Paper-Based Fluidic Sensing Platforms for β-Adrenergic Agonist Residue Point-of-Care Testing
Abstract
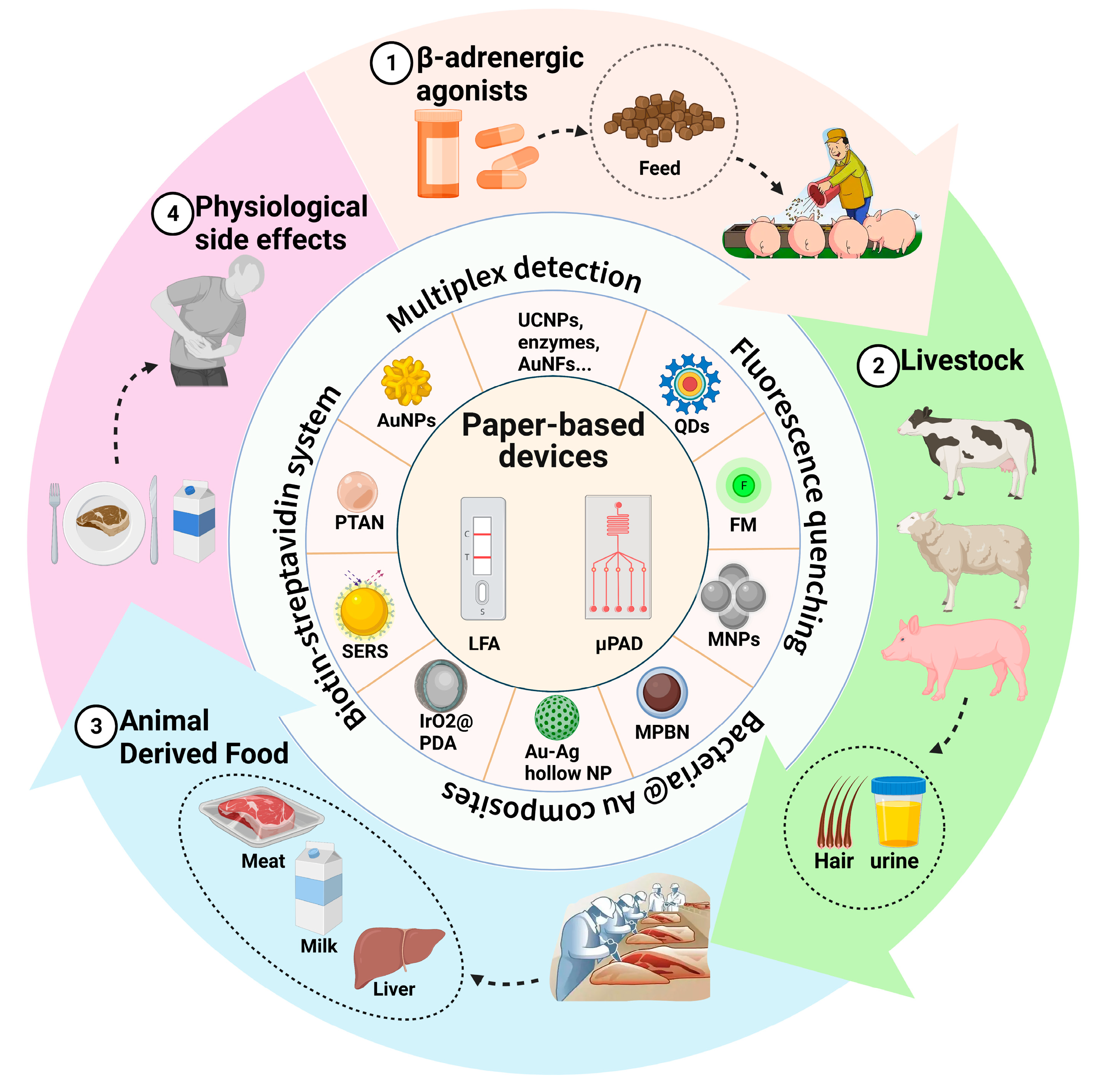
:1. Introduction
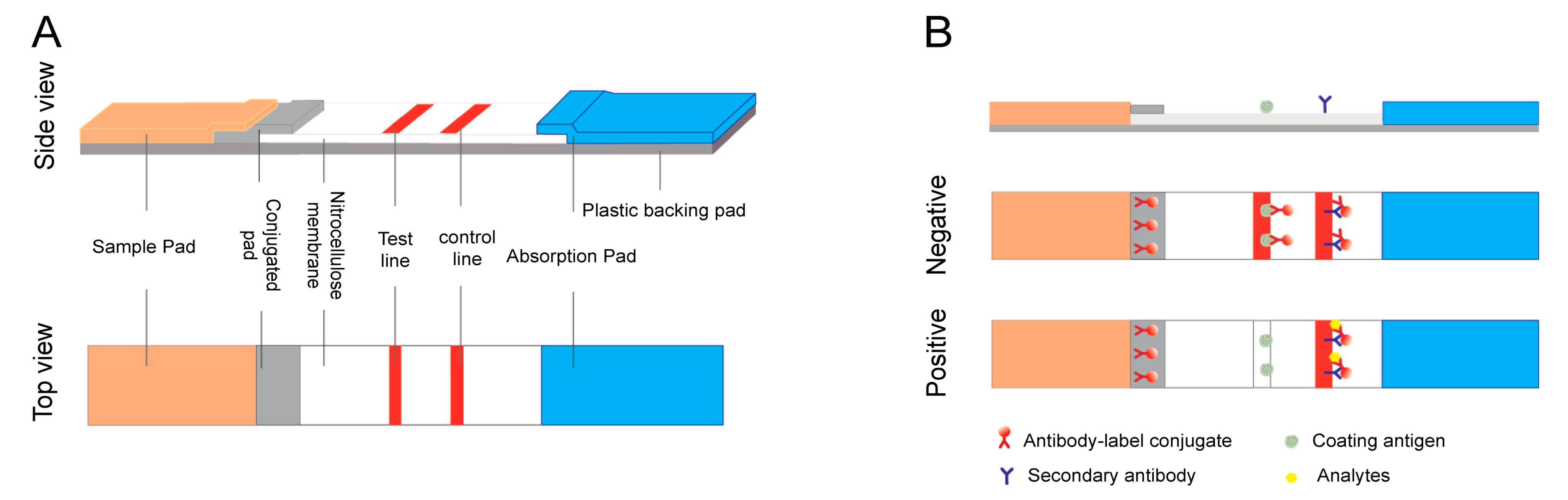
2. LFA Formats and Principles
2.1. Sandwich Format
2.2. Competitive Format
3. Applications for Detecting β-Adrenergic Agonist Residues
4. Novel Labels for Use in LFAs to Detect β-Adrenergic Agonist Residues
4.1. Quantum Dots
4.2. Luminescent Nanoparticles
4.3. Lanthanide
4.4. Up-Conversion Nanoparticles
4.5. Magnetic Nanoparticles
4.6. Enzymes and Nanozymes
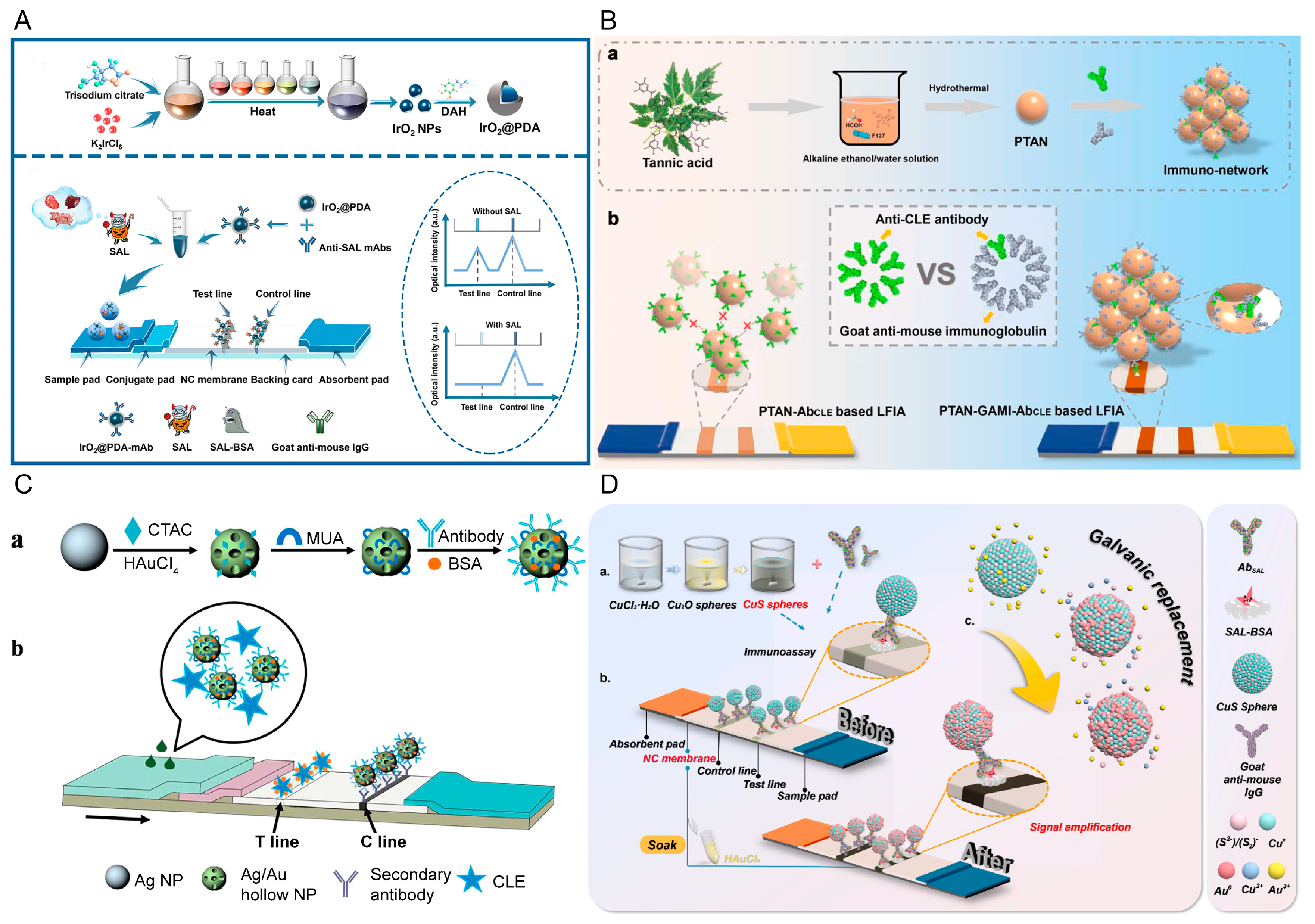
4.7. Surface-Enhanced Raman Scattering-Active Nanomaterials
4.8. Other Novel Labels
5. Strategies to Increase the Performance of LFA Systems
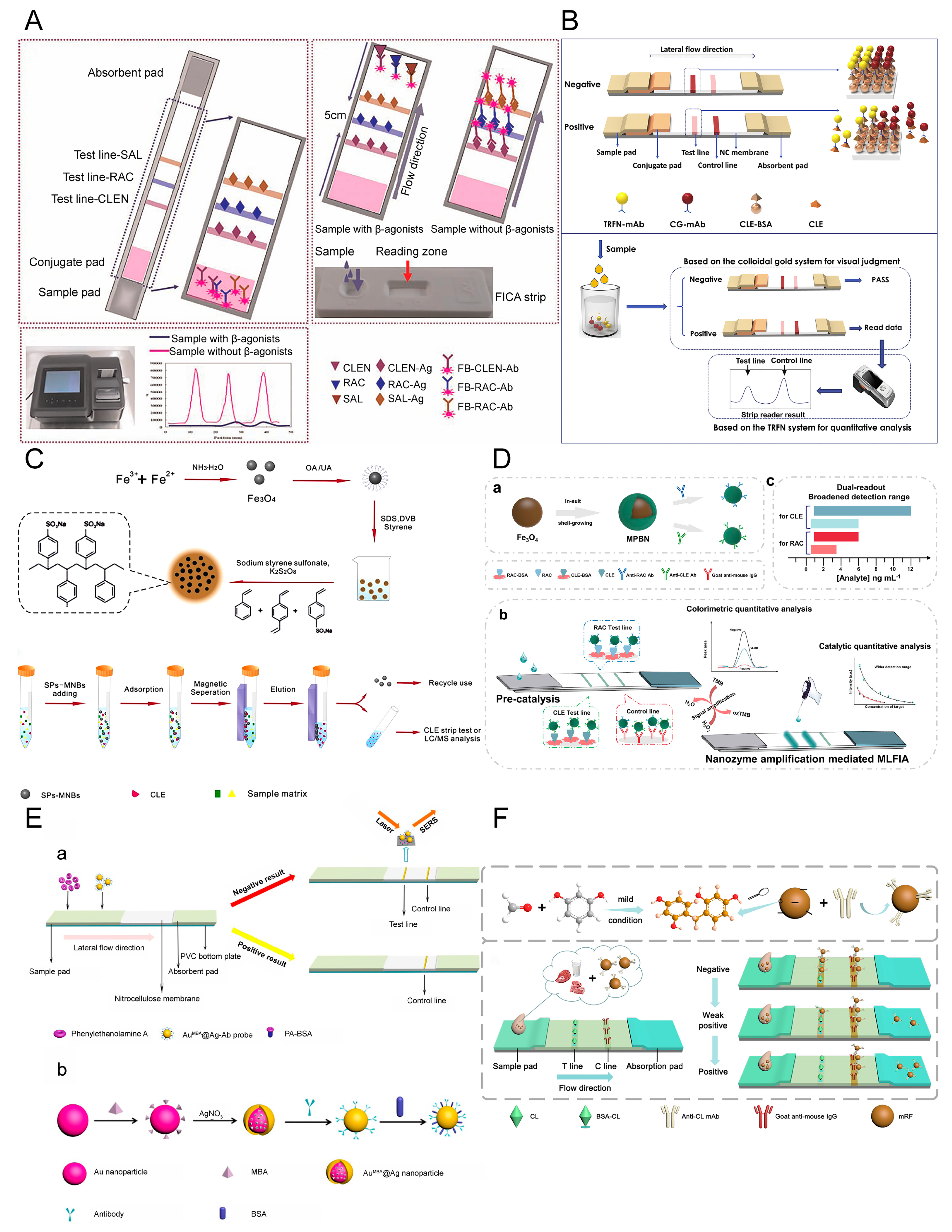
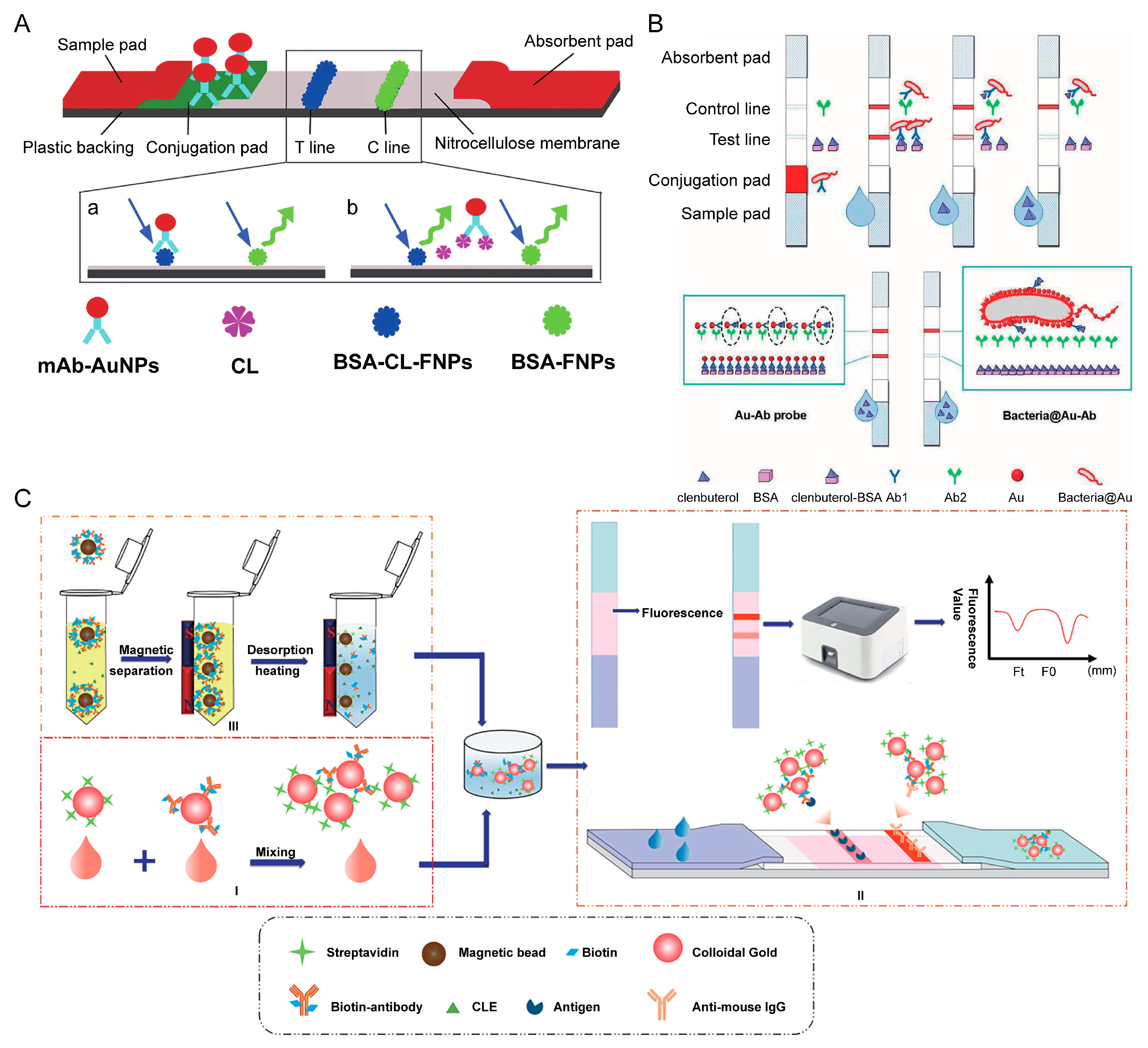
5.1. Novel RAC-BSA Carrier Conjugation
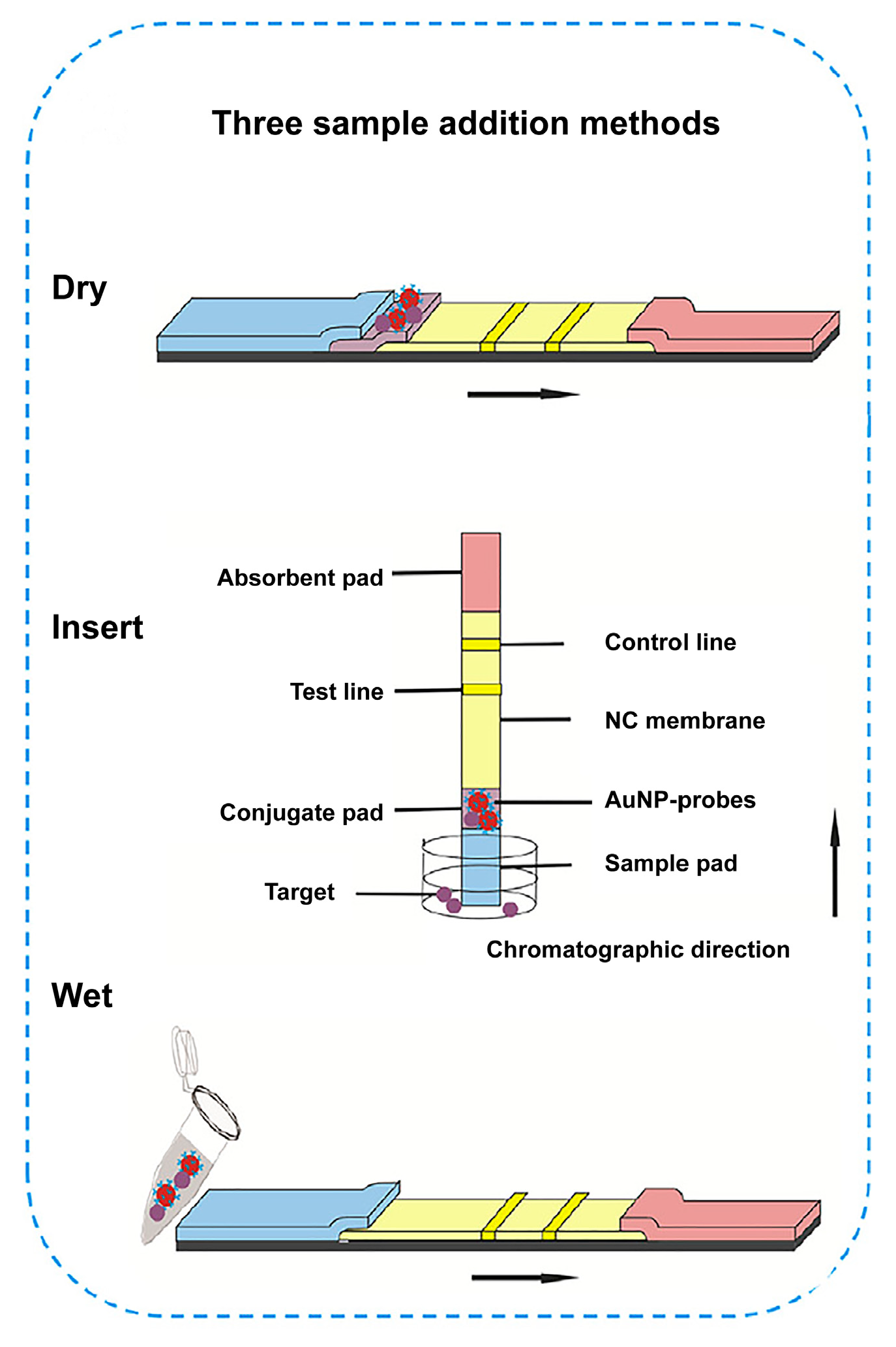
5.2. Different Sample Addition Methods
5.3. Multiplex Detection of β-Adrenergic Agonists
5.4. Fluorescence Quenching
5.5. Bacteria@ Au Composites
5.6. Biotin-Streptavidin System
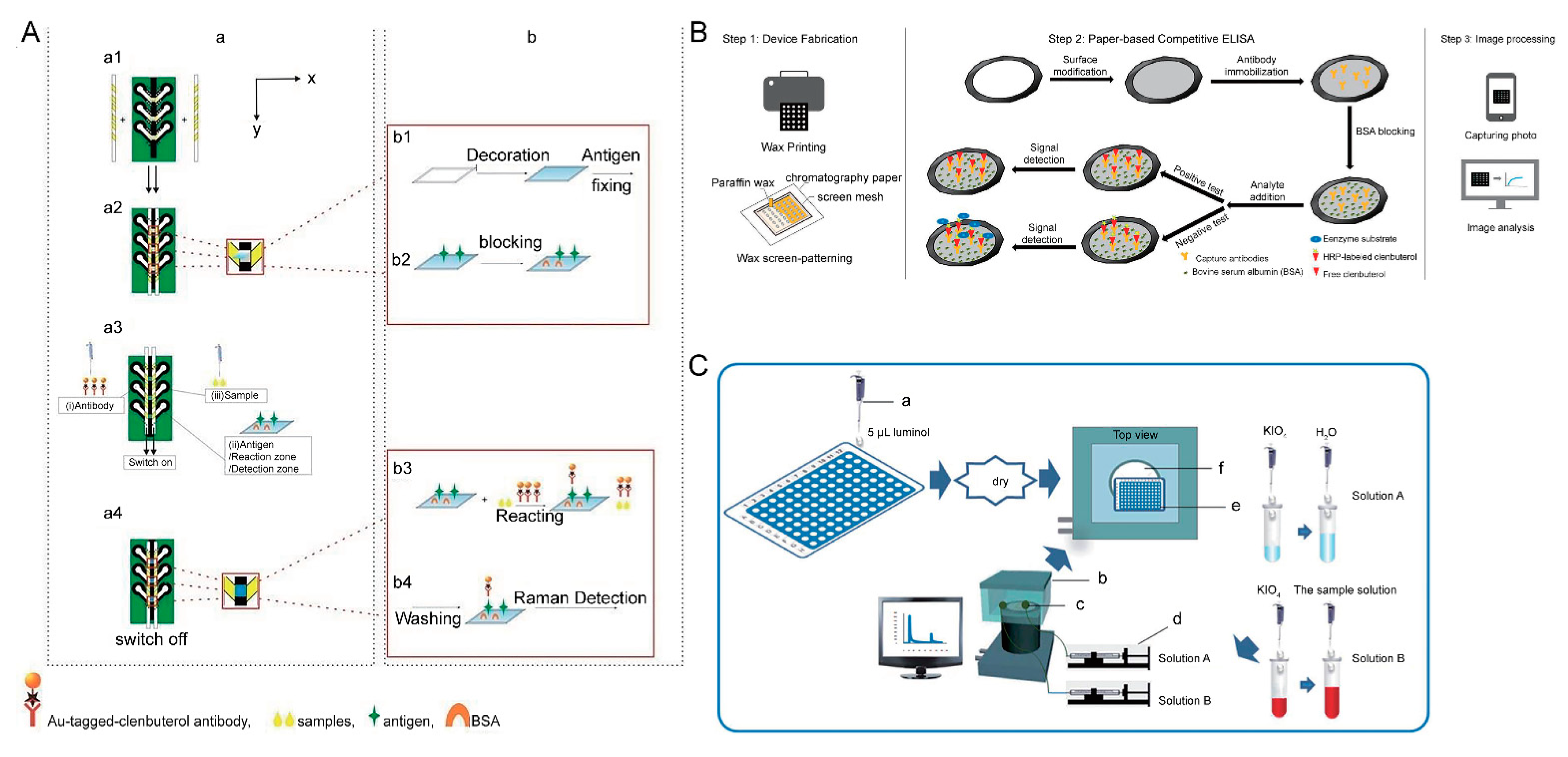
6. Microfluidic Paper-Based Analytical Devices
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, T.; Chapple, N. The animal health market. Nat. Rev. Drug Discov. 2002, 1, 937–938. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P. Even therapeutic antimicrobial use in animal husbandry may generate environmental hazards to human health. Environ. Microbiol. 2016, 18, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Mersmann, H.J. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J. Anim. Sci. 1998, 76, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, R.; Wu, E.; Wu, R.; Shen, W.; Ma, C.; Shi, R.; Guo, R.; Shao, M.; Liu, J. Sensitive detection of clenbuterol by hybrid iridium/silicon nanowire-enhanced laser desorption/ionization mass spectrometry. J. Mater. Chem. B 2020, 8, 7792–7800. [Google Scholar] [CrossRef]
- Barbosa, J.; Cruz, C.; Martins, J.; Silva, J.M.; Neves, C.; Alves, C.; Ramos, F.; Noronha Da Silveira, M.I. MIN: Food poisoning by clenbuterol in Portugal. Food Addit. Contam. 2005, 22, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, H.A.; Noordam, M.Y.; van Dooren-Flipsen, M.M.; Schilt, R.; Roos, A.H. Illegal use of beta-adrenergic agonists: European Community. J. Anim. Sci. 1998, 76, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhi, A.; Liu, Q.; Yang, J.; Jia, G.; Shervin, J.; Tang, L.; Hu, X.; Deng, R.; Xu, C.; et al. Rapid and sensitive detection of β-agonists using a portable fluorescence biosensor based on fluorescent nanosilica and a lateral flow test strip. Biosens. Bioelectron. 2013, 50, 62–65. [Google Scholar] [CrossRef]
- Bulletins, M. The Catalogue of Drug Banned to Use in the Feed and Animal Drinking Water. 2002. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-03/China-176.pdf (accessed on 17 June 2022).
- Zhang, X.; Zhao, H.; Xue, Y.; Wu, Z.; Zhang, Y.; He, Y.; Li, X.; Yuan, Z. Colorimetric sensing of clenbuterol using gold nanoparticles in the presence of melamine. Biosens. Bioelectron. 2012, 34, 112–117. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Wang, J.; Shi, X.; Ye, J. Determination of beta-agonists in pig feed, pig urine and pig liver using capillary electrophoresis with electrochemical detection. Meat Sci. 2010, 85, 302–305. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, R.; Li, Y.; Li, G. Investigation of ractopamine-imprinted polymer for dispersive solid-phase extraction of trace beta-agonists in pig tissues. J. Sep. Sci. 2010, 33, 2017–2025. [Google Scholar] [CrossRef]
- Wang, P.-L.; Fan, L.; Su, X.-O.; Ye, Z.-H. Determination of four kinds of β-agonists in swine urine by molecularly imprinted solid phase extraction followed gas chromatography coupled mass spectrometry. Chin. J. Anal. Chem. 2012, 40, 470–473. [Google Scholar] [CrossRef]
- Shao, B.; Jia, X.; Zhang, J.; Meng, J.; Wu, Y.; Duan, H.; Tu, X. Multi-residual analysis of 16 β-agonists in pig liver, kidney and muscle by ultra performance liquid chromatography tandem mass spectrometry. Food Chem. 2009, 114, 1115–1121. [Google Scholar] [CrossRef]
- Bocca, B.; Fiori, M.; Cartoni, C.; Brambilla, G. Simultaneous determination of Zilpaterol and other beta agonists in calf eye by gas chromatography/tandem mass spectrometry. JAOAC Int. 2003, 86, 8–14. [Google Scholar] [CrossRef] [Green Version]
- McPartlin, D.A.; O’Kennedy, R.J. Point-of-care diagnostics, a major opportunity for change in traditional diagnostic approaches: Potential and limitations. Expert Rev. Mol. Diagn. 2014, 14, 979–998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sarwar, M.; Yue, Q.; Chen, C.; Li, C.-Z. Biosensing of DNA oxidative damage: A model of using glucose meter for non-glucose biomarker detection. Int. J. Nanomed. 2017, 12, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-L.; Wang, L.; Hasi, C.-L.; Wang, Y.-P.; Khan, A.; Ren, B.-Z.; Liu, Z.-Z.; Hou, S.-L.; Yang, L.-H.; Zhang, L.-Y.; et al. A rapid colloidal gold immunochromatographic assay for the diagnosis of coronavirus disease 2019. Chin. Med. J. (Engl.) 2020, 133, 1986–1988. [Google Scholar] [CrossRef]
- Lin, L.-K.; Uzunoglu, A.; Stanciu, L.A. Aminolated and Thiolated PEG-Covered Gold Nanoparticles with High Stability and Antiaggregation for Lateral Flow Detection of Bisphenol A. Small 2018, 14, 1702828. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. Engl. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [Green Version]
- Clarke, O.; Goodall, B.; Hui, H.; Vats, N.; Brosseau, C. Development of a SERS-based rapid vertical flow assay for point-of-care diagnostics. Anal. Chem. 2017, 89, 1405–1410. [Google Scholar] [CrossRef]
- Raeisossadati, M.J.; Danesh, N.M.; Borna, F.; Gholamzad, M.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Lateral flow based immunobiosensors for detection of food contaminants. Biosens. Bioelectron. 2016, 86, 235–246. [Google Scholar] [CrossRef]
- Ngom, B.; Guo, Y.; Wang, X.; Bi, D. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: A review. Anal. Bioanal. Chem. 2010, 397, 1113–1135. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Huang, Z.; Hu, S.; Peng, J.; Liu, D.; Xiong, Y.; Xu, H.; Wei, H.; Lai, W. Invited review: Advancements in lateral flow immunoassays for screening hazardous substances in milk and milk powder. J. Dairy Sci. 2019, 102, 1887–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Zhang, W.; Wang, P.; Su, X. A paper-based competitive lateral flow immunoassay for multi β-agonist residues by using a single monoclonal antibody labelled with red fluorescent nanoparticles. Mikrochim. Acta 2018, 185, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.-Z.; Wang, M.-Z.; Chen, Z.-L.; Fang, J.-H.; Fang, M.-M.; Liu, J.; Yu, X.-P. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of clenbuterol and ractopamine in swine urine. Anal. Bioanal. Chem. 2009, 395, 2591–2599. [Google Scholar] [CrossRef]
- Lai, W.H.; Fung, D.Y.; Xu, Y.; Xiong, Y.H. Screening procedures for clenbuterol residue determination in raw swine livers using lateral-flow assay and enzyme-linked immunosorbent assay. J. Food Prot. 2008, 71, 865–869. [Google Scholar] [CrossRef]
- Lai, W.; Xu, Y.; Fung, D.Y.; Xiong, Y. Development of a lateral-flow assay for rapid screening of the performance-enhancing sympathomimetic drug clenbuterol used in animal production; food safety assessments. Asia Pac. J. Clin. Nutr. 2007, 16, 106–110. [Google Scholar]
- Zhang, G.P.; Wang, X.N.; Yang, J.F.; Yang, Y.Y.; Xing, G.X.; Li, Q.M.; Zhao, D.; Chai, S.J.; Guo, J.Q. Development of an immunochromatographic lateral flow test strip for detection of beta-adrenergic agonist Clenbuterol residues. J. Immunol. Methods 2006, 312, 27–33. [Google Scholar] [CrossRef]
- Wu, Q.; Song, Q.; Wang, X.; Yao, L.; Xu, J.; Lu, J.; Liu, G.; Chen, W. Simultaneous Detection of Multiple β-Adrenergic Agonists with 2-Directional Lateral Flow Strip Platform. Anal. Sci. 2020, 36, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Luo, W.; Xu, H.; Zhang, Q.; Xu, H.; Aguilar, Z.P.; Lai, W.; Wei, H.; Xiong, Y. Development of an immunochromatographic assay for rapid and quantitative detection of clenbuterol in swine urine. Food Control. 2013, 34, 725–732. [Google Scholar] [CrossRef]
- Khamta, Y.; Pattarawarapan, M.; Nangola, S.; Tayapiwatana, C. Development of immunochromatographic assay for the on-site detection of salbutamol. J. Immunoass. Immunochem. 2009, 30, 441–456. [Google Scholar] [CrossRef]
- Liu, A.; Lin, J.; Dai, M.; Wu, Y.; Fang, J.; Zhang, M. Development of a monoclonal antibody-based immunochromatographic assay detecting ractopamine residues in swine urine. Food Analytical. Methods 2016, 9, 2016–2025. [Google Scholar] [CrossRef]
- Zvereva, E.; Zherdev, A.; Xu, C.; Dzantiev, B. Highly sensitive immunochromatographic assay for qualitative and quantitative control of beta-agonist salbutamol and its structural analogs in foods. Food Control. 2018, 86, 50–58. [Google Scholar] [CrossRef]
- Ren, M.L.; Chen, X.L.; Li, C.H.; Xu, B.; Liu, W.J.; Xu, H.Y.; Xiong, Y.H. Lateral flow immunoassay for quantitative detection of ractopamine in swine urine. Biomed. Environ. Sci. 2014, 27, 134–137. [Google Scholar] [PubMed]
- Wang, Z.; Jing, J.; Ren, Y.; Guo, Y.; Tao, N.; Zhou, Q.; Zhang, H.; Ma, Y.; Wang, Y. Preparation and application of selenium nanoparticles in a lateral flow immunoassay for clenbuterol detection. Mater. Lett. 2019, 234, 212–215. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Q.; Guo, Y.; Hu, H.; Zheng, Z.; Li, S.; Wang, Y.; Ma, Y. Rapid Detection of Ractopamine and Salbutamol in Swine Urine by Immunochromatography Based on Selenium Nanoparticles. Int. J. Nanomed. 2021, 16, 2059–2070. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Hu, S.; Zhang, G.; Peng, J.; Xia, J.; Lai, W. Integrated immunochromatographic assay for qualitative and quantitative detection of clenbuterol. Anal. Biochem. 2019, 577, 45–51. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, G.; Dou, W. Immunochromatographic assay using brightly colored silica nanoparticles as visible label for point-of-care detection of clenbuterol. Sens. Actuators B Chemical. 2018, 266, 392–399. [Google Scholar] [CrossRef]
- Shelver, W.L.; Smith, D.J. Development of an immunochromatographic assay for the β-adrenergic agonist feed additive zilpaterol. Food Addit. Contam. Part A 2018, 35, 1519–1529. [Google Scholar] [CrossRef]
- Dai, M.; Gong, Y.; Liu, A.; Zhang, L.; Lin, J.; Zhang, M.; Yu, X. Development of a colloidal gold-based lateral-flow immunoassay for the rapid detection of phenylethanolamine A in swine urine. Anal. Methods 2015, 7, 4130–4137. [Google Scholar] [CrossRef]
- Jiang, W.; Zeng, L.; Liu, L.; Song, S.; Kuang, H. Development of an immunochromatographic assay for rapid detection of clorprenaline in pig urine. Food Agric. Immunol. 2018, 29, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Peng, T.; Zhang, F.S.; Yang, W.C.; Li, D.X.; Chen, Y.; Xiong, Y.H.; Wei, H.; Lai, W.H. Lateral-flow assay for rapid quantitative detection of clorprenaline residue in swine urine. J. Food Prot. 2014, 77, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, J.; Xu, X.; Guo, L.; Xu, L.; Sun, M.; Hu, S.; Kuang, H.; Xu, C.; Li, A. An Overview for the Nanoparticles-Based Quantitative Lateral Flow Assay. Small Methods 2022, 6, e2101143. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, X.; Sun, L.; Wu, A.; Song, S.; Kuang, H.; Xu, C. An ultrasensitive colloidal gold immunosensor to simultaneously detect 12 beta (2)-adrenergic agonists. J. Chromatogr. B 2022, 1191, 123119. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, H.; Xu, H.; Xiong, Y.; Wei, H.; Lai, W. Quantum dots-based lateral flow strip assay for rapid detection of clenbuterol. In Proceedings of the 2011 4th International Conference on Biomedical Engineering and Informatics (BMEI), Shanghai, China, 15–17 October 2011; pp. 1520–1524. [Google Scholar]
- Wang, P.; Wang, Z.; Su, X. A sensitive and quantitative fluorescent multi-component immuno-chromatographic sensor for β-agonist residues. Biosens. Bioelectron. 2015, 64, 511–516. [Google Scholar] [CrossRef]
- Wang, P.; Wang, R.; Zhang, W.; Su, X.; Luo, H. Novel fabrication of immunochromatographic assay based on up conversion phosphors for sensitive detection of clenbuterol. Biosens. Bioelectron. 2016, 77, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xiong, Z.; Chen, Y.; Hu, S.; Lai, W. Sensitive and matrix-tolerant lateral flow immunoassay based on fluorescent magnetic nanobeads for the detection of clenbuterol in swine urine. J. Agric. Food Chem. 2019, 67, 3028–3036. [Google Scholar] [CrossRef]
- Gao, H.; Han, J.; Yang, S.; Wang, Z.; Wang, L.; Fu, Z. Highly sensitive multianalyte immunochromatographic test strip for rapid chemiluminescent detection of ractopamine and salbutamol. Anal. Chim. Acta 2014, 839, 91–96. [Google Scholar] [CrossRef]
- Liu, S.; Dou, L.; Yao, X.; Zhang, W.; Zhao, M.; Yin, X.; Sun, J.; Zhang, D.; Wang, J. Nanozyme amplification mediated on-demand multiplex lateral flow immunoassay with dual-readout and broadened detection range. Biosens. Bioelectron. 2020, 169, 112610. [Google Scholar] [CrossRef]
- Li, M.; Yang, H.; Li, S.; Zhao, K.; Li, J.; Jiang, D.; Sun, L.; Deng, A. Ultrasensitive and quantitative detection of a new β-agonist phenylethanolamine A by a novel immunochromatographic assay based on surface-enhanced Raman scattering (SERS). J. Agric. Food Chem. 2014, 62, 10896–10902. [Google Scholar] [CrossRef]
- Lai, W.; Xiong, Z.; Huang, Y.; Su, F.; Zhang, G.; Huang, Z.; Peng, J.; Liu, D. Gold nanoflowers labelled lateral flow assay integrated with smartphone for highly sensitive detection of clenbuterol in swine urine. Food Agric. Immunol. 2019, 30, 1225–1238. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.L.; Shan, S.; Xu, C.L.; Liu, D.F.; Xiong, Y.H.; Wei, H.; Lai, W.H. Sample preincubation strategy for sensitive and quantitative detection of clenbuterol in swine urine using a fluorescent microsphere–based immunochromatographic assay. J. Food Prot. 2014, 77, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-M.; Luo, K.; Xia, J.; Xu, G.-M.; Wu, C.-H.; Han, J.-J.; Zhang, G.-G.; Liu, M.; Lai, W.-H. Advantages of time-resolved fluorescent nanobeads compared with fluorescent submicrospheres, quantum dots, and colloidal gold as label in lateral flow assays for detection of ractopamine. Biosens. Bioelectron. 2017, 91, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Guo, L.; Xu, W.; Xu, H.; Aguilar, Z.P.; Xu, G.; Lai, W.; Xiong, Y.; Wan, Y. Sulfonated polystyrene magnetic nanobeads coupled with immunochromatographic strip for clenbuterol determination in pork muscle. Talanta 2014, 129, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Su, X.; Ouyang, H.; Wang, L.; Fu, Z. A novel immunochromatographic assay based on a time-resolved chemiluminescence strategy for the multiplexed detection of ractopamine and clenbuterol. Anal. Chim. Acta 2016, 917, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chang, H.; Zhao, K.; Li, J.; Yang, H.; Mei, L.; Xu, S.; Deng, A. A novel immunochromatographic assay (ICA) based on surface-enhanced Raman scattering for the sensitive and quantitative determination of clenbuterol. Anal. Methods 2015, 7, 513–520. [Google Scholar] [CrossRef]
- Zhang, X.; Chu, Y.; Yang, H.; Zhao, K.; Li, J.; Du, H.; She, P.; Deng, A. Ultrasensitive and specific detection of salbutamol in swine feed, meat, and urine samples by a competitive immunochromatographic test integrated with surface-enhanced Raman scattering. Food Anal. Methods 2016, 9, 3396–3406. [Google Scholar] [CrossRef]
- Fu, X.; Chu, Y.; Zhao, K.; Li, J.; Deng, A. Ultrasensitive detection of the β-adrenergic agonist brombuterol by a SERS-based lateral flow immunochromatographic assay using flower-like gold-silver core-shell nanoparticles. Microchim. Acta 2017, 184, 1711–1719. [Google Scholar] [CrossRef]
- Su, L.; Hu, H.; Tian, Y.; Jia, C.; Wang, L.; Zhang, H.; Wang, J.; Zhang, D. Highly Sensitive Colorimetric/Surface-Enhanced Raman Spectroscopy Immunoassay Relying on a Metallic Core–Shell Au/Au Nanostar with Clenbuterol as a Target Analyte. Anal. Chem. 2021, 93, 8362–8369. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Huang, Y.; Dandapat, A.; Dai, L.; Zhang, G.; Lu, X.; Zhang, J.; Lai, W.; Chen, T. Hollow Au-Ag nanoparticles labeled immunochromatography strip for highly sensitive detection of clenbuterol. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Chen, X.; Huang, X.; Yang, W.; Liu, C.; Lai, W.; Xu, H.; Xiong, Y. Ru (phen) 32+ doped silica nanoparticle based immunochromatographic strip for rapid quantitative detection of β-agonist residues in swine urine. Talanta 2013, 114, 160–166. [Google Scholar] [CrossRef]
- Huang, Q.; Dang, L. Graphene-labeled synthetic antigen as a novel probe for enhancing sensitivity and simplicity in lateral flow immunoassay. Anal. Methods 2022, 14, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Huang, Q.; Dou, L.; Bu, T.; Chen, K.; Yang, Q.; Yan, L.; Wang, J.; Zhang, D. Prussian blue nanoparticles based lateral flow assay for high sensitive determination of clenbuterol. Sens. Actuators B Chem. 2018, 275, 223–229. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Yao, X.; Wang, Z.; Dou, L.; Su, L.; Zhao, M.; Sun, J.; Zhang, D.; Wang, J. Developing a simple immunochromatography assay for clenbuterol with sensitivity by one-step staining. J. Agric. Food Chem. 2020, 68, 15509–15515. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, Q.; Zhao, G.; Dou, W. Ultramarine blue nanoparticles as a label for immunochromatographic on-site determination of ractopamine. Microchim. Acta 2020, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Su, L.; Yao, X.; Wang, Z.; Zhao, M.; Sun, J.; Wang, J.; Zhang, D. Mild resorcinol formaldehyde resin polymer based immunochromatography assay for high-sensitive detection of clenbuterol. Sens. Actuators B Chem. 2021, 331, 129443. [Google Scholar] [CrossRef]
- Zhao, S.; Bu, T.; Yang, K.; Xu, Z.; Bai, F.; He, K.; Li, L.; Wang, L. Immunochromatographic assay based on polydopamine-decorated Iridium Oxide nanoparticles for the rapid detection of salbutamol in food samples. ACS Appl. Mater. Interfaces 2021, 13, 28899–28907. [Google Scholar] [CrossRef]
- Liu, S.; Shu, R.; Nie, C.; Li, Y.; Luo, X.; Ji, Y.; Yin, X.; Sun, J.; Zhang, D.; Wang, J. Bioresource-derived tannic acid-supported immuno-network in lateral flow immunoassay for sensitive clenbuterol monitoring. Food Chem. 2022, 382, 132390. [Google Scholar] [CrossRef]
- Shu, R.; Liu, S.; Xu, J.; Wang, S.; Ma, Y.; Chen, Y.; Li, Y.; Sun, J.; Zhang, D.; Wang, J. Galvanic replacement inspired signal amplification: Background-free and antibody-thrift in-situ growth immunochromatography. Chem. Eng. J. 2022, 437, 135362. [Google Scholar] [CrossRef]
- Shan, S.; Lai, W.; Xiong, Y.; Wei, H.; Xu, H. Novel strategies to enhance lateral flow immunoassay sensitivity for detecting foodborne pathogens. J. Agric. Food Chem. 2015, 63, 745–753. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, J.; Beloglazova, N.; Yang, S.; De Saeger, S.; Mari, G.M.; Zhang, S.; Shen, J.; Wang, Z.; Yu, X. Portable Multiplex Immunochromatographic Assay for Quantitation of Two Typical Algae Toxins Based on Dual-Color Fluorescence Microspheres. J. Agric. Food Chem. 2019, 67, 6041–6047. [Google Scholar] [CrossRef]
- Li, G.; Wang, D.; Zhou, A.; Sun, Y.; Zhang, Q.; Poapolathep, A.; Zhang, L.; Fan, Z.; Zhang, Z.; Li, P. Rapid, On-Site, Ultrasensitive Melamine Quantitation Method for Protein Beverages Using Time-Resolved Fluorescence Detection Paper. J. Agric. Food Chem. 2018, 66, 5671–5676. [Google Scholar] [CrossRef] [PubMed]
- Rostami, I.; Rezvani Alanagh, H.; Hu, Z.; Shahmoradian, S.H. Breakthroughs in medicine and bioimaging with up-conversion nanoparticles. Int. J. Nanomed. 2019, 14, 7759–7780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goryacheva, I.Y.; Lenain, P.; De Saeger, S. Nanosized labels for rapid immunotests. TrAC Trends Anal. Chem. 2013, 46, 30–43. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, S.; Xiong, Y.; Wei, H.; Xu, H.; Duan, H.; Lai, W. Application and development of superparamagnetic nanoparticles in sample pretreatment and immunochromatographic assay. TrAC Trends Anal. Chem. 2019, 114, 151–170. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Wang, Y.; Sun, J.; Yue, T.; Zhang, W.; Wang, J. One-pot bottom-up fabrication of a 2D/2D heterojuncted nanozyme towards optimized peroxidase-like activity for sulfide ions sensing. Sens. Actuators B Chem. 2020, 306, 127565. [Google Scholar] [CrossRef]
- Zhu, X.; Sarwar, M.; Zhu, J.-J.; Zhang, C.; Kaushik, A.; Li, C.-Z. Using a glucose meter to quantitatively detect disease biomarkers through a universal nanozyme integrated lateral fluidic sensing platform. Biosens. Bioelectron. 2019, 126, 690–696. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Y.; Zhang, D.; Wu, M.; Wu, L.; Huang, A.; Yang, H.; Liu, X.; Liu, J. Glypican-3 antibody functionalized Prussian blue nanoparticles for targeted MR imaging and photothermal therapy of hepatocellular carcinoma. J. Mater. Chem. B 2014, 2, 3686–3696. [Google Scholar] [CrossRef]
- Ye, H.; Liu, Y.; Zhan, L.; Liu, Y.; Qin, Z. Signal amplification and quantification on lateral flow assays by laser excitation of plasmonic nanomaterials. Theranostics 2020, 10, 4359. [Google Scholar] [CrossRef]
- Zhu, R.; Feng, H.; Li, Q.; Su, L.; Fu, Q.; Li, J.; Song, J.; Yang, H. Asymmetric Core–Shell Gold Nanoparticles and Controllable Assemblies for SERS Ratiometric Detection of MicroRNA. Angew. Chem. 2021, 133, 12668–12676. [Google Scholar] [CrossRef]
- Li, X.; Duan, X.; Li, L.; Ye, S.; Tang, B. An accurate and ultrasensitive SERS sensor with Au–Se interface for bioimaging and in situ quantitation. Chem. Commun. 2020, 56, 9320–9323. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Mei, R.; Yin, Y.; You, J.; Chen, L. Polystyrene encapsulated SERS tags as promising standard tools: Simple and universal in synthesis; highly sensitive and ultrastable for bioimaging. Anal. Chem. 2019, 91, 5270–5277. [Google Scholar] [CrossRef] [PubMed]
- Sheng, E.; Lu, Y.; Xiao, Y.; Li, Z.; Wang, H.; Dai, Z. Simultaneous and ultrasensitive detection of three pesticides using a surface-enhanced Raman scattering-based lateral flow assay test strip. Biosens. Bioelectron. 2021, 181, 113149. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Y.; Chen, Z.; Li, X.; Nogami, M. Fabricating Au–Ag core-shell composite films for surface-enhanced Raman scattering. J. Mater. Sci. 2008, 43, 5390–5393. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Tong, B.; Zhang, Y.; Sheng, W.; Pan, M.; Wang, S. Development and comparison of immunochromatographic strips with three nanomaterial labels: Colloidal gold, nanogold-polyaniline-nanogold microspheres (GPGs) and colloidal carbon for visual detection of salbutamol. Biosens. Bioelectron. 2016, 85, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zou, Y.; Li, Y.; Cheng, Y. Metal-containing polydopamine nanomaterials: Catalysis, energy, and theranostics. Small 2020, 16, 1907042. [Google Scholar] [CrossRef]
- Tian, M.; Lei, L.; Xie, W.; Yang, Q.; Li, C.M.; Liu, Y. Copper deposition-induced efficient signal amplification for ultrasensitive lateral flow immunoassay. Sens. Actuators B Chem. 2019, 282, 96–103. [Google Scholar] [CrossRef]
- Preechakasedkit, P.; Ngamrojanavanich, N.; Khongchareonporn, N.; Chailapakul, O. Novel ractopamine–protein carrier conjugation and its application to the lateral flow strip test for ractopamine detection in animal feed. J. Zhejiang Univ. Sci. B 2019, 20, 193–204. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Chen, X.; Huang, X.; Xiong, Y. Comparison of three sample addition methods in competitive and sandwich colloidal gold immunochromatographic assay. Anal. Chim. Acta 2020, 1094, 90–98. [Google Scholar] [CrossRef]
- Peng, T.; Wang, J.; Zhao, S.; Zeng, Y.; Zheng, P.; Liang, D.; Mari, G.M.; Jiang, H. Highly luminescent green-emitting Au nanocluster-based multiplex lateral flow immunoassay for ultrasensitive detection of clenbuterol and ractopamine. Anal. Chim. Acta 2018, 1040, 143–149. [Google Scholar] [CrossRef]
- Fu, Q.; Liang, J.; Lan, C.; Zhou, K.; Shi, C.; Tang, Y. Development of a novel dual-functional lateral-flow sensor for on-site detection of small molecule analytes. Sens. Actuators B Chem. 2014, 203, 683–689. [Google Scholar] [CrossRef]
- Shi, C.Y.; Deng, N.; Liang, J.J.; Zhou, K.N.; Fu, Q.Q.; Tang, Y. A fluorescent polymer dots positive readout fluorescent quenching lateral flow sensor for ractopamine rapid detection. Anal. Chim. Acta 2015, 854, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, M.; Liu, D.; Xiong, Y.; Feng, R.; Zhong, P.; Lai, W. Quantitative detection of β 2-adrenergic agonists using fluorescence quenching by immunochromatographic assay. Anal. Methods 2016, 8, 627–631. [Google Scholar] [CrossRef]
- Huang, Q.; Bu, T.; Zhang, W.; Yan, L.; Zhang, M.; Yang, Q.; Huang, L.; Yang, B.; Hu, N.; Suo, Y. An improved clenbuterol detection by immunochromatographic assay with bacteria@ Au composite as signal amplifier. Food Chem. 2018, 262, 48–55. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, D.; Zheng, P.; Peng, T.; Sun, S.; Mari, G.M.; Jiang, H. Immunochromatographic fluorometric determination of clenbuterol with enhanced sensitivity. Microchim. Acta 2019, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef]
- Lin, Y.; Gritsenko, D.; Feng, S.; Teh, Y.C.; Lu, X.; Xu, J. Detection of heavy metal by paper-based microfluidics. Biosens. Bioelectron. 2016, 83, 256–266. [Google Scholar] [CrossRef]
- Prasad, A.; Tran, T.; Gartia, M.R. Multiplexed paper microfluidics for titration and detection of ingredients in beverages. Sensors 2019, 19, 1286. [Google Scholar] [CrossRef] [Green Version]
- Rajasulochana, P.; Ganesan, Y.; Kumar, P.S.; Mahalaxmi, S.; Tasneem, F.; Ponnuchamy, M.; Kapoor, A. Based microfluidic colorimetric sensor on a 3D printed support for quantitative detection of nitrite in aquatic environments. Environ. Res. 2022, 208, 112745. [Google Scholar] [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef]
- Songjaroen, T.; Dungchai, W.; Chailapakul, O.; Laiwattanapaisal, W. Novel, simple and low-cost alternative method for fabrication of paper-based microfluidics by wax dipping. Talanta 2011, 85, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhang, Y.; Lin, L.; Zhou, C.; Li, S.; Zhang, L.; Li, J. Low-cost fabrication of paper-based microfluidic devices by one-step plotting. Anal. Chem. 2012, 84, 6331–6335. [Google Scholar] [CrossRef] [PubMed]
- Maejima, K.; Tomikawa, S.; Suzuki, K.; Citterio, D. Inkjet printing: An integrated and green chemical approach to microfluidic paper-based analytical devices. RSC Adv. 2013, 3, 9258–9263. [Google Scholar] [CrossRef]
- Bruzewicz, D.A.; Reches, M.; Whitesides, G.M. Low-cost printing of poly (dimethylsiloxane) barriers to define microchannels in paper. Anal. Chem. 2008, 80, 3387–3392. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tian, J.; Nguyen, T.; Shen, W. Based microfluidic devices by plasma treatment. Anal. Chem. 2008, 80, 9131–9134. [Google Scholar] [CrossRef]
- Chitnis, G.; Ding, Z.; Chang, C.-L.; Savran, C.A.; Ziaie, B. Laser-treated hydrophobic paper: An inexpensive microfluidic platform. Lab on A Chip 2011, 11, 1161–1165. [Google Scholar] [CrossRef]
- Curto, V.F.; Lopez-Ruiz, N.; Capitan-Vallvey, L.F.; Palma, A.J.; Benito-Lopez, F.; Diamond, D. Fast prototyping of paper-based microfluidic devices by contact stamping using indelible ink. RSC Adv. 2013, 3, 18811–18816. [Google Scholar] [CrossRef]
- Olkkonen, J.; Lehtinen, K.; Erho, T. Flexographically printed fluidic structures in paper. Anal. Chem. 2010, 82, 10246–10250. [Google Scholar] [CrossRef]
- Fenton, E.M.; Mascarenas, M.R.; López, G.P.; Sibbett, S.S. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS Appl. Mater. Interfaces 2009, 1, 124–129. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef]
- Fiorini, G.S.; Chiu, D.T. Disposable microfluidic devices: Fabrication, function, and application. BioTechniques 2005, 38, 429–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, E.; Gabriel, E.F.M.; Coltro, W.K.T.; Garcia, C.D. Rational selection of substrates to improve color intensity and uniformity on microfluidic paper-based analytical devices. Analyst 2014, 139, 2127–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paschoalino, W.J.; Kogikoski, S., Jr.; Barragan, J.T.; Giarola, J.F.; Cantelli, L.; Rabelo, T.M.; Pessanha, T.M.; Kubota, L.T. Emerging considerations for the future development of electrochemical paper-based analytical devices. ChemElectroChem 2019, 6, 10–30. [Google Scholar] [CrossRef]
- Ma, L.; Nilghaz, A.; Choi, J.R.; Liu, X.; Lu, X. Rapid detection of clenbuterol in milk using microfluidic paper-based ELISA. Food Chem. 2018, 246, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, Y.; Shi, B.; Liu, X.; Gao, Z.; Du, Y.; Zhao, W.; Lin, B. Chemiluminescence diminishment on a paper-based analytical device: High throughput determination of β-agonists in swine hair. Anal. Methods 2014, 6, 9684–9690. [Google Scholar] [CrossRef]
- Li, W.; Luo, Y.; Yue, X.; Wu, J.; Wu, R.; Qiao, Y.; Peng, Q.; Shi, B.; Lin, B.; Chen, X. A novel microfluidic paper-based analytical device based on chemiluminescence for the determination of β-agonists in swine hair. Anal. Methods 2020, 12, 2317–2322. [Google Scholar] [CrossRef]
- Zheng, T.; Gao, Z.; Luo, Y.; Liu, X.; Zhao, W.; Lin, B. Manual-slide-engaged paper chip for parallel SERS-immunoassay measurement of clenbuterol from swine hair. Electrophoresis 2016, 37, 418–424. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Y.; Zhou, Y. Selection of test samples for monitoring veterinary drug residue in bred animals. Agric. Sci. Technol. 2016, 17, 133. [Google Scholar]
- Chen, P.; Gates-Hollingsworth, M.; Pandit, S.; Park, A.; Montgomery, D.; AuCoin, D.; Gu, J.; Zenhausern, F. Based Vertical Flow Immunoassay (VFI) for detection of bio-threat pathogens. Talanta 2019, 191, 81–88. [Google Scholar] [CrossRef]
- Díaz-Amaya, S.; Lin, L.-K.; Deering, A.J.; Stanciu, L.A. Aptamer-based SERS biosensor for whole cell analytical detection of E. coli O157:H7. Anal. Chim. Acta 2019, 1081, 146–156. [Google Scholar] [CrossRef]
- Sha, J.; Lin, H.; Timira, V.; Sui, J. The construction and application of aptamer to simultaneous identification of enrofloxacin and ciprofloxacin residues in fish. Food Anal. Methods 2021, 14, 957–967. [Google Scholar] [CrossRef]
| Analyte | Label | Assay Format | Sample | Assay Time | LOD | Reference |
|---|---|---|---|---|---|---|
| CLE 1 and RAC 3 | AuNPs 2 | Competitive LFA | Swine urine | 5 min | 0.1 ± 0.01 ng/mL | [25] |
| CLE | AuNPs | Competitive LFA | Swine livers | 10 min | NA | [26] |
| CLE | AuNPs | Competitive LFA | Swine urine | 10 min | 3 ng/mL | [27] |
| CLE | AuNPs | Competitive LFA | Swine urine | 10 min | 0.1 ng/mL | [28] |
| CLE, RAC, SAL 4 | AuNPs | Competitive LFA | Swine urine | 10 min | 0.5 ng/mL | [29] |
| CLE | AuNPs | Competitive LFA | Swine urine | 10 min | 220 pg/mL | [30] |
| SAL | AuNPs | Competitive LFA | Swine urine | 10 min | 80 ng/mL | [31] |
| RAC | AuNPs | Competitive LFA | Swine urine | 5 min | 0.1 ng/mL | [32] |
| SAL | AuNPs | Competitive LFA | Meat and milk | 10 min | meat: 4.0 ng/g milk: 3.0 ng/g | [33] |
| RAC | AuNPs | Competitive LFA | Swine urine | 45 min | 0.13 ng/mL | [34] |
| CLE | SeNPs 5 | Competitive LFA | Swine urine | / | 3 ng/mL | [35] |
| RAC and SAL | SeNPs | Competitive LFA | Swine urine | 5 min | RAC: 1 ng/mL SAL: 3 ng/mL | [36] |
| CLE and RAC | SiNPs 6 | Competitive LFA | / | 10 min | CLE: 3 ng/mL RAC: 2 ng/mL | [37] |
| CLE | SiNPs | Competitive LFA | PBS, urine, and pork | 10 min | PBS: 3 ng/mL urine: 6 ng/mL pork: 5 ng/mL | [38] |
| Zilpaterol | AuNPs | Competitive LFA | Feed | 10 min | 20 ng/g | [39] |
| PA 7 | AuNPs | Competitive LFA | Swine urine | 10 min | 0.188 ng/mL | [40] |
| Clorprenaline | AuNPs | Competitive LFA | Swine urine | 3–5 min | 0.104 ng/mL | [41] |
| Clorprenaline | AuNPs | Competitive LFA | Swine urine | 9 min | 0.15 ng/mL | [42] |
| Labels | Advantages | Disadvantages | Reference |
|---|---|---|---|
| AuNPs | Ease of fabrication, good biocompatibility, direct observation, stability | Low sensitivity | [25] |
| QD | Strong resistance against photo-bleaching, narrow excitation spectrum, wide emission spectrum | Need reader for quantitation; toxic | [45] |
| FM | Strong fluorescence, low cost | Easy to photo-bleach; aggregation-caused quenching | [46] |
| Lanthanide | Large Stokes shift, low background signal interference, long fluorescence lifetime | Need reader for quantitation | [7] |
| UCNPs | High photo-stability, long fluorescence lifespan, low cost, low cytotoxicity | Need reader for quantitation | [47] |
| MNPs | Reduced matrix effects, increased the concentration of the target | Low signal intensity | [48] |
| Enzymes | Able to catalyze the redox reactions | Easy to decomposition, high cost | [49] |
| Nanozymes | Ease of fabrication, low cost, stability | Need reader for quantitation | [50] |
| SERS | Good biocompatibility, stability, simple preparation, high sensitivity | Need reader for quantitation | [51] |
| Analyte | Label | Sample | Linear Range | Assay Time | Detection Limit | Reference | |
|---|---|---|---|---|---|---|---|
| Qualitative | Quantitative | ||||||
| CLE | AuNFs 1 | Swine urine and PBS | 0.1–5.0 ng/mL | 10 min | / | 12.5 pg/mL | [52] |
| CLE | QD 2 | PBS | / | / | / | 30 ng/mL | [45] |
| CLE | FM 3 | Swine urine | / | 20 min | / | 0.01 pg/L | [53] |
| CLE, RAC, SAL | FM | Swine urine | 0.0–4.0 ng/mL | 10 min | / | CLE: 0.10 ng/mL RAC: 0.10 ng/mL SAL: 0.09 ng/mL | [46] |
| CLE | Fluorescent nanosilica | Swine urine | / | 8 min | 0.1 ng/mL | 0.037 ng/mL | [7] |
| CLE | AuNPs and fluorescent nanobead | Pork | 0.1–2.7 ng/mL | 5–20 min | 0.5 ng/mL | 0.04 ng/mL | [37] |
| RAC | TRFN 4 | Swine urine | 5–2500 pg/mL | 10 min | / | 7.2 pg/mL | [54] |
| CLE | UCNPs 5 | Swine urine | / | 10 min | 0.1 ng/mL | 0.01 ng/mL | [47] |
| CLE | spMNBs 6 and AuNPs | Pork muscle | 0.05–1.20 ng/mL | 40 min | 0.10 | 0.24 ng/g | [55] |
| CLE | FMNBs 7 | Swine urine | 0.25–5.00 ng/mL | 10 min | / | 0.22 ng/mL | [48] |
| RAC, SAL | HRP 8 | Swine urine | RAC: 0.5–40.0 ng/mL SAL: 0.1–50.0 ng/mL | 20 min | / | RAC: 0.20 ng/mL SAL: 0.040 ng/mL | [49] |
| RAC, SAL | HRP, ALP 9 | Swine urine | / | 20 min | / | RAC: 0.17 ng/mL CLE: 0.067 ng/mL | [56] |
| RAC, CLE | MPBN 10 | Pork and mutton | RAC: 1–6 ng/mL CLE: 1–12 ng/mL | 10 min | / | RAC: 0.12 ng/mL CLE: 0.20 ng/mL | [50] |
| PA | SERS 11 | Swine urine | / | 15 min | / | 0.32 pg/mL | [51] |
| CLE | SERS | Swine urine | 0–10 ng/mL | 15 min | / | 0.24 pg/mL | [57] |
| SAL | SERS | Swine feed, meat, and urine | 10−4–100 ng/mL | 15 min | / | 3.0 pg/mL | [58] |
| brombuterol | SERS | Pork and swine urine | / | 15 min | / | 0.5 pg/mL | [59] |
| CLE | SERS | Meat | 0–1 ng/mL | 8 min | 5 ng/mL | 0.05 ng/mL | [60] |
| CLE | Au-Ag NPs | PBS | / | 15 min | / | 2 ng/mL | [61] |
| SAL | Ru(phen)32+ doped silica NPs | Swine urine | 0.6–5.0 ng/mL | 15 min | / | 0.43 ng/mL | [62] |
| CLE | Graphene NPs | Meat | 0.1–2.0 ng/mL | 10 min | 0.1 ng/mL | 0.05 ng/mL | [63] |
| CLE | PBNPs 12 | Meat | 0.5–5.0 ng/mL | 15 min | 1.0 ng/mL | / | [64] |
| CLE | CBB 13 | Animal products | 2–10 ng/mL | 10 min | / | 2 ng/mL | [65] |
| RAC | Ultramarine blue NPs | Feed, pork | / | 10 min | feed: 2 ng/mL pork: 1 ng/mL | / | [66] |
| CLE | mRF 14 | Animal products | 0–2 ng/mL | 15 min | 1 ng/mL | / | [67] |
| SAL | IrO2@ PDA NPs 15 | Food samples | 0.02–3.00 ng/mL | 15 min | / | 0.002 ng/mL | [68] |
| CLE | PTAN 16 | Beef and pork liver | 0.0–0.9 ng/mL | 15 min | 0.6 ng/mL | 0.13 ng/mL | [69] |
| SAL | CuS@ Au 17 | Beef and pork | 5.0–12.0 ng/mL | 10 min | / | 4.0 μg/kg | [70] |
| Features | LFAs | μPADs |
|---|---|---|
| Sample flow | Capillary force | Capillary force |
| Washing steps | No | Yes |
| Multiplexing capability | Moderate | High |
| Hook effect | Yes | No |
| Easy-of-use | Easy | Moderate |
| Sensitivity | Moderate | High |
| Sample volume requirement | ∼µL | ∼µL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Liu, S.; Shi, L.; Li, Z.; Bai, Q.; Du, X.; Wang, L.; Zha, H.; Li, C. Paper-Based Fluidic Sensing Platforms for β-Adrenergic Agonist Residue Point-of-Care Testing. Biosensors 2022, 12, 518. https://doi.org/10.3390/bios12070518
Luo H, Liu S, Shi L, Li Z, Bai Q, Du X, Wang L, Zha H, Li C. Paper-Based Fluidic Sensing Platforms for β-Adrenergic Agonist Residue Point-of-Care Testing. Biosensors. 2022; 12(7):518. https://doi.org/10.3390/bios12070518
Chicago/Turabian StyleLuo, Hongzhi, Shan Liu, Lina Shi, Zhu Li, Qianwen Bai, Xiaoxin Du, Lijun Wang, He Zha, and Chenzhong Li. 2022. "Paper-Based Fluidic Sensing Platforms for β-Adrenergic Agonist Residue Point-of-Care Testing" Biosensors 12, no. 7: 518. https://doi.org/10.3390/bios12070518
APA StyleLuo, H., Liu, S., Shi, L., Li, Z., Bai, Q., Du, X., Wang, L., Zha, H., & Li, C. (2022). Paper-Based Fluidic Sensing Platforms for β-Adrenergic Agonist Residue Point-of-Care Testing. Biosensors, 12(7), 518. https://doi.org/10.3390/bios12070518







