Novel Amperometric Biosensor Based on Tyrosinase/Chitosan Nanoparticles for Sensitive and Interference-Free Detection of Total Catecholamine
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Synthesis of Chitosan Nanoparticles
2.4. Fabrication of TYR/ChitNPs-Modified Electrodes
2.4.1. Layer-by-Layer Method
- (1)
- A total of 6 μL of ChitNPs solution (1 mg mL−1 in a water solution) was drop-cast onto GPH/SPE and left to dry at room temperature in air for approximately 1 h;
- (2)
- A total of 6 μL of Tyr solution (0.5 mg mL−1 in PBS 0.1 M, pH 7.2, KCl 0.1 M) was drop-cast onto the GPH/SPE previously modified with ChitNPs and left to dry at room temperature in air for approximately 1 h.
2.4.2. Nanoprecipitation Method
2.5. Electrochemical Measurements
2.6. Preparation of Human Urine Samples
3. Results and Discussion
3.1. Characterization of ChitNPs
3.2. Electrochemical Characterization of the Tyr/ChitNPs Biosensor Platform
3.2.1. Optimization of Methods for Loading Tyr on ChitNPs
3.2.2. Optimization of Tyr Concentration
3.2.3. Tyrosinase Covalent Functionalization via EDC-NHS Coupling Chemistry
3.2.4. Electrochemical Impedance Spectroscopy Behavior of the Modified Electrodes
3.3. Amperometric Response of Dopamine
3.4. Amperometric Response of Epinephrine and Norepinephrine
3.5. Selectivity
3.6. Stability
3.7. Real Sample Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emran, M.Y.; Shenashen, M.A.; Abdelwahab, A.A.; Khalifa, H.; Mekawy, M.; Akhtar, N.; Abdelmottaleb, M.; EI-Safty, S.A.J. Design of hierarchical electrocatalytic mediator for one step, selective screening of biomolecules in biological fluid samples. Appl. Electrochem. 2018, 48, 529–542. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.Z.; Qian, Z.; Yang, Z.; Yang, K.; Zong, S.; Wang, Z.; Cui, Y. Ultra-sensitive surface enhanced Raman spectroscopy sensor for in-situ monitoring of dopamine release using zipper-like ortho-nanodimers. Biosens. Bioelectron. 2021, 180, 113100. [Google Scholar] [CrossRef] [PubMed]
- Liss, B.; Roeper, J. Individual dopamine midbrain neurons: Functional diversity and flexibility in health and disease. Brain Res. Rev. 2008, 58, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Dopamine outside the brain: The eye, cardiovascular system and endocrine pancreas. Pharmacol. Ther. 2019, 203, 107392. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Medina, M.Á.; Urdiales, J.L.; Rodríguez-Caso, C.; Ramírez, F.J.; Sánchez-Jiménez, F. Biogenic amines and polyamines: Similar biochemistry for different physiological missions and biomedical applications. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 23–59. [Google Scholar] [CrossRef]
- Marc, D.T.; Ailts, J.W.; Ailts Campeau, D.C.; Bull, M.J.; Olson, K.L. Neurotransmitters excreted in the urine as biomarkers of nervous system activity: Validity and clinical applicability. Neurosci. Biobehav. Rev. 2011, 35, 635–644. [Google Scholar] [CrossRef]
- Carrera, V.; Sabater, E.; Vilanova, E.; Sogorb, M.A. A simple and rapid HPLC–MS method for the simultaneous determination of epinephrine, norepinephrine, dopamine and 5-hydroxy- tryptamine: Application to the secretion of bovine chromaffin cell cultures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 847, 88–94. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Nishimori, D.; Kuwabara, T.; Takeuchi, M.; Iiyama, M.; Takayanagi, T. Track-etched membrane-based dual-electrode coulometric detector for microbore/capillary high-performance liquid chromatography. Anal. Chim. Acta 2020, 1102, 46–52. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- Moghzi, F.; Soleimannejad, J.; Sañudo, E.C.; Janczak, J. Dopamine Sensing Based on Ultrathin Fluorescent Metal–Organic Nanosheets. ACS Appl. Mater. Interfaces 2020, 12, 44499–44507. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xiang, X.; Fu, L.; Cao, Q.; Huang, R.; Liu, H.; Han, G.; Wu, L. Regenerative field effect transistor biosensor for in vivo monitoring of dopamine in fish brains. Biosens. Bioelectron. 2021, 188, 113340. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Fernandes, P.M.V.; Pereira, C.M.; Silva, F. Electrochemical sensors and biosensors for determination of catechola- mine neurotransmitters: A review. Talanta 2016, 160, 653–679. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Gu, C.; Xiao, Q.; Chen, J.; Yin, Z.Z.; Liu, H.; Fan, K.; Li, L. A highly selective and sensitive biosensor for dopamine based on a surface molecularly imprinted layer to coordinate nano-interface functionalized acupuncture needle. Chem. Eng. J. 2022, 436, 135203. [Google Scholar] [CrossRef]
- Rahman, S.F.; Min, K.; Park, S.H.; Park, J.H.; Yoo, J.C.; Park, D.H. Selective determination of dopamine with an amperometric biosensor using electrochemically pretreated and activated carbon/tyrosinase/Nafion®-modified glassy carbon electrode. Biotechnol. Bioprocess Eng. 2016, 21, 627–633. [Google Scholar] [CrossRef]
- Rand, E.; Periyakaruppan, A.; Tanaka, Z.; Zhang, D.A.; Marsh, M.P.; Andrews, R.J.; Lee, K.H.; Chen, B.; Meyyappan, M.; Koehne, J.E. A carbon nanofiber-based biosensor for simultaneous detection of dopamine and serotonin in the presence of ascorbic acid. Biosens. Bioelectron. 2013, 42, 434–438. [Google Scholar] [CrossRef] [Green Version]
- De-Qian, H.; Cheng, C.; Yi-Ming, W.; Hong, Z.; Liang-Quan, S.; Hua-Jie, X.; Zhao-Di, L. The Determination of Dopamine Using Glassy Carbon Electrode Pretreated by a Simple Electrochemical Method. J. Electrochem. Sci. 2012, 7, 5510–5520. [Google Scholar]
- Gao, F.; Cai, X.; Wang, X.; Gao, C.; Liu, S.; Gao, F.; Wang, Q. Highly sensitive and selective detection of dopamine in the presence of ascorbic acid at graphene oxide modified electrode. Sens. Actuators B Chem. 2013, 186, 380–387. [Google Scholar] [CrossRef]
- Qiu, H.J.; Zhou, G.P.; Ji, G.L.; Zhang, Y.; Huang, X.R.; Ding, Y. A novel nanoporous gold modified electrode for the selective determination of dopamine in the presence of ascorbic acid. Coll. Surf. B Biointerfaces 2008, 69, 105–108. [Google Scholar] [CrossRef]
- Üge, A.; Zeybek, D.K.; Zeybek, B. An electrochemical sensor for sensitive detection of dopamine based on MWCNTs/CeO2-PEDOT composite. J. Electroanal. Chem. 2018, 15, 134–142. [Google Scholar] [CrossRef]
- Yang, C.; Jacobs, C.B.; Nguyen, M.D.; Ganesana, M.; Zestos, A.G.; Ivanov, I.N.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; Venton, B.J. Carbon nanotubes grown on metal microelectrodes for the detection of dopamine. Anal. Chem. 2016, 88, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Deepika, J.; Sha, R.; Badhulika, S. A ruthenium (IV) disulfide based non-enzymatic sensor for selective and sensitive amperometric determination of dopamine. Microchim. Acta 2019, 186, 480. [Google Scholar] [CrossRef] [PubMed]
- Tortolini, C.; Cass, A.E.G.; Pofi, R.; Lenzi, A.; Antiochia, R. Microneedle-based nanoporous gold electrochemical sensor for real-time catecholamine detection. Microchim. Acta 2022, 189, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mphuthi, N.G.; Adekunle, A.S.; Ebenso, E.E. Electrocatalytic oxidation of epinephrine and norepinephrine at metal oxide doped phthalocyanine/MWCNT composite sensor. Sci. Rep. 2016, 6, 26938. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Martinez, T.; Yaniv, D.R.; McCormick, L.D. Scanning tunneling microscopic investigation of surface fouling of glassy carbon surfaces due to phenol oxidation. J. Electroanal. Chem. Interfacial Electrochem. 1991, 313, 129–140. [Google Scholar] [CrossRef]
- Lane, R.F.; Blaha, C.D. Detection of catecholamines in brain tissue: Surface-modified electrodes enabling in vivo investigations of dopamine function. Langmuir 1990, 6, 56–65. [Google Scholar] [CrossRef]
- Florescu, M.; David, M. Tyrosinase-based biosensors for selective dopamine detection. Sensors 2017, 17, 1314. [Google Scholar] [CrossRef] [Green Version]
- Apetrei, I.M.; Apetrei, C. Development of a novel biosensor based on tyrosinase/platinum nanoparticles/Chitosan/graphene nanostructured layer with applicability in bioanalysis. Materials 2019, 12, 1009. [Google Scholar] [CrossRef] [Green Version]
- Baluta, S.; Lesiak, A.; Cabaj, J. Simple and cost-effective electrochemical method for norepinephrine determination based on carbon dots and tyrosinase. Sensors 2020, 20, 4567. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Biosensor based on tyrosinase immobilized on a single-walled carbon nanotube-modified glassy carbon electrode for detection of epinephrine. Int. J. Nanomed. 2013, 8, 4391–4398. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhai, F.; Hasebe, Y.; Jia, H.; Zhang, Z. A highly sensitive electrochemical biosensor for phenol derivatives using a graphene oxide-modified tyrosinase electrode. Bioelectrochem 2018, 122, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Janegitz, B.C.; Medeiros, R.A.; Rocha-Fillo, R.C.; Fatibello-Filho, O. Direct electrochemistry of tyrosinase and biosensing for phenol based on gold nanoparticles electrodeposited on a boron-doped diamond electrode. Diam. Relat. Mater. 2012, 25, 128–133. [Google Scholar] [CrossRef]
- Yang, L.; Xiong, H.; Zhang, X.; Wang, S. A novel tyrosinase biosensor based on chitosan-carbon-coated nickel nanocomposite film. Bioelectrochem 2012, 84, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.C.; Janegitz, B.C.; Brett, C.M.A.; Fatibello-Filho, O. Tyrosinase biosensor based on a glassy carbon electrode modified with multi-walled carbon nanotubes and 1-butyl-3-methylimidazolium chloride within a dihexadecylphosphate film. Sens. Actuators B Chem. 2013, 188, 1101–1108. [Google Scholar] [CrossRef]
- Fartas, F.M.; Abdullah, J.; Yusof, N.A.; Sulamain, Y.; Saiman, M.I. Biosensor based on tyrosinase immobilized on graphene-decorated gold nanoparticle chitosan for phenolic detection in aqueous. Sensors 2017, 17, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apetrei, C.; Rodriguez-Mendez, M.L.; De Saja, J.A. Amperometric tyrosinase based biosensor using an electropolymerized phosphate-doped polypyrrole film as an immobilization support. Application for detection of phenolic compounds. Electrochim. Acta 2011, 56, 8919–8925. [Google Scholar] [CrossRef]
- Wee, Y.; Park, S.; Kwon, Y.H.; Ju, Y.; Yeon, K.M.; Kim, J. Tyrosinase-immobilized CNT based biosensor for highly-sensitive detection of phenolic compounds. Biosens. Bioelectron. 2019, 132, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.; Ghica, M.E.; Ajayi, R.F.; Iwuoha, E.I.; Brett, C.M.A. Tyrosinase based amperometric biosensor for determination of tyramine in fermented food and beverages with gold nanoparticles doped poly(8-anilino-1-naphtalene sulphonic acid) modified electrode. Food Chem. 2019, 282, 18–26. [Google Scholar] [CrossRef]
- Kim, D.S.; Kang, E.S.; Seungho, B.; Choo, S.S.; Chung, Y.H.; Lee, D.; Min, J.; Kim, T.H. Electrochemical detection of dopamine using periodic cylindrical gold nanoelectrode arrays. Sci. Rep. 2018, 8, 14049. [Google Scholar] [CrossRef] [Green Version]
- Sainio, S.; Palomaki, T.; Tujunen, N.; Prototopova, V.; Koene, J.; Kordas, K.; Koskinen, J.; Meyyappan, M.; Laurila, T. Integrated Carbon Nanostructures for Detection of Neurotransmitters. Mol. Neurobiol. 2015, 52, 859–866. [Google Scholar] [CrossRef]
- Sanchez-Ferrer, A.; Rodrìguez-Lòpez, J.N.; Garcìa-Cánovas, F.; Garcìa-Camona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Granero, A.M.; Fernandez, H.; Agostini, E.; Zon, M.L. An amperometric biosensor based on peroxidases from Brassica napus for the determination of the total polyphenolic content in wine and tea samples. Talanta 2010, 83, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Zejli, H.; de Cisneros, H.H.; Naranjo-Rodriguez, I.; Liu, B.; Temsamani, K.R.; Marty, J.L. Phenol biosensor based on Sonogel-Carbon transducer with tyrosinase alumina sol–gel immobilization. Anal. Chim. Acta 2008, 612, 198–203. [Google Scholar] [CrossRef]
- Shiddiky, M.J.A.; Torriero, A.A.J. Application of ionic liquids in electrochemical sensing systems. Bios. Bioelectron. 2011, 26, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, R.; Pasquali, M.; Scaramuzzo, F.A.; Curulli, A. Recent Advances in Electrochemical Chitosan-Based Chemosensors and Biosensors: Applications in Food Safety. Chemosensors 2021, 9, 254. [Google Scholar] [CrossRef]
- Karrat, A.; Amine, A. Recent advances in chitosan-based electrochemical sensors and biosensors. Arab. J. Chem. Environ. Res. 2020, 7, 66–93. [Google Scholar]
- Fisher, M.J.E. Amine coupling through EDC/NHS: A practical approach. Methods Mol. Biol. 2010, 627, 55–73. [Google Scholar]
- Ducker, R.E.; Montague, M.T.; Leggett, G.J. A comparative investigation of methods for protein immobilization on self-assembled monolayers using glutaraldehyde, carbodiimide, and anhydride reagents. Biointerphases 2008, 3, 59. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chou, Y.C.; Tsai, J.H.; Huang, T.M.; Chen, J.Z.; Yeh, Y.C. Tyrosinase/Chitosan/Reduced Graphene Oxide Modified Screen-Printed Carbon Electrode for Sensitive and Interference-Free Detection of Dopamine. Appl. Sci. 2019, 9, 622. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Liu, Y.; Huang, J.; Xian, Y.; Zhang, Z.; Jin, L. Fabrication of Tyrosinase Biosensor Based on Multiwalled Carbon Nanotubes-Chitosan Composite and Its Application to Rapid Determination of Coliforms. Electroanalysis 2008, 20, 1463–1469. [Google Scholar] [CrossRef]
- Wan, D.; Yuan, S.; Li, G.L.; Neoh, K.G.; Kang, E.T. Glucose biosensor from covalent Immobilization of Chitosan-Coupled Carbon Nanotubes on Polyaniline-Modified Gold Electrode. ACS Appl. Mater. Interfaces 2010, 2, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Lupu, S.; Lete, C.; Cătălin Balaure, P.; Ion Caval, D.; Mihailciuc, C.; Lakard, B.; Hihn, J.Y.; del Campo, F.J. Development of Amperometric Biosensors Based on Nanostructured Tyrosinase-Conducting Polymer Composite Electrodes. Sensors 2013, 13, 6759–6774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Wu, Y. Recent advances of chitosan-based nanoparticles for biomedical and biotechnological applications. Int. J. Biol. Macromol. 2022, 203, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Anusha, J.R.; Raj, C.J.; Cho, B.-B.; Fleming, A.T.; Yu, K.-H.; Kim, B.C. Amperometric glucose biosensor based on glucose oxidase immobilized over chitosan nanopartoicles from glaudius of Uroteuthis duvauceli. Sens. Actuators B Chem. 2015, 215, 536–543. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.S.; Kim, M.I. Poly-γ-glutamic Acid/Chitosan hydrogel nanoparticles entrapping glucose oxidase and magnetic nanoparticles for glucose biosensing. J. Nanosci. Nanotechnol. 2020, 20, 5333–5337. [Google Scholar] [CrossRef]
- Kalam, M.A. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 127–136. [Google Scholar] [CrossRef]
- Obaje, E.A.; Cummins, G.; Schulze, H.; Mahmood, S.; Desmulliez, M.P.; Bachmann, T.T. Carbon screen-printed electrodes on ceramic substrates for label-free molecular detection of antibiotic resistance. J. Interdiscip. Nanomed. 2016, 1, 93–109. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesaro, A. Chitosan nanoparticles: Preparation, size evolution, and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef]
- Oldham, K.B. Analytical expression for the reversible Randles-Sevcik function. J. Electroanal. Chem. Interfacial Electrochem. 1979, 105, 373–375. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. A calibration-base method for the evaluation of the detection limit of an electrochemical biosensor. Electroanalysis 2007, 19, 1127–1230. [Google Scholar] [CrossRef]
- Marroquin, J.B.; Rhee, K.Y.; Park, S.J. Chitosan nanocomposite films: Enhanced electrical conductivity, thernmal stability, and mechanical properties. Carbohydr. Polym. 2013, 92, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, B.Q.; Xu, B.; Cheng, G.J.; Dong, S.J. Amperometric quantification of polar organic solvents based on a tyrosinase biosensor. Anal. Chem. 2000, 72, 3455–3460. [Google Scholar] [CrossRef] [PubMed]
- Cosnier, S.; Popescu, I.C. Poly(amphilic pyrrole)-tyrosinase-peroxidase electrode for amplified flow injection-amperometric detection of phenol. Anal. Chim. Acta 1996, 319, 145–151. [Google Scholar] [CrossRef]
- Kamin, R.A.; Wilson, G.S. Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal. Chem. 1980, 52, 1198–1205. [Google Scholar] [CrossRef]
- Espin, J.C.; Varon, R.; Fenoll, L.G.; Gilabert, M.A.; Garcia-Ruiz, P.A.; Tudela, J.; Garcia-Canovas, F. Kinetic characterization of the substrate specificity and mechanism of mushroom tyrosinase. Eur. J. Biochem. 2000, 267, 1270–1279. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.S.; Lou, D.; Dou, K.; He, L.L.; Dong, Y.H.; Wang, S.L. Amperometric tyrosinase biosensor based on boron-doped nanocrystalline diamond film electrode for the detection of phenolic compounds. J. Solid State Electrochem. 2016, 20, 47–54. [Google Scholar] [CrossRef]
- Rahman, S.F.; Min, K.; Park, S.H.; Park, J.H.; Yoo, J.C.; Park, D.H. Highly sensitive and selective dopamine detection by an amperometric biosensor based on tyrosinase/MWNT/GCE. Korean J. Chem. Eng. 2016, 33, 3442–3447. [Google Scholar] [CrossRef]
- Li, H.Q.; Hu, X.; Zhu, H.M.; Zang, Y.; Xue, H.G. Amperometric phenol biosensor based on a new immobilization matrix: Polypyrrole nanotubes derived from methyl orange as dopant. Int. J. Electrochem. Sci. 2017, 12, 6714–6728. [Google Scholar] [CrossRef]
- Sethuraman, V.; Muthuraja, P.; Anandha Raj, J.; Manisankar, P. A highly sensitive electrochemical biosensor for catechol using conducting polymer reduced graphene oxide–metal oxide enzyme modified electrode. Biosens. Bioelectron. 2016, 84, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.C.; Garcia, L.L.C.; Figueiredo-Filho, L.C.S.; Janegitz, B.C.; Fatibello-Filho, O. A biosensor based on gold nanoparticles, dihexadecylphosphate, and tyrosinase for the determination of catechol in natural water. Enzym. Microb. Technol. 2016, 84, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Araque, E.; Villalonga, R.; Gamella, M.; Martínez-Ruiz, P.; Reviejo, J.; Pingarrón, J.M. Crumpled reduced graphene oxide–polyamidoamine dendrimer hybrid nanoparticles for the preparation of an electrochemical biosensor. J. Mater. Chem. B 2013, 1, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Borisova, B.; Ramos, J.; Díez, P.; Sánchez, A.; Parrado, C.; Araque, E.; Villalonga, R.; Pingarrón, J.M. A Layer-by-Layer biosensing architecture based on polyamidoamine dendrimer and carboxymethylcellulose-modified graphene oxide. Electroanalysis 2015, 27, 2131–2138. [Google Scholar] [CrossRef]
- Lete, C.; Lupu, S.; Lakard, B.; Hihn, J.Y.; del Campo, F.J. Multi-analyte determination of dopamine and catechol at single-walled carbon nanotubes—Conducting polymer—Tyrosinase based electrochemical biosensors. J. Electroanal. Chem. 2015, 744, 53–61. [Google Scholar] [CrossRef]
- Min, K.; Yoo, Y.J. Amperometric detection of dopamine based on tyrosinase-SWNTs-Ppy composite electrode. Talanta 2009, 80, 1007–1011. [Google Scholar] [CrossRef]
- Meloni, F.; Spychalska, K.; Zając, D.; Pilo, M.I.; Zucca, A.; Cabaj, J. Application of a Thiadiazole-derivative in a Tyrosinase-based Amperometric Biosensor for Epinephrine Detection. Electroanalysis 2021, 33, 1639–1645. [Google Scholar] [CrossRef]

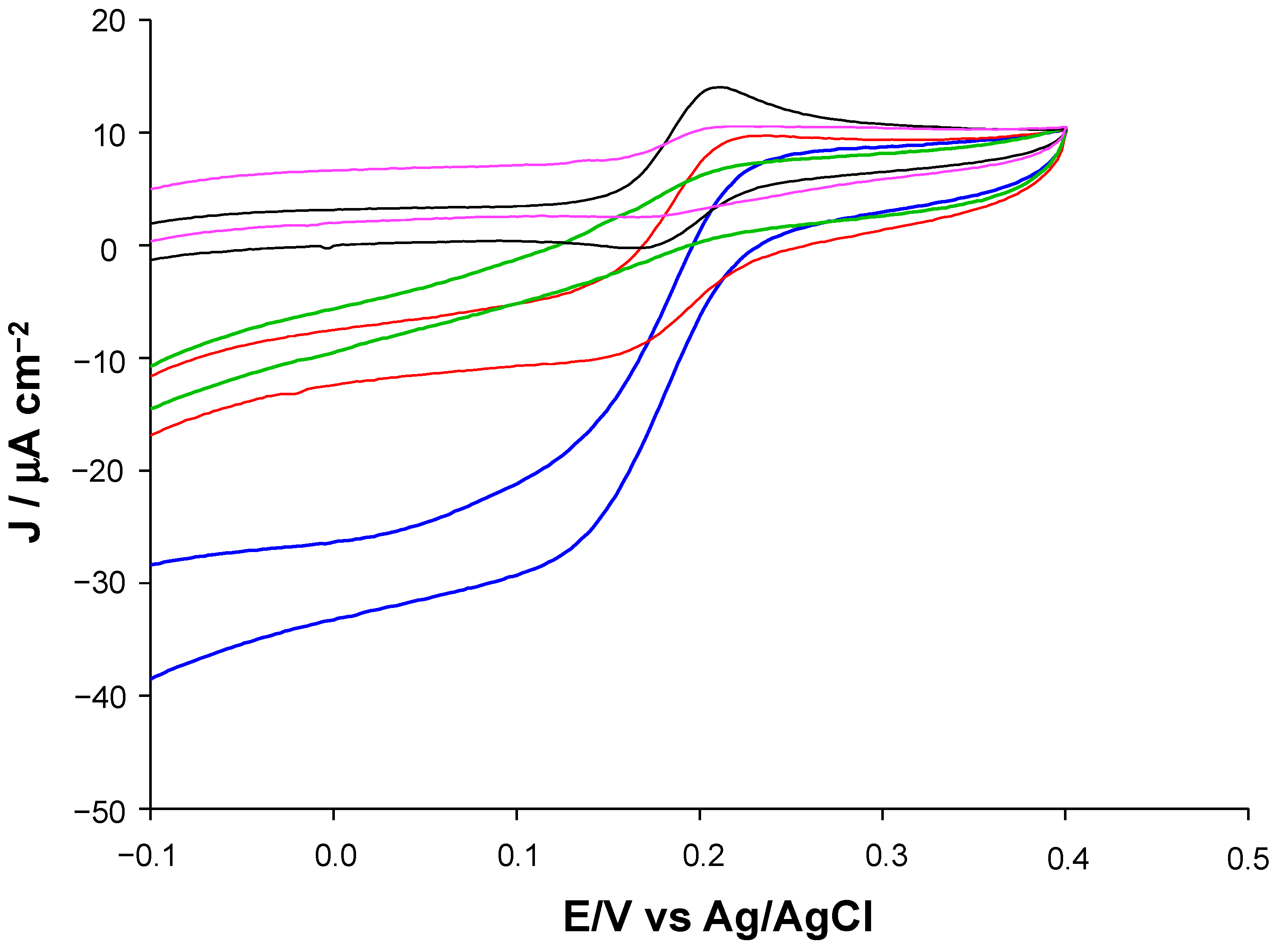

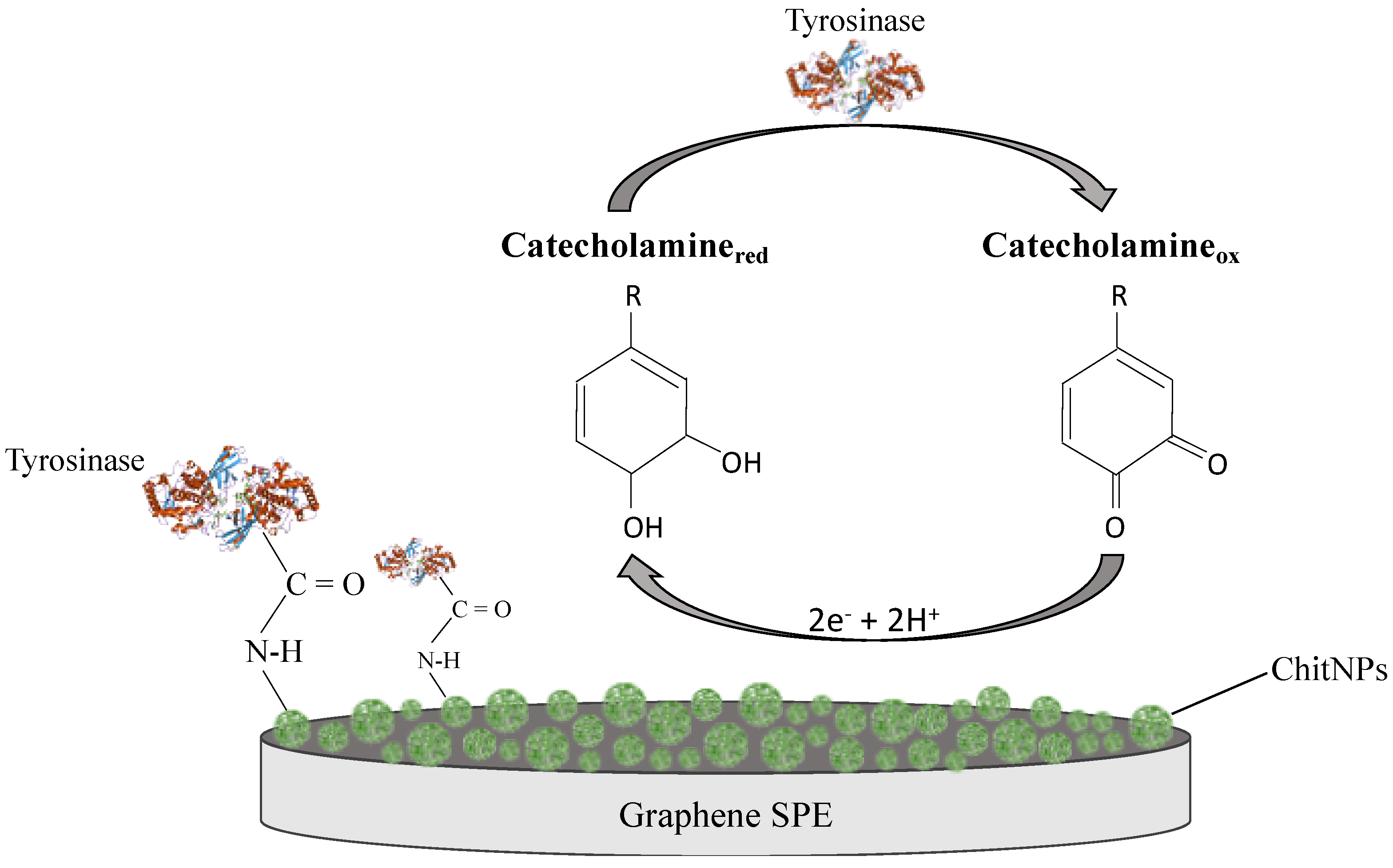
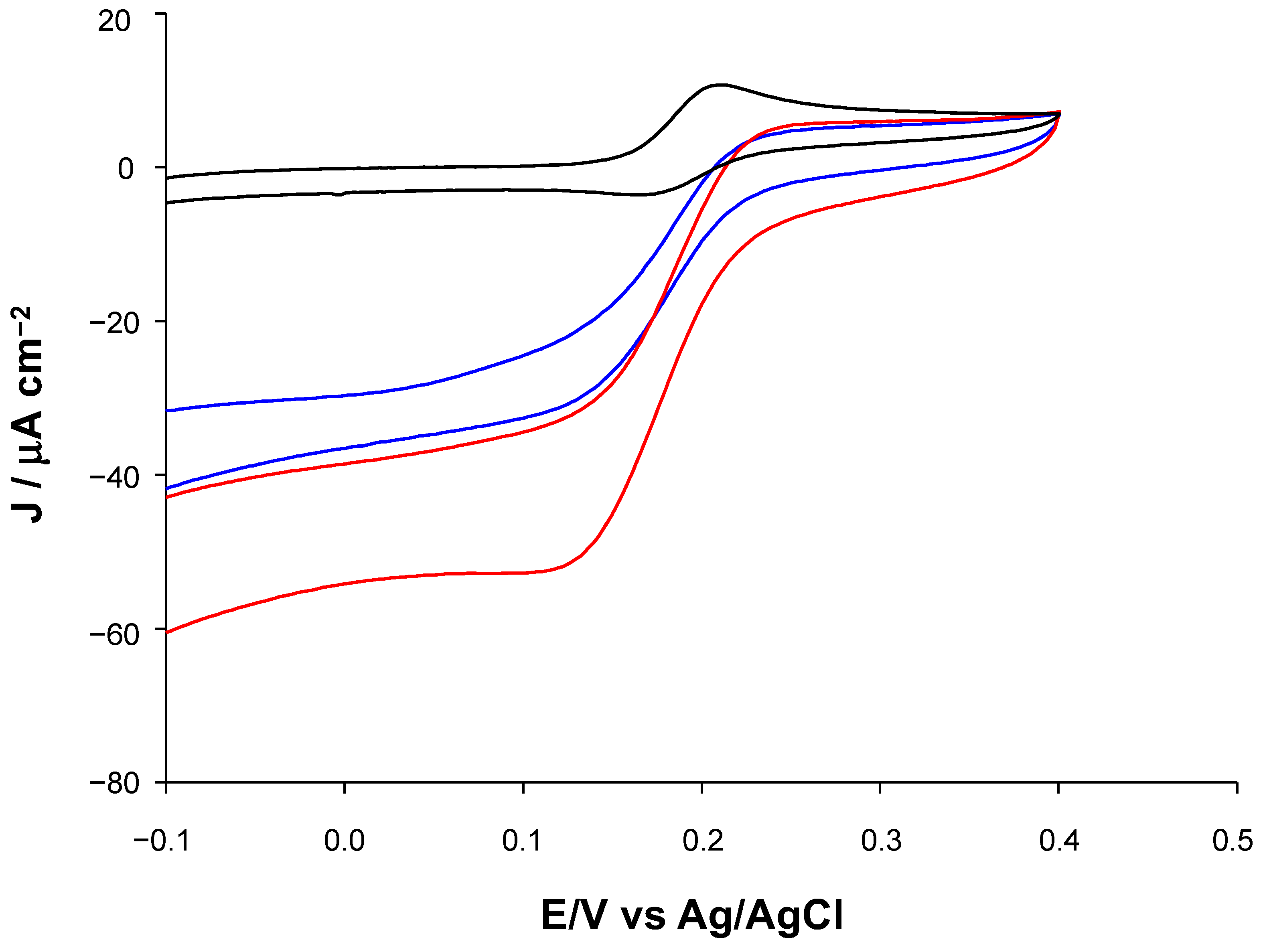
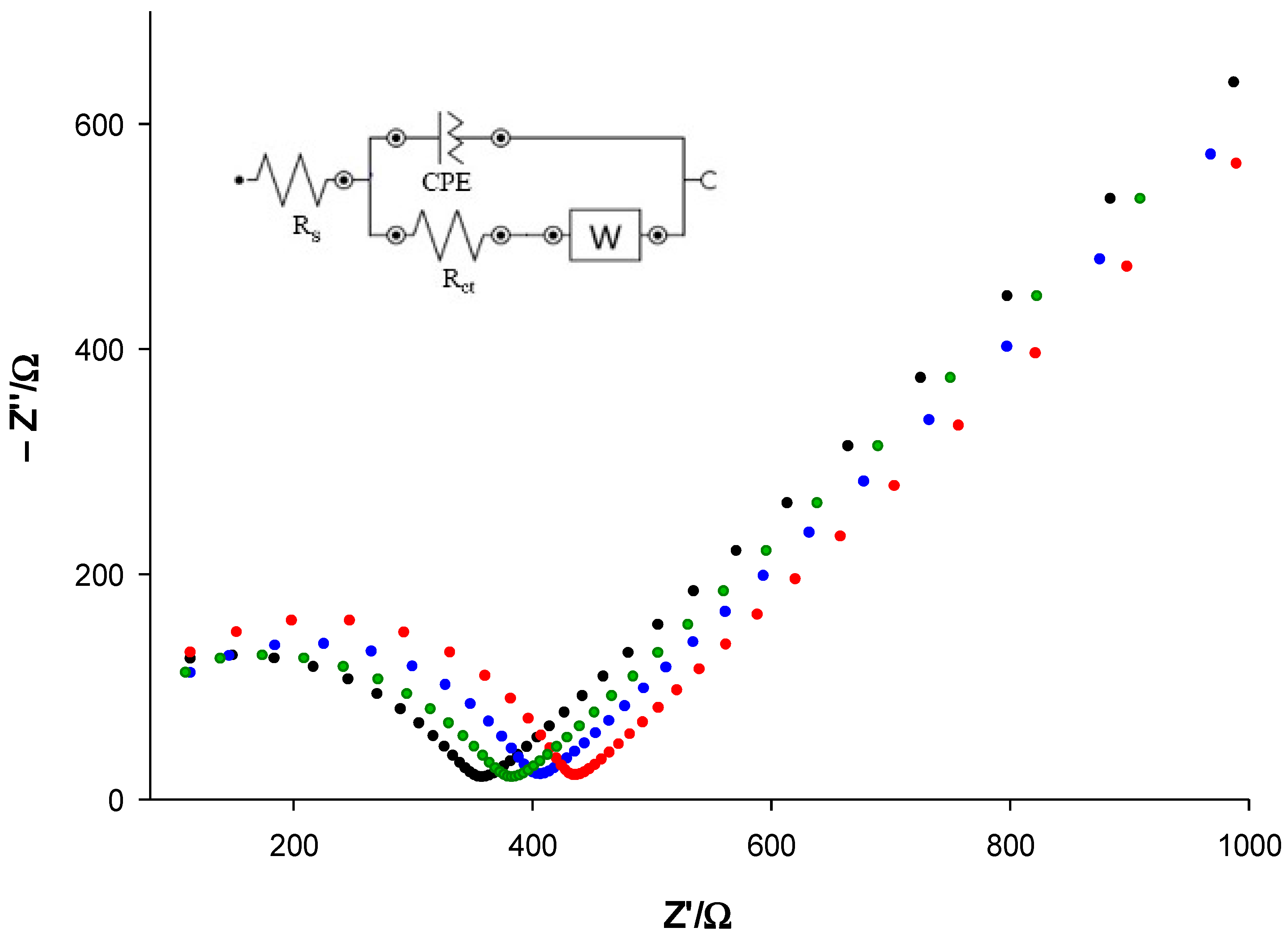
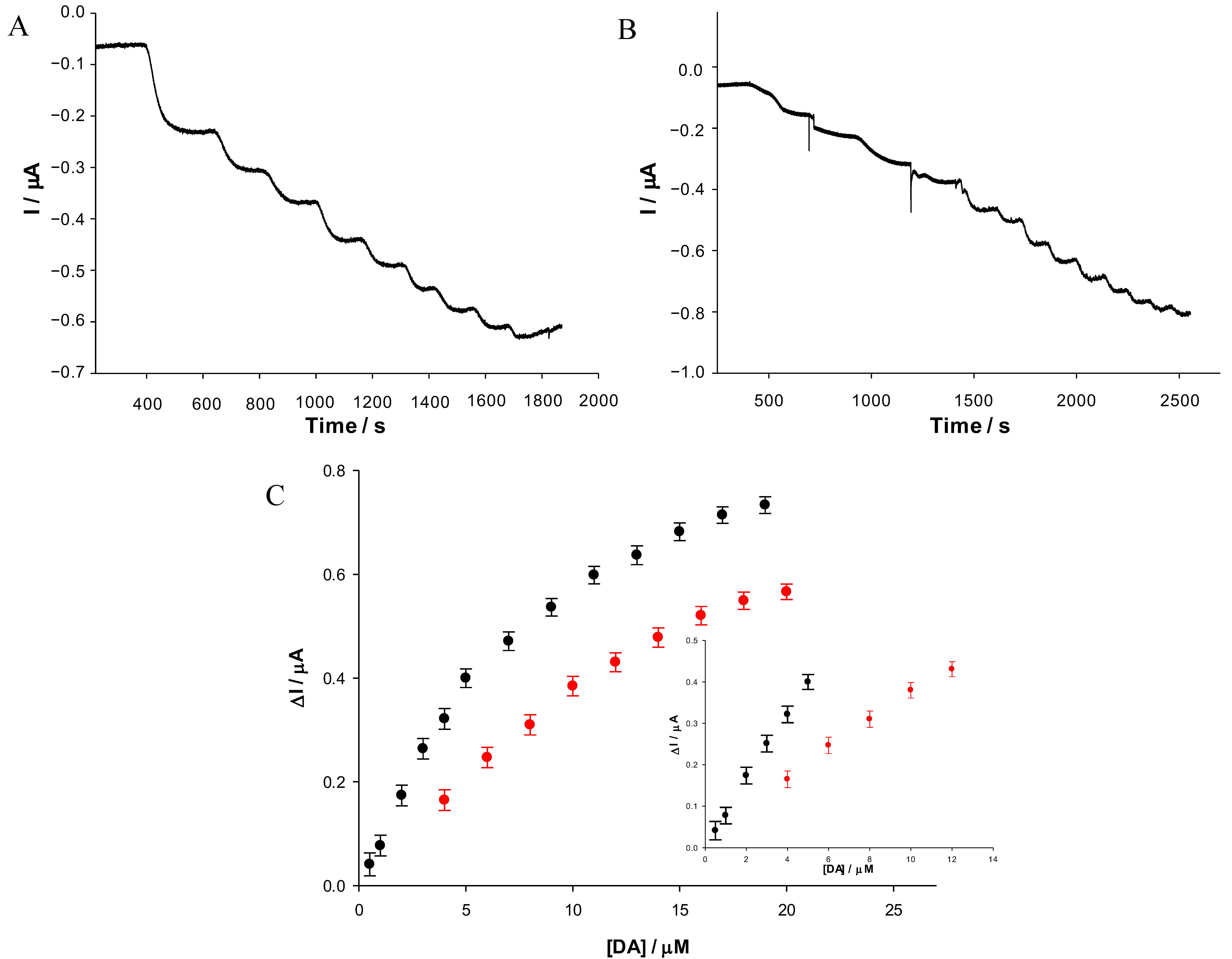
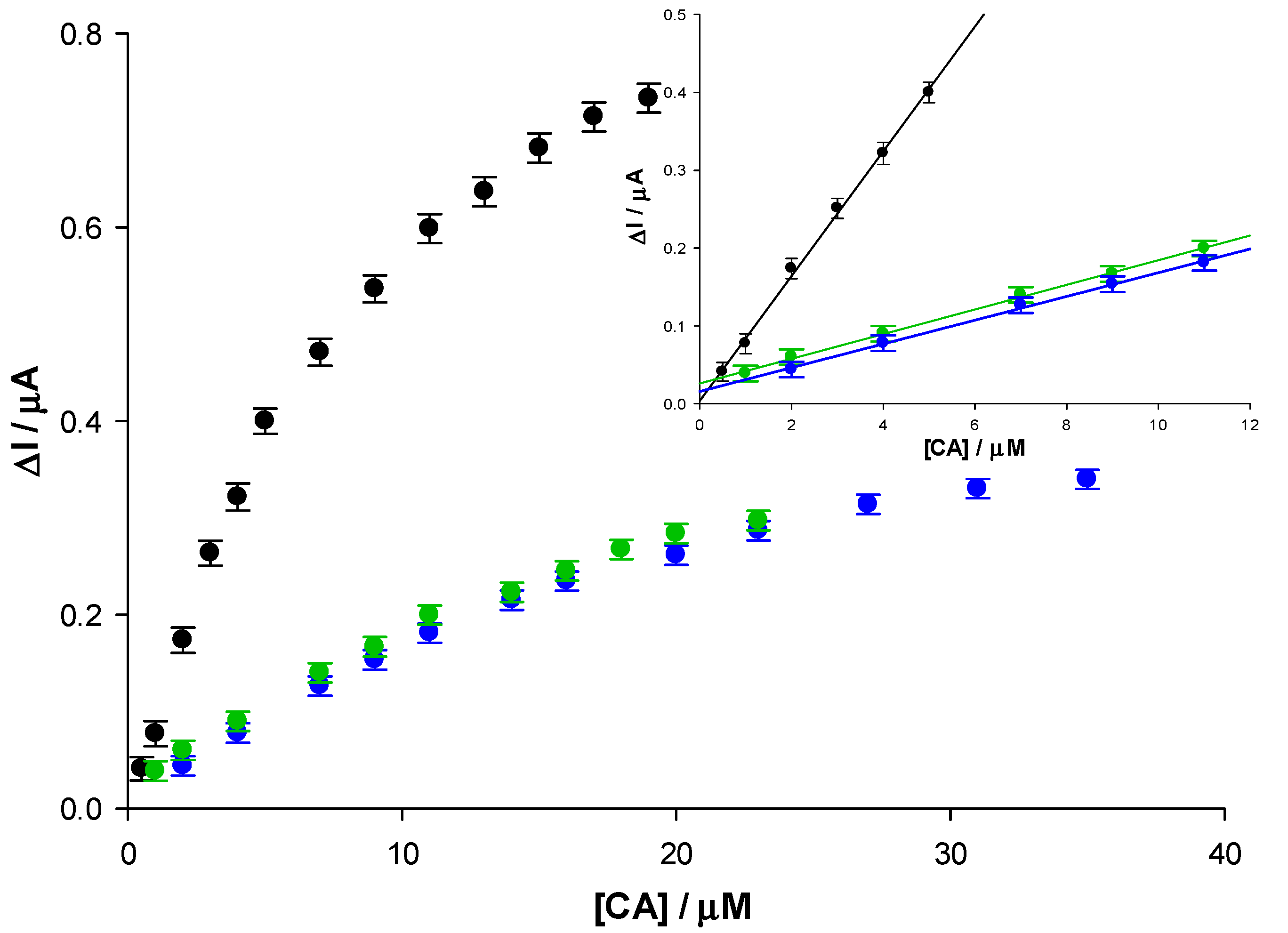
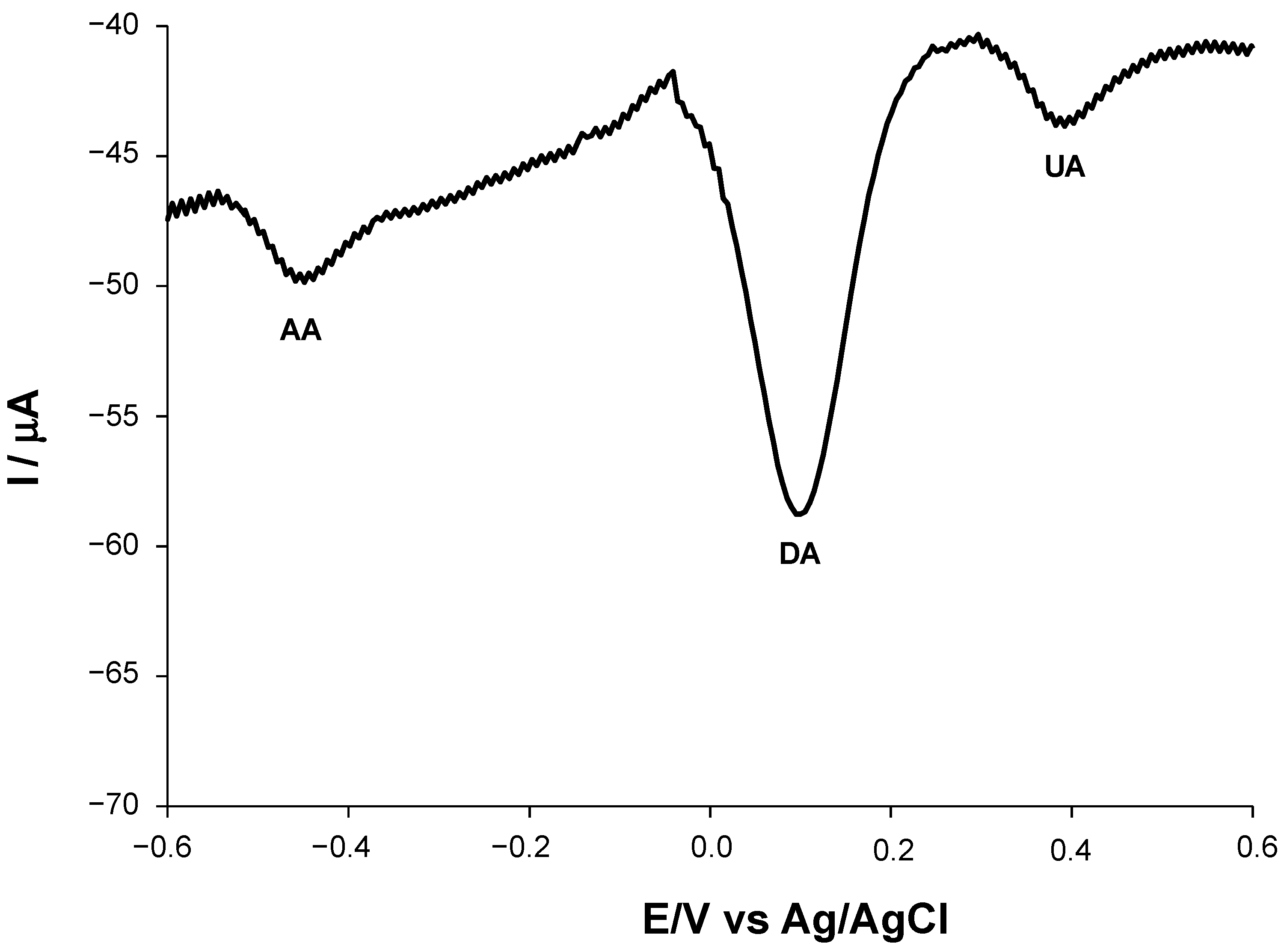
| Modified SPE | (μM) | Jmax (μA cm−2) | Linear Range (μM) | LOD (μM) | Sensitivity (μA μM−1 cm−2) | R2 |
|---|---|---|---|---|---|---|
| Tyr/ChitNPs/GPH | 25.59 ± 3.013 | 11.1 ± 0.84 | 4–12 | 1.36 | 0.275 | 0.994 |
| Tyr/EDC-NHS/ChitNPs/GPH | 9.51 ± 0.544 | 9.16 ± 0.009 | 0.5–5 | 0.17 | 0.583 | 0.996 |
| Catecholamine | (μM) | Jmax (μA cm−2) | Linear Range (μM) | LOD (μM) | Sensitivity (μA μM−1 cm−2) | R2 |
|---|---|---|---|---|---|---|
| DA | 9.51 ± 0.544 | 9.16 ± 0.009 | 0.5–5 | 0.17 | 0.583 | 0.996 |
| EP | 25.46 ± 1.095 | 4.95 ± 0.099 | 2–11 | 0.71 | 0.125 | 0.998 |
| NEP | 20.83 ± 2.2807 | 4.74 ± 0.323 | 1–11 | 0.40 | 0.132 | 0.995 |
| Catecholamine/Catechol | Biosensor Platform | Linear Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| catechol | Tyr/BOND/film electrode | 5–120 | 2.35 | [68] |
| DA | Tyr/MWNT/GCE | 50–1000 | 50 | [69] |
| catechol | PPy-NTs/PPO | 0.1–35 | 0.0012 | [70] |
| catechol | PEDOT-rGO-Fe2O3-PPO | 0.04–62 | 0.007 | [71] |
| catechol | Tyr-AuNPs-DHP/GCE | 2.5–95 | 0.17 | [72] |
| catechol | Tyr-PAMAM-Sil-rGO/GCE | 0.01–22 | 0.006 | [73] |
| catechol | Tyr/PAMAM/GO-CMC/GCE | 0.002–0.4 | 0.0009 | [74] |
| catechol | Tyr/GO/GA/GCE | 0.05–50 | 0.03 | [31] |
| NEP | Tyr-CDs-CA/Au-E | 1–200 | 0.196 | [29] |
| DA | Tyr-CoP-Au | 2–30 | 0.43 | [27] |
| DA | IDE/PEDOT-CNT-Tyr-GAD-HFR | 100–500 | 11 | [75] |
| DA | Tyr-SWNTs-Ppy | 5–50 | 5 | [76] |
| EP DA | Tyr/TBT-film/Au Tyr/EDC-NHS/ChitNPs/GPH | 0.1–50 0.5–5 | 0.06 0.17 | [77] this work |
| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (n = 3) (%) | Spectrophotometric Method (μM) |
|---|---|---|---|---|---|
| Sample 1 | 5 | 4.8 | 96 | 4.2 | 5.10 |
| Sample 2 | 5 | 4.9 | 98 | 2.1 | 5.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gigli, V.; Tortolini, C.; Capecchi, E.; Angeloni, A.; Lenzi, A.; Antiochia, R. Novel Amperometric Biosensor Based on Tyrosinase/Chitosan Nanoparticles for Sensitive and Interference-Free Detection of Total Catecholamine. Biosensors 2022, 12, 519. https://doi.org/10.3390/bios12070519
Gigli V, Tortolini C, Capecchi E, Angeloni A, Lenzi A, Antiochia R. Novel Amperometric Biosensor Based on Tyrosinase/Chitosan Nanoparticles for Sensitive and Interference-Free Detection of Total Catecholamine. Biosensors. 2022; 12(7):519. https://doi.org/10.3390/bios12070519
Chicago/Turabian StyleGigli, Valeria, Cristina Tortolini, Eliana Capecchi, Antonio Angeloni, Andrea Lenzi, and Riccarda Antiochia. 2022. "Novel Amperometric Biosensor Based on Tyrosinase/Chitosan Nanoparticles for Sensitive and Interference-Free Detection of Total Catecholamine" Biosensors 12, no. 7: 519. https://doi.org/10.3390/bios12070519
APA StyleGigli, V., Tortolini, C., Capecchi, E., Angeloni, A., Lenzi, A., & Antiochia, R. (2022). Novel Amperometric Biosensor Based on Tyrosinase/Chitosan Nanoparticles for Sensitive and Interference-Free Detection of Total Catecholamine. Biosensors, 12(7), 519. https://doi.org/10.3390/bios12070519







