Dostarlimab as a Miracle Drug: Rising Hope against Cancer Treatment
Abstract
:1. Introduction
2. Monoclonal Antibody-Based Cancer Immunotherapy
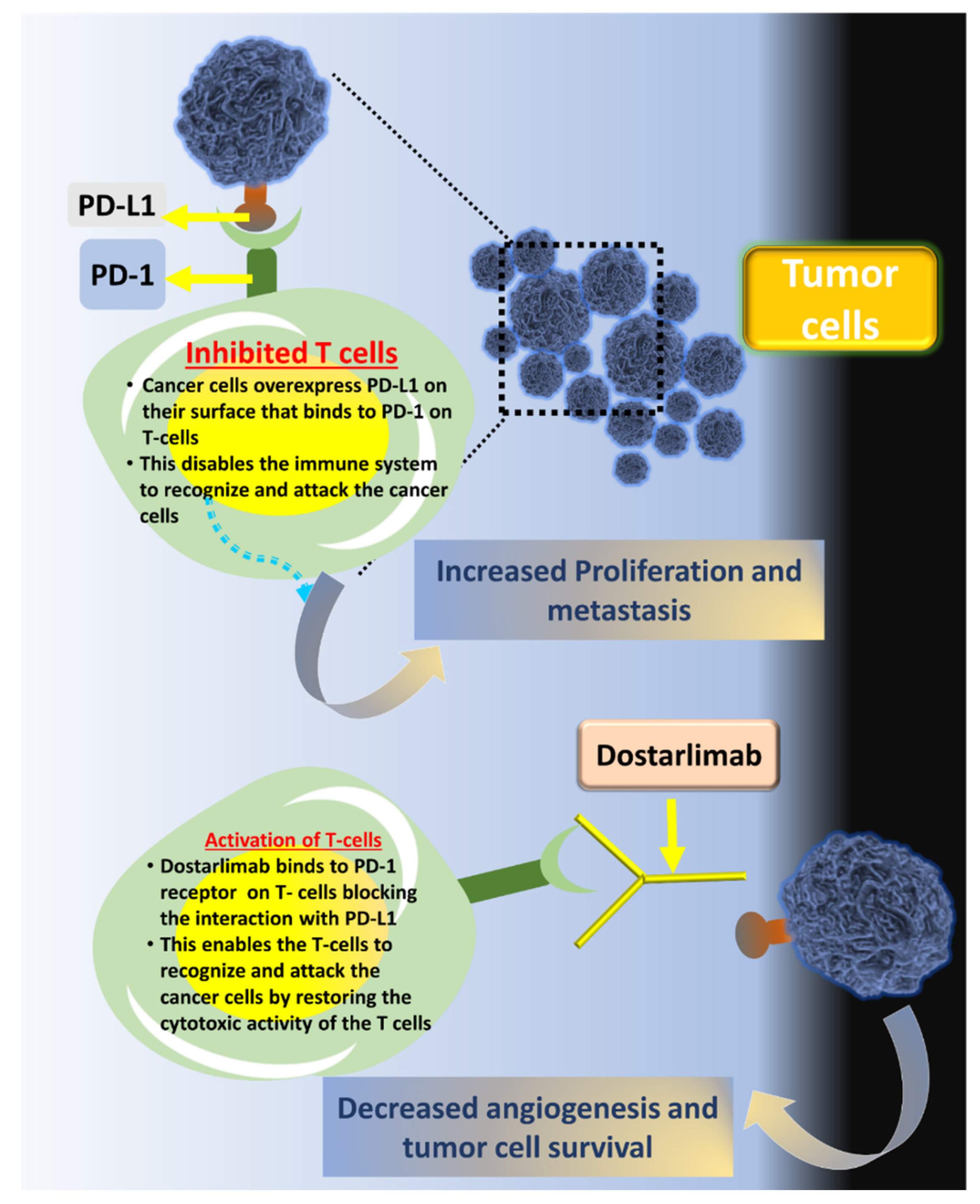
3. Dostarilimab and Mechanism of Action
4. Ongoing Clinical Trials for Dostarlimab
5. Dostarlimab and Other Combination Therapies under Trial
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Hussain, Z.; Rahim, M.A.; Jan, N.; Shah, H.; Rawas-Qalaji, M.; Khan, S.; Sohail, M.; Thu, H.E.; Ramli, N.A.; Sarfraz, R.M.; et al. Cell membrane cloaked nanomedicines for bio-imaging and immunotherapy of cancer: Improved pharmacokinetics, cell internalization and anticancer efficacy. J. Control. Release 2021, 335, 130–157. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Awal, A.; Khames, A.; Abourehab, M.A.S.; Samad, A.; Hassan, W.M.I.; Alam, R.; Osman, O.I.; Nur, S.M.; Molla, M.H.R.; et al. Computational Identification of Druggable Bioactive Compounds from Catharanthus roseus and Avicennia marina against Colorectal Cancer by Targeting Thymidylate Synthase. Molecules 2022, 27, 2089. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.; Ahmed, O.A.; Balata, G.F.; Almalki, W.H. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood–brain barrier. Int. J. Nanomed. 2018, 13, 3679–3687. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Tao, L.; Abourehab, M.A.; Hussain, Z. Design and development of novel hyaluronate-modified nanoparticles for combo-delivery of curcumin and alendronate: Fabrication, characterization, and cellular and molecular evidences of enhanced bone regeneration. Int. J. Biol. Macromol. 2018, 116, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, F.; Abourehab, M.A.; Hussain, Z. Hyaluronic acid decorated tacrolimus-loaded nanoparticles: Efficient approach to maximize dermal targeting and anti-dermatitis efficacy. Carbohydr. Polym. 2018, 197, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Al-Thubiani, A.S.; Maher, Y.A.; Fathi, A.; Abourehab, M.; Alarjah, M.; Khan, M.S.; Al Ghamdi, S.B. Identification and characterization of a novel antimicrobial peptide compound produced by Bacillus megaterium strain isolated from oral microflora. Saudi Pharm. J. 2018, 26, 1089–1097. [Google Scholar] [CrossRef]
- Fatima, M.; Sheikh, A.; Hasan, N.; Sahebkar, A.; Riadi, Y.; Kesharwani, P. Folic acid conjugated poly(amidoamine) dendrimer as a smart nanocarriers for tracing, imaging, and treating cancers over-expressing folate receptors. Eur. Polym. J. 2022, 170, 111156. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27 (Suppl. 2), S87–S97. [Google Scholar] [CrossRef]
- Singh, S.; Hassan, D.; Aldawsari, H.M.; Molugulu, N.; Shukla, R.; Kesharwani, P. Immune checkpoint inhibitors: A promising anticancer therapy. Drug Discov. Today 2019, 25, 223–229. [Google Scholar] [CrossRef]
- Shukla, A.; Mishra, V.; Kesharwani, P. Bilosomes in the context of oral immunization: Development, challenges and opportunities. Drug Discov. Today 2016, 21, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Numan, A.; Maddiboyina, B.; Arora, S.; Riadi, Y.; Shadab; Alhakamy, N.A.; Kesharwani, P. The emerging role of immune checkpoint inhibitors in the treatment of triple-negative breast cancer. Drug Discov. Today 2021, 26, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-W.; Chang, J.W.-C. Immune checkpoint inhibitors win the 2018 Nobel Prize. Biomed. J. 2019, 42, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kruger, S.; Ilmer, M.; Kobold, S.; Cadilha, B.L.; Endres, S.; Ormanns, S.; Schuebbe, G.; Renz, B.W.; D’Haese, J.G.; Schloesser, H.; et al. Advances in cancer immunotherapy 2019—Latest trends. J. Exp. Clin. Cancer Res. 2018, 38, 268. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Numan, A.; Agrawal, N.; Tambuwala, M.M.; Singh, V.; Kesharwani, P. Role of immune checkpoint inhibitors in the revolutionization of advanced melanoma care. Int. Immunopharmacol. 2020, 83, 106417. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, R.; Sahoo, D.; Naik, R.; Kesharwani, P.; Dandela, R. Pathogenesis, biology, and immunology of tuberculosis. In Nanotechnology Based Approaches for Tuberculosis Treatment; Academic Press: Cambridge, MA, USA, 2020; pp. 1–25. [Google Scholar] [CrossRef]
- Rahiman, N.; Markina, Y.V.; Kesharwani, P.; Johnston, T.P.; Sahebkar, A. Curcumin-based nanotechnology approaches and therapeutics in restoration of autoimmune diseases. J. Control. Release 2022, 348, 264–286. [Google Scholar] [CrossRef]
- Bandaru, R.; Rout, S.R.; Kamble, O.S.; Samal, S.K.; Gorain, B.; Sahebkar, A.; Ahmed, F.J.; Kesharwani, P.; Dandela, R. Clinical progress of therapeutics and vaccines: Rising hope against COVID-19 treatment. Process Biochem. 2022, 118, 154–170. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Monoclonal Antibodies (MABs) | Immunotherapy | Cancer Research UK, (n.d.). Available online: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/immunotherapy/types/monoclonal-antibodies (accessed on 16 June 2022).
- Weiner, G.J. Building better monoclonal antibody-based therapeutics. Nat. Cancer 2015, 15, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Kimiz-Gebologlu, I.; Gulce-Iz, S.; Biray-Avci, C. Monoclonal antibodies in cancer immunotherapy. Mol. Biol. Rep. 2018, 45, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- The Miraculous Cancer Cure: All you Wanted to Know about Dostarlimab|Lifestyle Health|English Manorama, (n.d.). Available online: https://www.onmanorama.com/lifestyle/health/2022/06/12/miraculous-cancer-cure-about-dostarlimab.html (accessed on 16 June 2022).
- Cancer Cure Finally Here? New Drug Dostarlimab Cures all Patients in Trial “First Time in History”|Mint, (n.d.). Available online: https://www.livemint.com/news/world/cancer-cure-finally-here-new-drug-dostarlimab-cures-all-patients-first-time-in-history-11654659187360.html (accessed on 16 June 2022).
- FDA; CDER. Highlights of Prescribing Information Tissue, Including the Following: Immune-Mediated Pneumonitis, (n.d.). Available online: www.fda.gov/medwatch (accessed on 16 June 2022).
- Home|GSK, (n.d.). Available online: https://www.gsk.com/en-gb/ (accessed on 16 June 2022).
- Jemperli | European Medicines Agency, (n.d.). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/jemperli (accessed on 16 June 2022).
- Lu, S.; Bowsher, R.R.; Clancy, A.; Rosen, A.; Zhang, M.; Yang, Y.; Koeck, K.; Gao, M.; Potocka, E.; Guo, W.; et al. An Integrated Analysis of Dostarlimab Immunogenicity. AAPS J. 2021, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Dostarlimab: First Approval. Drugs 2021, 81, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Kasherman, L.; Ahrari, S.; Lheureux, S. Dostarlimab in the treatment of recurrent or primary advanced endometrial cancer. Futur. Oncol. 2021, 17, 877–892. [Google Scholar] [CrossRef]
- Every Single Patient in This Small Experimental Drug Trial Saw Their Cancer Disappear, (n.d.). Available online: https://www.sciencealert.com/every-single-patient-in-this-small-experimental-drug-trial-saw-their-cancer-disappear (accessed on 16 June 2022).
- De Wilt, J.; Vermaas, M.; Ferenschild, F.; Verhoef, C. Management of Locally Advanced Primary and Recurrent Rectal Cancer. Clin. Colon Rectal Surg. 2007, 20, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.; Segal, M.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Study of Induction PD-1 Blockade in Subjects With Locally Advanced Mismatch Repair Deficient Solid Tumors—Full Text View—clinicaltrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04165772 (accessed on 15 June 2022).
- Redondo, A.; Gallego, A.; Mendiola, M. Dostarlimab for the treatment of advanced endometrial cancer. Expert Rev. Clin. Pharmacol. 2022, 15, 1–9. [Google Scholar] [CrossRef]
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouëlian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.-P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical Activity and Safety of the Anti–Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients With Recurrent or Advanced Mismatch Repair–Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020, 6, 1766–1772. [Google Scholar] [CrossRef]
- FDA Grants Accelerated Approval to Dostarlimab-Gxly for dMMR Endometrial Cancer | FDA, (n.d.). Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-endometrial-cancer (accessed on 15 June 2022).
- Oaknin, A.; Gilbert, L.; Tinker, A.V.; Brown, J.; Mathews, C.; Press, J.; Sabatier, R.; O’Malley, D.M.; Samouelian, V.; Boni, V.; et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: Interim results from GARNET—A phase I, single-arm study. J. Immunother. Cancer 2022, 10, e003777. [Google Scholar] [CrossRef]
- TSR-042 in Addition to Standard of Care Definitive Radiation for Inoperable Endometrial Cancer—Full Text View—clinicaltrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03955978 (accessed on 16 June 2022).
- Oaknin, A.; Iglesias, M.; Alarcon, J.; Javierre, G.V.; Garcia, L.G.; Santaballa, A.; Manso, L.; Romero, I.; Ginesta, M.B.; Churruca, C.; et al. 880TiP Randomized, open-label, phase II trial of dostarlimab (TSR-042), as maintenance therapy for patients with high-risk locally advanced cervical cancer after chemo-radiation: ATOMICC study. Ann. Oncol. 2020, 31, S645. [Google Scholar] [CrossRef]
- TSR-042 as Maintenance Therapy for Patients With High-risk Locally Advanced Cervical Cancer after Chemo-Radiation (ATOMICC)—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03833479 (accessed on 16 June 2022).
- Moreno, V.; Roda, D.; Pikiel, J.; Trigo, J.; Bosch-Barrera, J.; Drew, Y.; Kristeleit, R.; Hiret, S.; Bajor, D.L.; Cruz, P.; et al. Safety and Efficacy of Dostarlimab in Patients With Recurrent/Advanced Non–small Cell Lung Cancer: Results from Cohort E of the Phase I GARNET Trial. Clin. Lung Cancer 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Berton, D.; Curigliano, G.; Ellard, S.; Pérez, J.M.T.; Arkenau, H.-T.; Abdeddaim, C.; Moreno, V.; Guo, W.; Im, E.; et al. Safety and efficacy of anti–PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: Results from GARNET study. J. Clin. Oncol. 2021, 39, 9. [Google Scholar] [CrossRef]
- FDA Grants Accelerated Approval to Dostarlimab-Gxly for dMMR Advanced Solid Tumors|FDA, (n.d.). Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-advanced-solid-tumors (accessed on 15 June 2022).
- Berton, D.; Banerjee, S.N.; Curigliano, G.; Cresta, S.; Arkenau, H.-T.; Abdeddaim, C.; Kristeleit, R.S.; Redondo, A.; Leath, C.A.; Torres, A.A.; et al. Antitumor activity of dostarlimab in patients with mismatch repair-deficient/microsatellite instability–high tumors: A combined analysis of two cohorts in the GARNET study. J. Clin. Oncol. 2021, 39, 2564. [Google Scholar] [CrossRef]
- Study on TSR-042 in Advanced Clear Cell Sarcoma—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04274023 (accessed on 15 June 2022).
- Induction and Maintenance Treatment With PARP Inhibitor and Immunotherapy in HPV-Negative HNSCC—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04681469 (accessed on 16 June 2022).
- Swaminathan, S.; Padmapriyadarsini, C.; Venkatesan, P.; Narendran, G.; Kumar, S.R.; Iliayas, S.; Menon, P.A.; Selvaraju, S.; Pooranagangadevi, N.P.; Bhavani, P.K.; et al. Efficacy and Safety of Once-Daily Nevirapine- or Efavirenz-Based Antiretroviral Therapy in HIV-Associated Tuberculosis: A Randomized Clinical Trial. Clin. Infect. Dis. 2011, 53, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Niraparib and Dostarlimab for the Treatment of Small Cell Lung Cancer and Other High-Grade Neuroendocrine Carcinomas—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04701307 (accessed on 16 June 2022).
- Niraparib and Dostarlimab for the Treatment of Germline or Somatic BRCA1/2 and PALB2 Mutated Metastatic Pancreatic Cancer—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04493060 (accessed on 16 June 2022).
- Niraparib + TSR042 In BRCA Mutated Breast Cancer—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04584255 (accessed on 16 June 2022).
- Dose Escalation and Cohort Expansion Study of Niraparib and Dostarlimab in Pediatric Participants with Solid Tumors (SCOOP)—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04544995 (accessed on 16 June 2022).
- Clinical Trials Register, (n.d.). Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-000109-10/IT (accessed on 16 June 2022).
- Passiglia, F.; Bironzo, P.; Righi, L.; Listì, A.; Arizio, F.; Novello, S.; Volante, M.; Scagliotti, G.V. A Prospective Phase II Single-arm Study of Niraparib Plus Dostarlimab in Patients With Advanced Non–small-cell Lung Cancer and/or Malignant Pleural Mesothelioma, Positive for PD-L1 Expression and Germline or Somatic Mutations in the DNA Repair Genes: Rationale and Study Design. Clin. Lung Cancer 2020, 22, e63–e66. [Google Scholar] [CrossRef]
- Niraparib + Dostarlimab + RT in Pancreatic Cancer—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04409002 (accessed on 16 June 2022).
- Study of Niraparib and TSR-042 in Recurrent Endometrial Cancer—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03016338 (accessed on 16 June 2022).
- Recurrent Ovarian CarcinoSarcoma Anti-pd-1 Niraparib—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03651206 (accessed on 16 June 2022).
- A Phase 3 Comparison of Platinum-based Therapy With TSR-042 and Niraparib Versus Standard of Care (SOC) Platinum-based Therapy as First-line Treatment of Stage III or IV Nonmucinous Epithelial Ovarian Cancer—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03602859 (accessed on 16 June 2022).
- Study to Evaluate the Efficacy and Safety of the Combination of Niraparib and Dostarlimab (TSR-042) in Participants with Platinum Resistant Ovarian Cancer—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03955471 (accessed on 16 June 2022).
- Efficacy Comparison of Dostarlimab Plus Chemotherapy Versus Pembrolizumab Plus Chemotherapy in Participants with Metastatic Non-Squamous Non-Small Cell Lung Cancer (NSCLC)—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04581824 (accessed on 16 June 2022).
- Platform Trial of Novel Regimens Versus Standard of Care (SoC) in Participants with Non-Small Cell Lung Cancer (NSCLC)—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03739710 (accessed on 16 June 2022).
- Study of Niraparib, TSR-022, Bevacizumab, and Platinum-Based Doublet Chemotherapy in Combination with TSR-042—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://www.clinicaltrials.gov/ct2/show/NCT03307785 (accessed on 16 June 2022).
- Effects of Single Agent Niraparib and Niraparib Plus Programmed Cell Death-1 (PD-1) Inhibitors in Non-Small Cell Lung Cancer Participants—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03308942 (accessed on 16 June 2022).
- A Study of TSR-022 in Participants with Advanced Solid Tumors (AMBER)—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT02817633 (accessed on 16 June 2022).
- Dose Escalation and Expansion Study of GSK3359609 in Participants With Selected Advanced Solid Tumors (INDUCE-1)—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT02723955 (accessed on 16 June 2022).
- Study of TSR-033 with an Anti-Programmed Cell Death-1 Receptor (PD-1) in Participants with Advanced Solid Tumors—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03250832 (accessed on 16 June 2022).
- Radiation and TSR-042 in People With Endometrial Cancer After They Receive Surgery—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04774419 (accessed on 16 June 2022).
- Study of the Safety and Effectiveness of GSK6097608 in Participants with Advanced Solid Tumors—Full Text —ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04446351 (accessed on 16 June 2022).
- Study of TSR-042, an Anti-Programmed Cell Death-1 Receptor (PD-1) Monoclonal Antibody, in Participants with Advanced Solid Tumors—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT02715284 (accessed on 16 June 2022).
- Study Evaluating the Efficacy of Niraparib and Dostarlimab (TSR-042) in Recurrent/Metastatic HNSCC—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04313504 (accessed on 16 June 2022).
- SPY TRIAL: Neoadjuvant and Personalized Adaptive Novel Agents to Treat Breast Cancer—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT01042379 (accessed on 16 June 2022).
- Mesothelioma Stratified Therapy (MiST): A Multi-Drug Phase II Trial in Malignant Mesothelioma—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03654833 (accessed on 16 June 2022).
- A Study to Evaluate the Efficacy and Safety of Novel Treatment Combinations in Participants with Ovarian Cancer—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03574779 (accessed on 16 June 2022).
- Trial on NIraparib-TSR-042 (Dostarlimab) vs Physician’s Choice CHEmotherapy in Recurrent, Ovarian, Fallopian Tube or Primary Peritoneal Cancer Patients Not Candidate for Platinum Retreatment—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04679064 (accessed on 16 June 2022).
- Platform Study of Belantamab Mafodotin as Monotherapy and in Combination With Anti-Cancer Treatments in Participants with Relapsed/Refractory Multiple Myeloma (RRMM) (DREAMM 5)—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04126200 (accessed on 16 June 2022).
- Neoadjuvant PD-1 Inhibitor Dostarlimab (TSR-042), vs. Combination of Tim-3 Inhibitor Cobolimab (TSR-022) and PD-1 Inhibitor Dostarlimab (TSR-042) in Melanoma—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04139902 (accessed on 16 June 2022).
- A Study to Evaluate Dostarlimab Plus Carboplatin-paclitaxel Versus Placebo Plus Carboplatin-Paclitaxel in Participants With Recurrent or Primary Advanced Endometrial Cancer—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03981796 (accessed on 16 June 2022).

| Drug | Phase of Trial | Cancer Type | Start Date | End Date | No of Participants | Ref. |
|---|---|---|---|---|---|---|
| Dostarlimab | Phase II | Colorectal cancer | 11 December 2019 | 30 November 2025 | 30 | [35] |
| Dostarlimab | Phase I | Endometrial cancer | 15 October 2019 | 31 October 2024 | 12 | [40] |
| Dostarlimab | Phase II | Cervical cancer | 28 June 2019 | December 2024 | 132 | [42] |
| Dostarlimab | Phase II | Advanced clear cell sarcoma | 19 February 2021 | 1 May 2024 | 16 | [47] |
| Dostarlimab | Phase II | Endometrial cancer | 2 April 2021 | February 2023 | 31 | [68] |
| Dostarlimab | Phase I | Advanced solid tumors | 25 June 2020 | 29 August 2024 | 178 | [69] |
| Dostarlimab | Phase I | Advanced solid tumors | 7 March 2016 | 30 July 2024 | 740 | [70] |
| Dostarlimab, niraparib | Phase II | Head and neck squamous cell carcinoma (HNSCC) | 8 February 2021 | June 2028 | 49 | [48] |
| Dostarlimab, niraparib | Phase II | Neuroendocrine carcinomas | 1 February 2021 | 30 May 2025 | 48 | [50] |
| Dostarlimab, niraparib | Phase II | Pancreatic cancer | 28 December 2020 | 1 December 2022 | 20 | [51] |
| Dostarlimab, niraparib | Phase II | BRCA-mutated breast cancer | 18 December 2020 | 17 July 2029 | 62 | [52] |
| Dostarlimab, niraparib | Phase I | Pediatric solid tumors | 6 October 2020 | 15 March 2030 | 116 | [53] |
| Dostarlimab, niraparib | Phase II | Pancreatic cancer | 23 July 2020 | 1 October 2026 | 25 | [56] |
| Dostarlimab, niraparib | Phase II/III | Endometrial and ovarian cancer | 15 July 2020 | June 2025 | 196 | [58] |
| Dostarlimab, niraparib, pembrolizumab | Phase II | SCC, NSCLC | 29 September 2017 | 31 August 2021 | 53 | [64] |
| Dostarlimab, cobolimab, nivolumab, encelimab, docetaxel | Phase I | Solid tumors | 8 July 2016 | 3 October 2024 | 369 | [65] |
| Dostarlimab, bintrafusp alfa, cobolimab, feladilimab, GSK 3174998, pembrolizumab | Phase I | Solid tumors | 23 June 2016 | 20 June 2023 | 829 | [66] |
| Dostarlimab, bintrafusp alfa, cobolimab, feladilimab, GSK 3174998, pembrolizumab | Phase I | Solid tumors | 23 June 2016 | 20 June 2023 | 829 | [66] |
| Dostarlimab, niraparib | Phase II | Head and neck SCC | 4 November 2020 | 1 June 2027 | 23 | [71] |
| Dorstarlimab, paclitaxel, encequidar | Phase I | Breast cancer | 1 March 2010 | December 2031 | 4000 | [72] |
| Dostarlimab, niraparib | Phase II | Mesothelium | 28 January 2019 | 31 March 2023 | 200 | [73] |
| Dostarlimab, bevacizumab, niraparib | Phase II | Ovarian cancer | 15 November 2018 | 31 March 2026 | 125 | [74] |
| Dostarlimab, niraparib, doxorubicin, paclitaxel, gemcitabine, topotecan, bevacizumab | Phase III | Fallopian tube and ovarian cancer | 1 December 2020 | 1 January 2025 | 427 | [75] |
| Dostarlimab, belantamab, mafodotin, | Phase I/II | Multiple myeloma | 7 October 2019 | 23 February 2028 | 464 | [76] |
| Dostarlimab, Cobolimab | Phase II | Melanoma | 30 April 2020 | October 2027 | 56 | [77] |
| Dostarlimab, carboplatin, paclitaxel | Phase III | Endometrial cancer | 18 July 2019 | 23 December 2026 | 785 | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.; Sheikh, A.; Abourehab, M.A.S.; Kesharwani, P. Dostarlimab as a Miracle Drug: Rising Hope against Cancer Treatment. Biosensors 2022, 12, 617. https://doi.org/10.3390/bios12080617
Singh V, Sheikh A, Abourehab MAS, Kesharwani P. Dostarlimab as a Miracle Drug: Rising Hope against Cancer Treatment. Biosensors. 2022; 12(8):617. https://doi.org/10.3390/bios12080617
Chicago/Turabian StyleSingh, Vanshikha, Afsana Sheikh, Mohammed A. S. Abourehab, and Prashant Kesharwani. 2022. "Dostarlimab as a Miracle Drug: Rising Hope against Cancer Treatment" Biosensors 12, no. 8: 617. https://doi.org/10.3390/bios12080617
APA StyleSingh, V., Sheikh, A., Abourehab, M. A. S., & Kesharwani, P. (2022). Dostarlimab as a Miracle Drug: Rising Hope against Cancer Treatment. Biosensors, 12(8), 617. https://doi.org/10.3390/bios12080617





