttinen, J. Effect of pressure and padding on motion artifact of textile electrodes. Biomed. Eng. Online 2013, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meziane, N.; Webster, J.G.; Attari, M.; Nimunkar, J. Dry electrodes for electrocardiography. Physiol. Meas. 2013, 34, R47–R69. [Google Scholar] [CrossRef]
- Yokus, M.A.; Jur, J.S. Fabric-based wearable dry electrodes for body surface biopotential recording. IEEE Trans. Biomed. Eng. 2016, 63, 423–430. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, H.; Chen, K.; Zhang, Y.; Zhang, Y.; Liu, Y.; Zhu, C.; Ouyang, S.-C.; Kong, G.-W.; Yu, C.; et al. Epidermal impedance sensing sheets for precision hydration assessment and spatial mapping. IEEE Trans. Biomed. Eng. 2013, 60, 2848–2857. [Google Scholar] [CrossRef]
- Geddes, L.A.; Valentinuzzi, M.E. Temporal changes in electrode impedance while recording the electrocardiogram with ‘dry’ electrodes. Ann. Biomed. Eng. 1973, 1, 356–367. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.B.; Chen, X.; Chen, J.P. A flexible dry micro-dome electrode for ECG monitoring. Microsyst. Technol. 2015, 21, 1241–1248. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.; Chen, J. A flexible dry electrode based on APTES-anchored PDMS substrate for portable ECG acquisition system. Microsyst. Technol. 2016, 22, 2027–2034. [Google Scholar] [CrossRef]
- Beckmann, L.; Neuhaus, C.; Medrano, G.; Jungbecker, N.; Walter, M.; Gries, T.; Leonhardt, S. Characterization of textile electrodes and conductors using standardized measurement setups. Physiol Meas. 2010, 31, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Lobodzinski, S.M.; Laks, M.M. Biopotential fiber sensor. J. Electrocardiol. 2006, 39, S41–S46. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Beeck, M.O.D.; Vanderheyden, L.; Carrette, E.; Mihajlović, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; Hoof, C.V. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors 2014, 14, 23758–23780. [Google Scholar] [CrossRef] [Green Version]
- Reyes, B.A.; Posada-Quintero, H.F.; Bales, J.R.; Clement, A.L.; Pins, G.D.; Swiston, A.; Riistama, J.; Florian, J.P.; Shykoff, B.; Qin, M.; et al. Novel electrodes for underwater ECG monitoring. IEEE Trans. Biomed. Eng. 2014, 61, 1863–1876. [Google Scholar] [CrossRef] [PubMed]
- Marozas, V.; Petrenas, A.; Daukantas, S.; Lukosevicius, A. A comparison of conductive textile-based and silver/silver chloride gel electrodes in exercise electrocardiogram recordings. J. Electrocardiol. 2011, 44, 189–194. [Google Scholar] [CrossRef]
- Hsu, L.S.; Tung, S.W.; Kuo, C.H.; Yang, Y.J. Developing barbed microtip-based electrode arrays for biopotential measurement. Sensors 2014, 14, 12370–12386. [Google Scholar] [CrossRef] [Green Version]
- Griss, P.; Enoksson, P.; Tolvanen-Laakso, H.K.; Meriläinen, P.; Ollmar, S.; Stemme, G. Micromachined electrodes for biopotential measurements. J. Microelectromech. Syst. 2001, 10, 10–16. [Google Scholar] [CrossRef]
- Yu, L.M.; Tay, F.E.H.; Guo, D.G.; Xu, L.; Yap, K.L. A microfabricated electrode with hollow microneedles for ECG measurement. Sens. Actuators A Phys. 2009, 151, 17–22. [Google Scholar] [CrossRef]
- Lee, J.W.; Xu, R.; Lee, S.; Jang, K.-I.; Yang, Y.; Banks, A.; Yu, K.J.; Kim, J.; Xu, S.; Ma, S.; et al. Soft, thin skin-mounted power management systems and their use in wireless thermography. Proc. Natl. Acad. Sci. USA 2016, 113, 6131–6136. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Yan, Z.; Jang, K.-I.; Huang, W.; Fu, H.; Kim, J.; Wei, Z.; Flavin, M.; McCracken, J.; Wang, R.; et al. Assembly of micro/nanomaterials into complex, three-dimensional architectures by compressive buckling. Science 2015, 347, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Song, J. Mechanics of stretchable electronics. Curr. Opin. Solid State Mater. Sci. 2015, 19, 160–170. [Google Scholar] [CrossRef]
- Tuukkanen, S.; Hoikkanen, M.; Poikelispää, M.; Honkanen, M.; Vuorinen, T.; Kakkonen, M.; Vuorinen, J.; Lupo, D. Stretching of solution processed carbon nanotube and graphene nanocomposite films on rubber substrates. Synth. Met. 2014, 191, 28–35. [Google Scholar] [CrossRef]
- Khang, D.-Y.; Xiao, J.; Kocabas, C.; MacLaren, S.; Banks, T.; Jiang, H.; Huang, Y.Y.; Rogers, J.A. Molecular scale buckling mechanics in individual aligned single-wall carbon nanotubes on elastomeric substrates. Nano Lett. 2008, 8, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Yang, B.D.; Liu, Y.; Wang, Y.; Dagdeviren, C.; Liu, Z.; Carlson, A.; Li, J.; Huang, Y.; Rogers, J.A. Stretchable ferroelectric nanoribbons with wavy configurations on elastomeric substrates. ACS Nano 2011, 5, 3326–3332. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.Y.; Xiao, J.; Park, W.I.; Son, K.S.; Huang, Y.Y.; Paik, U.; Rogers, J.A. Lateral buckling mechanics in silicon nanowires on elastomeric substrates. Nano Lett. 2009, 9, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.M.; Song, J.; Khang, D.Y.; Jiang, H.; Huang, Y.Y.; Rogers, J.A. Biaxially stretchable ‘wavy’ silicon nanomembranes. Nano Lett. 2007, 7, 1655–1663. [Google Scholar] [CrossRef]
- Won, Y.; Kim, A.; Yang, W.; Jeong, S.; Moon, J. A highly stretchable, helical copper nanowire conductor exhibiting a stretchability of 700. NPG Asia Mater. 2014, 6, e132. [Google Scholar] [CrossRef] [Green Version]
- Khang, D.Y.; Jiang, H.; Huang, Y.; Rogers, J.A. A stretchable form of single-crystal silicon for high-performance electronics on rubber subtrates. Science 2006, 311, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Zhang, Y.; Jia, L.; Mathewson, K.E.; Jang, K.-I.; Kim, J.; Fu, H.; Huang, X.; Chava, P.; Wang, R.; et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 2014, 344, 70–74. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Cho, J.; Lee, J.; Huang, X.; Jia, L.; Fan, J.A.; Su, Y.; Su, J.; Zhang, H.; et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 2013, 4, 1543. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Huang, Y.; Li, Y. Extremely compliant and highly stretchable patterned graphene. Appl. Phys. Lett. 2014, 104, 173103. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Yihui, Z.; Qiang, M.; Fan, Z.; Haoran, F.; Keh-Chih, H.; Yonggang, H. A finite deformation model of planar serpentine interconnects for stretchable electronics. Int. J. Solids Struct. 2016, 91, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Fu, H.; Su, Y.; Xu, S.; Cheng, H.; Fan, J.A.; Hwang, K.-C.; Rogers, J.A.; Huang, Y. Mechanics of ultra-stretchable self-similar serpentine interconnects. Acta Mater. 2013, 61, 7816–7827. [Google Scholar] [CrossRef]
- Su, Y.; Wang, S.; Huang, Y.A.; Luan, H.; Dong, W.; Fan, J.A.; Yang, Q.; Rogers, J.A.; Huang, Y. Elasticity of Fractal Inspired Interconnects. Small 2015, 11, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.T.; Xiao, L.; Zhu, C.; Ye, D.; Wang, S.D.; Huang, Y.A.; Yin, Z.P. Theoretical and experimental study of 2D conformability of stretchable electronics laminated onto skin. Sci. China Technol. Sci. 2017, 60, 1415–1422. [Google Scholar] [CrossRef]
- Serteyn, A.; Lin, X.; Amft, Q. Reducing motion artifacts for robust QRS detection in capacitive sensor arrays. ACM Int. Conf. Proceeding Ser. 2011, 1–5. [Google Scholar]
- Gilchrist, J.M.; Sachs, G.M. Electrodiagnostic studies in the management and prognosis of neuromuscular disorders. Muscle Nerve 2004, 29, 165–190. [Google Scholar] [CrossRef]
- Castellini, C.; Smagt, P.V.D. Surface EMG in advanced hand prosthetics. Biol. Cybern. 2009, 100, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Bodéré, C.; Téa, S.H.; Giroux-Metges, M.A.; Woda, A. Activity of masticatory muscles in subjects with different orofacial pain conditions. Pain 2005, 116, 33–41. [Google Scholar] [CrossRef]
- Farina, D.; Arendt-Nielsen, L.; Graven-Nielsen, T. Experimental muscle pain decreases voluntary EMG activity but does not affect the muscle potential evoked by transcutaneous electrical stimulation. Clin. Neurophysiol. 2005, 116, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, A.; Salvo, P.; Vanfleteren, J. Reliable stretchable gold interconnects in biocompatible elastomers. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 773–776. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Green, T.A. Gold electrodeposition for microelectronic, optoelectronic and microsystem applications. Gold Bull. 2007, 40, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Shahandashti, P.F.; Pourkhevrollah, H.; Jahanshahi, A.; Ghafoorifard, H. Fabrication of Stretchable Interconnects Embedded in Biocompatible Elastomers. In Proceedings of the 26th Iranian Conference on Electrical Engineering (ICEE), Mashhad, Iran, 8–10 May 2018. [Google Scholar]
- Williams, K.R.; Gupta, K.; Wasilik, M. Etch rates for micromachining processing—Part II. J. Microelectromech. Syst. 2003, 12, 761–778. [Google Scholar] [CrossRef] [Green Version]
- Roland, T.; Wimberger, K.; Amsuess, S.; Russold, M.F.; Baumgartner, W. An insulated flexible sensor for stable electromyography detection: Application to prosthesis control. Sensors 2019, 19, 961. [Google Scholar] [CrossRef] [Green Version]
- Lou, C.; Li, R.; Li, Z.; Liang, T.; Wei, Z.; Run, M.; Yan, X.; Liu, X. Flexible graphene electrodes for prolonged dynamic ECG monitoring. Sensors 2016, 16, 1833. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.L.; Reaz, M.B.I. Characterization of textile-insulated capacitive biosensors. Sensors 2017, 17, 574. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Heo, J.; Lee, W.K.; Lim, Y.G.; Kim, Y.H.; Park, K.S. Flexible capacitive electrodes for minimizing motion artifacts in ambulatory electrocardiograms. Sensors 2014, 14, 14732–14743. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Kim, J.H.; Park, C.; Hwang, J.-Y.; Hong, J.S.; Lee, K.H.; Lee, S.H. Self-adhesive and capacitive carbon nanotube-based electrode to record electroencephalograph signals from the hairy scalp. IEEE Trans. Biomed. Eng. 2016, 63, 138–147. [Google Scholar] [CrossRef]
- Wang, L.F.; Liu, J.Q.; Dong, Y.; Yang, B.; Chen, X.; Yang, C.S. Polydimethyl-siloxane film for biomimetic dry adhesive integrated with capacitive biopotentials sensing. Sens. Actuators B Chem. 2014, 205, 168–175. [Google Scholar] [CrossRef]
- Chen, C.; Xue, M.; Wen, Y.; Yao, G.; Cui, Y.; Liao, F.; Yan, Z.; Huang, L.; Khan, S.A.; Gao, M.; et al. A Ferroelectric Ceramic/Polymer Composite-Based Capacitive Electrode Array for In Vivo Recordings. Adv. Healthc. Mater. 2017, 6, 1700305. [Google Scholar] [CrossRef] [PubMed]
- Plonsey, R. The Active Fiber in a Volume Conductor. IEEE Trans. Biomed. Eng. 1974, 21, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.J.; Lee, H.J.; Lim, Y.G.; Park, K.S. Conductive polymer foam surface improves the performance of a capacitive EEG electrode. IEEE Trans. Biomed. Eng. 2012, 59, 3422–3431. [Google Scholar] [CrossRef]
- Taji, B.; Chan, A.D.C.; Shirmohammadi, S. Effect of Pressure on Skin-Electrode Impedance in Wearable Biomedical Measurement Devices. IEEE Trans. Instrum. Meas. 2018, 67, 1900–1912. [Google Scholar] [CrossRef]
- Huang, Y.A.; Dong, W.; Zhu, C.; Xiao, L. Electromechanical Design of Self-Similar Inspired Surface Electrodes for Human-Machine Interaction. Complexity 2018, 2018, 3016343. [Google Scholar] [CrossRef]
- Jha, C.K.; Kolekar, M.H. Cardiac arrhythmia classification using tunable Q-wavelet transform based features and support vector machine classifier. Biomed. Signal Process. Control 2020, 59, 101875. [Google Scholar] [CrossRef]
- Kassanos, P.; Rosa, B.G.; Keshavarz, M.; Yang, G.Z. From wearables to implantables—clinical drive and technical challenges. In Wearable Sensors: Fundamentals, Implementation and Applications, 1st ed.; Sazonov, S., Ed.; Associated Press: New York, NY, USA, 2021; pp. 29–84. [Google Scholar]
- Lai, D.; Bu, Y.; Su, Y.; Zhang, X.; Ma, C.-S. A Flexible Multilayered Dry Electrode and Assembly to Single-Lead ECG Patch to Monitor Atrial Fibrillation in a Real-Life Scenario. IEEE Sens. J. 2020, 20, 12295–12306. [Google Scholar] [CrossRef]
- Bu, Y.; Hassan, M.F.U.; Lai, D. The Embedding of Flexible Conductive Silver-Coated Electrodes into ECG Monitoring Garment for Minimizing Motion Artefacts. IEEE Sens. J. 2021, 21, 14454–14465. [Google Scholar] [CrossRef]
- Lai, D.; Bu, Y.; Su, Y.; Zhang, X.; Ma, C.-S. Non-Standardized Patch-Based ECG Lead Together With Deep Learning Based Algorithm for Automatic Screening of Atrial Fibrillation. IEEE J. Biomed. Health Inf. 2020, 24, 1569–1578. [Google Scholar] [CrossRef]
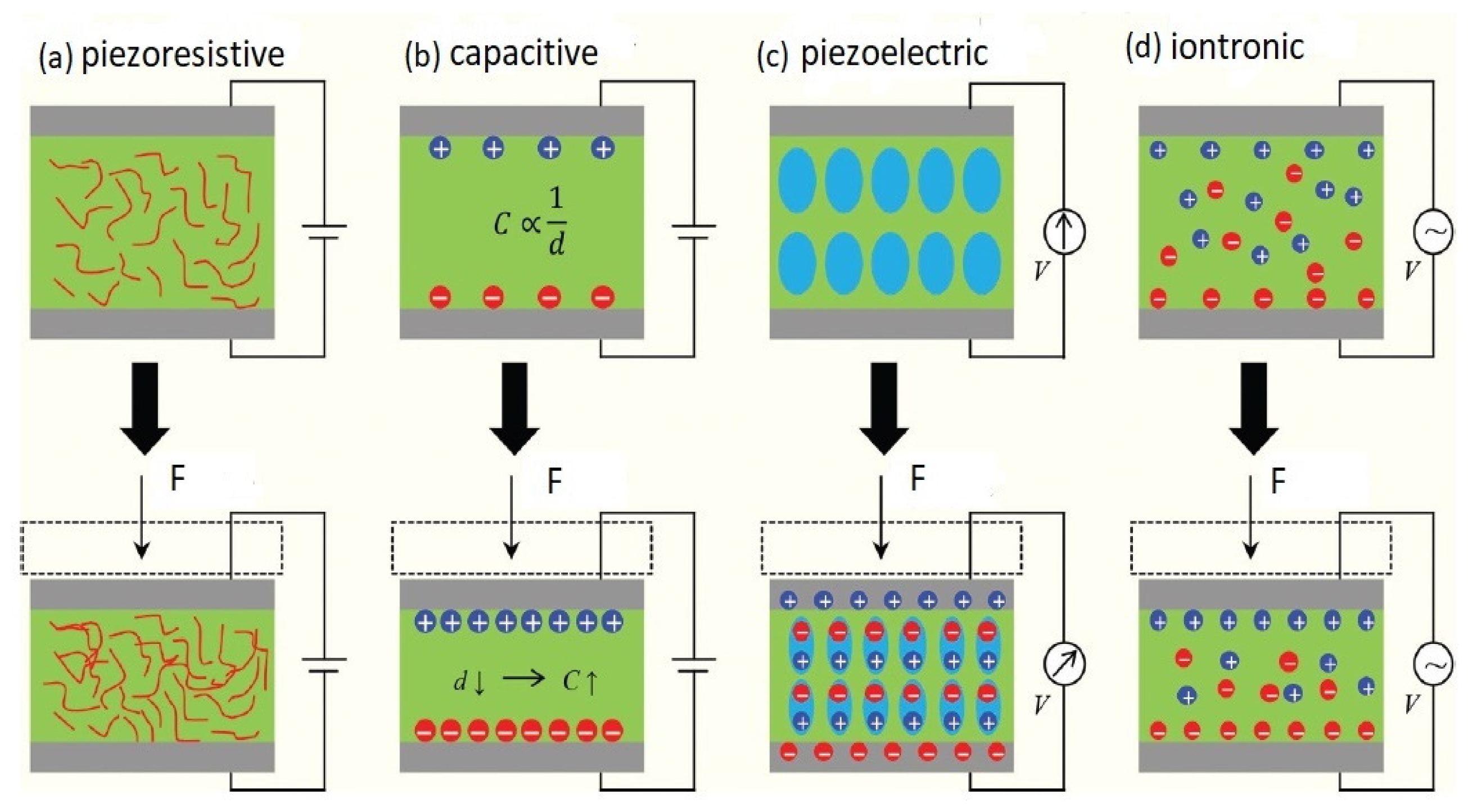
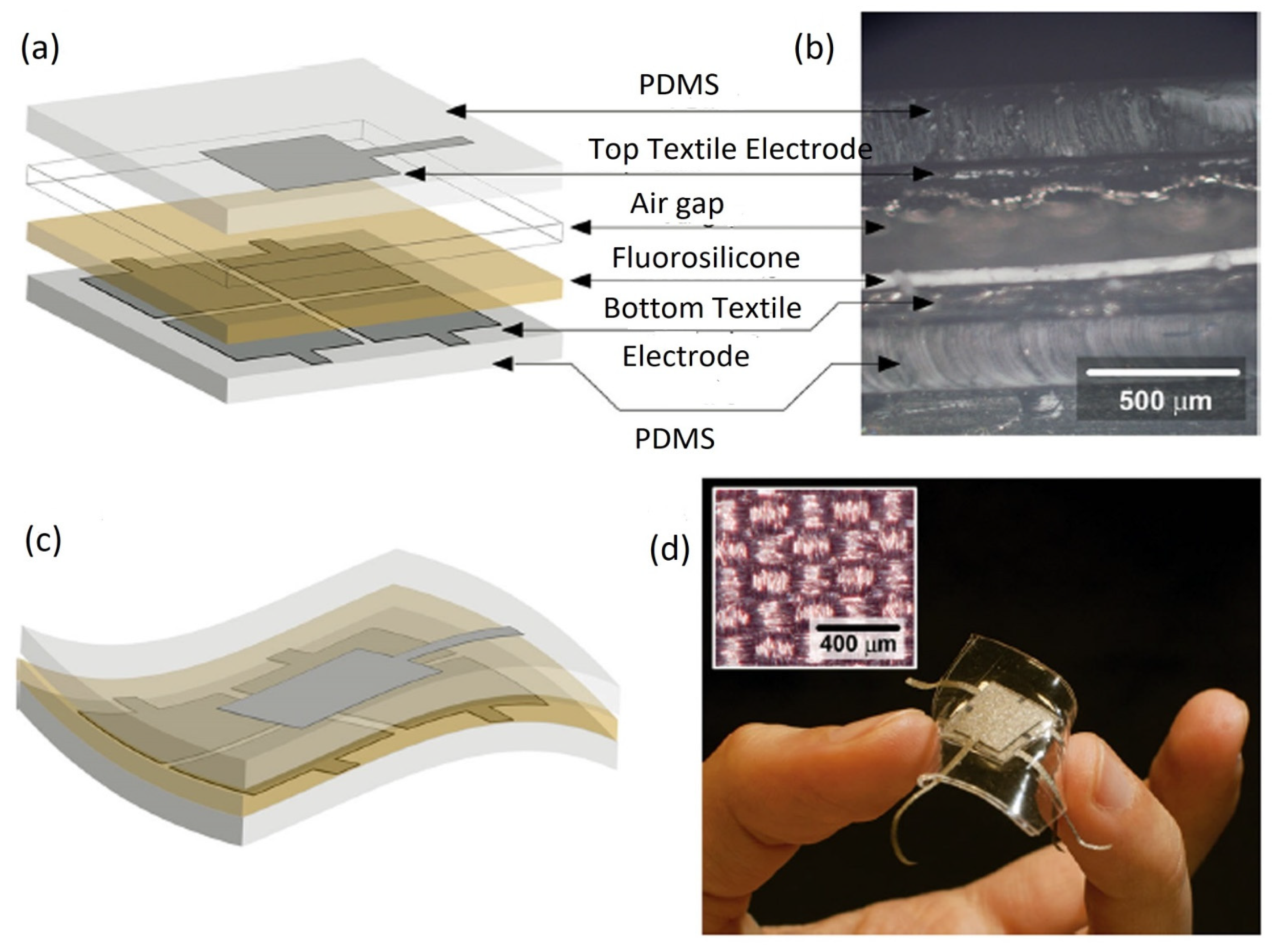
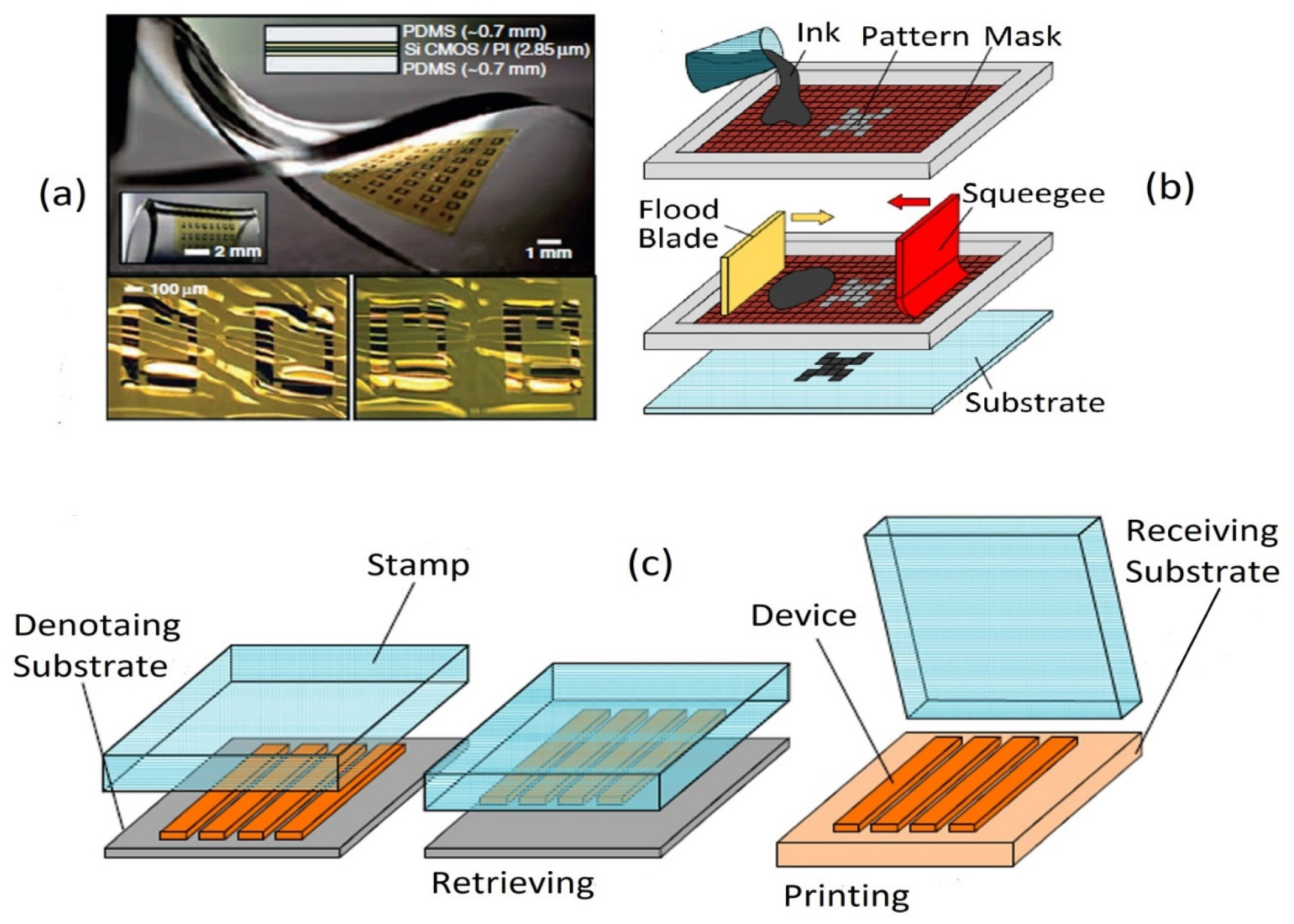

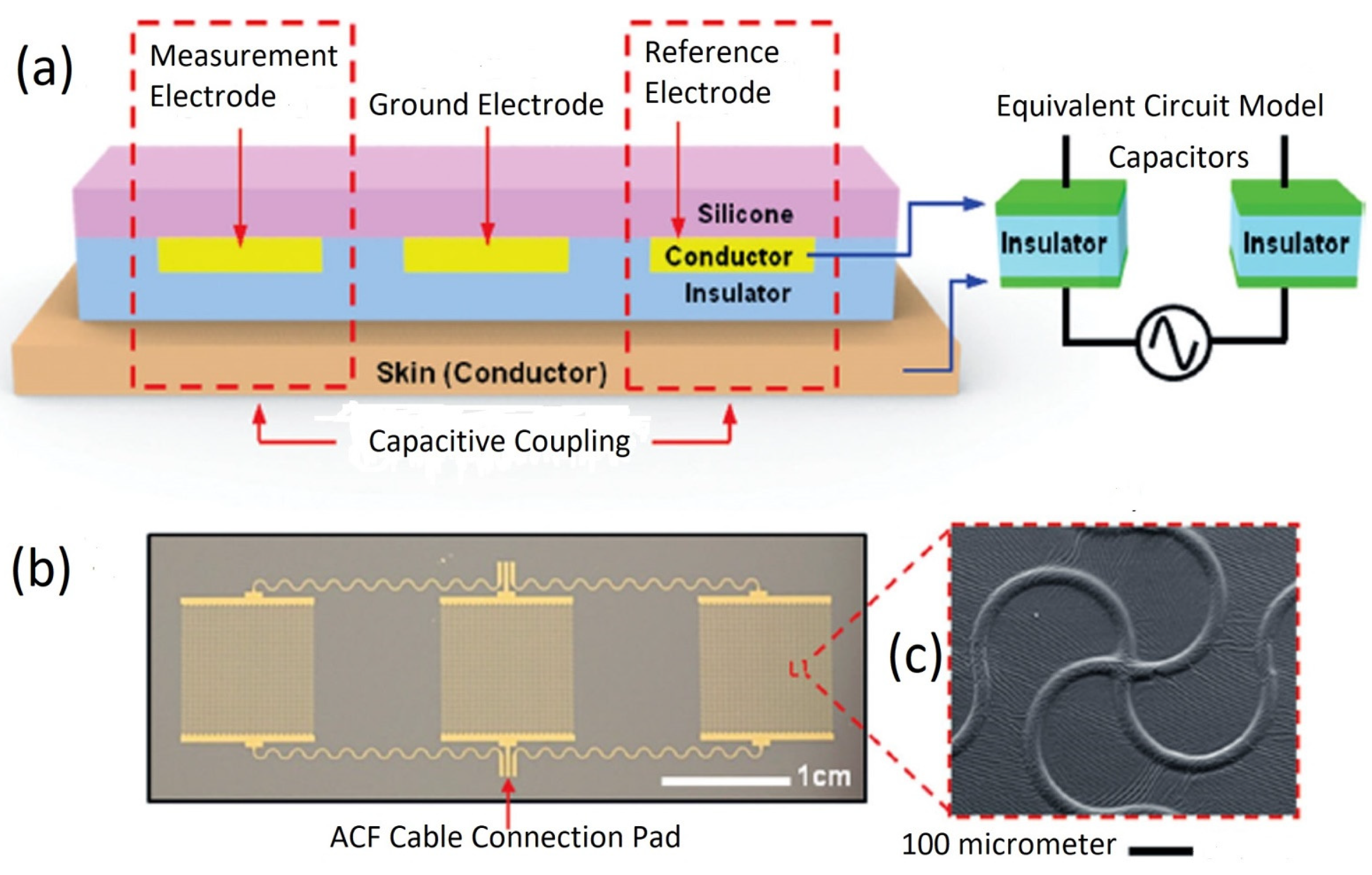
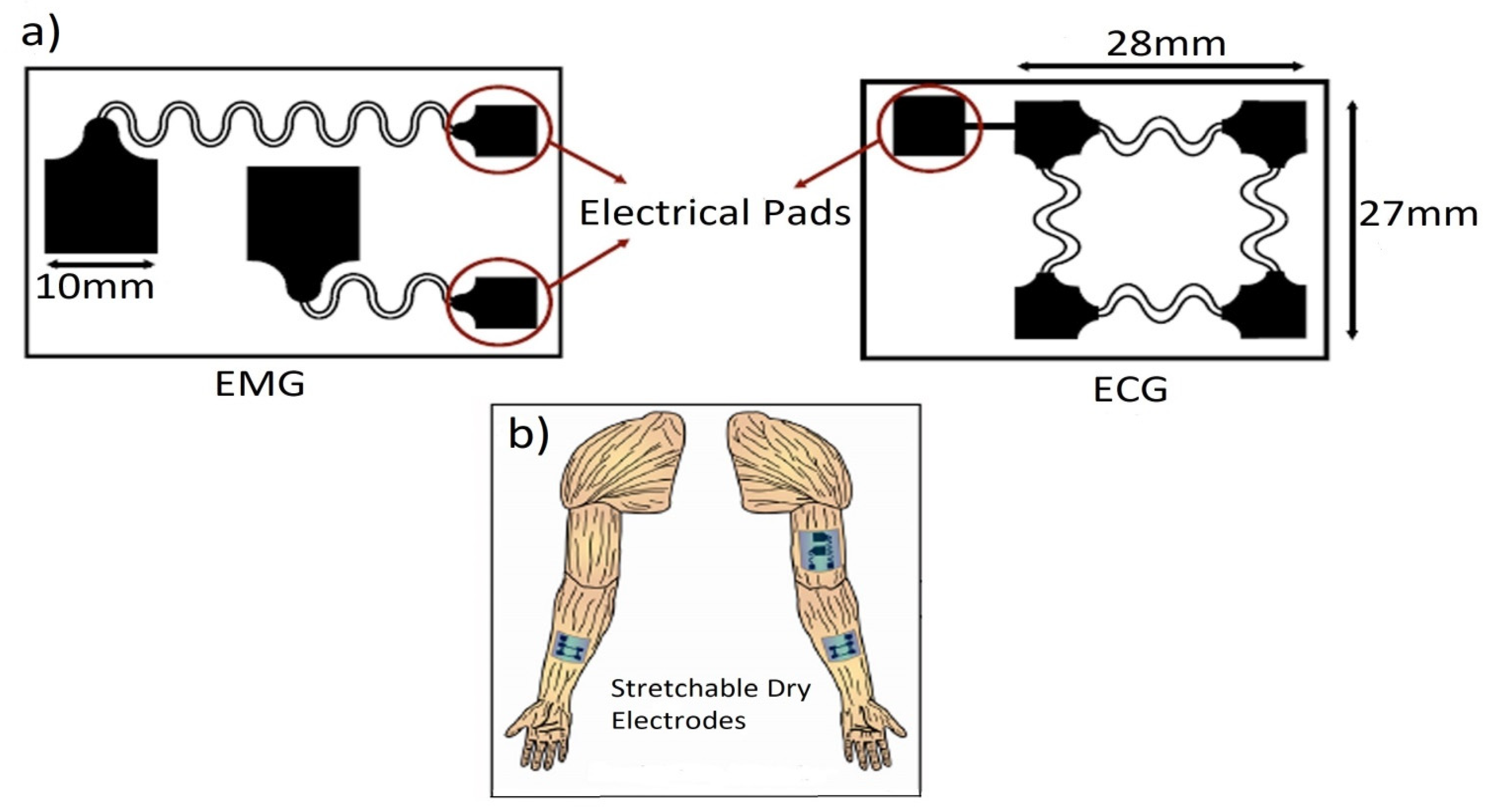

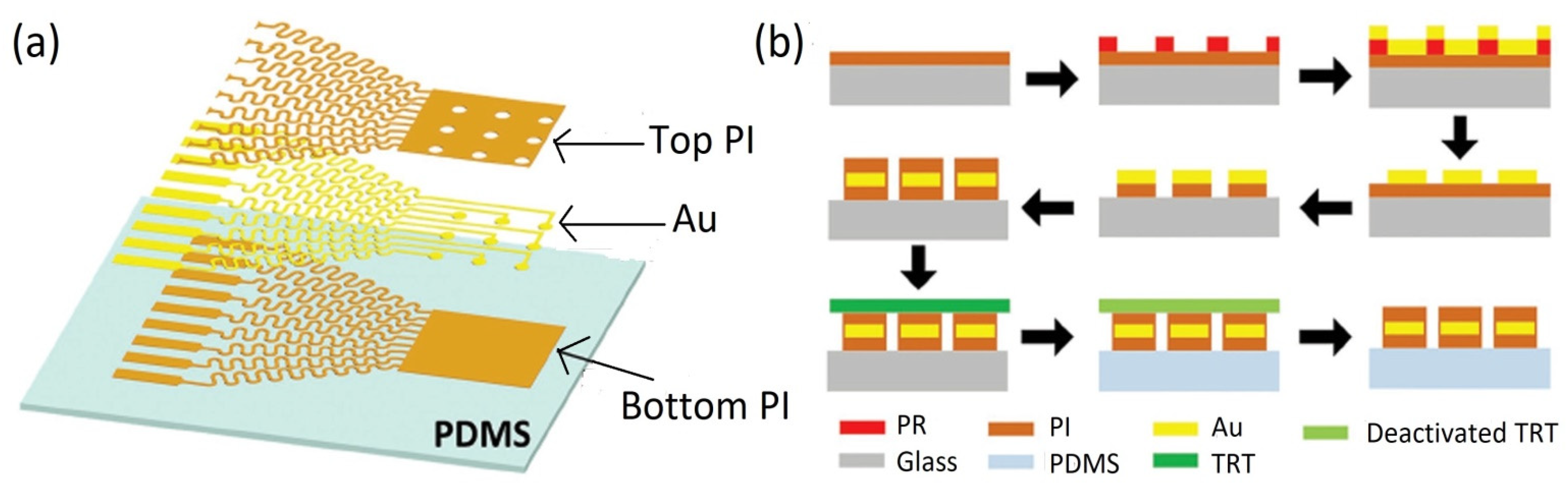
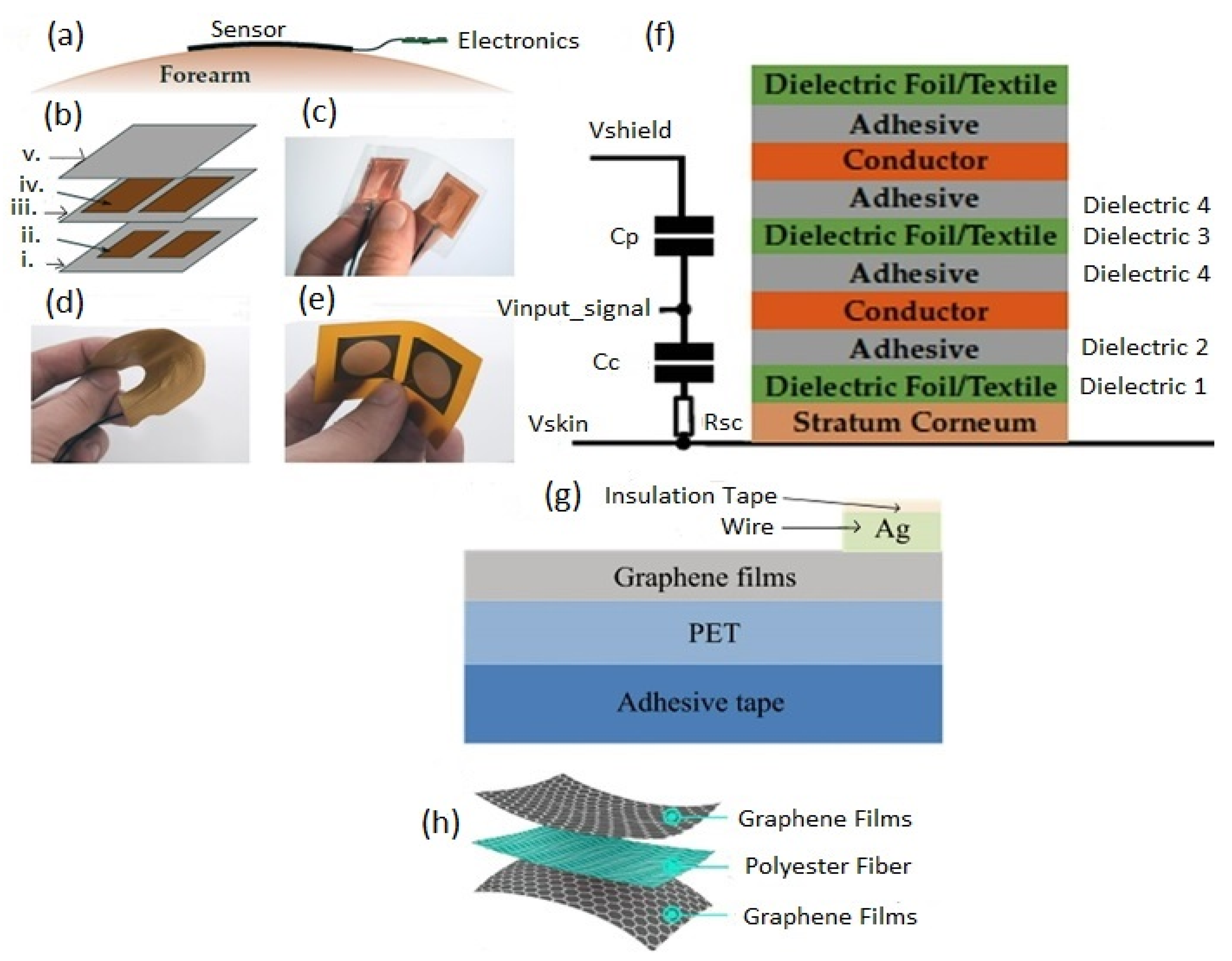

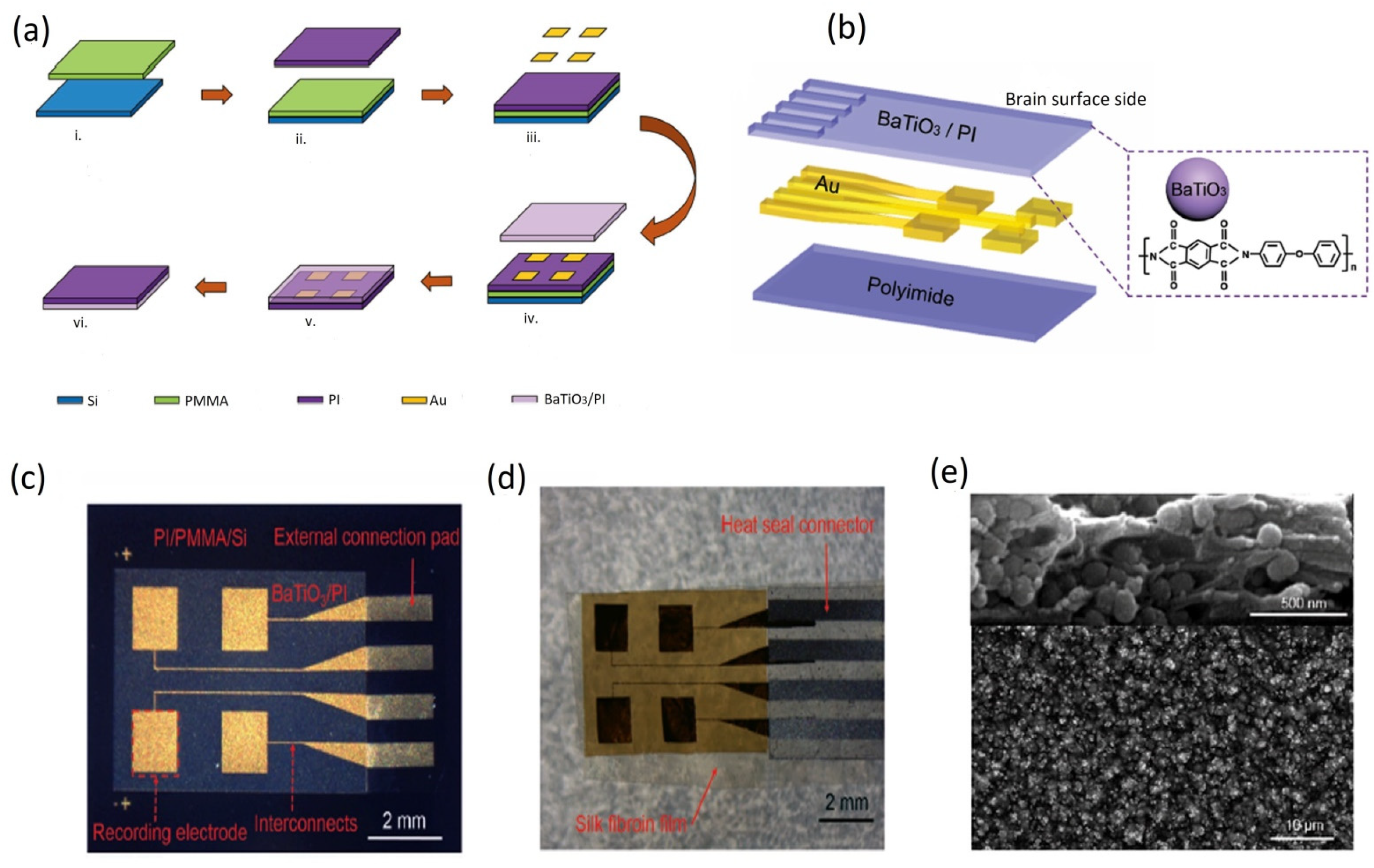
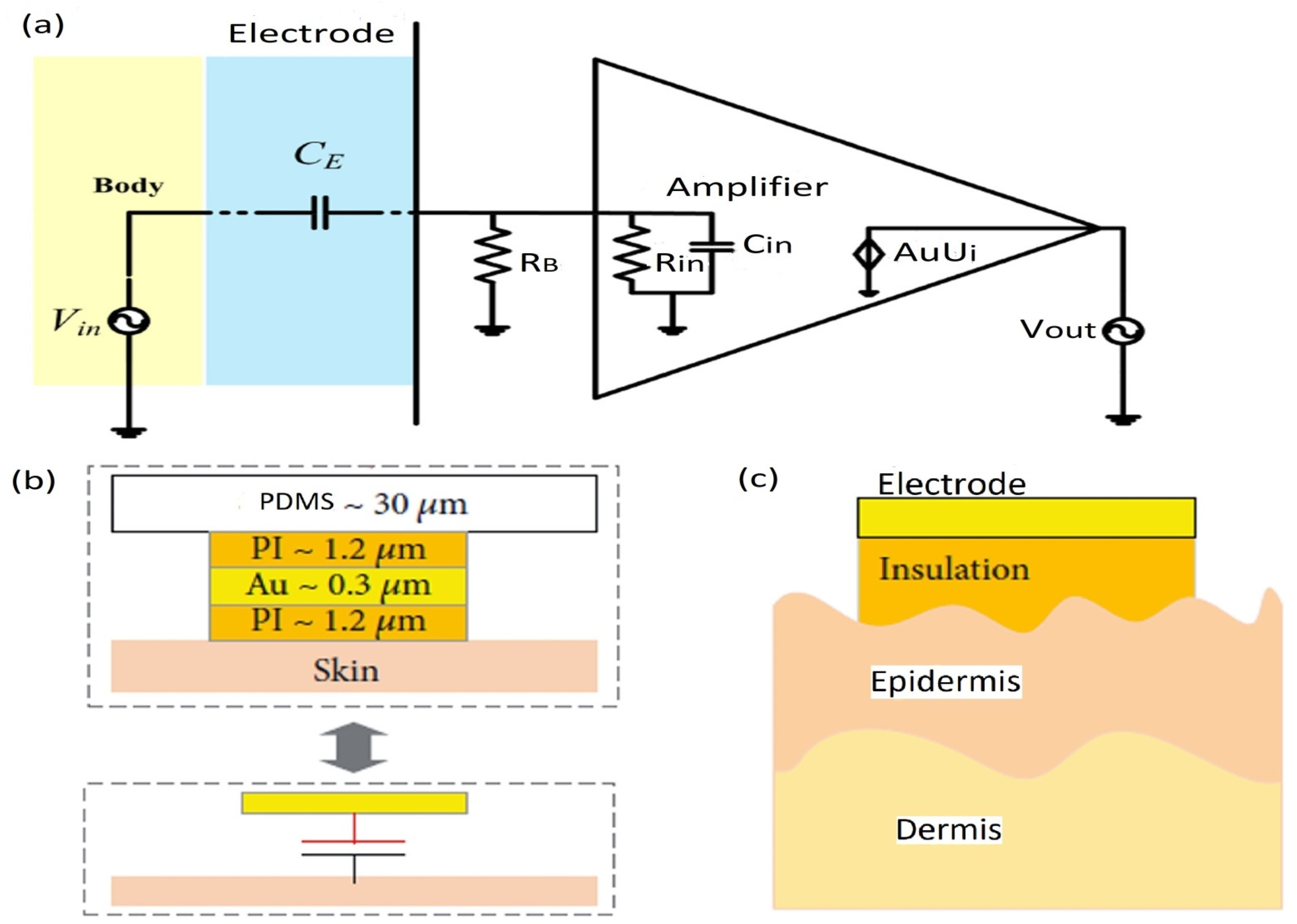
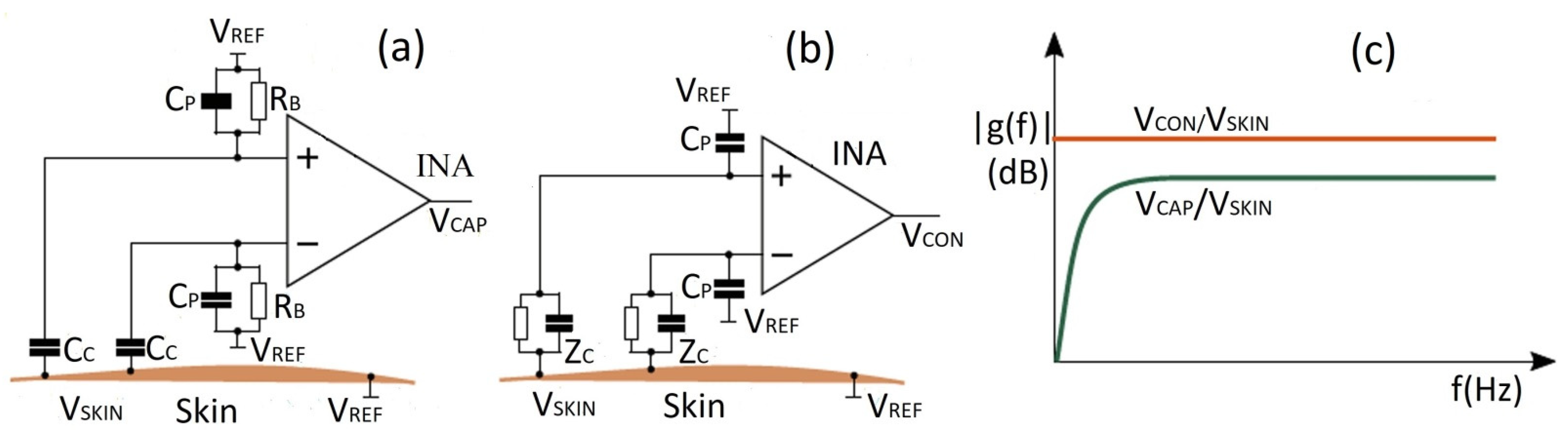
| Substrate Materials | Pros | Cons | Young’s Modulus |
|---|---|---|---|
| PDMS [96,175] | Commercially available, cheap, biocompatible, transparent, non-flammable, low autofluorescence, chemically inert, and easy processing | Difficult to integrate electrodes on the skin, absorb small hydrophobic molecules, adsorption of proteins on its surface | 0.5–3 MPa |
| PI [64,182,183] | Good wear and low creep resistance, low flammability, high thermal stability, high tensile strength, good flexibility, and infusible | Expensive, low impact strength, poor resistance to hydrolysis and alkalies, and attacked by concentrated acids | 2.3 GPa |
| Ecoflex silicone [149,182,184,185] | Safe for skin, highly stretchable with low modulus, excellent printability, good transparency, and good heat and creep resistance | Poor tear strength, comparably high cost, ultimate tensile and tear are declined with thinner, and poor transparency | 50–100 kPa |
| PMMA [186] | Excellent optical clarity, good UV and abrasion resistance, low temperature, good track and arc resistance, low fatigue, low smoke emission, low water absorption | Poor solvent and fatigue resistance, notch sensitive, limited chemical resistance, poor abrasion and wear resistance, cracked under load, prone to attack by organic solvents | 2000 MPa |
| Polyamide (PA) [187] | High abrasion resistance, good thermal resistance, good fatigue resistance, high machinability, noise dampening ability | Water absorption, chemical resistance, high shrinkage, and lacks of dimensional stability | 4750 MPa |
| Liquid Crystal Polymer (LCP) [188] | High heat resistance, flame retardant, good dimensional stability, moldability, low viscosity, adhesion, weldable, wide processing window, excellent organic solvent, and heat aging resistance | Weak weld lines, chemical resistance, high anisotropic properties, high Z-axis thermal expansion coefficient, less cost-effectiveness, and knit line strength | 10.6 GPa |
| Thermoplastic polyurethane (TPU) [189] | Excellent abrasion resistance, good impact strength, rubber-like elasticity, toughness but good flexibility, good resistance with abrasion, oil, and grease | Short shelf life, less cost-effective, drying is needed before processing, easily degrades with sunlight or UV exposure, easy fracturing feature | 3.6–88.8 MPa |
| Polyethylene terephthalate (PET) [190] | Inexpensive and available, high resistant to moisture, high strength to weight ratio, high chemical resistance to water and organic materials, highly shatterproof and transparent, easily recycled | Low heat resistance, resins and susceptible to oxides, lower impact strength, lower moldability, more sensitive to high temperatures (>60 °C), highly affected by tough bases, boiling water, and alkalis | 2.5 GPa |
| Electrode Materials | Advantages | Disadvantages | Applications | Conductivity/Thermal Conductivity | Young’s Modulus |
|---|---|---|---|---|---|
| PEDOT [234] | Optically transparent, high stability, moderate band gap, and low redox potential | Poor solubility, acidity, anisotropic charge injection, hygroscopicity | Biomedicine (drug delivery, tissue engineering), wearable electronics (biosensors), industry (optoelectronic/thermoelectric devices, fuel cells) | 1200 S/cm | 2.6 ± 1.4 GPa |
| PANI [235] | Controllable and wide range of conductivity, transparent and colored electrically conductive products, environmental stability, reversible doping, and pH change properties, simple synthesis | Low processing capacity, inflexibility, lack of biodegradability, poor solubility | Renewable energy storage devices (Li–ion batteries, supercapacitors, Li–sulfur batteries), medicine (delivery systems, neural biotic abiotic/prosthesis interfaces, scaffolds), electrochromic glasses, electroluminescence | 5 S/cm | 2–4 GPa |
| PPy [236] | Biocompatibility, easy synthesis, the inspiration of proliferation and cell attachment, good electrical conductivity, environmental friendliness | Non-thermoplastic, brittle, rigid, non-degradable, and insoluble in some common solvents (for example: acetone, methanol, ethanol) | Optical, medical, electronics, electrochemical and biological applications (as the sensors), catalyst support of fuel cells, micro-actuators | 40–200 S/cm | 430–800 MPa |
| Polythiophene (PT) [237] | Low cost, good electrical, mechanical, and optical properties, high thermal and environmental constancy, smaller band gap energy (2.0 eV) compared to PANI and PPy | Poor solubility with ordinary solvents, hard to synthesize, poor chemical stability and processibility | Biosensors, solar cells, thermoelectric applications, OLEDS, FETS, batteries, memories, electroluminescent devices | 10–100 S/cm | 3 GPa |
| Graphene [238,239] | Mechanically strength, more energy storing for a long time and fast charging capability, lightweight, good thermal and electrical behavior, flexibility, chemically inert | An expensive, complex process that cannot be switched off easily, susceptibility of catalyst to oxide environments, toxic chemicals are required to grow it | Aerospace, mobile devices, building materials, heat sinks, microelectronics, batteries, fuel cells, supercapacitors, flexible solar panels, flexible displays, drug delivery, DNA sequencing | ~4000 W/mK | 1 TPa |
| Diamond [240] | Low affinity and friction coefficient with non-ferrous metals, high thermal conductivity, good quality machined surface, good anti-adhesion, excellent cutting performance, tool durability | Low thermal stability, poor toughness, chemical reaction contacting with iron group of elements, grinding of diamond tools is costly and difficult, carbonization at 700~800 °C | Industry, medicine, engraving, audio equipment, beauty products, heat sinks, medical devices, super lasers, surgical tools, windows, jewelry | 1000 W/mK | ≈103 GPa |
| Carbon nanofiber (CNF) [241,242] | Low density, good thermal stability, high modulus, large aspect ratio, high strength, high conductivity, compact structure ability | Lack of solubility with aqueous media, hydrophobicity, large surface area, insolubility, non-uniform morphological structure | Filtration, tissue engineering, nanocomposites, water treatment, packaging, sensing, energy devices, drug delivery, photocatalytic | 2000 W/mK | 6–207 GPa |
| Glassy carbon [243,244] | Reproducible features, high-temperature resistance, extreme resistance with chemical attack, versatility in miniaturized devices, excellent electrical, chemical, thermal, mechanical properties | Concoidal fracture surface, brittle, dimensional shrinkage, impermeability in liquids and gases, high costs in large-scale structure production | Antistatic packaging, Electrode material in electrochemistry, tissue engineering, electrochemical sensors, biomedical, energy storage sectors, pharmaceutical, encapsulation for nuclear waste | 4.6–6.3 W/mK | 20 GPa |
| Measuring Instrument | Features | Purposes | Used Software | Reference |
|---|---|---|---|---|
| Instrumentation amplifier: AD620 (Analog Devices, USA) | Easy to use, low-cost, gain range with 1 external resistor 1 to 10,000, low-noise (9 nV/√Hz @ 1 kHz), input voltage noise (0.28, ac characteristics: μV p-p noise (0.1 Hz to 10 Hz)), 120 kHz bandwidth (G = 100). | Suppressing the common-mode noises | Lab VIEW software (National Instruments, USA) | [18] |
| NI USB-6363 (National Instruments, USA) | Sample rate (max for single channel (2.00 M Sample/s), max for multi-channel (aggregate) (1.00 M Sample/s)), resolution timing (10 ns), accuracy timing (50 ppm), max working voltage for the analog inputs (signal + common mode) (±11 V), CMRR (DC to 60 Hz) 100 dB. | Digitization of acquired signals for converting analog to digital | Lab VIEW software (National Instruments, USA) | [18] |
| Instrumentation amplifier: AD622 (Analog Devices, USA) | Easy to use, low cost solution, gain range with 1 external resistor 2 to 2000, Unity gain with no external resistor, 66 dB (min CMRR) (G = 1), low noise (12 nV/√Hz @ 1 kHz), input voltage noise (0.60 μV p-p noise (0.1 Hz to 10 Hz)) (G = 10), ac characteristics: 10 μs settling time to 0.1% G = 1 to 100, 800 kHz bandwidth: G = 10, 10, 1.2 V/μs slew rate. | Suppressing the common-mode noises | Matlab (Mathworks, Inc., USA) | [22] |
| Operational amplifier: TLC2272 (Texas Instruments, Inc., USA) | Low noise: 9 nV/√Hz at f = 1 kHz (typical), low-input bias current (1–60 pA), high-gain bandwidth: 2.2 MHz (typical), high slew rate: 3.6 V/µs (typical), low input offset voltage: 950 µV max at TA = 25 °C, output current (2.2 mA), min CMRR (70 dB), Offset Drift (2 uV/C), temperature range: 40 to 125/0 to 70 C. | Amplification and filtering | Matlab (Mathworks, Inc., USA) | [22] |
| AD8232 (Analog Devices, USA)) | Typical low supply current (170 µA) (typical), CMRR-80 dB (dc to 60 Hz), high signal gain (G = 100) with the dc blocking capabilities, single-supply operation (2.0 V to 3.5 V). | Signal to acquire, amplify, and filtering | Matlab (Mathworks, Inc., USA) | [22] |
| OPA124 (Texas Instruments, USA) | Typical low input capacitance (1 pf), high input resistance (1013 Ω), low noise: 6 nV/√Hz at f = 10 kHz, low bias current: 1 pA (max), low offset: 250 mv (max), low drift: 2 mv/°C (max), high open-loop gain: 120 dB (min), high common-mode rejection (min): 100 dB. | Reducing the high input impedance effect | Matlab (Math-Works, Inc., USA) | [111,339] |
| MP150, BioPAC systems (Aero Camino, Goleta, CA 93117, USA) | Band-pass filter (0.5–35 Hz), no-load power consumption (<150 mW), output power (2 W) (max), output current (<120 (DCM)/200 (CCM) mA) (max), Internal high-voltage current source. | Amplification and digitization | Matlab (Math-Works, Inc., USA) | [340] |
| INA118 (Texas Instruments Inc., USA) | Low offset voltage: 50 µV (max), low drift: 0.5 µV/°C (max), low input bias current: 5 nA (max), high CMR-110 dB (min), supply range (±1.35 to ±18 V), low quiescent current: 350 µA. | Amplification | Matlab (Math-Works, Inc., USA) | [341] |
| LMP7702(Texas Instruments Inc., USA) | Input offset voltage: ±220 µV (max), input bias current: ±200 fA, The specified low-offset voltage of less than ±200 µV, input voltage noise: 9 nV/√Hz, CMRR: 130 dB, open- loop gain (130 dB), temperature Range (−40 °C to 125 °C), Unity-gain bandwidth: 2.5 MHz, supply current-1.5 mA, supply voltage range: 2.7 V to 12 V. | Reducing the high input impedance effect | Matlab (Math-Works, Inc., USA) | [341] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, H.; Wahab, M.A.; Will, G.; Karim, M.R.; Pan, T.; Gao, M.; Lai, D.; Lin, Y.; Miraz, M.H. Recent Advances in Stretchable and Wearable Capacitive Electrophysiological Sensors for Long-Term Health Monitoring. Biosensors 2022, 12, 630. https://doi.org/10.3390/bios12080630
Ullah H, Wahab MA, Will G, Karim MR, Pan T, Gao M, Lai D, Lin Y, Miraz MH. Recent Advances in Stretchable and Wearable Capacitive Electrophysiological Sensors for Long-Term Health Monitoring. Biosensors. 2022; 12(8):630. https://doi.org/10.3390/bios12080630
Chicago/Turabian StyleUllah, Hadaate, Md A. Wahab, Geoffrey Will, Mohammad R. Karim, Taisong Pan, Min Gao, Dakun Lai, Yuan Lin, and Mahdi H. Miraz. 2022. "Recent Advances in Stretchable and Wearable Capacitive Electrophysiological Sensors for Long-Term Health Monitoring" Biosensors 12, no. 8: 630. https://doi.org/10.3390/bios12080630
APA StyleUllah, H., Wahab, M. A., Will, G., Karim, M. R., Pan, T., Gao, M., Lai, D., Lin, Y., & Miraz, M. H. (2022). Recent Advances in Stretchable and Wearable Capacitive Electrophysiological Sensors for Long-Term Health Monitoring. Biosensors, 12(8), 630. https://doi.org/10.3390/bios12080630








