Measurement of Interfacial Adhesion Force with a 3D-Printed Fiber-Tip Microforce Sensor
Abstract
1. Introduction
2. Materials and Methods
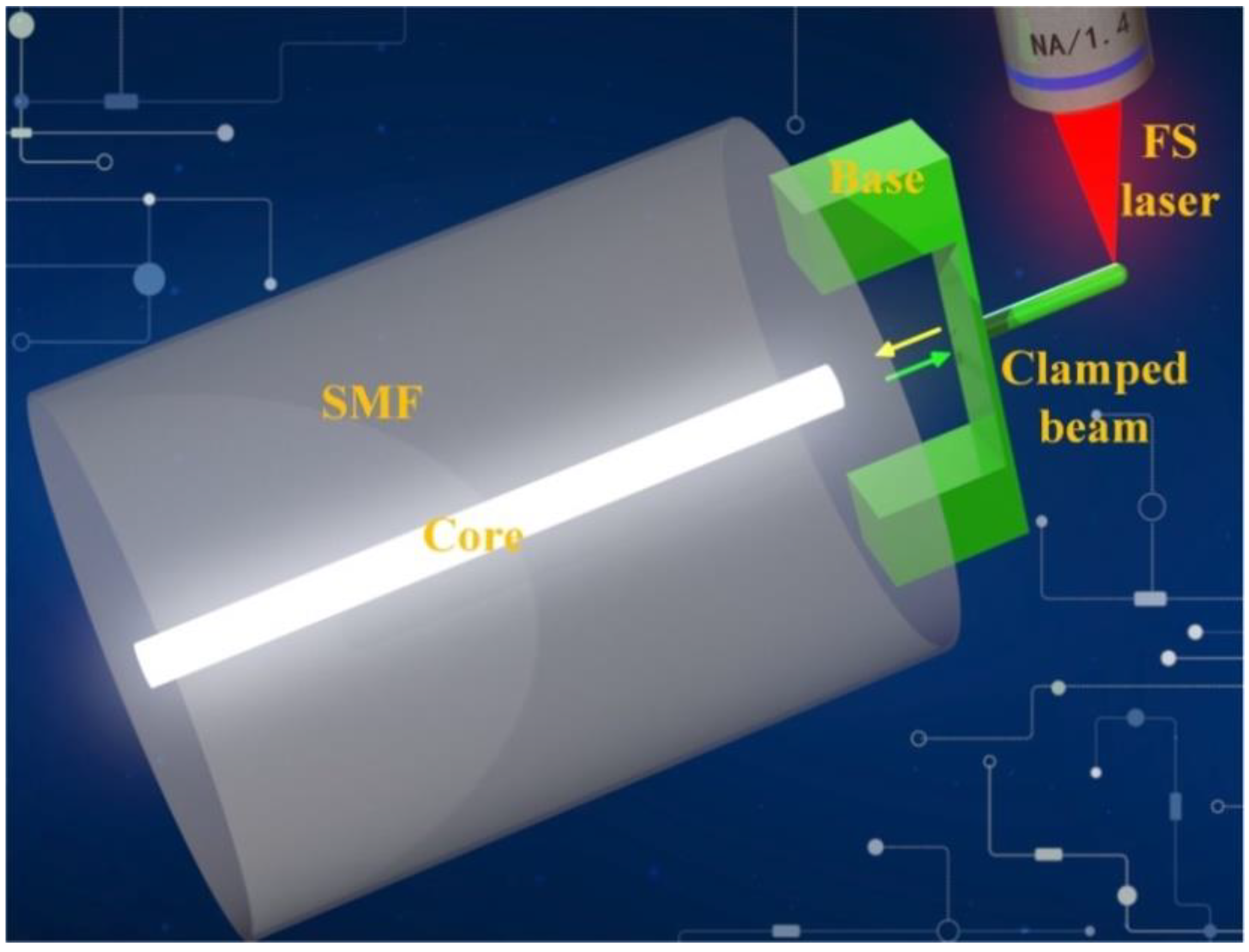
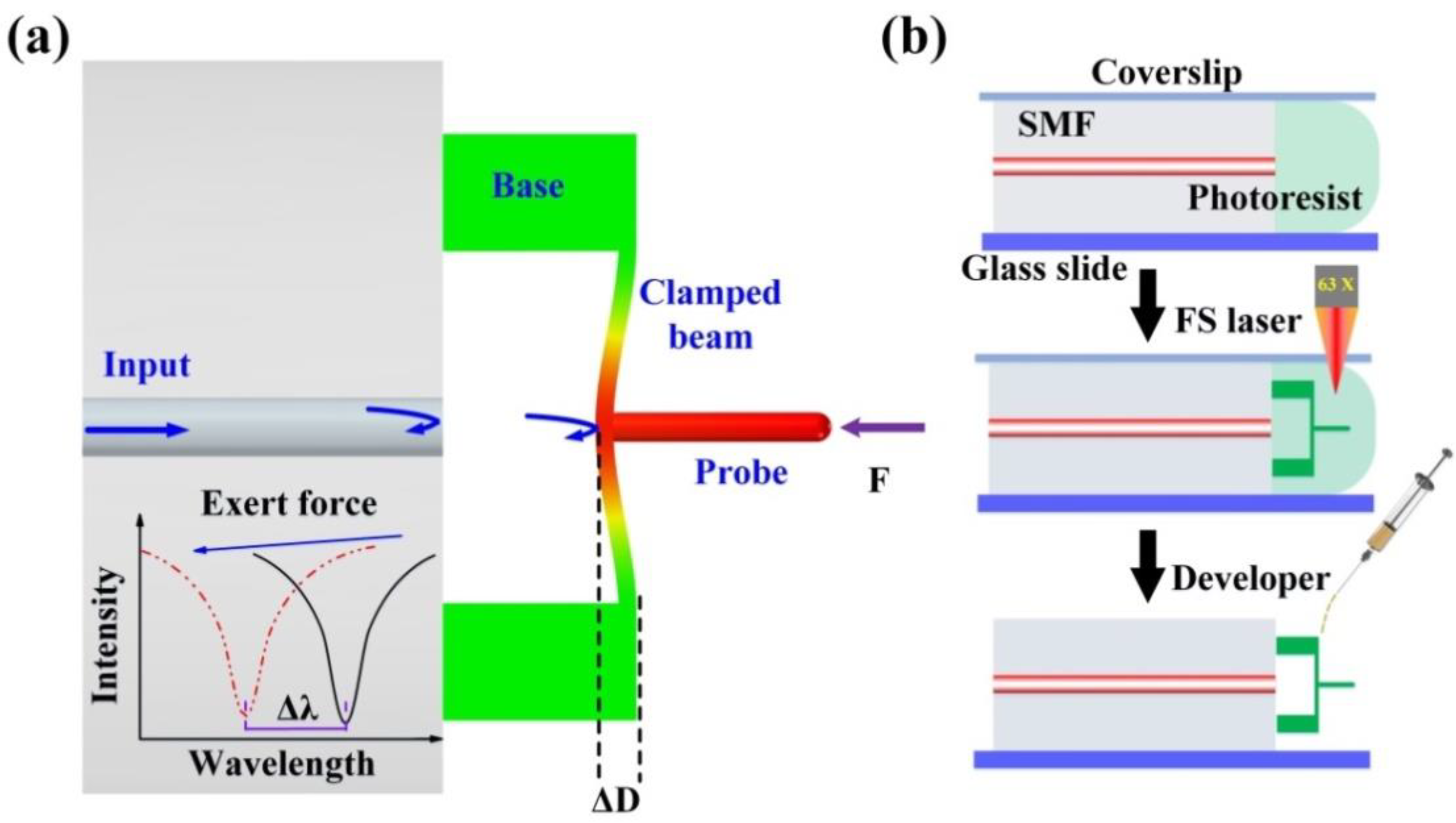
2.1. Sensing Principle and Fabrication
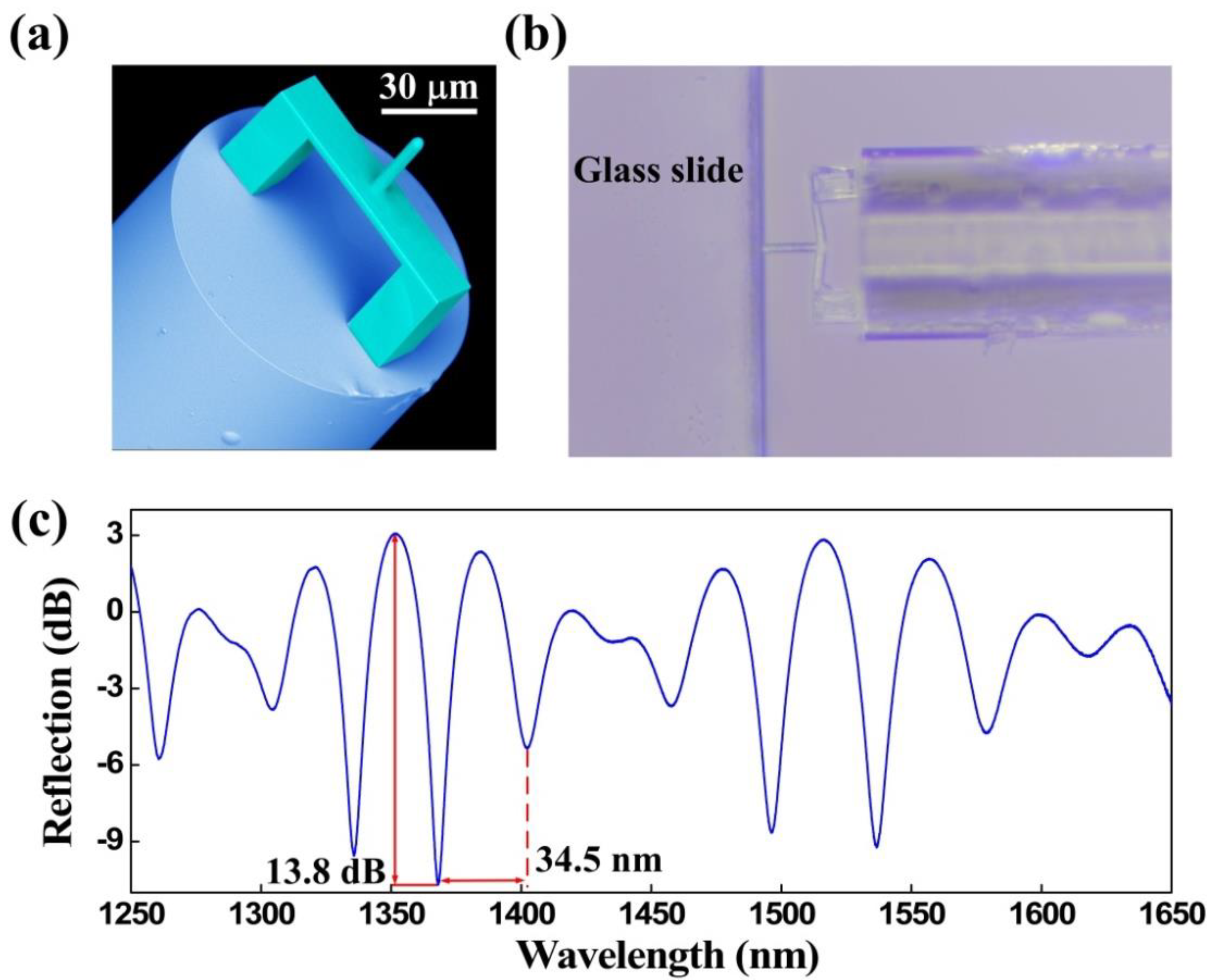
2.2. Characterization
3. Results
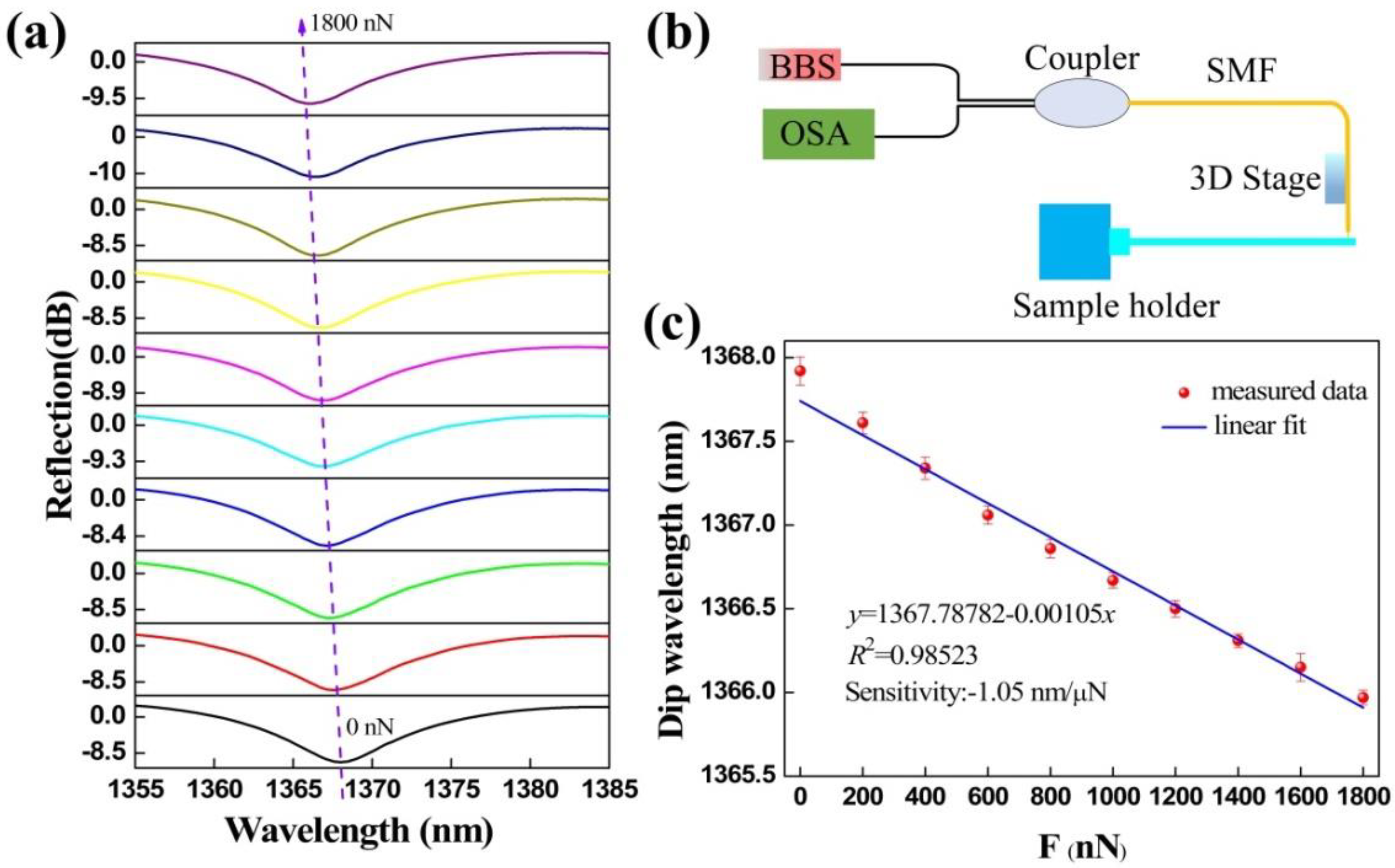
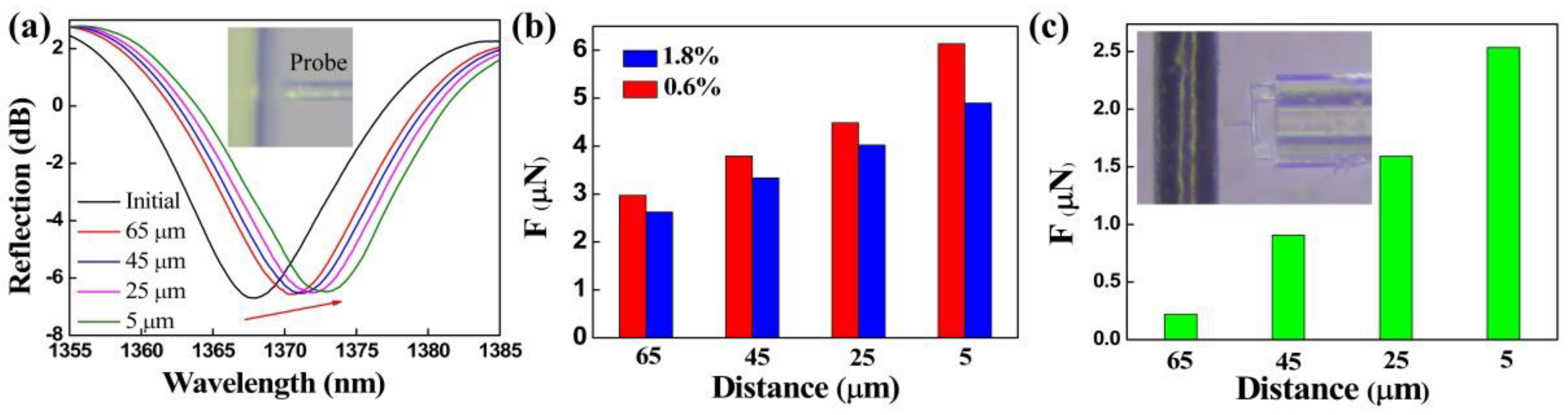
3.1. Force Measurement
3.2. Interface Adhesion Force Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, T.; Chen, Y.; Sun, J.; Guo, M. Material-related contact time dependence of adhesion force revealed by an AFM cantilever in a humid environment. Appl. Surf. Sci. 2021, 550, 149357. [Google Scholar] [CrossRef]
- Takeuchi, M. Adhesion forces of charged particles. Chem. Eng. Sci. 2006, 61, 2279–2289. [Google Scholar] [CrossRef]
- Stegemann, B.; Backhaus, H.; Kloß, H.; Santner, E. Spherical AFM probes for adhesion force measurements on metal single crystals. Mod. Res. Educ. Top. Microsc. Ser. 2007, 3, 820–827. [Google Scholar]
- Li, G.H.; Laboriante, I.; Liu, F.; Shavezipur, M.; Bush, B.; Carraro, C.; Maboudian, R. Measurement of adhesion forces between polycrystalline silicon surfaces via a MEMS double-clamped beam test structure. J. Micromech. Microeng. 2010, 20, 095015. [Google Scholar] [CrossRef]
- Erath, J.; Schmidt, S.; Fery, A. Characterization of adhesion phenomena and contact of surfaces by soft colloidal probe AFM. Soft Matter 2010, 6, 1432. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.; Kong, L. Theoretical study of the effect of probe shape on adhesion force between probe and substrate in Atomic Force Microscope experiment. J. Theor. Appl. Mech. 2015, 53, 235–241. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Huang, S.; Wei, C.; Wu, C.; Mochalin, V.N. Adhesion of two-dimensional titanium carbides (MXenes) and graphene to silicon. Nat. Commun. 2019, 10, 3014. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, T.; Chandrashekhar, A.; Prasat, S.V.; Reddy, I.R. Effect of Substrate Surface Roughness on Adhesion of Titanium Nitride Coatings Deposited by Physical Vapour Deposition Technique. IOP Conf. Ser. Mater. Sci. Eng. 2020, 981, 042022. [Google Scholar] [CrossRef]
- Spahiu, T.; Al-Arabiyat, M.; Martens, Y.; Ehrmann, A.; Piperi, E.; Shehi, E. Adhesion of 3D printing polymers on textile fabrics for garment production. IOP Conf. Ser. Mater. Sci. Eng. 2018, 459, 012065. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Vera, A.M.; Bernardi, R.C.; Tinnefeld, P.; Nash, M.A. High force catch bond mechanism of bacterial adhesion in the human gut. Nat. Commun. 2020, 11, 4321. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Hoshiba, T.; Yoshikawa, C.; Sakakibara, K. Characterization of initial cell adhesion on charged polymer substrates in serum-containing and serum-free media. Langmuir 2018, 34, 4043–4051. [Google Scholar] [CrossRef]
- Batista Ponce, M.; Vazquez-Martinez, J.M.; Davim, J.P.; Salguero Gomez, J. Analysis of secondary adhesion wear mechanism on hard machining of titanium aerospace alloy. Materials 2019, 12, 2015. [Google Scholar] [CrossRef]
- Rokni, H.; Wei, L. Direct measurements of interfacial adhesion in 2D materials and van der Waals heterostructures in ambient air. Nat. Commun. 2020, 11, 5607. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, R.; Zhao, K. Micromechanics of material detachment during adhesive wear: A numerical assessment of Archard’s wear model. Wear 2021, 476, 203739. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Russell, A.P.; Stark, A.Y.; Higham, T.E. The integrative biology of gecko adhesion: Historical review, current understanding, and grand challenges. Integr. Comp. Biol. 2019, 59, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Jain, D.; Zoltowski, C.M.; Voleti, S.; Stark, A.Y.; Niewiarowski, P.H.; Dhinojwala, A. Direct evidence of acid-base interactions in gecko adhesion. Sci. Adv. 2021, 7, eabd9410. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, M.; David, R.; Fornof, A.; Gaub, H.E. Electrically controlled DNA adhesion. Nat. Nanotechnol. 2010, 5, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Dvir, H.; Jopp, J.; Gottlieb, M. Estimation of polymer–surface interfacial interaction strength by a contact AFM technique. J. Colloid Interface Sci. 2006, 304, 58–66. [Google Scholar] [CrossRef]
- Vlassov, S.; Oras, S.; Antsov, M.; Sosnin, I.; Polyakov, B.; Shutka, A.; Krauchanka, M.Y.; Dorogin, L.M. Adhesion and mechanical properties of PDMS-based materials probed with AFM: A review. Rev. Adv. Mater. Sci. 2018, 56, 62–78. [Google Scholar] [CrossRef]
- Lai, T.; Meng, Y. Behaviors of time-dependent and time-independent adhesion forces revealed using an AFM under different humidities and measuremental protocols. Int. J. Adhes. Adhes. 2017, 78, 121–134. [Google Scholar] [CrossRef]
- Qian, H.; Lee, J.; Arce, F.T.; Yoon, I.; Angsantikul, P.; Liu, J.; Shi, Y.; Villanueva, J.; Thamphiwatana, S.; Ma, X. Nanofibre optic force transducers with sub-piconewton resolution via near-field plasmon–dielectric interactions. Nat. Photonics 2017, 11, 352–355. [Google Scholar] [CrossRef]
- Sun, L.; Gu, H.; Liu, X.; Ni, H.; Gu, Z. 3D-printed cellular tips for tuning fork atomic force microscopy in shear mode. Nature Commun. 2020, 11, 5732. [Google Scholar] [CrossRef]
- Gissibl, T.; Thiele, S.; Herkommer, A.; Giessen, H. Two-photon direct laser writing of ultracompact multi-lens objectives. Nat. Photonics 2016, 10, 554–560. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, K.; Wu, J.; Li, Y.; Yang, Y.; Huang, S.; Zhao, J.; Tweedle, T.; Carpenter, D.; Zheng, G. Femtosecond laser fabrication of nanograting-based distributed fiber sensors for extreme environmental applications. Int. J. Extrem. Manuf. 2021, 3, 025401. [Google Scholar] [CrossRef]
- Liao, C.; Xiong, C.; Zhao, J.; Zou, M.; Zhao, Y.; Li, B.; Ji, P.; Cai, Z.; Gan, Z.; Wang, Y. Design and realization of 3D printed fiber-tip microcantilever probes applied to hydrogen sensing. Light Adv. Manuf. 2022, 3, 1–11. [Google Scholar] [CrossRef]
- Li, B.; Liao, C.; Cai, Z.; Zhou, J.; Zhao, C.; Jing, L.; Wang, J.; Xiong, C.; Xu, L.; Wang, Y. Femtosecond laser 3D printed micro objective lens for ultrathin fiber endoscope. Fundam. Res. 2022; in press. [Google Scholar] [CrossRef]
- Shen, C.; Zhong, C.; Liu, D.; Lian, X.; Zheng, J.; Wang, J.; Yuliya, S.; Gerald, F.; Jacques, A.; Donegan, J.F. Measurements of milli-Newton surface tension forces with tilted fiber Bragg gratings. Opt. Lett. 2018, 43, 255–258. [Google Scholar] [CrossRef]
- Zou, M.; Liao, C.; Liu, S.; Xiong, C.; Zhao, C.; Zhao, J.; Gan, Z.; Chen, Y.; Yang, K.; Liu, D. Fiber-tip polymer clamped-beam probe for high-sensitivity nanoforce measurements. Light Sci. Appl. 2021, 10, 171. [Google Scholar] [CrossRef]
- Pevec, S.; Donlagic, D. Miniature all-fiber force sensor. Opt. Lett. 2020, 45, 5093–5096. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Zhou, J.; Liao, C.; Zhu, M.; Wang, Y.; Liu, S.; Li, C.; Zhang, Y.; Zhao, Y.; Gan, Z. Fiber-tip polymer microcantilever for fast and highly sensitive hydrogen measurement. ACS Appl. Mater. Interfaces 2020, 12, 33163–33172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xing, L.; Wu, Z.; Cai, L.; Zhang, Z.; Zhao, J. High-sensitivity photonic crystal fiber force sensor based on Sagnac interferometer for weighing. Opt. Laser Technol. 2020, 123, 105939. [Google Scholar] [CrossRef]
- Xue, B.; Gu, J.; Li, L.; Yu, W.; Yin, S.; Qin, M.; Jiang, Q.; Wang, W.; Cao, Y. Hydrogel tapes for fault-tolerant strong wet adhesion. Nat. Commun. 2021, 12, 7156. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, S.; Liu, X.; Qin, Z.; Yang, Y.; Zang, J.; Zhao, X. Fatigue-resistant adhesion of hydrogels. Nat. Commun. 2020, 11, 1071. [Google Scholar] [CrossRef]
- Fu, J. Hydrogel properties and applications. J. Mater. Chem. B 2019, 7, 1523–1525. [Google Scholar] [CrossRef]
- Kabir, S.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.; Ali, A.; Islam, M. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-based sensor networks: Compositions, properties, and applications—A review. ACS Appl. Bio Mater. 2020, 4, 140–162. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, X.; Ding, J.; Huang, B.; Wang, P.; Zhao, Y.; Mu, Q.; Zhang, S.; Ren, C.; Xu, W. Hydrogels for underwater adhesion: Adhesion mechanism, design strategies and applications. J. Mater. Chem. A 2022, 10, 11823–11853. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.K.; Zhao, B.; Pinnaratip, R. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, M.; Liao, C.; Chen, Y.; Gan, Z.; Liu, S.; Liu, D.; Liu, L.; Wang, Y. Measurement of Interfacial Adhesion Force with a 3D-Printed Fiber-Tip Microforce Sensor. Biosensors 2022, 12, 629. https://doi.org/10.3390/bios12080629
Zou M, Liao C, Chen Y, Gan Z, Liu S, Liu D, Liu L, Wang Y. Measurement of Interfacial Adhesion Force with a 3D-Printed Fiber-Tip Microforce Sensor. Biosensors. 2022; 12(8):629. https://doi.org/10.3390/bios12080629
Chicago/Turabian StyleZou, Mengqiang, Changrui Liao, Yanping Chen, Zongsong Gan, Shen Liu, Dejun Liu, Li Liu, and Yiping Wang. 2022. "Measurement of Interfacial Adhesion Force with a 3D-Printed Fiber-Tip Microforce Sensor" Biosensors 12, no. 8: 629. https://doi.org/10.3390/bios12080629
APA StyleZou, M., Liao, C., Chen, Y., Gan, Z., Liu, S., Liu, D., Liu, L., & Wang, Y. (2022). Measurement of Interfacial Adhesion Force with a 3D-Printed Fiber-Tip Microforce Sensor. Biosensors, 12(8), 629. https://doi.org/10.3390/bios12080629






