Recent Advancements in Electrochemical Biosensors for Monitoring the Water Quality
Abstract
:1. Introduction
2. Electrochemical Biosensors
3. Surface Modification Technique
3.1. Adsorption
3.2. Self-Assembled Monolayers (SAMs)
3.3. Covalent Attachment
3.4. Electrodeposition
4. Biorecognition Elements
4.1. Enzyme-Based Bio-Recognition Materials
4.2. Antibody
4.3. Nucleic Acid-Based Bio-Recognition Materials
4.4. Whole Cell-Based Bio-Recognition Materials
| Recognition Element | Analyte/Pollutant | Electrode/Sensing Material | Type of Transducers | Limit of Detection | Response Time | Response Range | References |
|---|---|---|---|---|---|---|---|
| An enzyme (HRP) | phenol | electrochemically reduced graphene oxide/glass carbon electrode | differential pulse voltammetry | 2.19 μM | - | 3.0–100.0 μM | [90] |
| Enzyme (BChE) | paraoxon | Prussian Blue Nanoparticles/screen-printed electrodes | amperometric | 1 μg L−1 | 10 min | 2.0–10 μg L−1 | [91] |
| Enzyme (AChE) | Chlorpyrifos | ZrO2/RGO/ITO glass electrode | amperometric | 100 fM | - | 0.1–1000 pM | [92] |
| An enzyme (tyrosinase) | PhOH | ZnO Nanoparticles/screen-printed carbon electrodes | amperometric | 19.8 nM | <10 s | 0.1–14 μM | [93] |
| Antibodies (anti-Microcystin-leucine arginine) | Microcystin-leucine arginine | cysteamine/gold electrode | electrochemical impedance spectroscopy | 570 pg L−1 | - | 3.3 × 10−4–10−7 g L−1 | [94] |
| Antibodies (anti-alkylphenols) | 4-nonylphenol | single-walled carbon nanotubes/gold electrode | field effect transistors | 5 µg L−1 | - | 5–500 µg L−1 | [21] |
| Aptamers | atrazine | platinum nanoparticles microwires | electrochemical impedance spectroscopy | 10 pM | 10 min | 100 pM–1 μM | [57] |
| Aptamers | Vibrio alginolyticus | magnetic beads with a solid-contact polycation-sensitive membrane | potentiometric | 10 CFU mL−1 | 1 min | 10–100 CFU mL−1 | [95] |
| Whole cell (Shewanella cells) | riboflavin | Shewanella oneidensis MR-1 | amperometric | 0.85 ± 0.09 nM | - | 2–100 nM | [96] |
5. Type of Transducers
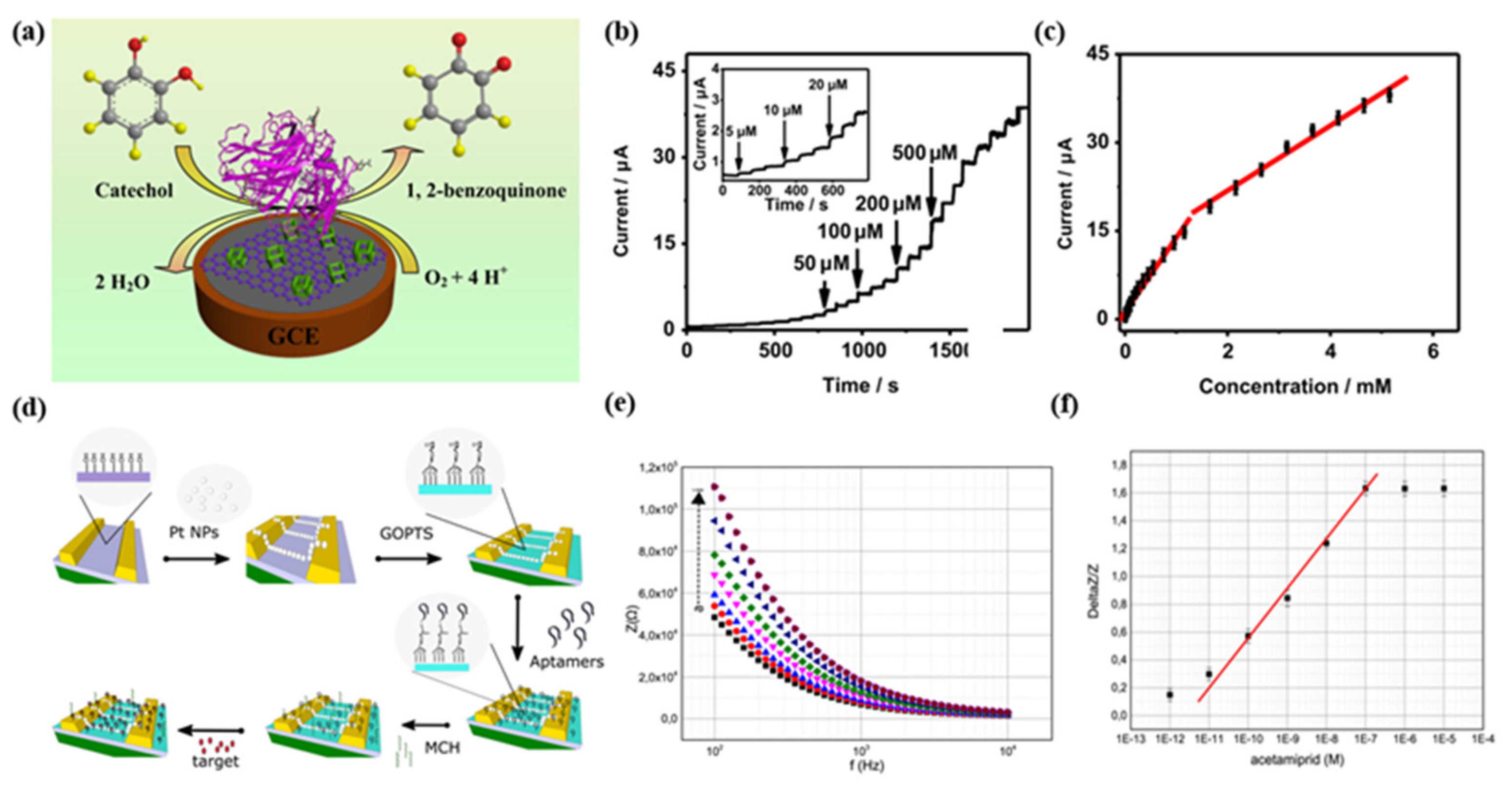
5.1. Voltammetric/Amperometric Biosensors
| Characteristics | Bio-Recognition Element | Detection Range | LOD | Response Time | Application | Ref |
|---|---|---|---|---|---|---|
| indium tin oxide (ITO) nanoparticles, hexaammineruthenium (III) chloride (RUT), and chitosan (CH) modified glassy carbon electrode (GCE) | horseradish peroxidase (HRP) enzyme | 0.009–0.301 M (Pb2+), 0.011–0.368 M (Ni2+), and 0.008–0.372 M (Cd2+). | 8 nM (Pb2+), 3 nM (Ni2+), and 1 nM (Cd2+) | 10 s | Heavy metal detection in water, with good selectivity, stability, and reproducibility | [104] |
| glassy carbon electrode with gold nanoparticles | Pb (II)-DNAzyme | 1 pM–1000 nM | 0.42 pM | - | Heavy metal detection in water. High sensitivity, excellent specificity, good stability and acceptable reproducibility | [105] |
| glassy carbon electrode | E. coli cells immobilization using bovine serum albumin (BSA) | 4.99 × 10−10 to 4.99 × 10−3 mol/L for mercury, 8.89 × 10−10 mol/L to 8.89 × 10−3 mol/L for cadmium, and 15.29 × 10−10 mol/L to 15.29 × 10−3 mol/L for zinc. | 5.58 × 10(−11) mol/L for mercury ion, 5.10 × 10(−10) mol/L for cadmium ion, and 1.38 × 10(−9) mol/L for zinc ion. | - | Heavy metal detection in water and lowcost and easy availability | [106] |
| glassy carbon electrode (GCE) modified with multiwalled carbon nanotubes (MWCNT) | choline oxidase enzyme | 0.1 to 1.0 nM (Pb2+) | 0.04 nM | 5 min | Heavy metal detection in tap water | [107] |
| Pt/CeO2/urease electrode | ceria (CeO2) nano-interface | 0.5–2.2 (Pb2+) and 0.02–0.8 μM (Hg2+) | 0.019 ± 0.001 μM (Pb2+) and 0.018 ± 0.003 μM (Hg2+) | <1 s | Heavy metal detection in river water and good repeatability and reproducibility | [108] |
5.2. Impedimetric Biosensors
5.3. Capacitive Biosensors
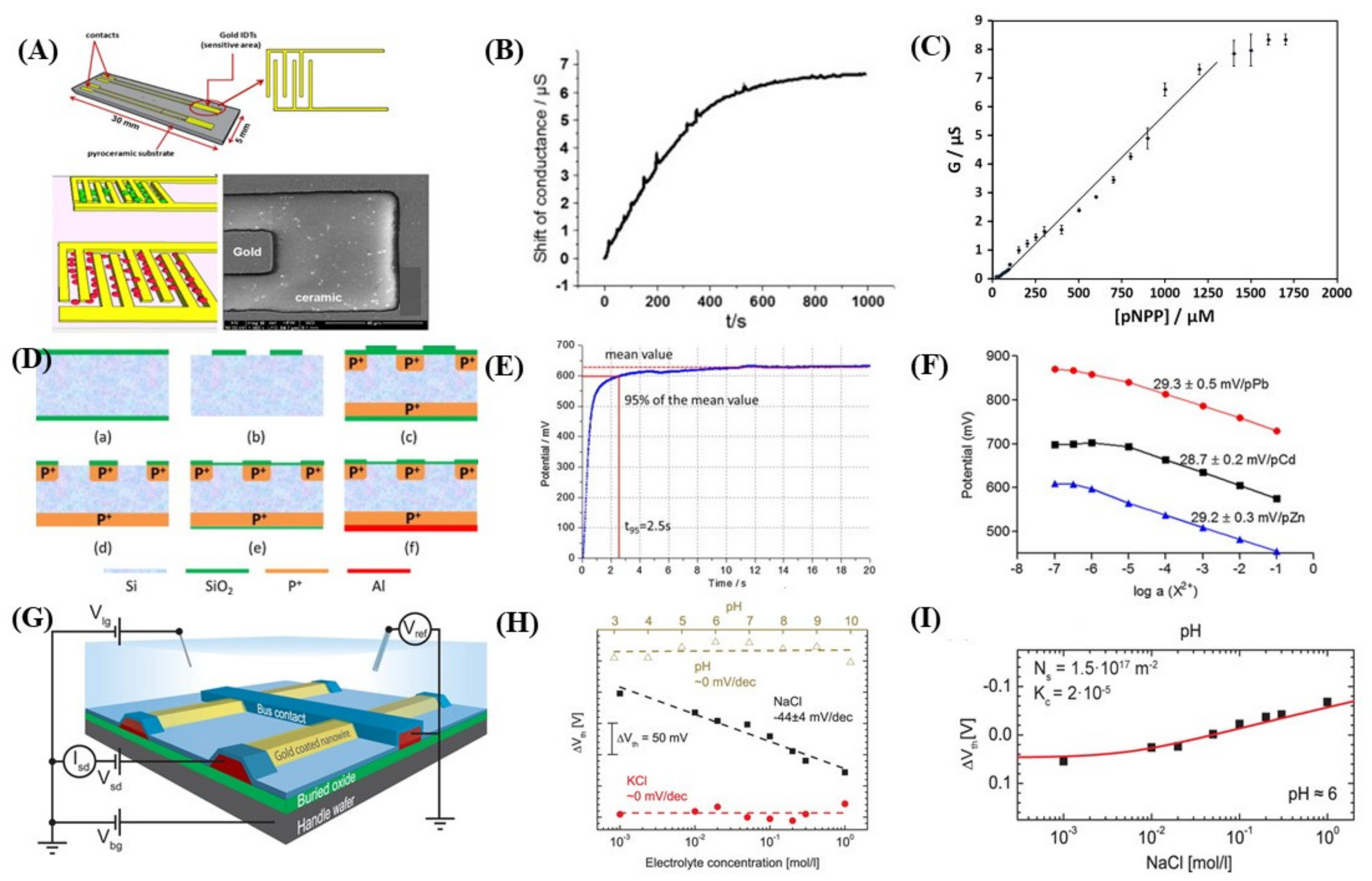
5.4. Conductometric Biosensors
5.5. Potentiometric Biosensors
5.6. Ion-Selective Field-Effect Transistors (ISFET) Based Biosensors
6. Signal Amplification Strategy
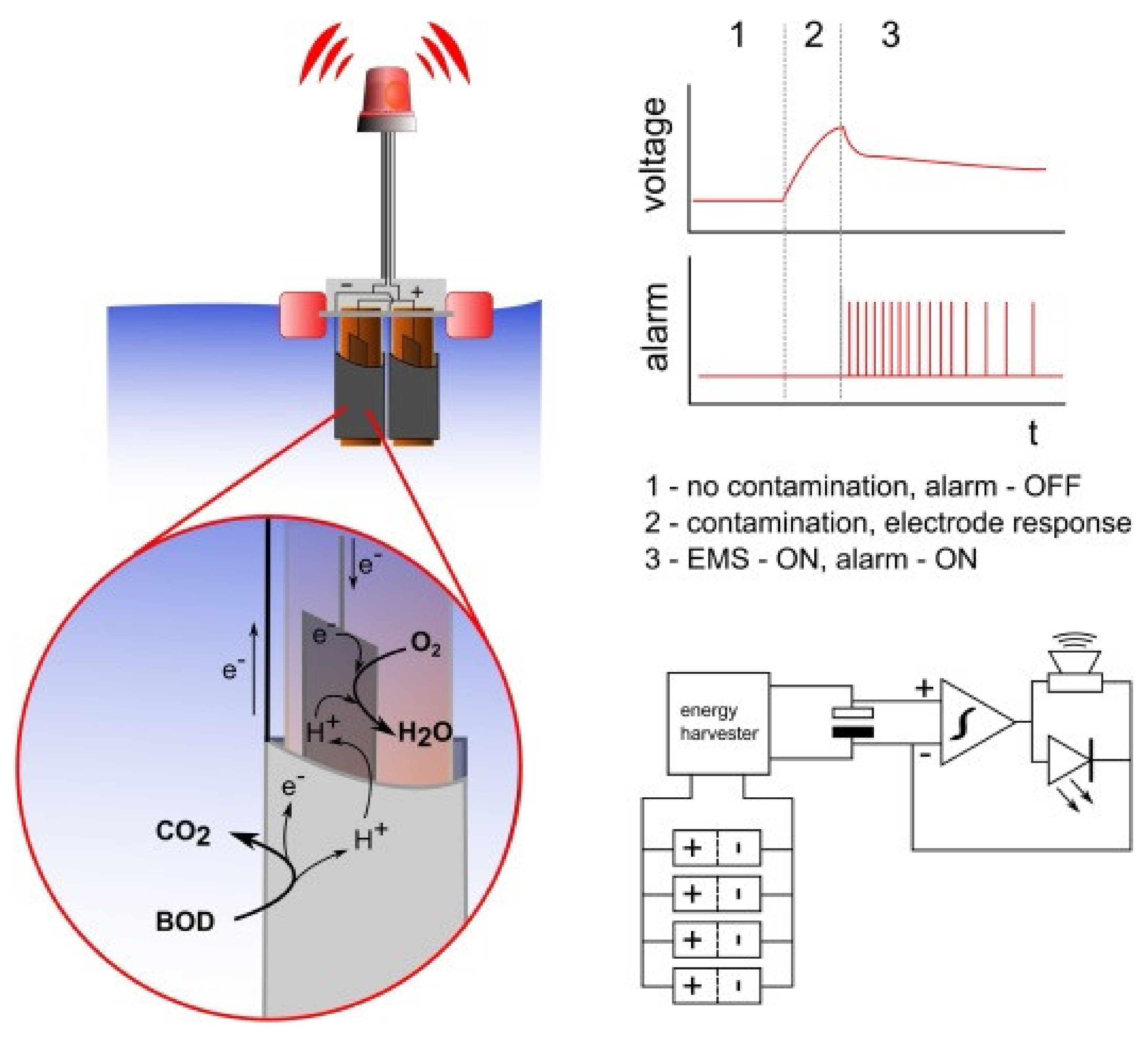
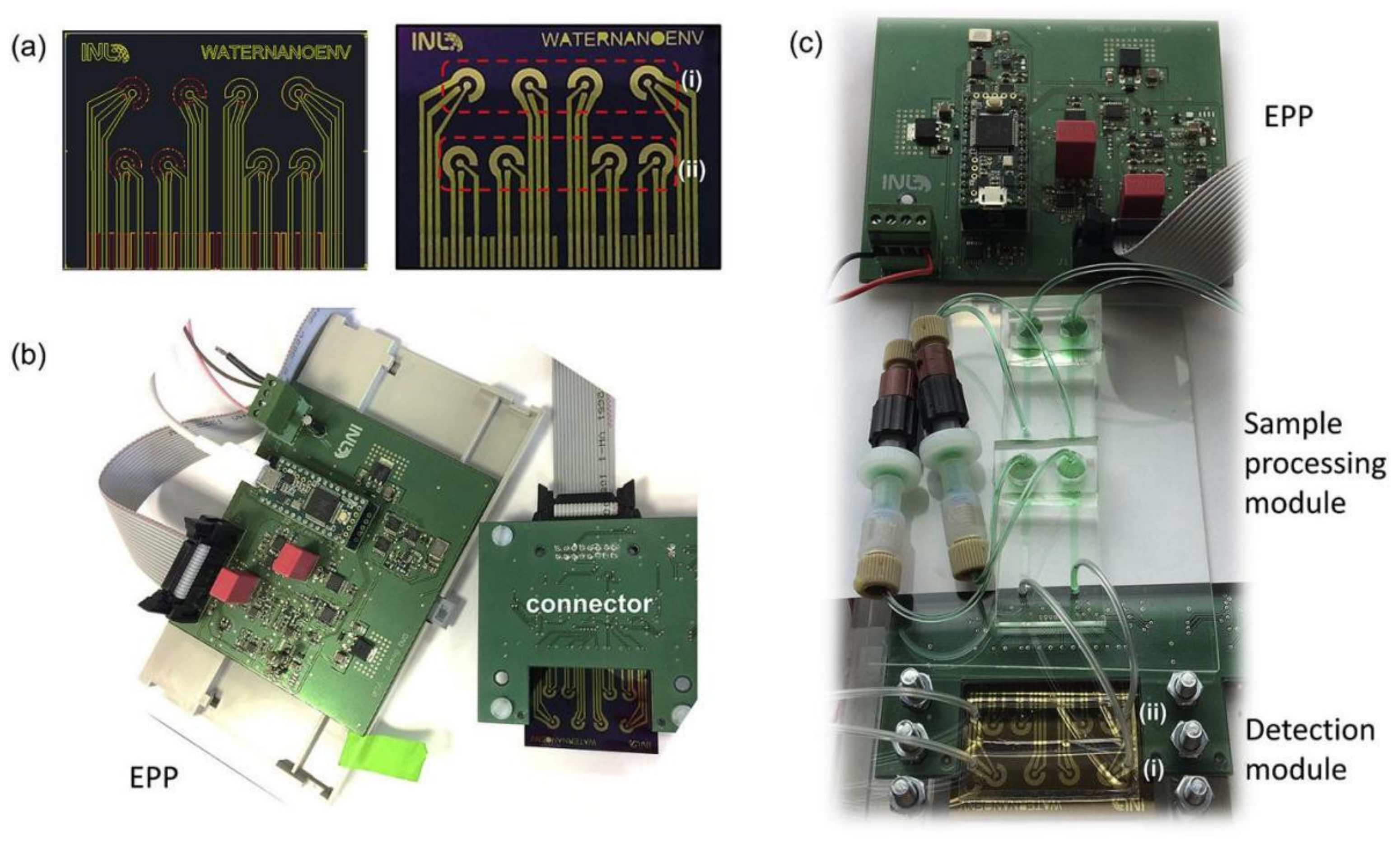
7. In Situ Monitoring System
8. Challenges and Future Work
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.-A.; Soozanipour, A.; Low, Z.-X.; Asadnia, M.; Taheri-Kafrani, A.; Razmjou, A. Biosensors for wastewater monitoring: A review. Biosens. Bioelectron. 2018, 118, 66–79. [Google Scholar] [CrossRef] [PubMed]
- W.H. Organization. Water, Sanitation and Hygiene (WASH). Available online: https://www.who.int/health-topics/water-sanitation-and-hygiene-wash (accessed on 10 May 2022).
- Bereza-Malcolm, L.T.; Mann, G.L.; Franks, A.E. Environmental sensing of heavy metals through whole cell microbial biosensors: A synthetic biology approach. ACS Synth. Biol. 2015, 4, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, M.M.; Hoekstra, A.Y. Sustainability: Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korostynska, O.; Mason, A.; Al-Shamma’a, A. Monitoring Pollutants in Wastewater: Traditional Lab Based versus Modern Real-Time Approaches. In Smart Sensors for Real-Time Water Quality Monitoring; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–24. [Google Scholar]
- Michael-Kordatou, I.; Iacovou, M.; Frontistis, Z.; Hapeshi, E.; Dionysiou, D.; Fatta-Kassinos, D. Erythromycin oxidation and ERY-resistant Escherichia coli inactivation in urban wastewater by sulfate radical-based oxidation process under UV-C irradiation. Water Res. 2015, 85, 346–358. [Google Scholar] [CrossRef]
- Sun, J.; Gan, Y.; Liang, T.; Zhou, S.; Wang, X.; Wan, H.; Wang, P. Signal enhancement of electrochemical DNA biosensors for the detection of trace heavy metals. Curr. Opin. Electrochem. 2019, 17, 23–29. [Google Scholar] [CrossRef]
- Uniyal, S.; Sharma, R.K. Technological advancement in electrochemical biosensor based detection of Organophosphate pesticide chlorpyrifos in the environment: A review of status and prospects. Biosens. Bioelectron. 2018, 116, 37–50. [Google Scholar] [CrossRef]
- Jouanneau, S.; Recoules, L.; Durand, M.; Boukabache, A.; Picot, V.; Primault, Y.; Lakel, A.; Sengelin, M.; Barillon, B.; Thouand, G. Methods for assessing biochemical oxygen demand (BOD): A review. Water Res. 2014, 49, 62–82. [Google Scholar] [CrossRef]
- Akhter, F.; Nag, A.; Alahi, M.E.E.; Liu, H.; Mukhopadhyay, S.C. Electrochemical detection of calcium and magnesium in water bodies. Sens. Actuators A Phys. 2020, 305, 111949. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, D.; Liu, Y.; Liu, R.; Wang, X.; Yu, Y.; Zhi, J. Problems analysis and new fabrication strategies of mediated electrochemical biosensors for wastewater toxicity assessment. Biosens. Bioelectron. 2018, 108, 82–88. [Google Scholar] [CrossRef]
- Justino, C.I.; Duarte, A.C.; Rocha-Santos, T.A. Recent progress in biosensors for environmental monitoring: A review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.; Li, S.-P.; Lisak, G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2020, 179, 112913. [Google Scholar] [CrossRef]
- Hassan, S.H.; Van Ginkel, S.W.; Hussein, M.A.; Abskharon, R.; Oh, S.-E. Toxicity assessment using different bioassays and microbial biosensors. Environ. Int. 2016, 92, 106–118. [Google Scholar] [CrossRef]
- Yang, P.; Xia, J.; Zhan, C.; Qiao, Y.; Wang, Y. Monitoring the spatio-temporal changes of terrestrial water storage using GRACE data in the Tarim River basin between 2002 and 2015. Sci. Total Environ. 2017, 595, 218–228. [Google Scholar] [CrossRef]
- Asadnia, M.; Myers, M.; Akhavan, N.D.; O’Donnell, K.; Umana-Membreno, G.A.; Mishra, U.; Nener, B.; Baker, M.; Parish, G. Mercury (II) selective sensors based on AlGaN/GaN transistors. Anal. Chim. Acta 2016, 943, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Biswas, P.; Karn, A.K.; Balasubramanian, P.; Kale, P.G. Biosensor for detection of dissolved chromium in potable water: A review. Biosens. Bioelectron. 2017, 94, 589–604. [Google Scholar] [CrossRef]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
- Dou, Y.; Li, Z.; Su, J.; Song, S. A Portable Biosensor Based on Au Nanoflower Interface Combined with Electrochemical Immunochromatography for POC Detection of Prostate-Specific Antigen. Biosensors 2022, 12, 259. [Google Scholar] [CrossRef]
- Sohrabi, H.; Hemmati, A.; Majidi, M.R.; Eyvazi, S.; Jahanban-Esfahlan, A.; Baradaran, B.; Adlpour-Azar, R.; Mokhtarzadeh, A.; de la Guardia, M. Recent advances on portable sensing and biosensing assays applied for detection of main chemical and biological pollutant agents in water samples: A critical review. TrAC Trends Anal. Chem. 2021, 143, 116344. [Google Scholar] [CrossRef]
- Belkhamssa, N.; da Costa, J.P.; Justino, C.I.; Santos, P.S.; Cardoso, S.; Duarte, A.C.; Rocha-Santos, T.; Ksibi, M. Development of an electrochemical biosensor for alkylphenol detection. Talanta 2016, 158, 30–34. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2019, 126, 346–354. [Google Scholar] [CrossRef]
- Adekunle, A.; Raghavan, V.; Tartakovsky, B. A comparison of microbial fuel cell and microbial electrolysis cell biosensors for real-time environmental monitoring. Bioelectrochemistry 2019, 126, 105–112. [Google Scholar] [CrossRef]
- Zheng, H.; Yan, Z.; Wang, M.; Chen, J.; Zhang, X. Biosensor based on polyaniline-polyacrylonitrile-graphene hybrid assemblies for the determination of phenolic compounds in water samples. J. Hazard. Mater. 2019, 378, 120714. [Google Scholar] [CrossRef]
- Nag, A.; Alahi, M.E.E.; Feng, S.; Mukhopadhyay, S.C. IoT-based sensing system for phosphate detection using Graphite/PDMS sensors. Sens. Actuators A Phys. 2019, 286, 43–50. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Jalili Ghazizadeh, A.; Hashemi, P.; Afkhami, A.; Arduini, F.; Bagheri, H. An overview to electrochemical biosensors and sensors for the detection of environmental contaminants. J. Iran. Chem. Soc. 2020, 17, 2429–2447. [Google Scholar] [CrossRef]
- Guo, J. Uric acid monitoring with a smartphone as the electrochemical analyzer. Anal. Chem. 2016, 88, 11986–11989. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Ma, X. Simultaneous monitoring of glucose and uric acid on a single test strip with dual channels. Biosens. Bioelectron. 2017, 94, 415–419. [Google Scholar] [CrossRef]
- Tsopela, A.; Laborde, A.; Salvagnac, L.; Ventalon, V.; Bedel-Pereira, E.; Séguy, I.; Temple-Boyer, P.; Juneau, P.; Izquierdo, R.; Launay, J. Development of a lab-on-chip electrochemical biosensor for water quality analysis based on microalgal photosynthesis. Biosens. Bioelectron. 2016, 79, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Song, Y.; Zhang, Y.; Xing, Y.; Zhao, H.; Xie, J.; Xu, S.; Gao, F.; Wang, M.; Xing, G. Microelectrode arrays modified with nanocomposites for monitoring dopamine and spike firings under deep brain stimulation in rat models of parkinson’s disease. ACS Sens. 2019, 4, 1992–2000. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, P.; Chen, Y.; Bai, X.; Du, L.; Chen, W.; Wu, C.; Wang, P. Piezoelectric aptasensor with gold nanoparticle amplification for the label-free detection of okadaic acid. Sens. Actuators B Chem. 2021, 346, 130446. [Google Scholar] [CrossRef]
- Yang, F.; Chang, T.-L.; Liu, T.; Wu, D.; Du, H.; Liang, J.; Tian, F. Label-free detection of Staphylococcus aureus bacteria using long-period fiber gratings with functional polyelectrolyte coatings. Biosens. Bioelectron. 2019, 133, 147–153. [Google Scholar] [CrossRef]
- Khorshid, M.; Sichani, S.B.; Cornelis, P.; Wackers, G.; Wagner, P. The hot-wire concept: Towards a one-element thermal biosensor platform. Biosens. Bioelectron. 2021, 179, 113043. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P. Biosensors—Sense and sensitivity. Science 2000, 290, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Bezaatpour, A.; Jafari, H.; Boukherroub, R.; Szunerits, S. Electrochemical methodologies for the detection of pathogens. ACS Sens. 2018, 3, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Y.; Erf, G.F. Interdigitated array microelectrode-based electrochemical impedance immunosensor for detection of Escherichia coli O157: H7. Anal. Chem. 2004, 76, 1107–1113. [Google Scholar] [CrossRef]
- Bhadra, P.; Shajahan, M.; Bhattacharya, E.; Chadha, A. Studies on varying n-alkanethiol chain lengths on a gold coated surface and their effect on antibody–antigen binding efficiency. RSC Adv. 2015, 5, 80480–80487. [Google Scholar] [CrossRef]
- Lu, Z.; Zhao, W.; Wu, L.; He, J.; Dai, W.; Zhou, C.; Du, H.; Ye, J. Tunable electrochemical of electrosynthesized layer-by-layer multilayer films based on multi-walled carbon nanotubes and metal-organic framework as high-performance electrochemical sensor for simultaneous determination cadmium and lead. Sens. Actuators B Chem. 2021, 326, 128957. [Google Scholar] [CrossRef]
- Konishi, T.; Hashimoto, T.; Sato, N.; Nakajima, K.; Yamaguchi, K. Substituent effects at the benzyl position and aromatic ring of silane-coupling agents containing 2-nitrobenzyl esters on photosensitivity and hydrophobic surface of a self-assembled monolayer (SAM). Bull. Chem. Soc. Jpn. 2016, 89, 125–134. [Google Scholar] [CrossRef]
- Jia, S.; Bian, C.; Sun, J.; Tong, J.; Xia, S. A wavelength-modulated localized surface plasmon resonance (LSPR) optical fiber sensor for sensitive detection of mercury (II) ion by gold nanoparticles-DNA conjugates. Biosens. Bioelectron. 2018, 114, 15–21. [Google Scholar] [CrossRef]
- Bizid, S.; Mlika, R.; Said, A.H.; Chemli, M.; Youssoufi, H.K. Investigations of poly (p-phenylene) modified with ferrocene and their application in electrochemical DNA sensing. Sens. Actuators B Chem. 2016, 226, 370–380. [Google Scholar] [CrossRef]
- Lian, Y.; He, F.; Wang, H.; Tong, F. A new aptamer/graphene interdigitated gold electrode piezoelectric sensor for rapid and specific detection of Staphylococcus aureus. Biosens. Bioelectron. 2015, 65, 314–319. [Google Scholar] [CrossRef]
- Rahmanian, R.; Mozaffari, S.A. Electrochemical fabrication of ZnO-polyvinyl alcohol nanostructured hybrid film for application to urea biosensor. Sens. Actuators B Chem. 2015, 207, 772–781. [Google Scholar] [CrossRef]
- Hui, Y.; Bian, C.; Xia, S.; Tong, J.; Wang, J. Synthesis and electrochemical sensing application of poly(3,4-ethylenedioxythiophene)-based materials: A review. Anal. Chim. Acta 2018, 1022, 1–19. [Google Scholar] [CrossRef]
- Armistead, P.M.; Thorp, H.H. Modification of indium tin oxide electrodes with nucleic acids: Detection of attomole quantities of immobilized DNA by electrocatalysis. Anal. Chem. 2000, 72, 3764–3770. [Google Scholar] [CrossRef]
- Azek, F.; Grossiord, C.; Joannes, M.; Limoges, B.; Brossier, P. Hybridization assay at a disposable electrochemical biosensor for the attomole detection of amplified human cytomegalovirus DNA. Anal. Biochem. 2000, 284, 107–113. [Google Scholar] [CrossRef]
- Schöning, M.J.; Wagner, T.; Poghossian, A.; Miyamoto, K.; Werner, C.; Krause, S.; Yoshinobu, T. Light-Addressable Potentiometric Sensors for (Bio-) Chemical Sensing and Imaging. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–308. [Google Scholar]
- Evtugyn, G. Biosensors: Essentials; Springer: Berlin/Heidelberg, Germany, 2014; Volume 84. [Google Scholar]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Jijie, R.; Kahlouche, K.; Barras, A.; Yamakawa, N.; Bouckaert, J.; Gharbi, T.; Szunerits, S.; Boukherroub, R. Reduced graphene oxide/polyethylenimine based immunosensor for the selective and sensitive electrochemical detection of uropathogenic Escherichia coli. Sens. Actuators B Chem. 2018, 260, 255–263. [Google Scholar] [CrossRef]
- Jain, A.; Cheng, K. The principles and applications of avidin-based nanoparticles in drug delivery and diagnosis. J. Control. Release 2017, 245, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Feng, M.; Yan, H.; Sun, W.; Shi, Z.; Lin, Q. Fabrication of myoglobin-sodium alginate-graphene composite modified carbon ionic liquid electrode via the electrodeposition method and its electrocatalysis toward trichloroacetic acid. Int. J. Electrochem. Sci. 2017, 12, 11633–11645. [Google Scholar] [CrossRef]
- Ben Messaoud, N.; Ghica, M.E.; Dridi, C.; Ben Ali, M.; Brett, C.M. A novel amperometric enzyme inhibition biosensor based on xanthine oxidase immobilised onto glassy carbon electrodes for bisphenol A determination. Talanta 2018, 184, 388–393. [Google Scholar] [CrossRef]
- Bettazzi, F.; Romero Natale, A.; Torres, E.; Palchetti, I. Glyphosate determination by coupling an immuno-magnetic assay with electrochemical sensors. Sensors 2018, 18, 2965. [Google Scholar] [CrossRef] [Green Version]
- Azri, F.A.; Sukor, R.; Selamat, J.; Abu Bakar, F.; Yusof, N.A.; Hajian, R. Electrochemical immunosensor for detection of aflatoxin B1 based on indirect competitive ELISA. Toxins 2018, 10, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Fernández, B.; Mercader, J.V.; Checa-Orrego, B.I.; De La Escosura-Muñiz, A.; Costa-García, A. A monoclonal antibody-based immunosensor for the electrochemical detection of imidacloprid pesticide. Analyst 2019, 144, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Madianos, L.; Tsekenis, G.; Skotadis, E.; Patsiouras, L.; Tsoukalas, D. A highly sensitive impedimetric aptasensor for the selective detection of acetamiprid and atrazine based on microwires formed by platinum nanoparticles. Biosens. Bioelectron. 2018, 101, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Campaña, A.L.; Florez, S.L.; Noguera, M.J.; Fuentes, O.P.; Ruiz Puentes, P.; Cruz, J.C.; Osma, J.F. Enzyme-based electrochemical biosensors for microfluidic platforms to detect pharmaceutical residues in wastewater. Biosensors 2019, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Ayenimo, J.G.; Adeloju, S.B. Rapid amperometric detection of trace metals by inhibition of an ultrathin polypyrrole-based glucose biosensor. Talanta 2016, 148, 502–510. [Google Scholar] [CrossRef]
- Ghanavati, M.; Azad, R.R.; Mousavi, S.A. Amperometric inhibition biosensor for the determination of cyanide. Sens. Actuators B Chem. 2014, 190, 858–864. [Google Scholar] [CrossRef]
- Hervas, M.; Lopez, M.A.; Escarpa, A. Electrochemical immunosensing on board microfluidic chip platforms. Trends Anal. Chem. 2012, 31, 109–128. [Google Scholar] [CrossRef]
- González-Techera, A.; Zon, M.A.; Molina, P.G.; Fernández, H.; González-Sapienza, G.; Arévalo, F.J. Development of a highly sensitive noncompetitive electrochemical immunosensor for the detection of atrazine by phage anti-immunocomplex assay. Biosens. Bioelectron. 2015, 64, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Li, Q.; Pang, X.; Liu, Y.; Wang, X.; Chen, G. A sensitive electrochemical impedance immunosensor for determination of malachite green and leucomalachite green in the aqueous environment. Anal. Bioanal. Chem. 2016, 408, 5593–5600. [Google Scholar] [CrossRef]
- Rengaraj, S.; Cruz-Izquierdo, Á.; Scott, J.L.; Di Lorenzo, M. Impedimetric paper-based biosensor for the detection of bacterial contamination in water. Sens. Actuators B Chem. 2018, 265, 50–58. [Google Scholar] [CrossRef]
- Tan, A.; Lim, C.; Zou, S.; Ma, Q.; Gao, Z. Electrochemical nucleic acid biosensors: From fabrication to application. Anal. Methods 2016, 8, 5169–5189. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J.L. Aptamer based electrochemical sensors for emerging environmental pollutants. Front. Chem. 2014, 2, 41. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, J.; Liu, J.; Wang, X.; Chen, B. Disease-related detection with electrochemical biosensors: A review. Sensors 2017, 17, 2375. [Google Scholar] [CrossRef]
- Díaz-Serrano, M.; Rosado, A.; Del Pilar, J.; Vega, E.Z.; Guadalupe, A.R. Synthesis, Characterization and Use of Ru-Fc Intercalation Complex as an Electrochemical Label for the Detection of Pathogen-DNA. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2013. [Google Scholar]
- Lv, X.; Ge, W.; Li, Q.; Wu, Y.; Jiang, H.; Wang, X. Rapid and ultrasensitive electrochemical detection of multidrug-resistant bacteria based on nanostructured gold coated ITO electrode. ACS Appl. Mater. Interfaces 2014, 6, 11025–11031. [Google Scholar] [CrossRef]
- Ligaj, M.; Tichoniuk, M.; Gwiazdowska, D.; Filipiak, M. Electrochemical DNA biosensor for the detection of pathogenic bacteria Aeromonas hydrophila. Electrochim. Acta 2014, 128, 67–74. [Google Scholar] [CrossRef]
- Zhan, S.; Wu, Y.; Wang, L.; Zhan, X.; Zhou, P. A mini-review on functional nucleic acids-based heavy metal ion detection. Biosens. Bioelectron. 2016, 86, 353–368. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Corduneanu, O.; Oliveira, S.C.B.; Diculescu, V.C.; Oliveira-Brett, A.M. Electrochemical and AFM evaluation of hazard compounds-DNA interaction. Electrochim. Acta 2009, 54, 1978–1985. [Google Scholar] [CrossRef]
- Torigoe, H.; Ono, A.; Kozasa, T. HgII ion specifically binds with T:T mismatched base pair in duplex DNA. Chem. Eur. J. 2010, 16, 13218–13225. [Google Scholar] [CrossRef]
- Xiao, Z.; Meng, H.; Qin, X.; Sang, X.; Zhang, Y.; Yuan, Y. The functionalization of gold nanoparticles as a novel platform for the highly efficient electrochemical detection of silver ions. Analyst 2021, 146, 597–604. [Google Scholar] [CrossRef]
- Xu, G.; Huo, D.; Hou, C.; Zhao, Y.; Bao, J.; Yang, M.; Fa, H. A regenerative and selective electrochemical aptasensor based on copper oxide nanoflowers-single walled carbon nanotubes nanocomposite for chlorpyrifos detection. Talanta 2018, 178, 1046–1052. [Google Scholar] [CrossRef]
- Lan, L.; Liu, Y.; Chen, X.; Zhang, T.; Dong, N.; Miao, P. Preparation of a novel iron cryptate as an electrochemical probe for biosensing. Electrochem. Commun. 2019, 98, 92–95. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Q.; Guo, Z.; Wang, S.; Du, C.; Zhai, C. In situ grown DNA nanotail-templated silver nanoclusters enabling label-free electrochemical sensing of terminal deoxynucleotidyl transferase activity. Biosens. Bioelectron. 2017, 98, 91–99. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Housaindokht, M.R.; Verdian, A.; Bozorgmehr, M.R. Simultaneous detection and determination of mercury (II) and lead (II) ions through the achievement of novel functional nucleic acid-based biosensors. Biosens. Bioelectron. 2018, 116, 130–147. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Ye, Z.; Peng, D.; He, L.; Yan, F.; Yang, Y.; Zhang, H.; Zhang, Z. A gold electrode modified with amino-modified reduced graphene oxide, ion specific DNA and DNAzyme for dual electrochemical determination of Pb (II) and Hg (II). Microchim. Acta 2015, 182, 2251–2258. [Google Scholar] [CrossRef]
- Saini, R.; Hegde, K.; Brar, S.K.; Verma, M. Advances in Whole Cell-Based Biosensors in Environmental Monitoring. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Lagarde, F.; Jaffrezic-Renault, N. Cell-based electrochemical biosensors for water quality assessment. Anal. Bioanal. Chem. 2011, 400, 947–964. [Google Scholar] [CrossRef]
- Gutiérrez, J.C.; Amaro, F.; Martín-González, A. Microbial Biosensors for Metal(loid)s. In Microbial Ecotoxicology; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Alpat, Ş.; Alpat, S.K.; Çadirci, B.H.; Yaşa, I.; Telefoncu, A. A novel microbial biosensor based on Circinella sp. modified carbon paste electrode and its voltammetric application. Sens. Actuators B Chem. 2008, 134, 175–181. [Google Scholar] [CrossRef]
- Yüce, M.; Nazir, H.; Dönmez, G. An advanced investigation on a new algal sensor determining Pb(II) ions from aqueous media. Biosens. Bioelectron. 2010, 26, 321–326. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Wang, J.; Bian, C.; Tong, J.; Li, Y.; Xia, S. A single-layer structured microbial sensor for fast detection of biochemical oxygen demand. Biochem. Eng. J. 2016, 112, 219–225. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Wang, J.; Bian, C.; Tong, J.; Li, Y.; Xia, S. A microbial electrode based on the co-electrodeposition of carboxyl graphene and Au nanoparticles for BOD rapid detection. Biochem. Eng. J. 2017, 123, 86–94. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Bian, C.; Tong, J.; Fang, Y.; Xia, S. Ultramicroelectrode array modified with magnetically labeled Bacillus subtilis, palladium nanoparticles and reduced carboxy graphene for amperometric determination of biochemical oxygen demand. Microchim. Acta 2017, 184, 763–771. [Google Scholar] [CrossRef]
- Hooi, K.B.; Ismail, A.K.; Ahamad, R.; Shahir, S. A redox mediated UME biosensor using immobilized Chromobacterium violaceum strain R1 for rapid biochemical oxygen demand measurement. Electrochim. Acta 2015, 176, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Kaffash, A.; Zare, H.R.; Rostami, K. Highly sensitive biosensing of phenol based on the adsorption of the phenol enzymatic oxidation product on the surface of an electrochemically reduced graphene oxide-modified electrode. Anal. Methods 2018, 10, 2731–2739. [Google Scholar] [CrossRef]
- Arduini, F.; Neagu, D.; Scognamiglio, V.; Patarino, S.; Moscone, D.; Palleschi, G. Automatable flow system for paraoxon detection with an embedded screen-printed electrode tailored with butyrylcholinesterase and prussian blue nanoparticles. Chemosensors 2015, 3, 129–145. [Google Scholar] [CrossRef] [Green Version]
- Mogha, N.K.; Sahu, V.; Sharma, M.; Sharma, R.K.; Masram, D.T. Biocompatible ZrO2-reduced graphene oxide immobilized AChE biosensor for chlorpyrifos detection. Mater. Des. 2016, 111, 312–320. [Google Scholar] [CrossRef]
- Haddaoui, M.; Raouafi, N. Chlortoluron-induced enzymatic activity inhibition in tyrosinase/ZnO NPs/SPCE biosensor for the detection of ppb levels of herbicide. Sens. Actuators B Chem. 2015, 219, 171–178. [Google Scholar] [CrossRef]
- Dos Santos, M.B.; Queirós, R.B.; Geraldes, Á.; Marques, C.; Vilas-Boas, V.; Dieguez, L.; Paz, E.; Ferreira, R.; Morais, J.; Vasconcelos, V. Portable sensing system based on electrochemical impedance spectroscopy for the simultaneous quantification of free and total microcystin-LR in freshwaters. Biosens. Bioelectron. 2019, 142, 111550. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, J.; Yu, H.; Yin, T.; Qin, W. Potentiometric aptasensing of Vibrio alginolyticus based on DNA nanostructure-modified magnetic beads. Sensors 2016, 16, 2052. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Wang, J.-X.; Si, R.-W.; Yang, Y.; Zhang, C.-L.; Yong, Y.-C. Sensitive amperometric detection of riboflavin with a whole-cell electrochemical sensor. Anal. Chim. Acta 2017, 985, 148–154. [Google Scholar] [CrossRef]
- Bozal-Palabiyik, B. Foodborne Diseases || Biosensor-Based Methods for the Determination of Foodborne Pathogens. Available online: https://www.sciencedirect.com/science/article/pii/B9780128114445000129 (accessed on 10 May 2022).
- Mei, L.; Feng, J.; Wu, L.; Zhou, J.; Chen, J.; Wang, A. Novel phenol biosensor based on laccase immobilized on reduced graphene oxide supported palladium-copper alloyed nanocages. Biosens. Bioelectron. 2015, 74, 347–352. [Google Scholar] [CrossRef]
- Albareda-Sirvent, M.; Merkoçi, A.; Alegret, S. Pesticide determination in tap water and juice samples using disposable amperometric biosensors made using thick-film technology. Anal. Chim. Acta 2001, 442, 35–44. [Google Scholar] [CrossRef]
- Badea, M.; Amine, A.; Palleschi, G.; Moscone, D.; Curulli, A. New electrochemical sensors for detection of nitrites and nitrates. J. Electroanal. Chem. 2001, 509, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Biagiotti, V.; Valentini, F.; Tamburri, E.; Terranova, M.L.; Moscone, D.; Palleschi, G. Synthesis and characterization of polymeric films and nanotubule nets used to assemble selective sensors for nitrite detection in drinking water. Sens. Actuators B Chem. 2007, 122, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Stoytcheva, M.; Zlatev, R.; Gochev, V.; Velkova, Z.; Montero, G.; Beleno, M.T. Amperometric biosensors precision improvement. Application to phenolic pollutants determination. Electrochim. Acta 2014, 147, 25–30. [Google Scholar] [CrossRef]
- Pan, Y.; Shang, L.; Zhao, F.; Zeng, B. A novel electrochemical 4-nonyl-phenol sensor based on molecularly imprinted poly (o-phenylenediamine-co-o-toluidine)—Nitrogen-doped graphene nanoribbons—Ionic liquid composite film. Electrochim. Acta 2015, 151, 423–428. [Google Scholar] [CrossRef]
- Dalkıran, B. Amperometric determination of heavy metal using an HRP inhibition biosensor based on ITO nanoparticles-ruthenium (III) hexamine trichloride composite: Central composite design optimization. Bioelectrochemistry 2020, 135, 107569. [Google Scholar] [CrossRef]
- Mu, Q.; Liu, G.; Yang, D.; Kou, X.; Cao, N.; Tang, Y.; Miao, P. Ultrasensitive Detection of DNA Based on Exonuclease III-Assisted Recycling Amplification and DNAzyme Motor. Bioconjugate Chem. 2018, 29, 3527–3531. [Google Scholar] [CrossRef]
- Han, X.; Li, C.; Yong, D. Microbial Electrode Sensor for Heavy-metal Ions. Sens. Mater. 2019, 31, 4103–4111. [Google Scholar] [CrossRef] [Green Version]
- Magar, H.S.; Ghica, M.E.; Abbas, M.N.; Brett, C.M.A. Highly Sensitive Choline Oxidase Enzyme Inhibition Biosensor for Lead Ions Based on Multiwalled Carbon Nanotube Modified Glassy Carbon Electrodes. Electroanalysis 2017, 29, 1741–1748. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Krishnan, U.M.; Rayappan, J.B.B. Design and development of amperometric biosensor for the detection of lead and mercury ions in water matrix—A permeability approach. Anal. Bioanal. Chem. 2017, 409, 4257–4266. [Google Scholar] [CrossRef]
- Rezaei, B.; Askarpour, N.; Ensafi, A.A. A novel sensitive doxorubicin impedimetric immunosensor based on a specific monoclonal antibody—Gold nanoparticle—Sol—Gel modified electrode. Talanta 2014, 119, 164–169. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Ferreira, R.; Uria, N.; Abramova, N.; Gargallo, R.; Muñoz-Pascual, F.-X.; Bratov, A. Novel impedimetric aptasensor for label-free detection of Escherichia coli O157: H7. Sens. Actuators B Chem. 2018, 255, 2988–2995. [Google Scholar] [CrossRef] [Green Version]
- Hnaien, M.; Bourigua, S.; Bessueille, F.; Bausells, J.; Errachid, A.; Lagarde, F.; Jaffrezic-Renault, N. Impedimetric microbial biosensor based on single wall carbon nanotube modified microelectrodes for trichloroethylene detection. Electrochim. Acta 2011, 56, 10353–10358. [Google Scholar] [CrossRef]
- Lin, Z.; Li, X.; Kraatz, H.B. Impedimetric immobilized DNA-based sensor for simultaneous detection of Pb2+, Ag+, and Hg2+. Anal. Chem. 2011, 83, 6896–6901. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Shi, J.; Li, Z.; Huang, X.; Zou, X.; Tan, W.; Zhang, X.; Hu, X.; Wang, X.; et al. Impedimetric aptasensor based on highly porous gold for sensitive detection of acetamiprid in fruits and vegetables. Food Chem. 2020, 322, 126762. [Google Scholar] [CrossRef]
- Beloglazova, N.V.; Lenain, P.; De Rycke, E.; Goryacheva, I.Y.; Knopp, D.; De Saeger, S. Capacitive sensor for detection of benzo (a) pyrene in water. Talanta 2018, 190, 219–225. [Google Scholar] [CrossRef]
- Graniczkowska, K.; Pütz, M.; Hauser, F.M.; De Saeger, S.; Beloglazova, N.V. Capacitive sensing of N-formylamphetamine based on immobilized molecular imprinted polymers. Biosens. Bioelectron. 2017, 92, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Mugo, S.M.; Lu, W.; Dhanjai, D. A pathogen imprinted hybrid polymer capacitive sensor for selective Escherichia coli detection. Med. Devices Sens. 2020, 3, e10071. [Google Scholar] [CrossRef]
- Razavi, H.; Janfaza, S. Medical nanobiosensors: A tutorial review. Nanomed. J. 2015, 2, 74–87. [Google Scholar]
- Zhylyak, G.; Dzyadevich, S.; Korpan, Y.; Soldatkin, A.; El’Skaya, A. Application of urease conductometric biosensor for heavy-metal ion determination. Sens. Actuators B Chem. 1995, 24, 145–148. [Google Scholar] [CrossRef]
- Tekaya, N.; Saiapina, O.; Ouada, H.B.; Lagarde, F.; Ouada, H.B.; Jaffrezic-Renault, N. Ultra-sensitive conductometric detection of heavy metals based on inhibition of alkaline phosphatase activity from Arthrospira platensis. Bioelectrochemistry 2013, 90, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Soldatkin, O.; Kucherenko, I.; Pyeshkova, V.; Kukla, A.; Jaffrezic-Renault, N.; El’Skaya, A.; Dzyadevych, S.; Soldatkin, A. Novel conductometric biosensor based on three-enzyme system for selective determination of heavy metal ions. Bioelectrochemistry 2012, 83, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, L.S.B.; Verma, N. Alkaline phosphatase inhibition based conductometric biosensor for phosphate estimation in biological fluids. Biosens. Bioelectron. 2015, 68, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dzyadevych, S.V.; Chovelon, J.-M.; Renault, N.J.; Chen, L.; Xia, S.; Zhao, J. Development of a conductometric nitrate biosensor based on Methyl viologen/Nafion® composite film. Electrochem. Commun. 2006, 8, 201–205. [Google Scholar] [CrossRef]
- Ha, D.; Hu, N.; Wu, C.; Kirsanov, D.; Legin, A.; Khaydukova, M.; Wang, P. Novel structured light-addressable potentiometric sensor array based on PVC membrane for determination of heavy metals. Sens. Actuators B Chem. 2012, 174, 59–64. [Google Scholar] [CrossRef]
- Wipf, M.; Stoop, R.L.; Tarasov, A.; Bedner, K.; Fu, W.; Wright, I.A.; Martin, C.J.; Constable, E.C.; Calame, M.; Schonenberger, C. Selective sodium sensing with gold-coated silicon nanowire field-effect transistors in a differential setup. ACS Nano 2013, 7, 5978–5983. [Google Scholar] [CrossRef]
- Khadro, B.; Namour, P.; Bessueille, F.; Leonard, D.; Jaffrezic-Renault, N. Validation of a conductometric bienzyme biosensor for the detection of proteins as marker of organic matter in river samples. J. Environ. Sci. 2009, 21, 545–551. [Google Scholar] [CrossRef]
- Chouteau, C.; Dzyadevych, S.; Chovelon, J.-M.; Durrieu, C. Development of novel conductometric biosensors based on immobilised whole cell Chlorella vulgaris microalgae. Biosens. Bioelectron. 2004, 19, 1089–1096. [Google Scholar] [CrossRef]
- Fabry, P.; Siebert, E. The CRC Handbook of Solid State Electrochemistry. In Electrochemical Sensors; CRC Press: Boca Raton, FL, USA, 1997; pp. 329–365. [Google Scholar]
- Compton, R.G.; Banks, C.E. Understanding Voltammetry; World Scientific: London, UK, 2018. [Google Scholar]
- Huang, M.R.; Rao, X.W.; Li, X.G.; Ding, Y.B. Lead ion-selective electrodes based on polyphenylenediamine as unique solid ionophores. Talanta 2011, 85, 1575–1584. [Google Scholar] [CrossRef]
- Alexander, S. Multiwalled carbon nanotube based molecular imprinted polymer for trace determination of 2,4-dichlorophenoxyaceticacid in natural water samples using a potentiometric method. Appl. Surf. Sci. 2014, 303, 180–186. [Google Scholar]
- Mashhadizadeh, M.H.; Khani, H.; Shockravi, A. Used a new aza-thia-macrocycle as a suitable carrier in potentiometric sensor of copper (II). J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 219–227. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 1, 70–71. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Knopfmacher, O.; Tarasov, A.; Fu, W.; Wipf, M.; Niesen, B.; Calame, M.; Schonenberger, C. Nernst limit in dual-gated Si-nanowire FET sensors. Nano Lett. 2010, 10, 2268–2274. [Google Scholar] [CrossRef]
- Chen, S.; Bomer, J.G.; Carlen, E.T.; van den Berg, A. Al2O3/silicon nanoISFET with near ideal Nernstian response. Nano Lett. 2011, 11, 2334–2341. [Google Scholar] [CrossRef]
- Sudhölter, E.; Van der Wal, P.; Skowronska-Ptasinska, M.; Van den Berg, A.; Reinhoudt, D. Ion-sensing using chemically-modified ISFETs. Sens. Actuators 1989, 17, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Rocher, V.; Jaffrezic-Renault, N.; Perrot, H.; Chevalier, Y.; Le Perchec, P. Nitrate-sensitive field-effect transistor with silica gate insulator modified by chemical grafting. Anal. Chim. Acta 1992, 256, 251–255. [Google Scholar] [CrossRef]
- Reinhoudt, D.N.; Engbersen, J.F.; Brzozka, Z.; van der Vlekkert, H.H.; Honig, G.W.; Holterman, H.A.; Verkerk, U.H. Development of durable K+-selective chemically modified field effect transistors with functionalized polysiloxane membranes. Anal. Chem. 1994, 66, 3618–3623. [Google Scholar] [CrossRef] [Green Version]
- Park, L.-S.; Hur, Y.-J.; Sohn, B.-K. Effect of membrane structure on the performance of field-effect transistor potassium-sensitive sensor. Sens. Actuators A Phys. 1996, 57, 239–243. [Google Scholar] [CrossRef]
- Gao, A.; Lu, N.; Wang, Y.; Dai, P.; Li, T.; Gao, X.; Wang, Y.; Fan, C. Enhanced sensing of nucleic acids with silicon nanowire field effect transistor biosensors. Nano Lett. 2012, 12, 5262–5268. [Google Scholar] [CrossRef]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef]
- Stern, E.; Vacic, A.; Rajan, N.K.; Criscione, J.M.; Park, J.; Ilic, B.R.; Mooney, D.J.; Reed, M.A.; Fahmy, T.M. Label-free biomarker detection from whole blood. Nat. Nanotechnol. 2010, 5, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Li, Y.; Rajan, N.K.; Routenberg, D.A.; Modis, Y.; Reed, M.A. Quantification of the affinities and kinetics of protein interactions using silicon nanowire biosensors. Nat. Nanotechnol. 2012, 7, 401–407. [Google Scholar] [CrossRef]
- Duan, X.; Gao, R.; Xie, P.; Cohen-Karni, T.; Qing, Q.; Choe, H.S.; Tian, B.; Jiang, X.; Lieber, C.M. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nat. Nanotechnol. 2012, 7, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Rothberg, J.M.; Hinz, W.; Rearick, T.M.; Schultz, J.; Mileski, W.; Davey, M.; Leamon, J.H.; Johnson, K.; Milgrew, M.J.; Edwards, M. An integrated semiconductor device enabling non-optical genome sequencing. Nature 2011, 475, 348–352. [Google Scholar] [CrossRef]
- Luo, L.; Jie, J.; Zhang, W.; He, Z.; Wang, J.; Yuan, G.; Zhang, W.; Wu, L.C.M.; Lee, S.-T. Silicon nanowire sensors for Hg2+ and Cd2+ ions. Appl. Phys. Lett. 2009, 94, 193101. [Google Scholar] [CrossRef]
- Gokhale, A.A.; Lu, J.; Weerasiri, R.R.; Yu, J.; Lee, I. Amperometric Detection and Quantification of Nitrate Ions Using a Highly Sensitive Nanostructured Membrane Electrocodeposited Biosensor Array. Electroanalysis 2015, 27, 1127–1137. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Tshikalaha, P.; Arotiba, O.A. Dendrimer supported electrochemical immunosensor for the detection of cholera toxin in water. Int. J. Electrochem. Sci. 2015, 10, 10083–10092. [Google Scholar]
- Salihov, S.V.; Ivanenkov, Y.A.; Krechetov, S.P.; Veselov, M.S.; Sviridenkova, N.V.; Savchenko, A.G.; Klyachko, N.L.; Golovin, Y.I.; Chufarova, N.V.; Beloglazkina, E.K.; et al. Recent advances in the synthesis of Fe3O4@AU core/shell nanoparticles. J. Magn. Magn. Mater. 2015, 394, 173–178. [Google Scholar] [CrossRef]
- Kholafazad Kordasht, H.; Pazhuhi, M.; Pashazadeh-Panahi, P.; Hasanzadeh, M.; Shadjou, N. Multifunctional aptasensors based on mesoporous silica nanoparticles as an efficient platform for bioanalytical applications: Recent advances. TrAC Trends Anal. Chem. 2020, 124, 115778. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Chen, M.; Fang, X.; Pang, P.; Wang, H.; Wu, Z.; Yang, W. Ultrasensitive electrochemical biosensor for silver ion based on magnetic nanoparticles labeling with hybridization chain reaction amplification strategy. Sens. Actuators B Chem. 2017, 249, 431–438. [Google Scholar] [CrossRef]
- Eguílaz, M.; Villalonga, R.; Rivas, G. Electrochemical biointerfaces based on carbon nanotubes-mesoporous silica hybrid material: Bioelectrocatalysis of hemoglobin and biosensing applications. Biosens. Bioelectron. 2018, 111, 144–151. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Song, Y.; Zhang, S.; Wang, M.; Jia, C.; Tian, J.Y.; He, L.; Zhang, X.; Liu, C.S. Fe(III)-based metal–organic framework-derived core–shell nanostructure: Sensitive electrochemical platform for high trace determination of heavy metal ions. Biosens. Bioelectron. 2017, 94, 358–364. [Google Scholar] [CrossRef]
- Hod, I.; Sampson, M.D.; Deria, P.; Kubiak, C.P.; Farha, O.K.; Hupp, J.T. Fe-Porphyrin-Based Metal-Organic Framework Films as High-Surface Concentration, Heterogeneous Catalysts for Electrochemical Reduction of CO2. ACS Catal. 2015, 5, 6302–6309. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Xie, L.; Mukhopadhyay, S.; Burkitt, L. A temperature compensated smart nitrate-sensor for agricultural industry. IEEE Trans. Ind. Electron. 2017, 64, 7333–7341. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C.; Burkitt, L. Imprinted polymer coated impedimetric nitrate sensor for real-time water quality monitoring. Sens. Actuators B Chem. 2018, 259, 753–761. [Google Scholar] [CrossRef]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Self-powered, autonomous Biological Oxygen Demand biosensor for online water quality monitoring. Sens. Actuators B Chem. 2017, 244, 815–822. [Google Scholar] [CrossRef]
- Quek, S.B.; Cheng, L.; Cord-Ruwisch, R. Microbial fuel cell biosensor for rapid assessment of assimilable organic carbon under marine conditions. Water Res. 2015, 77, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lu, X.; Chen, J. Development of biosensor technologies for analysis of environmental contaminants. Trends Environ. Anal. Chem. 2014, 2, 25–32. [Google Scholar] [CrossRef]
- Verma, N.; Kaur, H.; Kumar, S. Whole cell based electrochemical biosensor for monitoring lead ions in milk. Biotechnology 2011, 10, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Abdulhalim, I.; Karabchevsky, A.; Patzig, C.; Rauschenbach, B.; Fuhrmann, B.; Eltzov, E.; Marks, R.; Xu, J.; Zhang, F.; Lakhtakia, A. Surface-enhanced fluorescence from metal sculptured thin films with application to biosensing in water. Appl. Phys. Lett. 2009, 94, 63106. [Google Scholar] [CrossRef] [Green Version]
- Woznica, M.; Kowalska, P.; Lysek, R.; Masnyk, M.; Gorecki, M.; Kwit, M.; Furche, F.; Frelek, J. Stereochemical assingment of β-lactam antibiotics and their analogues by electronic circular dichroism spectroscopy. Curr. Org. Chem. 2010, 14, 1022–1036. [Google Scholar] [CrossRef]
- McAuley, J.; Daly, P.; Curtis, C. A preliminary investigation of a novel design of visual cue glasses that aid gait in Parkinson’s disease. Clin. Rehabil. 2009, 23, 687–695. [Google Scholar] [CrossRef] [Green Version]
- An, F.; Bai, J.; Balantekin, A.; Band, H.; Beavis, D.; Beriguete, W.; Bishai, M.; Blyth, S.; Boddy, K.; Brown, R. Observation of electron-antineutrino disappearance at Daya Bay. Phys. Rev. Lett. 2012, 108, 171803. [Google Scholar] [CrossRef] [Green Version]
- Kumlanghan, A.; Kanatharana, P.; Asawatreratanakul, P.; Mattiasson, B.; Thavarungkul, P. Microbial BOD sensor for monitoring treatment of wastewater from a rubber latex industry. Enzym. Microb. Technol. 2008, 42, 483–491. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, S.I.; Lim, D.; Park, K.; Oh, S.; Kwon, H.; Hwang, I.; Lee, S.; Nam, E.; Shin, D. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J. Clin. Oncol. 2011, 30, 1513–1518. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.; He, W.; Yan, C. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 91001. [Google Scholar] [CrossRef] [Green Version]
- Akki, S.U.; Werth, C.J. Critical review: DNA aptasensors, are they ready for monitoring organic pollutants in natural and treated water sources? Environ. Sci. Technol. 2018, 52, 8989–9007. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Kougianos, E. Biosensors: A tutorial review. IEEE Potentials 2006, 25, 35–40. [Google Scholar] [CrossRef]
- Jung, J.K.; Alam, K.K.; Verosloff, M.S.; Capdevila, D.A.; Desmau, M.; Clauer, P.R.; Lee, J.W.; Nguyen, P.Q.; Pastén, P.A.; Matiasek, S.J. Cell-free biosensors for rapid detection of water contaminants. Nat. Biotechnol. 2020, 38, 1451–1459. [Google Scholar] [CrossRef]



| Surface Modification Technique | Immobilization Site | Spatial Orientation | Accessibility | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|---|---|
| Adsorption | random | random | low | simple and direct | low immobilization efficiency | [45,46] |
| Encapsulation in polymers or gel | random | random | low | abundant BRE | necessary surface treatment and low immobilization efficiency | [47] |
| Chemical crosslinking | random | random | low | simple and high stability | the strict control of conditions and nonspecific interaction | [48] |
| Self-assembled monolayers | active terminal | orientation | high | simple and controllable BRE density | possible nonspecific interaction | [49] |
| Covalent linking | terminal activation | orientation | high | high stability | necessary surface treatment and low immobilization efficiency | [50] |
| Affinity | biotinylated terminal | orientation | high | simple and high stability | necessary surface treatment and possible nonspecific interaction | [51] |
| Electrodeposition | random | random | high | Reliable, cost-effective, and easy fabrication and maintenance | possible nonspecific interaction | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, Y.; Huang, Z.; Alahi, M.E.E.; Nag, A.; Feng, S.; Mukhopadhyay, S.C. Recent Advancements in Electrochemical Biosensors for Monitoring the Water Quality. Biosensors 2022, 12, 551. https://doi.org/10.3390/bios12070551
Hui Y, Huang Z, Alahi MEE, Nag A, Feng S, Mukhopadhyay SC. Recent Advancements in Electrochemical Biosensors for Monitoring the Water Quality. Biosensors. 2022; 12(7):551. https://doi.org/10.3390/bios12070551
Chicago/Turabian StyleHui, Yun, Zhaoling Huang, Md Eshrat E. Alahi, Anindya Nag, Shilun Feng, and Subhas Chandra Mukhopadhyay. 2022. "Recent Advancements in Electrochemical Biosensors for Monitoring the Water Quality" Biosensors 12, no. 7: 551. https://doi.org/10.3390/bios12070551
APA StyleHui, Y., Huang, Z., Alahi, M. E. E., Nag, A., Feng, S., & Mukhopadhyay, S. C. (2022). Recent Advancements in Electrochemical Biosensors for Monitoring the Water Quality. Biosensors, 12(7), 551. https://doi.org/10.3390/bios12070551









