Pyrene-Based AIE Active Materials for Bioimaging and Theranostics Applications
Abstract
:1. Introduction
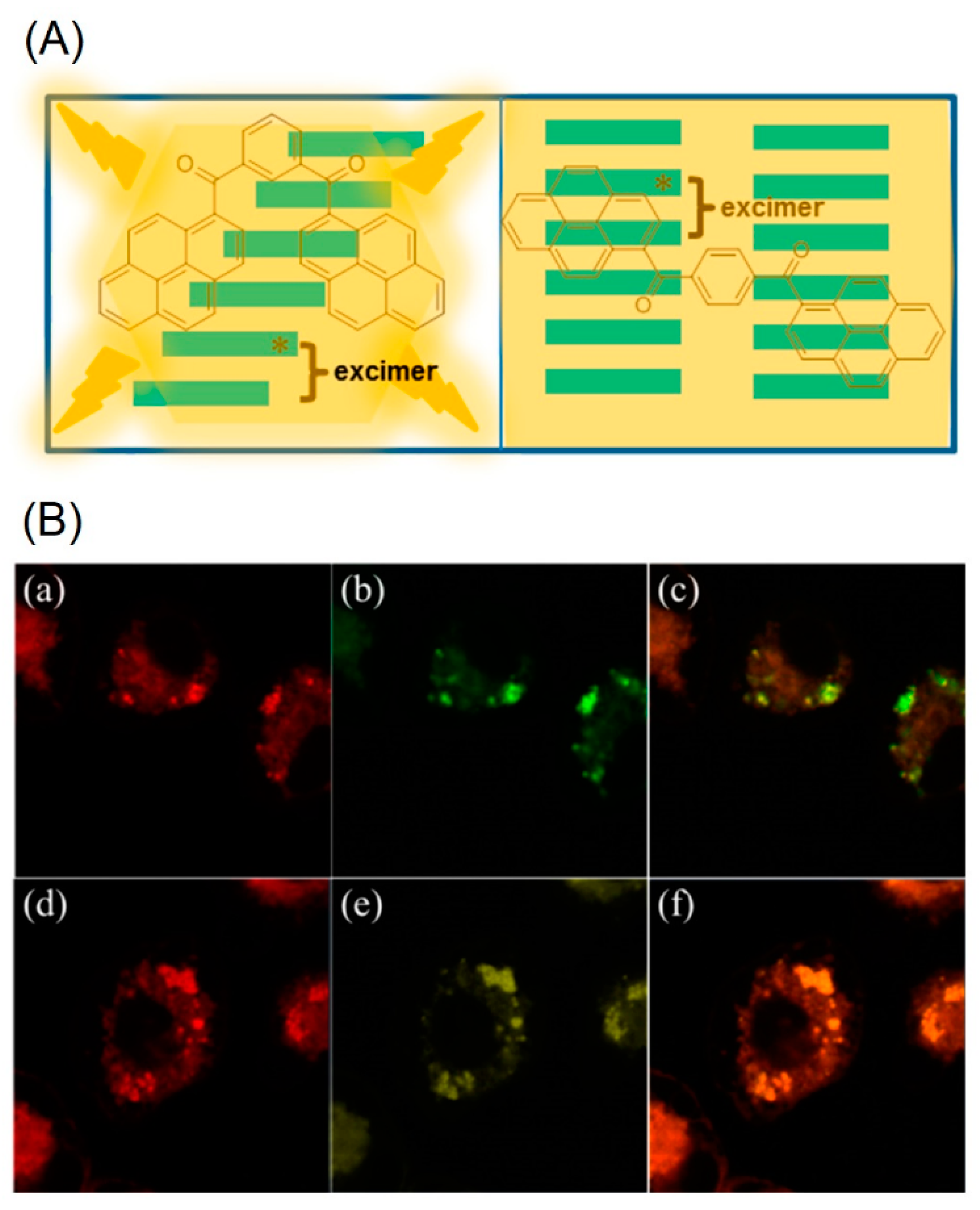
2. Bis-Pyrene Derivatives for Bioimaging and Theranostics
3. AIE Active Pyrene Conjugates for Bioimaging
4. AIE-Tuned Bioimaging from Pyrene-Based Sensory Probes
5. Design Requirements, Advantages and Limitations
5.1. Design Requirements
- (1)
- The molecules must be hydrophobic in nature to be able to induce the AIE in diverse water fractions. Attachment of more hydrophilic units like peptides and cationic salts generation can lead to nanostructures formation; however, careful optimization is required to avoid loss of the AIE features.
- (2)
- Since the molecules are designed for the AIE-facilitated bioimaging studies, they should possess low toxicity and are viable in biological environment. If the molecules are designed for the long-term tracking purpose, biocompatibility of the molecules must be justified by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT) assay for extended time intervals.
- (3)
- Introducing the peptides and polypeptides may enhance biocompatibility of the designed moiety; however, its stability in cellular environment must be attested before subjecting to any imaging investigation. Multiple cleavage of the peptide/polypeptide linked pyrene molecules may lead to loss of imaging or affect the cell culture environment, which results cell death.
- (4)
- For efficient energy transfer from the donor pyrene moiety to the acceptor dye in FRET and AIE-tuned two-photon imaging studies, selection of the suitable biocompatible acceptor is necessary. Similarly, for the composited pyrene-moiety/acceptor dye-based FRET/AIE system, optimization of the dye ratio is mandatory in determining the suitable composition for applicability in bioimaging studies.
- (5)
- For reactive AIE-based cancerous bio-analytes (such as GSH, Cys and Hcy) detection in cells, the pyrene-based probes must be designed with essential binding units or cleaving units (in FRET mechanism) to initiate the AIE in biological environment.
- (6)
- Lastly, if the pyrene moiety is designed for the theranostics drug delivery and bioimaging studies, the molecule should have the higher drug loading ability for conveying drug into specified tumor environment and possess the AIE property for imaging the tumor suppression.
5.2. Advantages
- (1)
- Due to existence of the fused aromatic structure, π–π stacking and self-assembling nature, probability of AIEE occurring by the pyrene containing molecules is high. Under certain circumstance they tend to form emissive nanostructures, which are comparable with recently developed nanomaterials, such as nanocluster, quantum dots, MOFs, etc. [112,113,114,115,116].
- (2)
- By attaching biocompatible units, such as peptides, polypeptide and inorganic nanostructures, toxicity of the AIE-active pyrene derivatives can be reduced to be comparable to other therapeutic nanomaterials, such as conjugated polymers, MOFs, carbon-dots, nanoclusters, etc. [117,118,119,120,121,122].
- (3)
- Since cell culture is majorly conducted in aqueous environment, the pyrene-based molecules can easily tune their AIE properties at certain water fractions, thereby is effective in bioimaging studies.
- (4)
- By means of the AIE mechanism, the pyrene-based small molecules can detect theranostics biothiols, such as GSH, Cys and HCy, as demonstrated in intracellular imaging in in vivo and in vitro studies. Similarly, the DNA conjugated pyrene scaffold was found to be effective towards the cancerous miRNA detection.
- (5)
- In the FRET and AIE-based bioimaging studies, the pyrene containing molecules acted as efficient donors in the energy transfer process due to strong π-electronic clouds, which can be noted as an additional advantage over other aromatic systems.
- (6)
- By conjugating the pyrene moieties with low toxic inorganic/polymeric nanostructures, effective drug delivery and tumor suppression were witnessed via intracellular imaging by means of the AIE of pyrene derivatives.
5.3. Limitations
- (1)
- Precipitation may form at higher water fractions due to the hydrophobic nature of the pyrene-based AIE-active materials, which will affect the intracellular environment and long-term tracking studies.
- (2)
- Covalent linking of excess hydrophilic units with the pyrene derivatives may affect the AIE properties and imaging ability.
- (3)
- Self-aggregation/self-assembly of the pyrene containing cationic dye may also lead to aggregation-induced quenching (ACQ), which limits the design towards bioimaging studies.
- (4)
- In hybrid nano-drug delivery systems, high concentrated loading of the pyrene derivatives over the proposed nanomaterials may lead to loss of biocompatibility.
- (5)
- The NIR dye and pyrene composites proposed in the FRET and AIE-based bioimaging and theranostics studies are limited by the composition ratios, toxicity and biocompatibility.
- (6)
- Therapeutic applicability of the pyrene-conjugates is also limited by the pathological and physiological conditions, which need careful optimization.
6. Conclusions and Perspectives
- (1)
- The majority of reports on the pyrene-conjugated systems applied in bioimaging and theranostics studies were described based on the hydrophobic and self-assembly process rather than the AIE effect. For example, the J-type nanoaggregates were formed from the bis-pyrene derivatives to induce emission enhancement in intracellular studies. This phenomenon was generally explained based on the self-assembly but not the AIE effect. Future investigations should rectify this misinterpretation.
- (2)
- To authenticate the bis-pyrene-conjugated probes as potential candidates in the AIE-tuned state-of-the-art pH-responsive endocytosis process, attention and supportive reports are required.
- (3)
- Applying the bis-pyrene-conjugated cyanine dye in photoacoustic studies appears to be a major research trend, and thus continuous research must be conducted toward the direction of developing commercialized biomedical devices.
- (4)
- FRET and AIE-tuned two-photon imaging and therapeutic studies from the bis-pyrene derivatives still need in-depth investigations in the following fields in the future, including biocompatibility, tumor suppression and mice-based theranostics investigations.
- (5)
- Reports on the metal ions regulated AIE effect on the pyrene-conjugated system towards the cancerous cell imaging and therapeutics are insufficient, thereby, requiring more attention.
- (6)
- Few reports on applying the cancerous analyte- (such as biothiols) induced AIE of pyrene derivatives in bioimaging studies are available, which should be the focus for researchers.
- (7)
- Thus far, only one report can be found in applying the pyrene-conjugated system to detect intracellular autophagy via the AIE mechanism. This subject requires greater attention.
- (8)
- The pyrene-based derivatives and their hybrid systems towards the AIE-tuned photo thermal and photo dynamic therapies still lack strong evidence and mechanistic explanations, which requires great efforts to rectify.
- (9)
- Fewer reports are available to justify the AIE effect of the pyrene-DNA conjugate applied in the cancerous miRNA detection. Concerning its impact on cancer cell detection, similar innovative designs must be continuously pursued.
- (10)
- In-depth discussions are missing regarding the intracellular imaging applications using the AIE-active pyrene-based small molecular probes and rotaxanes, which should be addressed with clarity in the future.
- (11)
- More experiments are mandatory to justify the AIE effect of the pyrene derivatives and their cationic dye salts towards antibacterial imaging, bioimaging and therapeutic utilities.
- (12)
- Many pyrene-containing probes with stimuli/analyte responsive features have not been investigated on the AIE effect, which should be clarified for the research community.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suman, G.R.; Pandey, M.; Chakravarthy, A.S.J. Review on New Horizons of Aggregation Induced Emission: From Design to Development. Mater. Chem. Front. 2021, 5, 1541–1584. [Google Scholar]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, X.; Yang, B.; Liu, M.; Liu, W.; Chen, Y.; Wei, Y. Polymerizable Aggregation-Induced Emission Dye-Based Fluorescent Nanoparticles for Cell Imaging Applications. Polym. Chem. 2014, 5, 356–360. [Google Scholar] [CrossRef]
- Cai, Y.; Qiu, Z.; Lin, X.; Zeng, W.; Cao, Y.; Liu, W.; Liu, Y. Self-Assembled Nanomaterials Based on Aggregation-Induced Emission of AuNCs: Fluorescence and Colorimetric Dual-Mode Biosensing of Organophosphorus Pesticides. Sens. Actuators B 2020, 321, 128481. [Google Scholar] [CrossRef]
- Arshad, F.; Pal, A.; Sk, M.P. Review-Aggregation-Induced Emission in Carbon Dots for Potential Applications. ECS J. Solid State Sci. Technol. 2021, 10, 021001. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Wang, D.; Tang, B.Z. Inorganic–Organic Nanocomposites Based on Aggregation-Induced Emission Luminogens. Adv. Funct. Mater. 2021, 31, 2006952. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Phenomenon, Mechanism and Applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Tu, Y.; Zhao, Z.; Lam, J.W.Y.; Tang, B.Z. Mechanistic Connotations of Restriction of Intramolecular Motions (RIM). Nat. Sci. Rev. 2021, 8, nwaa260. [Google Scholar] [CrossRef]
- Peng, Q.; Shuai, Z. Molecular Mechanism of Aggregation-Induced Emission. Aggregate 2021, 2, e91. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- Zalmi, G.A.; Gawade, V.K.; Nadimetla, D.N.; Bhosale, S.V. Aggregation Induced Emissive Luminogens for Sensing of Toxic Elements. Chem. Open 2021, 10, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.H.; Shah, K.W.; Zhou, H.; Xu, J. Recent Advances in Aggregation-Induced Emission Chemosensors for Anion Sensing. Molecules 2019, 24, 2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Huang, R.; Guo, J.; Zhao, Z.; Tang, B.Z. Promising Applications of Aggregation-Induced Emission Luminogens in Organic Optoelectronic Devices. PhotoniX 2020, 1, 11. [Google Scholar] [CrossRef]
- He, Z.; Tian, S.; Gao, Y.; Meng, F.; Luo, L. Luminescent AIE Dots for Anticancer Photodynamic Therapy. Front. Chem. 2021, 9, 672917. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Cao, H.; Li, Q.; Li, Y.; Han, M.; Wang, H.; Li, J. Photodynamic Therapy with Liposomes Encapsulating Photosensitizers with Aggregation-Induced Emission. Nano Lett. 2019, 19, 1821–1826. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. AIE-Based Cancer Theranostics. Coord. Chem. Rev. 2020, 402, 213076. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. Aggregation-Induced Emission Probes for Cancer Theranostics. Drug Discov. Today 2017, 22, 1288–1294. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Gao, Z.; Zhang, J.; Ou, H.; Gao, H.; Kwok, R.T.K.; Ding, D.; Tang, B.Z. A Wearable AIEgen-Based Lateral Flow Test Strip for Rapid Detection of SARS-CoV-2 RBD Protein and N Protein. Cell Rep. Phys. Sci. 2022, 3, 100740. [Google Scholar] [CrossRef]
- Qiao, S.-L.; Ma, Y.; Wang, Y.; Lin, Y.-X.; An, H.-W.; Li, L.-L.; Wang, H. General Approach of Stimuli-Induced Aggregation for Monitoring Tumor Therapy. ACS Nano 2017, 11, 7301–7311. [Google Scholar] [CrossRef]
- Ma, D. Status and Prospects of Aggregation-Induced Emission Materials in Organic Optoelectronic Devices. Top. Curr. Chem. 2021, 379, 16. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhao, X.; Chen, S. AIEgen-Based Fluorescent Nanomaterials: Fabrication and Biological Applications. Molecules 2018, 23, 419. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Liu, P.; Li, D.; Wang, D.; Tang, B.Z. NIR-II Aggregation-Induced Emission Luminogens for Tumor Phototheranostics. Biosensors 2022, 12, 46. [Google Scholar] [CrossRef]
- Khan, I.M.; Niazi, S.; Iqbal Khan, M.K.; Pasha, I.; Mohsin, A.; Haider, J.; Iqbal, M.W.; Rehman, A.; Yue, L.; Wang, Z. Recent Advances and Perspectives of Aggregation-Induced Emission as An Emerging Platform for Detection and Bioimaging. TrAC Trends Anal. Chem. 2019, 119, 115637. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, H.; Huang, Y.; Zhao, R. Recent Advances in AIEgens for Metal Ion Biosensing and Bioimaging. Molecules 2019, 24, 4593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, J.; Tang, B.Z. AIE Luminogens for Bioimaging and Theranostics: From Organelles to Animals. Chem 2017, 3, 56–91. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.; Xia, T.; He, Y. A Tetraphenylethylene-Based Aggregation-Induced Emission Sensor: Ultrasensitive “Turn-On” Fluorescent Sensing for Phosphate Anion in Pure Water. Talanta 2021, 221, 121434. [Google Scholar] [CrossRef]
- Sharath Kumar, K.S.; Girish, Y.R.; Ashrafizadeh, M.; Mirzaei, S.; Rakesh, K.P.; Hossein Gholami, M.; Zabolian, A.; Hushmandi, K.; Orive, G.; Kadumudi, F.B.; et al. AIE-Featured Tetraphenylethylene Nanoarchitectures in Biomedical Application: Bioimaging, Drug Delivery and Disease Treatment. Coord. Chem. Rev. 2021, 447, 214135. [Google Scholar] [CrossRef]
- Alifu, N.; Dong, X.; Li, D.; Sun, X.; Zebibula, A.; Zhang, D.; Zhang, G.; Qian, J. Aggregation-Induced Emission Nanoparticles as Photosensitizer for Two-Photon Photodynamic Therapy. Mater. Chem. Front. 2017, 1, 1746–1753. [Google Scholar] [CrossRef]
- Chen, J.; Zou, Z.; Ke, Z.; Zhang, X.; Feng, J.; Jing, Y.; Peng, L.; Yang, J.; Dai, Y.; Zou, D. Dimerization of Heavy Atom Free Tetraphenylethylene with Aggregation Induced Emission for Boosting Photodynamic Therapy. New J. Chem. 2020, 44, 7029–7034. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. AIE Luminogens: Emission Brightened by Aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Murase, H.; Nagata, Y.; Akahori, S.; Shinokubo, H.; Miyake, Y. Aggregation-Induced Emission in Tetrathia [8] circulene Octaoxides via Restriction of the Dynamic Motion of their Negatively Curved π-Frameworks. Chem. Asian J. 2020, 15, 3873–3877. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, L.; Wang, J.; Zhou, Y.; Hu, H.; Ye, X.; Liu, G.; Xu, Z.; Xu, H.; Yang, W.; et al. Aggregation Caused Quenching to Aggregation Induced Emission Transformation: A Precise Tuning Based on BN-Doped Polycyclic Aromatic Hydrocarbons Toward Subcellular Organelle Specific Imaging. Chem. Sci. 2022, 13, 3129–3139. [Google Scholar] [CrossRef] [PubMed]
- Kalva, N.; Tran, C.H.; Lee, M.W.; Augustine, R.; Lee, S.J.; Kim, I. Aggregation-Induced Emission-Active Hyperbranched Polymers Conjugated with Tetraphenylethylene for Nitroaromatic Explosive Detection. Dyes Pig. 2021, 194, 109617. [Google Scholar] [CrossRef]
- Islam, M.M.; Hu, Z.; Wang, Q.; Redshaw, C.; Feng, X. Pyrene-Based Aggregation-Induced Emission Luminogens and Their Applications. Mater. Chem. Front. 2019, 3, 762–781. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, J.; Yin, J.; Liu, S.H. Multiple Photoluminescent Processes from Pyrene Derivatives with Aggregation- and Mechano-Induced Excimer Emission. Chem. Asian J. 2019, 14, 2903–2910. [Google Scholar] [CrossRef]
- Wang, J.; Dang, Q.; Gong, Y.; Liao, Q.; Song, G.; Li, Q.; Li, Z. Precise Regulation of Distance between Associated Pyrene Units and Control of Emission Energy and Kinetics in Solid State. CCS Chem. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Ge, Y.; Wen, Y.; Liu, H.; Lu, T.; Yu, Y.; Zhang, X.; Li, B.; Zhang, S.-T.; Li, W.; Yang, B. A Key Stacking Factor for the Effective Formation of Pyrene Excimer in Crystals: Degree of π–π Overlap. J. Mater. Chem. C 2020, 8, 11830–11838. [Google Scholar] [CrossRef]
- Gou, Z.; Wang, A.; Tian, M.; Zuo, Y. Pyrene-Based Monomer-Excimer Dual Response Organosilicon Polymer for the Selective Detection of 2,4,6-Trinitrotoluene (TNT) and 2,4,6-Trinitrophenol (TNP). Mater. Chem. Front. 2022, 6, 607–612. [Google Scholar] [CrossRef]
- Kathiravan, A.; Sundaravel, K.; Jaccob, M.; Dhinagaran, G.; Rameshkumar, A.; Arul Ananth, D.; Sivasudha, T. Pyrene Schiff Base: Photophysics, Aggregation Induced Emission, and Antimicrobial Properties. J. Phys. Chem. B 2014, 118, 13573–13581. [Google Scholar] [CrossRef]
- Singh, G.; Singh, P.K. Stimulus-Responsive Supramolecular Host–Guest Assembly of a Cationic Pyrene Derivative with Sulfated β-Cyclodextrin. Langmuir 2019, 35, 14628–14638. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, P.K. Complexation of A Cationic Pyrene Derivative with Sulfobutylether Substituted β-Cyclodextrin: Towards A Stimulus-Responsive Supramolecular Material. J. Mol. Liq. 2020, 305, 112840. [Google Scholar] [CrossRef]
- Ibáñez, S.; Poyatos, M.; Peris, E. Preparation and Self-Aggregation Properties of a Series of Pyrene-Imidazolylidene Complexes of Gold (I). J. Organomet. Chem. 2020, 917, 121284. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Ling, Q.-H.; Wang, W.; Xu, L. Pyrene-Based Metallocycles and Metallocages: More than Fluorophores. Mater. Chem. Front. 2020, 4, 3190–3200. [Google Scholar] [CrossRef]
- Kinik, F.P.; Ortega-Guerrero, A.; Ongari, D.; Ireland, C.P.; Smit, B. Pyrene-Based Metal Organic Frameworks: From Synthesis to Applications. Chem. Soc. Rev. 2021, 50, 3143–3177. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, Q.; Zhang, X.; Huang, K.; Qin, D. Tetra-Imidazole Functionalized Pyrene for Constructing Co-MOF and Its Application for Sensing of Cyanide Ion. J. Solid State Chem. 2021, 300, 122258. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, J.; Li, J.; Yang, X.; Feng, M.; Cai, P.; Zhou, H.-C. Visible-Light Harvesting Pyrene-Based MOFs as Efficient ROS Generators. Chem. Sci. 2019, 10, 8455–8460. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Chi, W.; Han, X.; Wang, C.; Liu, S.; Liu, X.; Yin, J. Aggregation-Induced Emission or Aggregation-Caused Quenching? Impact of Covalent Bridge Between Tetraphenylethene and Naphthalimide. Chinese Chem. Lett. 2021, 32, 1790–1794. [Google Scholar] [CrossRef]
- Shellaiah, M.; Wu, Y.-H.; Singh, A.; Raju, M.V.R.; Lin, H.-C. Novel Pyrene- and Anthracene-Based Schiff Base Derivatives as Cu2+ and Fe3+ Fluorescence Turn-On Sensors and for Aggregation Induced Emissions. J. Mater. Chem. A 2013, 1, 1310–1318. [Google Scholar] [CrossRef]
- Suzuki, S.; Sasaki, S.; Sairi, A.S.; Iwai, R.; Tang, B.Z.; Konishi, G.-I. Principles of Aggregation-Induced Emission: Design of Deactivation Pathways for Advanced AIEgens and Applications. Angew. Chem. Int. Ed. 2020, 59, 9856–9867. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Chi, Z.; Zhu, W.; Tang, B.Z.; Li, Z. Aggregation-Induced Emission: A Coming-of-Age Ceremony at the Age of Eighteen. Sci. China Chem. 2019, 62, 1090–1098. [Google Scholar] [CrossRef]
- Wang, A.; Fan, R.; Dong, Y.; Song, Y.; Zhou, Y.; Zheng, J.; Du, X.; Xing, K.; Yang, Y. Novel Hydrogen-Bonding Cross-Linking Aggregation-Induced Emission: Water as a Fluorescent “Ribbon” Detected in a Wide Range. ACS Appl. Mater. Interfaces 2017, 9, 15744–15757. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Murale, D.P.; Yoon, H.Y.; Arun, V.; Choi, S.; Kim, E.; Lee, J.-S.; Kim, S. Recent Trends in Molecular Aggregates: An Exploration of Biomedicine. Aggregate 2022, 3, e159. [Google Scholar] [CrossRef]
- Gierschner, J.; Lüer, L.; Milián-Medina, B.; Oelkrug, D.; Egelhaaf, H.-J. Highly Emissive H-Aggregates or Aggregation-Induced Emission Quenching? The Photophysics of All-Trans para-Distyrylbenzene. J. Phys. Chem. Lett. 2013, 4, 2686–2697. [Google Scholar] [CrossRef]
- Shellaiah, M.; Raju, M.V.R.; Singh, A.; Lin, H.-C.; Wei, K.-H.; Lin, H.-C. Synthesis of Novel Platinum Complex Core as A Selective Ag+ Sensor and Its H-Bonded Tetrads Self-Assembled with Triarylamine Dendrimers for Electron/Energy Transfers. J. Mater. Chem. A 2014, 2, 17463–17476. [Google Scholar] [CrossRef]
- Fang, H.-P.; Shellaiah, M.; Singh, A.; Raju, M.V.R.; Wu, Y.-H.; Lin, H.-C. Naked Eye and Fluorescent Detections of Hg2+ Ions and Cysteine via J-aggregation and Deaggregation of a Perylene Bisimide Derivative. Sens. Actuators B 2014, 194, 229–237. [Google Scholar] [CrossRef]
- Hestand, N.J.; Spano, F.C. Expanded Theory of H- and J-Molecular Aggregates: The Effects of Vibronic Coupling and Intermolecular Charge Transfer. Chem. Rev. 2018, 118, 7069–7163. [Google Scholar] [CrossRef]
- Ayyavoo, K.; Velusamy, P. Pyrene Based Materials as Fluorescent Probes in Chemical and Biological Fields. New J. Chem. 2021, 45, 10997–11017. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, X.; Cheng, S.; Xie, A.; Dong, W. Pyrene Derived Aggregation-Induced Emission Sensor for Highly Selective Detection of Explosive CL-20. J. Luminescence 2021, 233, 117871. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Ran, H.; Zhang, J.; Chen, L.; Han, R.; Duan, X.; Sun, H.; Hu, J.-Y. New Pyrene-Based Butterfly-Shaped Blue AIEgens: Synthesis, Structure, Aggregation-Induced Emission and Their Nondoped Blue OLEDs. Dye. Pigment. 2020, 173, 107881. [Google Scholar] [CrossRef]
- Kumari, R.; Sunil, D. Emerging Trends in Aggregation Induced Emissive Luminogens as Bacterial Theranostics. J. Drug Target. 2021, 29, 793–807. [Google Scholar] [CrossRef]
- Ren, C.; Wang, Z.; Wang, Q.; Yang, C.; Liu, J. Self-Assembled Peptide-Based Nanoprobes for Disease Theranostics and Disease-Related Molecular Imaging. Small 2020, 4, 1900403. [Google Scholar] [CrossRef]
- Yu, H.; Chen, B.; Huang, H.; He, Z.; Sun, J.; Wang, G.; Gu, X.; Tang, B.Z. AIE-Active Photosensitizers: Manipulation of Reactive Oxygen Species Generation and Applications in Photodynamic Therapy. Biosensors 2022, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- He, P.-P.; Li, X.-D.; Wang, L.; Wang, H. Bispyrene-Based Self-Assembled Nanomaterials: In Vivo Self-Assembly, Transformation, and Biomedical Effects. Acc. Chem. Res. 2019, 52, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Lu, J.; Zhao, Y.-X.; Fan, G.; Zhang, J.-P.; Wang, H. Supramolecular Nano-Aggregates Based on Bis(Pyrene) Derivatives for Lysosome-Targeted Cell Imaging. J. Phys. Chem. C 2013, 117, 26811–26820. [Google Scholar] [CrossRef]
- Qiao, Z.-Y.; Hou, C.-Y.; Zhao, W.-J.; Zhang, D.; Yang, P.-P.; Wang, L.; Wang, H. Synthesis of Self-reporting Polymeric Nanoparticles for In Situ Monitoring of Endocytic Microenvironmental pH. Chem. Commun. 2015, 51, 12609–12612. [Google Scholar] [CrossRef]
- Duan, Z.; Gao, Y.-J.; Qiao, Z.-Y.; Qiao, S.; Wang, Y.; Hou, C.; Wang, L.; Wang, H. pH-Sensitive Polymer Assisted Self-Aggregation of Bis(pyrene) in Living Cells In Situ with Turn-On Fluorescence. Nanotechnology 2015, 26, 355703. [Google Scholar] [CrossRef]
- An, H.-W.; Qiao, S.-L.; Hou, C.-Y.; Lin, Y.-X.; Li, L.-L.; Xie, H.-Y.; Wang, Y.; Wang, L.; Wang, H. Self-Assembled NIR Nanovesicles for Long-Term Photoacoustic Imaging in Vivo. Chem. Commun. 2015, 51, 13488–13491. [Google Scholar] [CrossRef]
- Yang, P.-P.; Yang, Y.; Gao, Y.-J.; Wang, Y.; Zhang, J.-C.; Lin, Y.-X.; Dai, L.; Li, J.; Wang, L.; Wang, H. Unprecedentedly High Tissue Penetration Capability of Co-Assembled Nanosystems for Two-Photon Fluorescence Imaging In Vivo. Adv. Opt. Mater. 2015, 3, 646–651. [Google Scholar] [CrossRef]
- Yang, P.-P.; Zhai, Y.-G.; Qi, G.-B.; Lin, Y.-X.; Luo, Q.; Yang, Y.; Xu, A.-P.; Yang, C.; Li, Y.-S.; Wang, L.; et al. NIR Light Propulsive Janus-like Nanohybrids for Enhanced Photothermal Tumor Therapy. Small 2016, 12, 5423–5430. [Google Scholar] [CrossRef]
- Xu, A.-P.; Yang, P.-P.; Yang, C.; Gao, Y.-J.; Zhao, X.-X.; Luo, Q.; Li, X.-D.; Li, L.-Z.; Wang, L.; Wang, H. Bio-Inspired Metal Ions Regulate the Structure Evolution of Self-Assembled Peptide-Based Nanoparticles. Nanoscale 2016, 8, 14078–14083. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.-L.; Wang, Y.; Lin, Y.-X.; An, H.-W.; Ma, Y.; Li, L.-L.; Wang, L.; Wang, H. Thermo-Controlled in Situ Phase Transition of Polymer–Peptides on Cell Surfaces for High-Performance Proliferative Inhibition. ACS Appl. Mater. Interfaces 2016, 8, 17016–17022. [Google Scholar] [CrossRef] [PubMed]
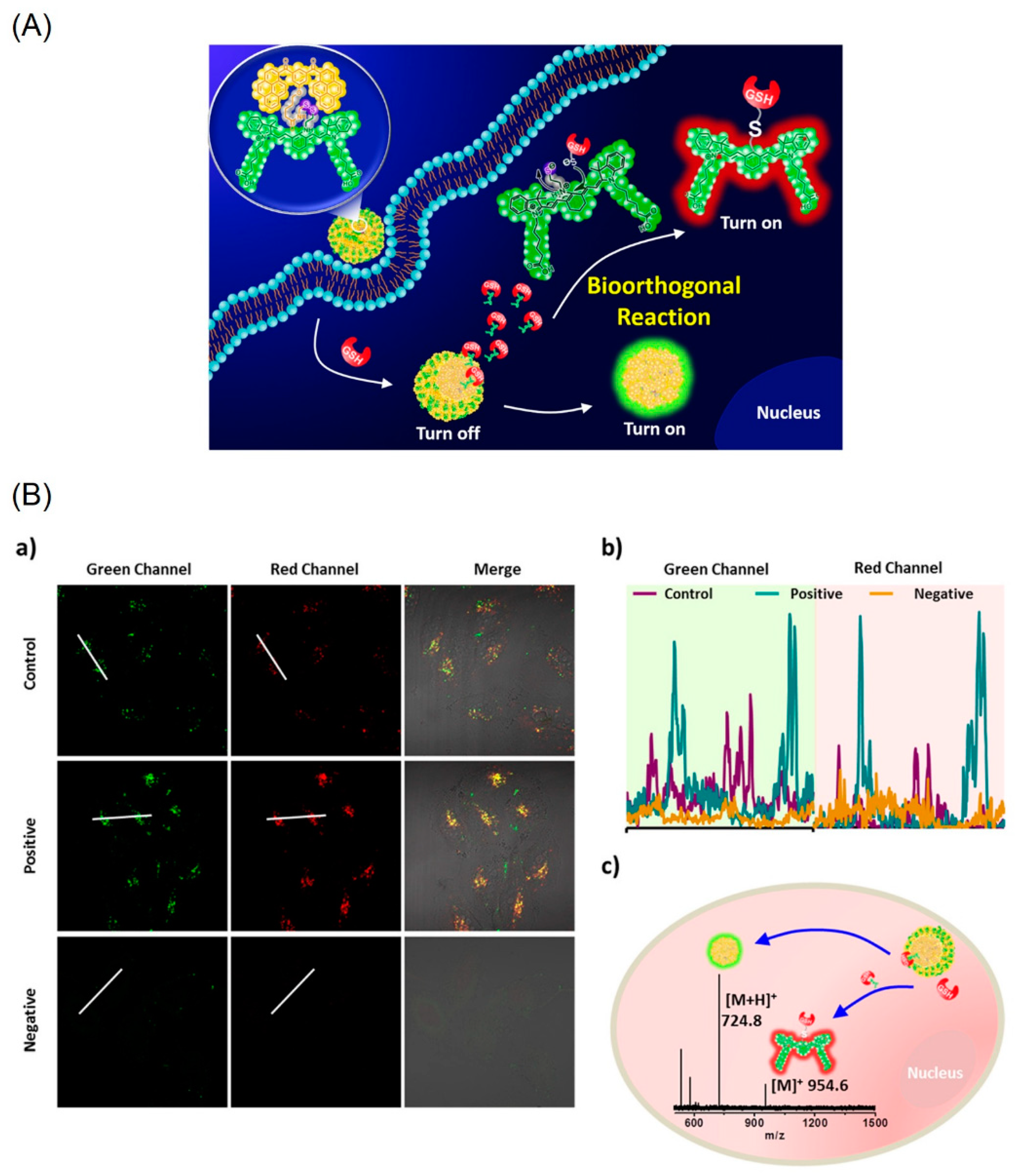
- An, H.-W.; Qiao, S.-L.; Li, L.-L.; Yang, C.; Lin, Y.-X.; Wang, Y.; Qiao, Z.-Y.; Wang, L.; Wang, H. Bio-orthogonally Deciphered Binary Nanoemitters for Tumor Diagnostics. ACS Appl. Mater. Interfaces 2016, 8, 19202–19207. [Google Scholar] [CrossRef]
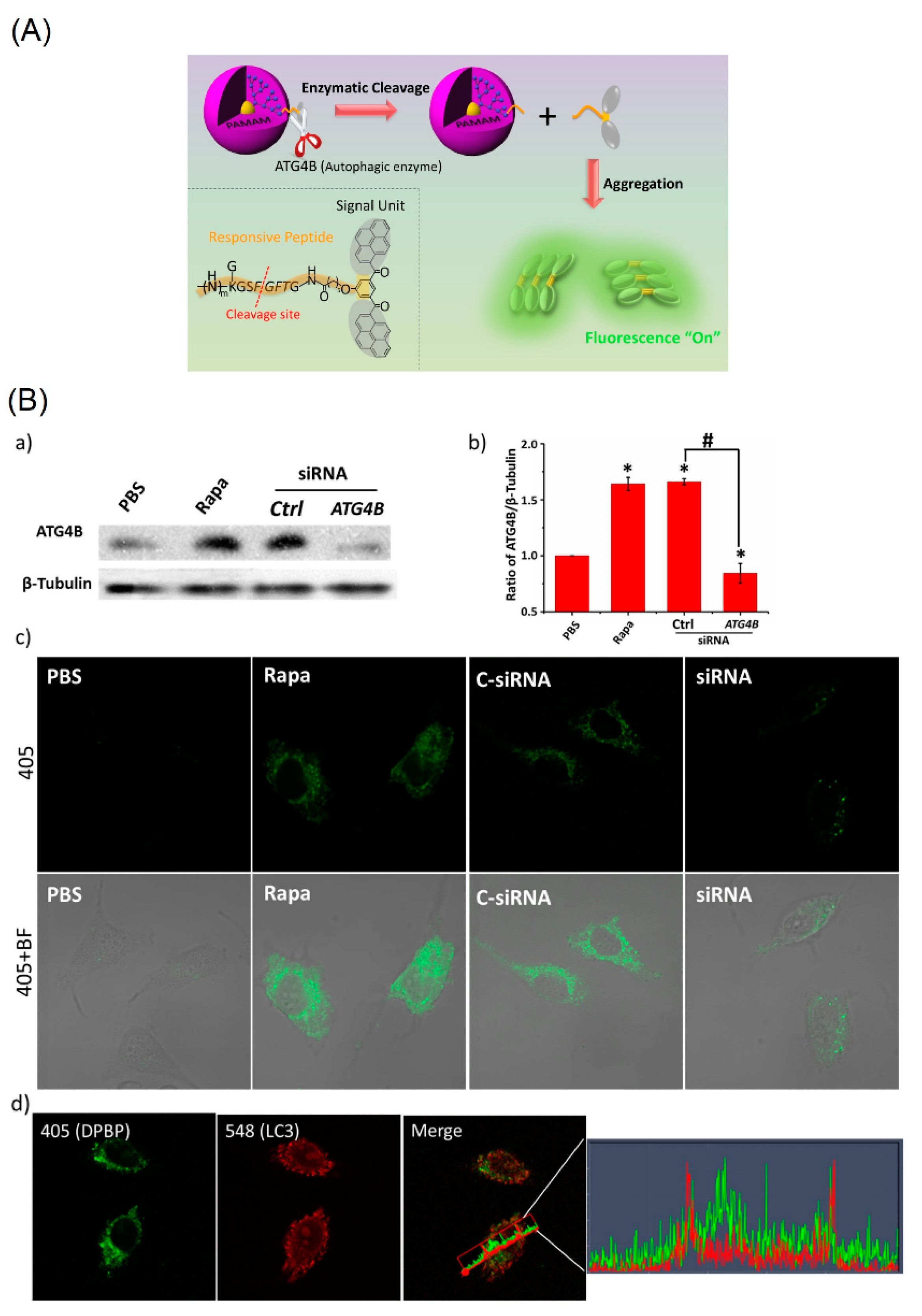
- Lin, Y.-X.; Qiao, S.-L.; Wang, Y.; Zhang, R.-X.; An, H.-W.; Ma, Y.; Rajapaksha, R.P.Y.J.; Qiao, Z.-Y.; Wang, L.; Wang, H. An in Situ Intracellular Self-Assembly Strategy for Quantitatively and Temporally Monitoring Autophagy. ACS Nano 2017, 11, 1826–1839. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, L.; Li, Q.; He, P.; Liu, H.; Wang, H.; Yang, Y.; Li, J. Bis(pyrene)-Doped Cationic Dipeptide Nanoparticles for Two-Photon-Activated Photodynamic Therapy. Biomacromolecules 2017, 18, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Lu, J.; Zhang, J.-P.; Wang, H. Bis-Pyrene Carbocations for Chromogenic and Fluorogenic Dual-Detection of Fluoride Anion In Situ. Tetrahedron 2014, 70, 3172–3177. [Google Scholar] [CrossRef]
- Li, W.; Yang, P.-P.; Wang, L.; Wang, H. Bis-Pyrene Based Reversibly Multi-Responsive Fluorescence Switches. J. Mater. Chem. C 2015, 3, 3783–3789. [Google Scholar] [CrossRef]
- Jana, A.; Saha, B.; Ikbal, M.; Ghosh, S.K.; Singh, N.D.P. 1-(Hydroxyacetyl)pyrene A New Fluorescent Phototrigger for Cell Imaging and Caging of Alcohols, Phenol and Adenosine. Photochem. Photobiol. Sci. 2012, 11, 1558–1566. [Google Scholar] [CrossRef]
- Ghosh, A.; Sengupta, A.; Chattopadhyay, A.; Das, D. Lysine Triggered Ratiometric Conversion of Dynamic to Static Excimer of a Pyrene Derivative: Aggregation-Induced Emission, Nanomolar Detection and Human Breast Cancer Cell (MCF7) Imaging. Chem. Commun. 2015, 51, 11455–11458. [Google Scholar] [CrossRef]
- Singh, V.K.; Prasad, R.; Koch, B.; Hasan, S.H.; Dubey, M. Pyrene–Fluorescein-Based Colour-Tunable AIE-Active Hybrid Fluorophore Material for Potential Live Cell Imaging Applications. New J. Chem. 2017, 41, 5114–5120. [Google Scholar] [CrossRef]
- Lalitha, K.; Nagarajan, S. Strongly Fluorescent Organogels and Self-Assembled Nanostructures from Pyrene Coupled Coumarin Derivatives: Application in Cell Imaging. J. Mater. Chem. B 2015, 3, 5690–5701. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhu, H.-Y.; Wang, J.-F.; Chen, X.; Wang, K.-R.; Li, X.-L. Supramolecular Nanofiber of Pyrene-Lactose Conjugates and Its Two-Photon Fluorescence Imaging. Bioorg. Chem. 2018, 79, 126–130. [Google Scholar] [CrossRef]
- Nie, H.; Liang, Y.; Han, C.; Zhang, R.; Zhang, X.; Yan, H. Rational Design of Cyanovinyl-Pyrene Dual-Emission AIEgens for Potential Application in Dual-Channel Imaging and Ratiometric Sensing in Living Cells. Dyes Pig. 2019, 168, 42–48. [Google Scholar] [CrossRef]
- Panigrahi, A.; Are, V.N.; Jain, S.; Nayak, D.; Giri, S.; Sarma, T.K. Cationic Organic Nanoaggregates as AIE Luminogens for Wash-Free Imaging of Bacteria and Broad-Spectrum Antimicrobial Application. ACS Appl. Mater. Interfaces 2020, 12, 5389–5402. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Lai, H.; Liu, Y.; Li, Q.; Chen, H.; Ji, S.; Zhang, J.; Huo, Y. Highly Selective Isomer Fluorescent Probes for Distinguishing Homo-/Cysteine from Glutathione Based on AIE. Talanta 2020, 206, 120177. [Google Scholar] [CrossRef]
- Kundu, S.; Das, S.; Dutta, A.; Patra, A. Three in One: Stimuli-Responsive Fluorescence, Solid-State Emission, and Dual-Organelle Imaging Using a Pyrene-Benzophenone Derivative. J. Phys. Chem. B 2022, 126, 691–701. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Wijesinghe, K.J.; Stahelin, R.V.; Pang, Y. Red-Emitting Pyrene–Benzothiazolium: Unexpected Selectivity to Lysosomes for Real-Time Cell Imaging Without Alkalinizing Effect. Chem. Commun. 2019, 55, 3469–3472. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Wijesinghe, K.J.; Stahelin, R.V.; Pang, Y. Lysosome Imaging in Cancer Cells by Pyrene-Benzothiazolium Dyes: An Alternative Imaging Approach for LAMP-1 Expression-Based Visualization Methods to Avoid Background Interference. Bioorg. Chem. 2019, 91, 103144. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Wijesinghe, K.J.; Plescia, C.B.; Fisher, L.S.; Goodson Iii, T.; Stahelin, R.V.; Pang, Y. A Pyrene-Based Two-Photon Excitable Fluorescent Probe to Visualize Nuclei in Live Cells. Photochem. Photobiol. Sci. 2020, 19, 1152–1159. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, J.; Xiao, F.; Huang, Y.; Zhang, X.; Tian, L. A Self-Delivery DNA Nanoprobe for Reliable MicroRNA Imaging in Live Cells by Aggregation Induced Red-Shift-Emission. Chem. Commun. 2020, 56, 1501–1504. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, B.H. Pyrene-Modified Guanine Cluster Probes Forming DNA/RNA Hybrid Three-Way Junctions for Imaging of Intracellular MicroRNAs. ACS Appl. Bio Mater. 2021, 4, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Y. Self-Assembled Nanorods of Phenylboronic Acid Functionalized Pyrene for In Situ Two-Photon Imaging of Cell Surface Sialic Acids and Photodynamic Therapy. Anal. Chem. 2021, 93, 7029–7036. [Google Scholar] [CrossRef] [PubMed]
- Arumugaperumal, R.; Shellaiah, M.; Srinivasadesikan, V.; Awasthi, K.; Sun, K.W.; Lin, M.-C.; Ohta, N.; Chung, W.-S. Diversiform Nanostructures Constructed from Tetraphenylethene and Pyrene-Based Acid/Base Controllable Molecular Switching Amphiphilic [2]Rotaxanes with Tunable Aggregation-Induced Static Excimers. ACS Appl. Mater. Interfaces 2020, 12, 45222–45234. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.-Y.; Lee, G.-Y.; Zheng, J.-H.; Huang, J.-H.; Cho, E.-C.; Lee, K.-C. Intercalating Pyrene with Polypeptide as A Novel Self-Assembly Nano-Carrier for Colon Cancer Suppression In Vitro and In Vivo. Mater. Sci. Eng. C 2020, 109, 110593. [Google Scholar] [CrossRef]
- Srinivasan, V.; Jhonsi, M.A.; Dhenadhayalan, N.; Lin, K.-C.; Ananth, D.A.; Sivasudha, T.; Narayanaswamy, R.; Kathiravan, A. Pyrene-Based Prospective Biomaterial: In Vitro Bioimaging, Protein Binding Studies and Detection of Bilirubin and Fe3+. Spectrochim. Acta A 2019, 221, 117150. [Google Scholar] [CrossRef]
- Srinivasan, V.; Jhonsi, M.A.; Dhenadhayalan, N.; Lin, K.-C.; Jaccob, M.; Kathiravan, A. AIE Nanodots Obtained from a Pyrene Schiff Base and Their Applications. ChemistrySelect 2017, 2, 1353–1359. [Google Scholar] [CrossRef]
- Panigrahi, A.; Sahu, B.P.; Mandani, S.; Nayak, D.; Giri, S.; Sarma, T.K. AIE Active Fluorescent Organic Nanoaggregates for Selective Detection of Phenolic-Nitroaromatic Explosives and Cell imaging. J. Photochem. Photobiol. A 2019, 374, 194–205. [Google Scholar] [CrossRef]
- Singh, A.; Singh, R.; Shellaiah, M.; Prakash, E.C.; Chang, H.-C.; Raghunath, P.; Lin, M.-C.; Lin, H.-C. A New Pyrene-Based Aggregation Induced Ratiometric Emission Probe for Selective Detections of Trivalent Metal Ions and Its Living Cell Application. Sens. Actuators B 2015, 207, 338–345. [Google Scholar] [CrossRef]
- Shellaiah, M.; Simon, T.; Srinivasadesikan, V.; Lin, C.-M.; Sun, K.W.; Ko, F.-H.; Lin, M.-C.; Lin, H.-C. Novel Pyrene Containing Monomeric and Dimeric Supramolecular AIEE Active Nano-Probes Utilized in Selective “Off–On” Trivalent Metal and Highly Acidic pH Sensing with Live Cell Applications. J. Mater. Chem. C 2016, 4, 2056–2071. [Google Scholar] [CrossRef]
- Shellaiah, M.; Chen, Y.-T.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Pyrene-Based AIEE Active Nanoprobe for Zn2+ and Tyrosine Detection Demonstrated by DFT, Bioimaging, and Organic Thin-Film Transistor. ACS Appl. Mater. Interfaces 2021, 13, 28610–28626. [Google Scholar] [CrossRef]
- Wawi, M.J.; Bijoux, A.; Inguimbert, N.; Mahler, C.; Wagner, S.; Marder, T.B.; Ribou, A.-C. Peptide Vectors Carry Pyrene to Cell Organelles Allowing Real-Time Quantification of Free Radicals in Mitochondria by Time-Resolved Fluorescence Microscopy. ChemBioChem 2021, 22, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Rajan, Y.C.; Balu, P.; Murugan, A. A Pyrene Based Schiff Base Probe for Selective Fluorescence Turn-On Detection of Hg2+ Ions with Live Cell Application. New J. Chem. 2015, 39, 2523–2531. [Google Scholar] [CrossRef]
- Dolai, B.; Nayim, S.; Hossain, M.; Pahari, P.; Kumar Atta, A. A Triazole Linked C-Glycosyl Pyrene Fluorescent Sensor for Selective Detection of Au3+ in Aqueous Solution and Its Application in Bioimaging. Sens. Actuators B 2019, 279, 476–482. [Google Scholar] [CrossRef]
- Prabhu, J.; Velmurugan, K.; Raman, A.; Duraipandy, N.; Kiran, M.S.; Easwaramoorthi, S.; Tang, L.; Nandhakumar, R. Pyrene-Phenylglycinol Linked Reversible Ratiometric Fluorescent Chemosensor for the Detection of Aluminium in Nanomolar Range and Its Bio-imaging. Anal. Chim. Acta 2019, 1090, 114–124. [Google Scholar] [CrossRef]
- Kim, I.; Jeong, H.-H.; Kim, Y.-J.; Lee, N.-E.; Huh, K.-m.; Lee, C.-S.; Kim, G.H.; Lee, E. A “Light-up” 1D Supramolecular Nanoprobe for Silver Ions Based on Assembly of Pyrene-Labeled Peptide Amphiphiles: Cell-Imaging and Antimicrobial Activity. J. Mater. Chem. B 2014, 2, 6478–6486. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.K.; Liu, S.-R.; Wang, H.-F.; Wu, S.-P. A Pyrene-Based Highly Selective Turn-On Fluorescent Chemosensor for Iron(III) Ions and Its Application in Living Cell Imaging. J. Fluoresc. 2013, 23, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Jana, D.; Boxi, S.; Parui, P.P.; Ghorai, B.K. Planar–Rotor Architecture Based Pyrene–Vinyl–Tetraphenylethylene Conjugated Systems: Photophysical Properties and Aggregation Behavior. Org. Biomol. Chem. 2015, 13, 10663–10674. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Mao, X.; Wang, Q.; Mu, Z.; An, L.; Zhang, W.; Feng, X.; Redshaw, C.; Cao, C.; et al. Pyrene-Based Aggregation-Induced Emission Luminogens (AIEgens) with Less Colour Migration for Anti-Counterfeiting Applications. J. Mater. Chem. C 2021, 9, 12828–12838. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Yu, Z.-D.; Zhao, W.-X.; Yang, K.; Noda, Y.; Zhao, Y.; Feng, X.; Elsegood, M.R.J.; Teat, S.J.; Redshaw, C.; et al. Pyrene-Fused Hexaarylbenzene Luminogens: Synthesis, Characterization, and Aggregation-Induced Emission Enhancement. Dyes Pig. 2021, 192, 109452. [Google Scholar] [CrossRef]
- Li, J.; Yang, C.; Huang, C.; Wan, Y.; Lai, W.-Y. Tuning Circularly Polarized Luminescence of an AIE-Active Pyrene Luminogen from Fluidic Solution to Solid Thin Film. Tetrahed. Lett. 2016, 57, 1256–1260. [Google Scholar] [CrossRef]
- Jana, P.; Kanvah, S. Aggregation-Induced Emission and Organogels with Chiral and Racemic Pyrene-Substituted Cyanostyrenes. Langmuir 2020, 36, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.W. Luminescent Metal Nanoclusters for Potential Chemosensor Applications. Chemosensors 2017, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Shellaiah, M.; Simon, T.; Thirumalaivasan, N.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-Capped Gold-Copper Nanoclusters for Fluorometric Determination and Imaging of Chromium(VI) and Dopamine. Microchim. Acta 2019, 186, 788. [Google Scholar] [CrossRef]
- Shellaiah, M.; Awasthi, K.; Chandran, S.; Aazaad, B.; Sun, K.W.; Ohta, N.; Wu, S.-P.; Lin, M.-C. Methylammonium Tin Tribromide Quantum Dots for Heavy Metal Ion Detection and Cellular Imaging. ACS Appl. Nano Mater. 2022, 5, 2859–2874. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A Review on Syntheses, Properties, Characterization and Bioanalytical Applications of Fluorescent Carbon Dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.-W. Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors 2021, 9, 101. [Google Scholar] [CrossRef]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef]
- Peng, H.; Liu, X.; Wang, G.; Li, M.; Bratlie, K.M.; Cochran, E.; Wang, Q. Polymeric Multifunctional Nanomaterials for Theranostics. J. Mater. Chem. B 2015, 3, 6856–6870. [Google Scholar] [CrossRef] [Green Version]
- Hiremath, N.; Kumar, R.; Hwang, K.C.; Banerjee, I.; Thangudu, S.; Vankayala, R. Near-Infrared Light Activatable Two-Dimensional Nanomaterials for Theranostic Applications: A Comprehensive Review. ACS Appl. Nano Mater. 2022, 5, 1719–1733. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, X.; Fu, K.; Zhang, X.; Shang, L.; Su, Z. Highly Fluorescent Carbon Dots as Novel Theranostic Agents for Biomedical Applications. Nanoscale 2021, 13, 17236–17253. [Google Scholar] [CrossRef]
- Xu, Q.; Li, C.; Chen, Y.; Zhang, Y.; Lu, B. Metal-organic framework-based intelligent drug delivery systems for cancer theranostic: A review. Front. Mater. Sci. 2021, 15, 374–390. [Google Scholar] [CrossRef]
- Villela Zumaya, A.L.; Mincheva, R.; Raquez, J.-M.; Hassouna, F. Nanocluster-Based Drug Delivery and Theranostic Systems: Towards Cancer Therapy. Polymers 2022, 14, 1188. [Google Scholar] [CrossRef] [PubMed]



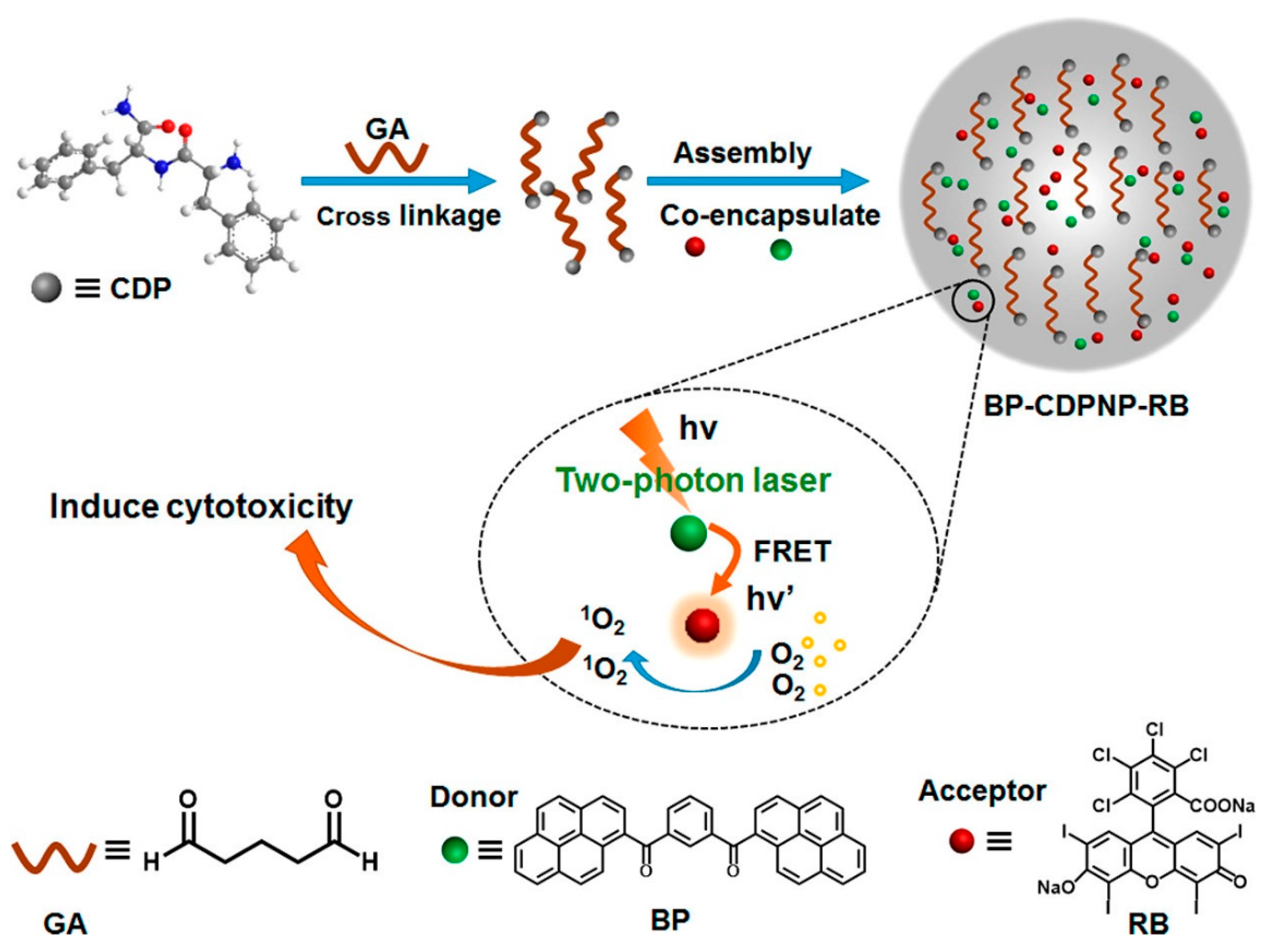
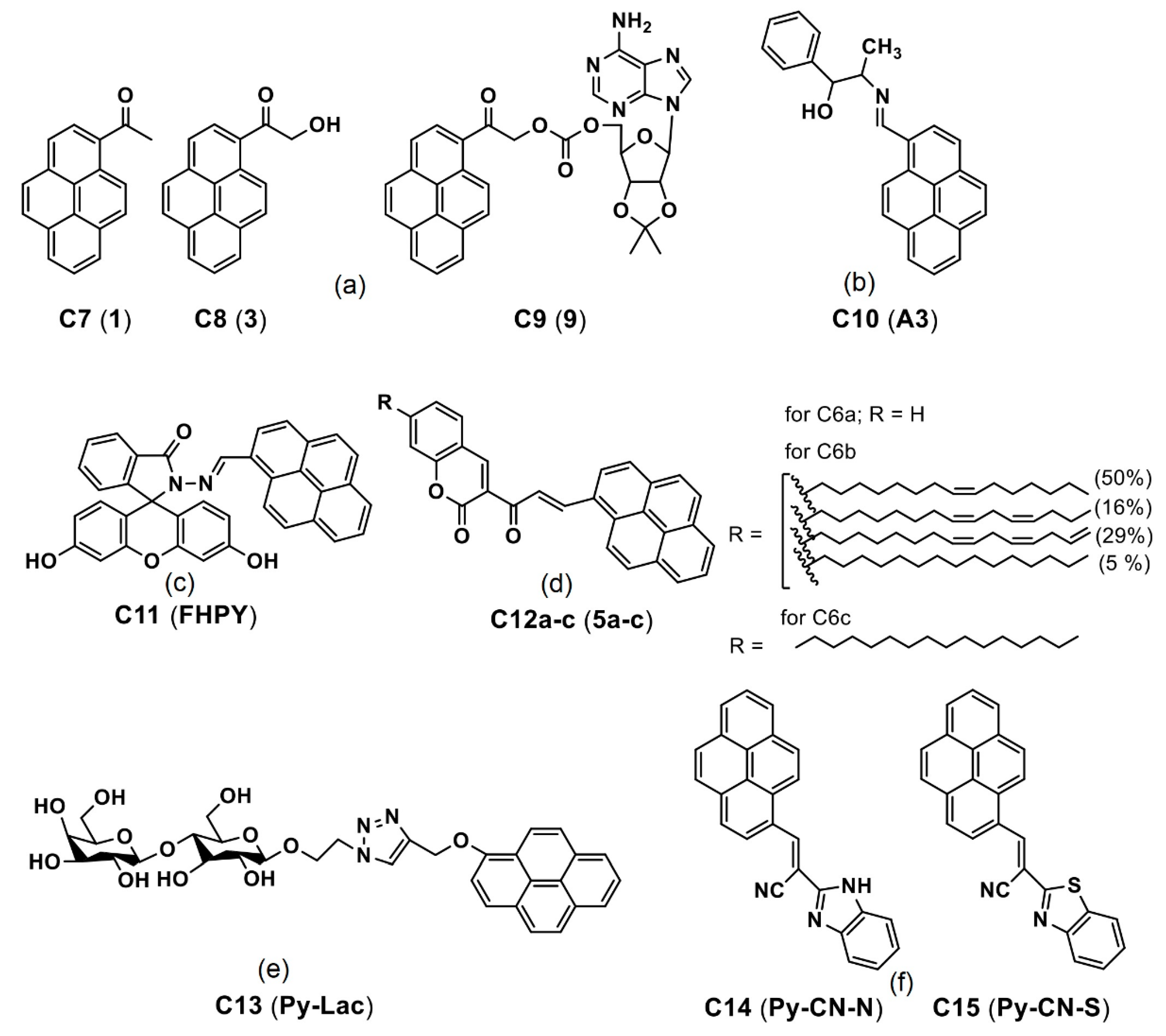
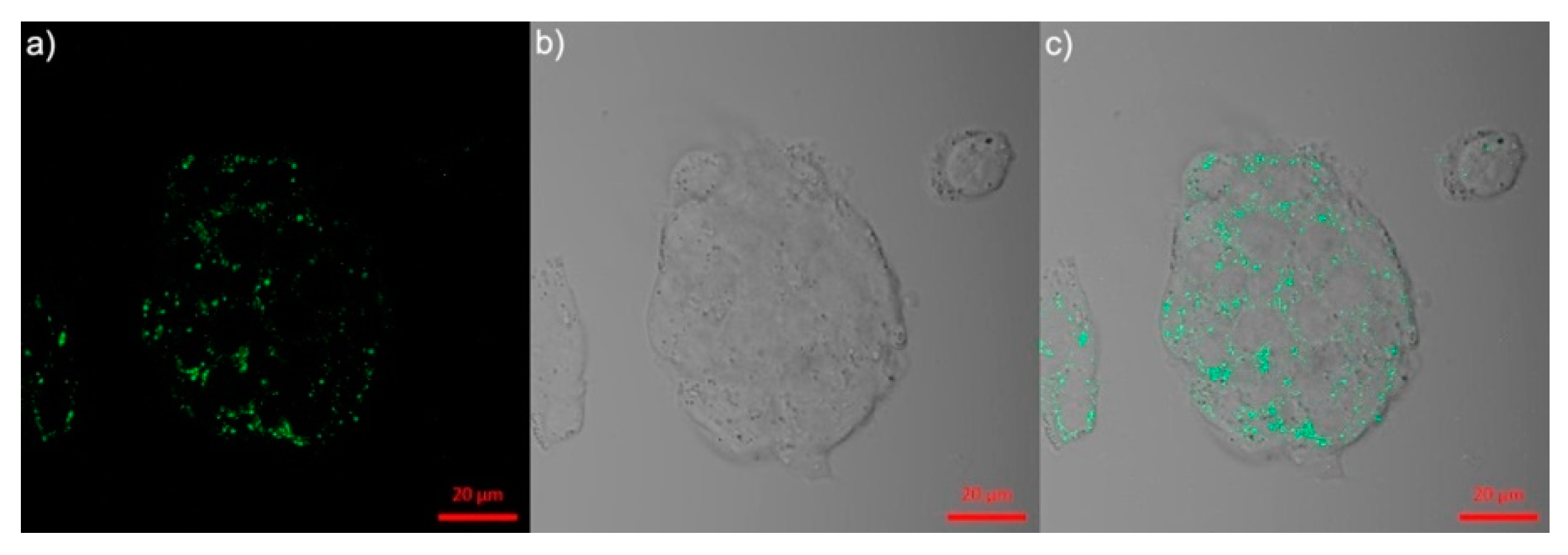
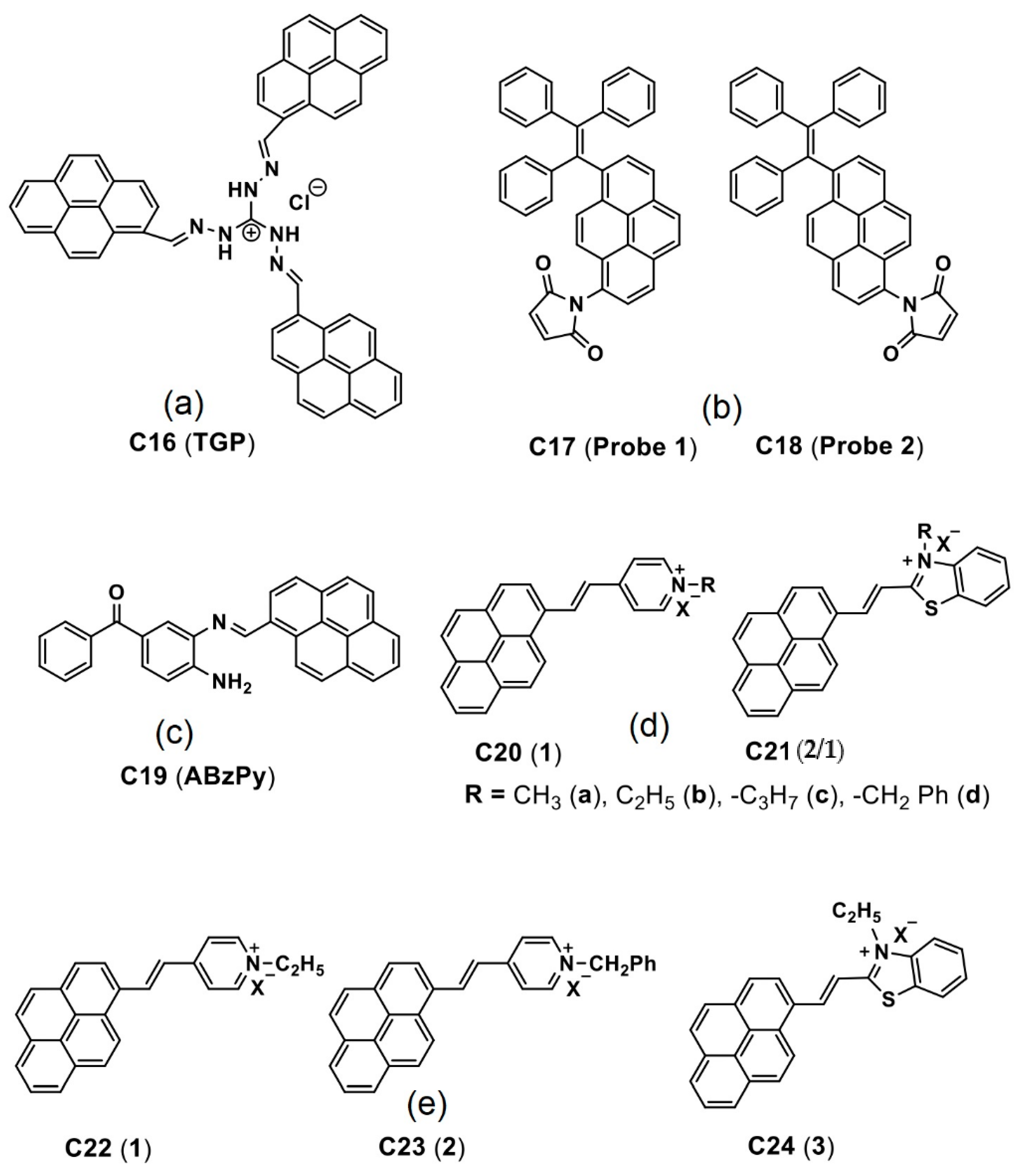

| Compound/System | λabs (nm) | λem (nm) | λem Stokes Shift (nm) | Φem (%) | Applications | Ref |
|---|---|---|---|---|---|---|
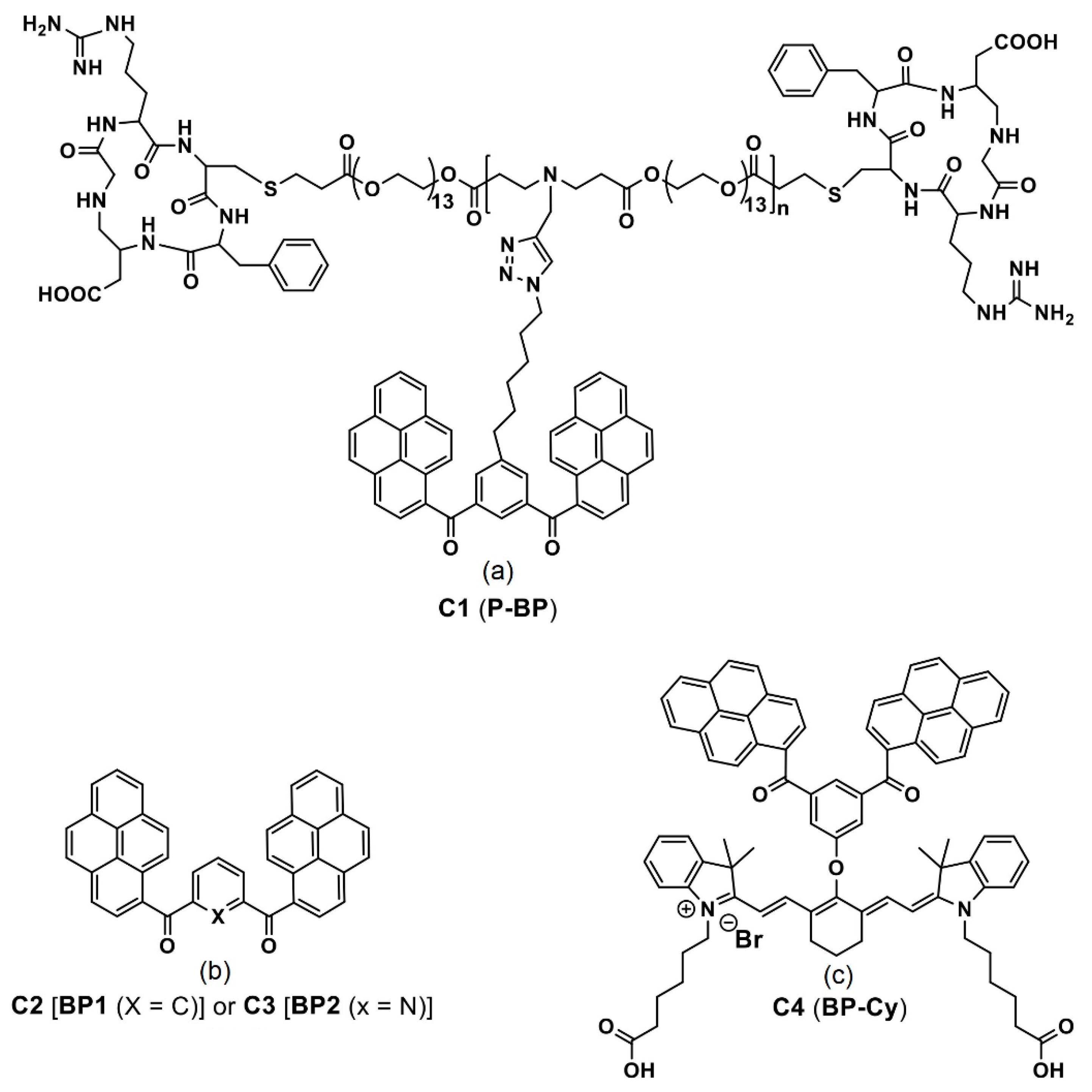
| BP1 and BP2 | 342 and 378 a 418 (for both) b | 506 and 394 a 512 and 542 b | n/a 6 and 48 b | 1.1 (for both) a 32.6 and 10.5 b | Lysosome imaging | [65] |
| C1 (P-BP) | NA NA | 537 a 418 b | n/a 119 b | NA NA | Lysosome endocytic pH imaging | [66] |
| C2 and C3 (BP1 and BP2) with polymer | 342 and 378 a NA | 506 and 394 a 512 and 533 b | n/a 6 and 39 b | 1.1 (for both) a NA | Lysosome endocytic pH imaging | [67] |
| C4 (BP-Cy) | 790 a 850 | 812 a >825 b | n/a >13 b | 24.6 NA | In vivo PA imaging | [68] |
| C6 (BKR) | 395 a 411 b | 519 a 528 b | n/a 9 b | NA NA | Cancer cell imaging | [71] |
| DPBP | NA | 525 a 525 b | n/a NA | NA NA | Intracellular autophagy imaging | [74] |
| Compound | λabs (nm) | λem (nm) | λem Stokes Shift (nm) | Φem (%) | Applications | Ref |
|---|---|---|---|---|---|---|
| C10 (A3) | 385 a 394 b | 404 a 505 b | n/a 101 b | NA NA | Cancer cell imaging | [79] |
| C11 (FHPY) | 371 a 391 b | 451 a 470 b | n/a 19 b | 12 97 | Cancer cell imaging | [80] |
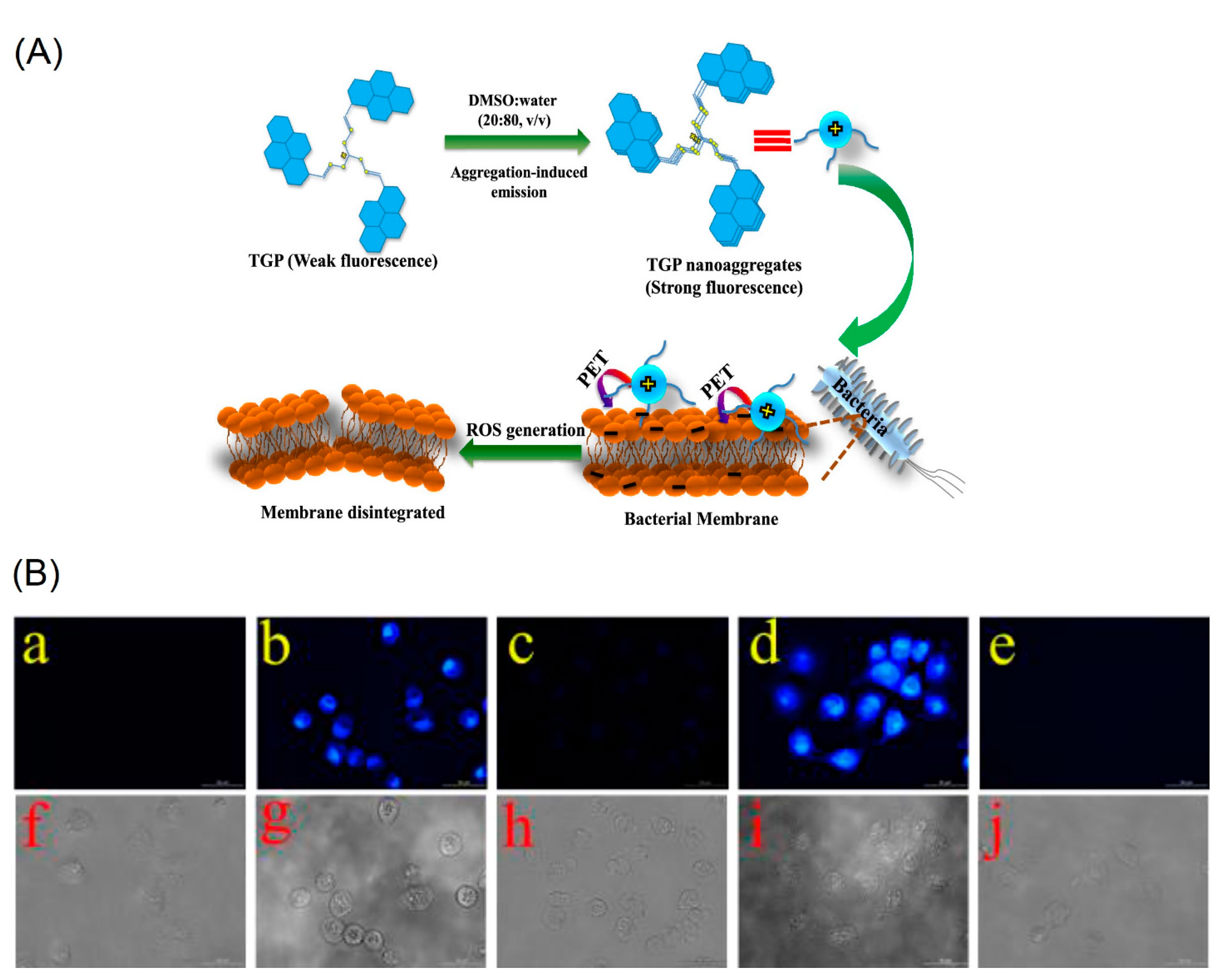
| C16 (TGP) | 365 a 419 b | 413 a 467 b | n/a 54 b | 11.7 36 | Bacterial imaging | [84] |
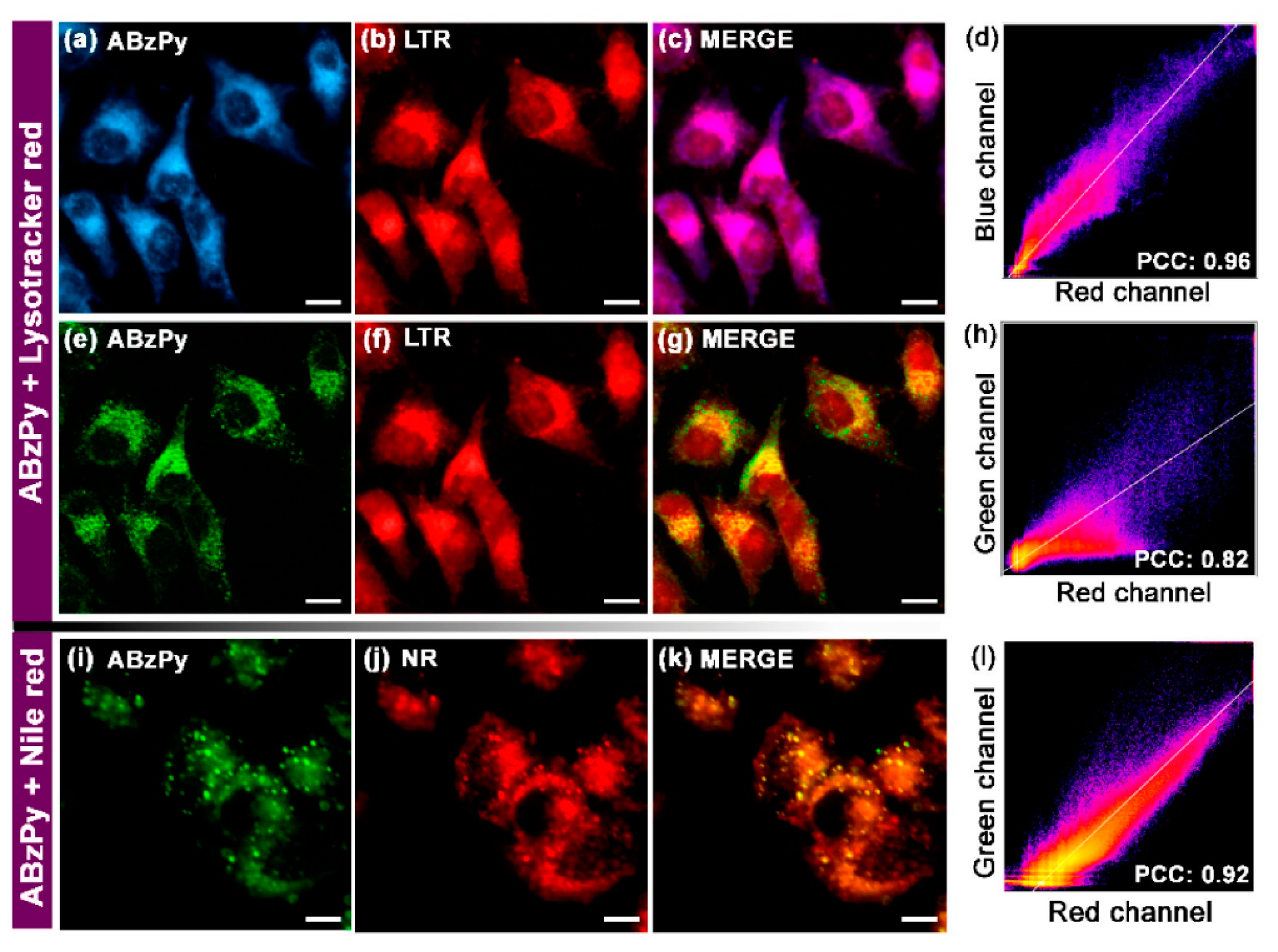
| C19 (ABzPy) | 365 a 420 b | 450 a 575 b | n/a 125 b | <1 >4 | Cancer cell imaging | [86] |
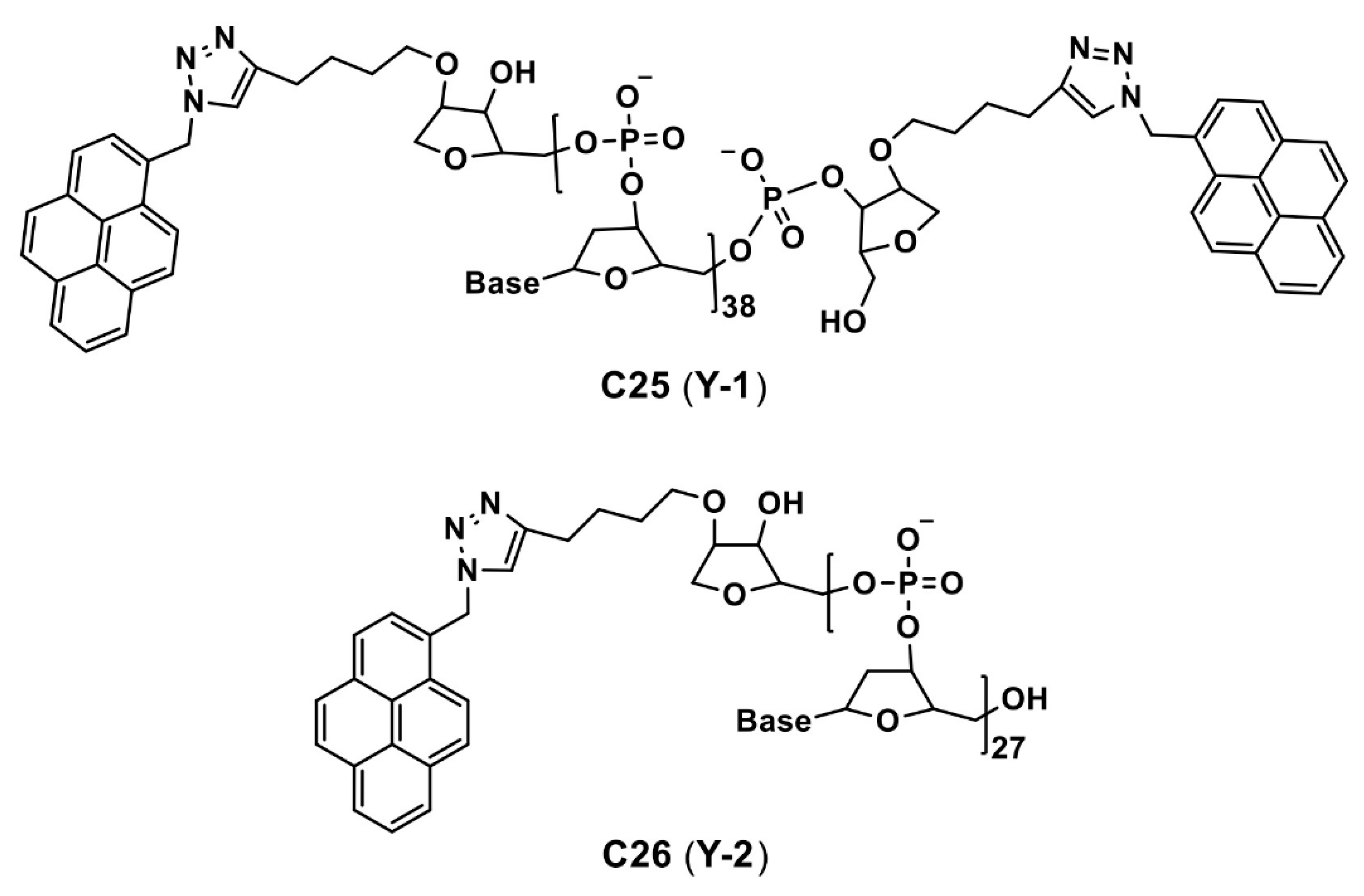
| C25 and C26 (Y-1 and Y-2) | 260 (for both) a 260 (for both) b | 400 a (for both) a 480 (for both) b | n/a 80 b | NA NA | Cancer cell imaging | [90] |
| Compound | λabs (nm) | λem (nm) | λem Stokes Shift (nm) | Φem (%) | Applications | Ref |
|---|---|---|---|---|---|---|
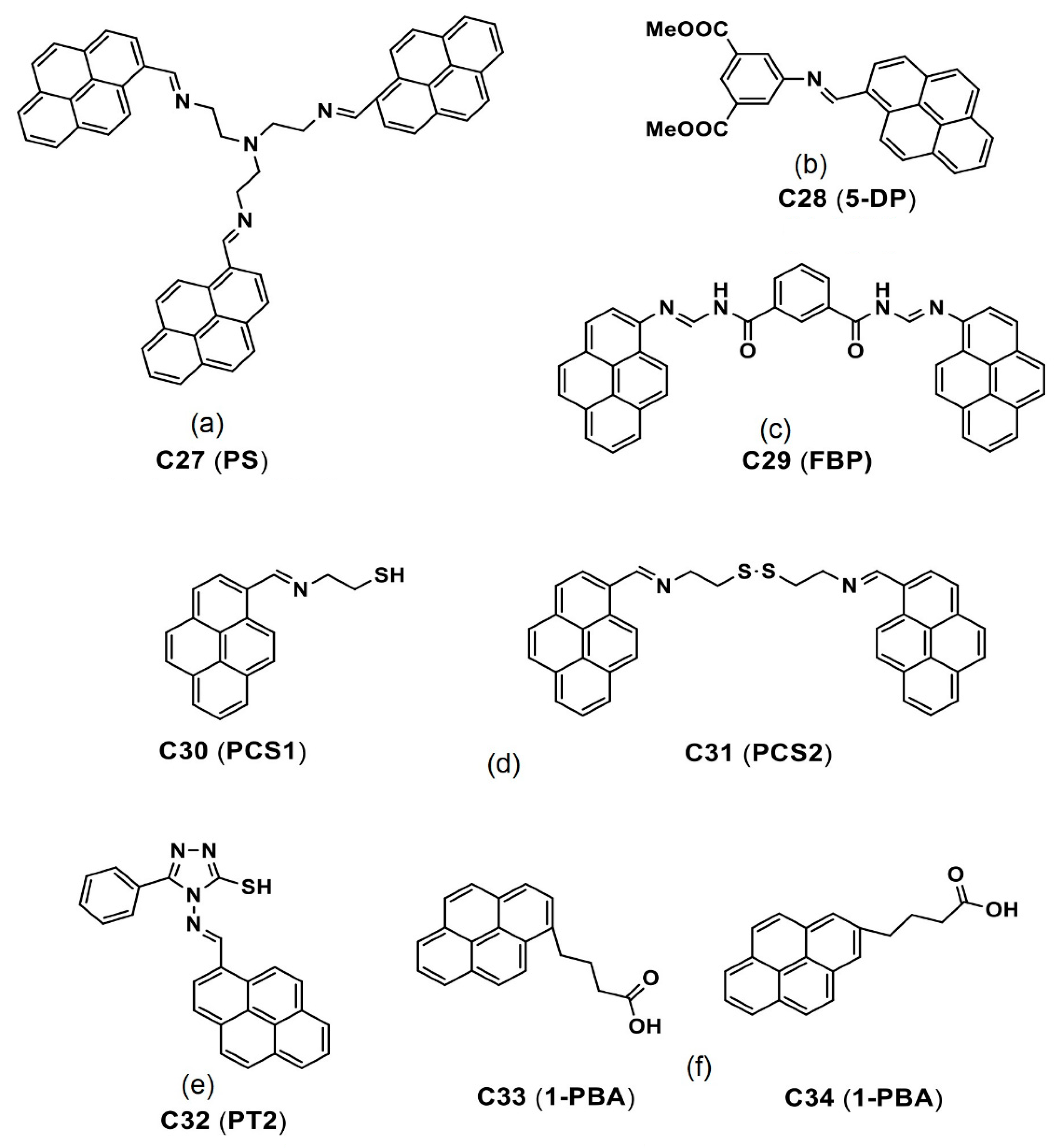
| C27 (PS) | 350–370 a 390–420 b | 430–435 a 475 b | n/a >35 b | 0.7/0.1 a 48/54 b | Cancer cell imaging | [95,96] |
| C28 (5-DP) | 378 a 365 b | 407 a 469 b | n/a 62 b | 1 a 67 b | Cancer cell imaging | [97] |
| C30 (PCS1) and C31 (PCS2) | 356 and 352 a 364 and 357 b | 421 and 425 a 465 and 469 b | n/a 44 (for both) b | 1.1 and 1.5 a 55 and 85 | Cancer cell imaging | [98] |
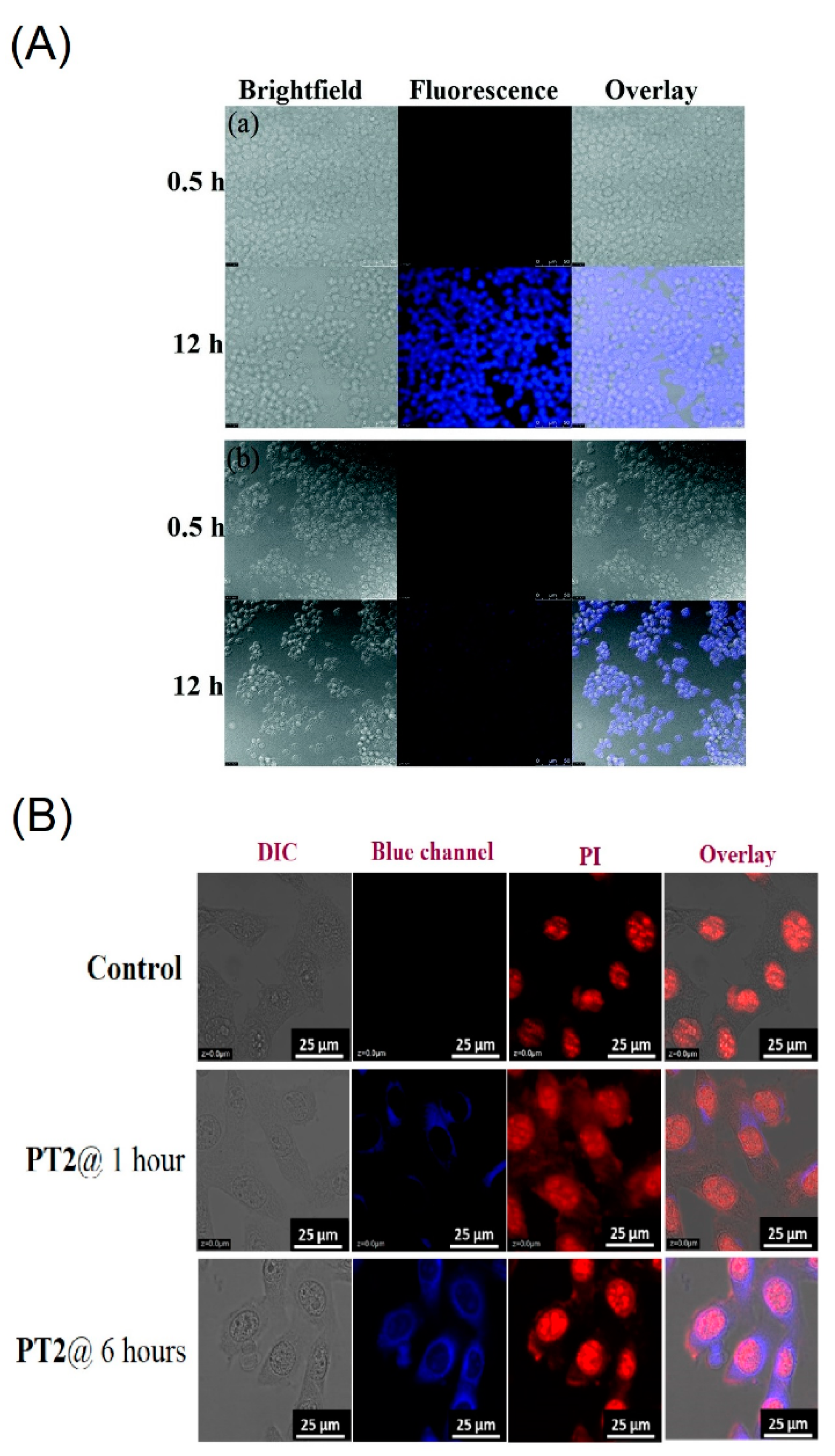
| C32 (PT2) | 401 a 413 b | 453 a 468 b | n/a 15 | 1 a 68 b | Cancer cell imaging | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shellaiah, M.; Sun, K.-W. Pyrene-Based AIE Active Materials for Bioimaging and Theranostics Applications. Biosensors 2022, 12, 550. https://doi.org/10.3390/bios12070550
Shellaiah M, Sun K-W. Pyrene-Based AIE Active Materials for Bioimaging and Theranostics Applications. Biosensors. 2022; 12(7):550. https://doi.org/10.3390/bios12070550
Chicago/Turabian StyleShellaiah, Muthaiah, and Kien-Wen Sun. 2022. "Pyrene-Based AIE Active Materials for Bioimaging and Theranostics Applications" Biosensors 12, no. 7: 550. https://doi.org/10.3390/bios12070550
APA StyleShellaiah, M., & Sun, K.-W. (2022). Pyrene-Based AIE Active Materials for Bioimaging and Theranostics Applications. Biosensors, 12(7), 550. https://doi.org/10.3390/bios12070550






