Development of GO/Co/Chitosan-Based Nano-Biosensor for Real-Time Detection of D-Glucose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
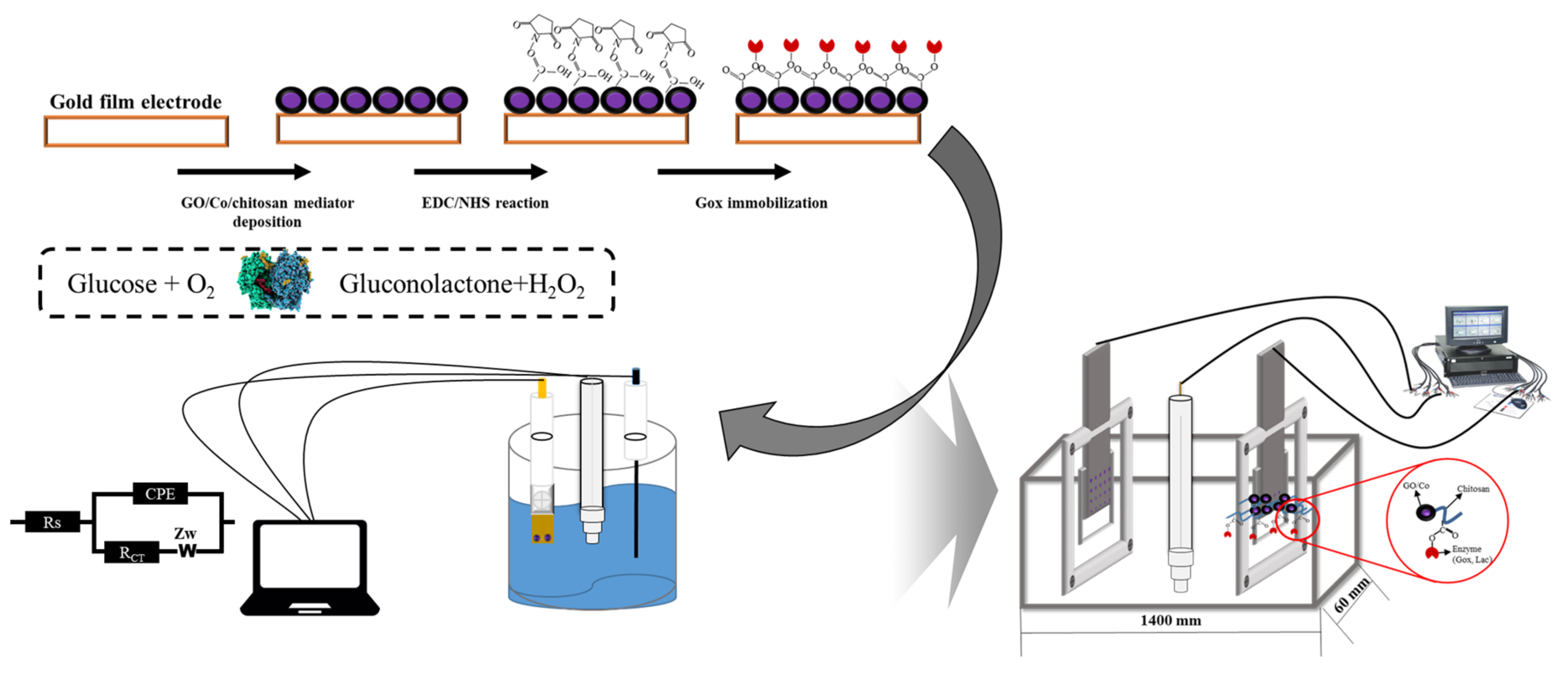
2.2. Preparation of Electron Transfer Mediator and Immobilization Process of Glucose Oxidase on an Au Film Electrode
2.3. Surface Analysis of Modified Electrode
2.4. Electrochemical Analysis of Developed Biosensor
3. Results and Discussion
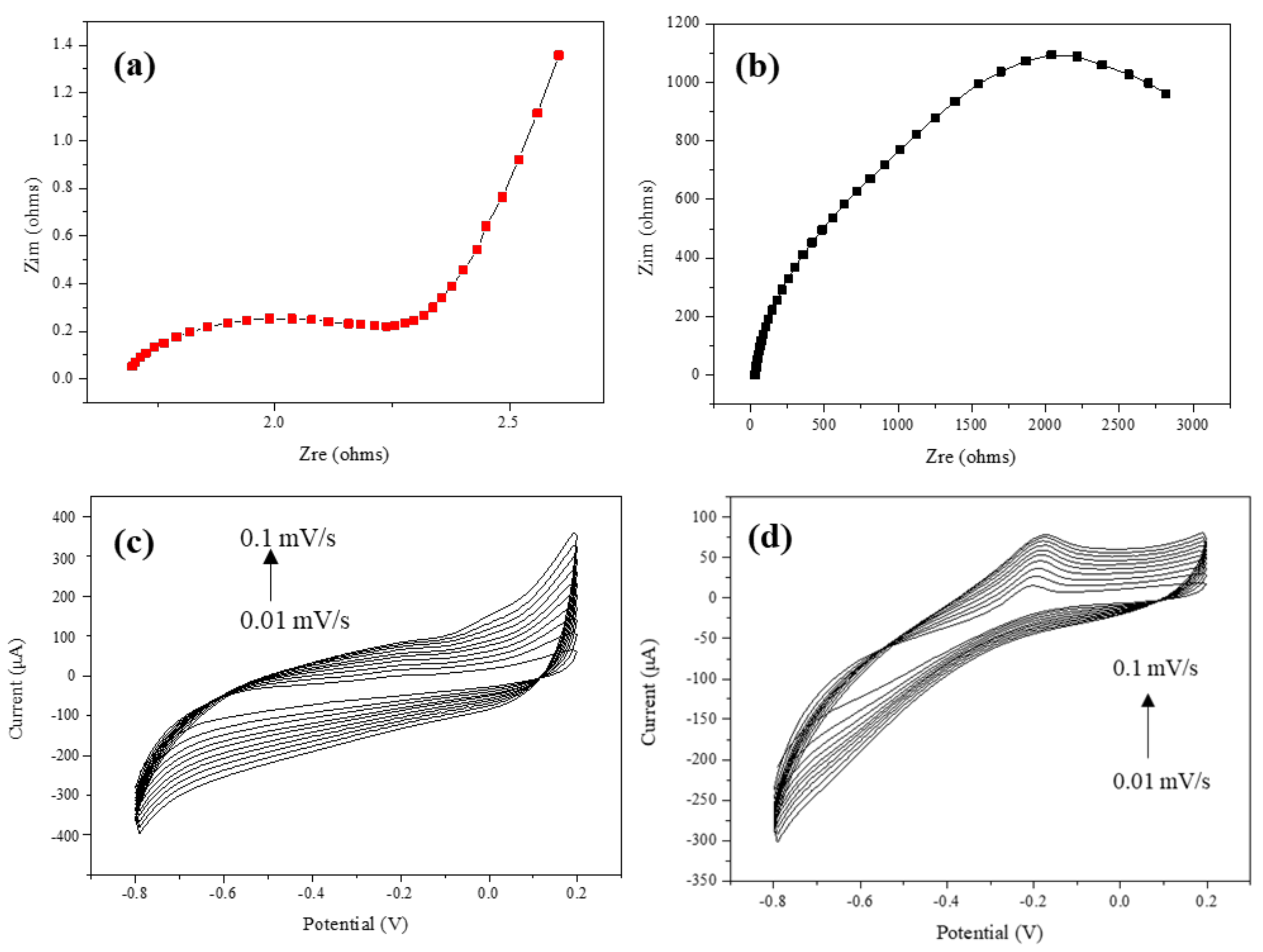
3.1. Surface Analysis of GO/Co/Chitosan Modified Film-Typed Electrode
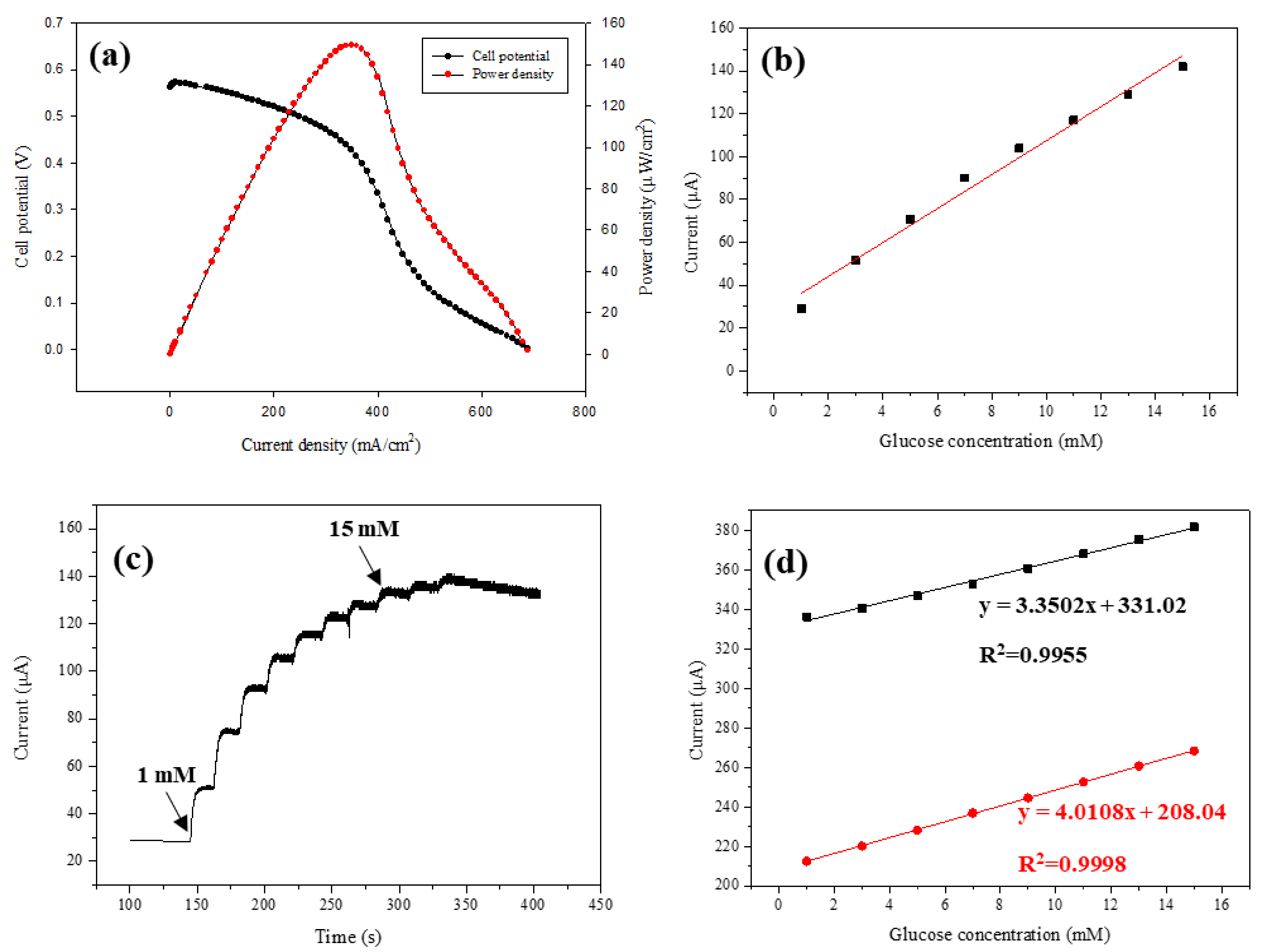
3.2. Electrochemical Property of the Developed GO/Co/Chitosan-Based Nano-Biosensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conghaile, P.; Kumar, R.; Ferrer, M.L.; Leech, D. Glucose oxidation by enzyme electrodes using genipin to crosslink chitosan, glucose oxidase and amine-containing osmium redox complexes. Electrochem. Commun. 2020, 113, 106703. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, W.; Sun, Y.; Sun, C. Enzyme-free glucose sensor based on a three-dimensional gold film electrode. Sens. Actuators B Chem. 2008, 134, 471–476. [Google Scholar] [CrossRef]
- Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Development Perspective of Bioelectrocatalysis-Based Biosensors. Sensors 2020, 20, 4826. [Google Scholar] [CrossRef] [PubMed]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme production of D-gluconic acid and glucose oxidase: Successful tales of cascade reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Otero, F.; Magner, E. Biosensors—Recent Advances and Future Challenges in Electrode Materials. Sensors 2020, 20, 3561. [Google Scholar] [CrossRef]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Karimi, F.; Shabani-Nooshabadi, M.; Alizadeh, M.; Al-Othman, A.; Erk, N.; Raman, P.K.Y.; Karaman, C. Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere 2021, 287, 132187. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Zhu, L.; Shen, H.; Jia, N. Electrochemical sensing based on graphene oxide/Prussian blue hybrid film modified electrode. Electrochim. Acta 2011, 56, 1239–1245. [Google Scholar] [CrossRef]
- Xie, F.; Huang, Z.; Chen, C.; Xie, Q.; Huang, Y.; Qin, C.; Liu, Y.; Su, Z.; Yao, S. Preparation of Au-film electrodes in glucose-containing Au-electroplating aqueous bath for high-performance nonenzymatic glucose sensor and glucose/O2 fuel cell. Electrochem. Commun. 2012, 18, 108–111. [Google Scholar] [CrossRef]
- Wang, C.; Shim, E.; Chang, H.-K.; Lee, N.; Kim, H.R.; Park, J. Sustainable and high-power wearable glucose biofuel cell using long-term and high-speed flow in sportswear fabrics. Biosens. Bioelectron. 2020, 169, 112652. [Google Scholar] [CrossRef]
- Barbhuiya, N.H.; Misra, U.; Singh, S.P. Biocatalytic membranes for combating the challenges of membrane fouling and micropollutants in water purification: A review. Chemosphere 2021, 286, 131757. [Google Scholar] [CrossRef]
- Strakosas, X.; Selberg, J.; Pansodtee, P.; Yonas, N.; Manapongpun, P.; Teodorescu, M.; Rolandi, M. A non-enzymatic glucose sensor enabled by bioelectronic pH control. Sci. Rep. 2019, 9, 10844. [Google Scholar] [CrossRef] [Green Version]
- Hamouda, I.M.; Ibrahim, D.A.; Alwakeel, E.E. Influence of sport beverages on the properties of dental restorative glass ionomers. Int. J. Dent. Oral Health 2016, 2, 1–8. [Google Scholar]
- Amir, M.N.I.; Halilu, A.; Julkapli, N.M.; Ma’Amor, A. Gold-graphene oxide nanohybrids: A review on their chemical catalysis. J. Ind. Eng. Chem. 2020, 83, 1–13. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, S.B.; Yang, X.; Lee, J.H.; Yoo, H.Y.; Chun, Y.; Cho, J.; Park, C.; Lee, J.; Kim, S.W. Development of electron transfer mediator using modified graphite oxide/cobalt for enzymatic fuel cell. Electrochem. Soc. 2015, 162, G113–G118. [Google Scholar] [CrossRef]
- Sobhan, A.; Oh, J.H.; Park, M.K.; Kim, S.W.; Park, C.; Lee, J. Assessment of peanut allergen Ara h1 in processed foods using a SWCNTs-based nano-biosensor. Biosci. Biotechnol. Biochem. 2018, 82, 1134–1142. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Choi, H.S.; Yang, X.; Yang, J.H.; Lee, J.H.; Yoo, H.Y.; Lee, J.; Park, C.; Kim, S.W. Improvement of power generation of enzyme fuel cell by novel GO/Co/chitosan electrodeposition. J. Ind. Eng. Chem. 2020, 81, 108–114. [Google Scholar] [CrossRef]
- Gholami, F.; Navaee, A.; Salimi, A.; Ahmadi, R.; Korani, A.; Hallaj, R. Direct Enzymatic Glucose/O2 Biofuel Cell based on Poly-Thiophene Carboxylic Acid alongside Gold Nanostructures Substrates Derived through Bipolar Electrochemistry. Sci. Rep. 2018, 8, 15103. [Google Scholar] [CrossRef]
- Ozyilmaz, G. Glucose Oxidase Applications and Comparison of the Activity Assays. Nat. Eng. Sci. 2019, 4, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Aberer, F.; Theiler-Schwetz, V.; Ziko, H.; Hausegger, B.; Wiederstein-Grasser, I.; Hochfellner, D.A.; Eller, P.; Tomberger, G.; Ellmerer, M.; Mader, J.K.; et al. Accuracy and stability of an arterial sensor for glucose monitoring in a porcine model using glucose clamp technique. Sci. Rep. 2020, 10, 6604. [Google Scholar] [CrossRef]
- Libansky, M.; Zima, J.; Barek, J.; Reznickova, A.; Svorcik, V.; Dejmkova, H. Basic electrochemical properties of sputtered gold film electrodes. Electrochim. Acta 2017, 251, 452–460. [Google Scholar] [CrossRef]
- Ilginis, A.; Griškonis, E. Modification of Graphite Felt with Lead (II) Formate and Acetate—An Approach for Preparation of Lightweight Electrodes for a Lead-Acid Battery. Processes 2020, 8, 1248. [Google Scholar] [CrossRef]
- Du Toit, H.; Di Lorenzo, M. Glucose Oxidase Directly Immobilized onto Highly Porous Gold Electrodes for Sensing and Fuel Cell applications. Electrochim. Acta 2014, 138, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, R.; Kanagavalli, P.; Senthilkumar, S.; Mathiyarasu, J. Au Nanoparticle-decorated Nanoporous PEDOT Modified Glassy Carbon Electrode: A New Electrochemical Sensing Platform for the Detection of Glutathione. Biotechnol. Bioprocess Eng. 2020, 25, 715–723. [Google Scholar] [CrossRef]
- Nasar, A.; Rahman, M.M. Applications of chitosan (CHI)-reduced graphene oxide (rGO)-polyaniline (PAni) conducting composite electrode for energy generation in glucose biofuel cell. Sci. Rep. 2020, 10, 10428. [Google Scholar]
- Surya, S.G.; Khatoon, S.; Lahcen, A.A.; Nguyen, A.T.H.; Dzantiev, B.B.; Tarannum, N.; Salama, K.N. A chitosan gold nanoparticles molecularly imprinted polymer based ciprofloxacin sensor. RSC Adv. 2020, 10, 12823–12832. [Google Scholar] [CrossRef]
- Lee, J. Significant Effect of Sample Pretreatment on Ara h1 Extraction and Improved Sensitive SWCNT-Based Detection through Optimization. Processes 2020, 8, 1420. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Razak, K.A.; Lockman, Z. Fabrication and Characterization of Glucose Biosensors by Using Hydrothermally Grown ZnO Nanorods. Sci. Rep. 2018, 8, 13722. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Tasca, F.; Antiochia, R. Minimally Invasive Glucose Monitoring Using a Highly Porous Gold Microneedles-Based Biosensor: Characterization and Application in Artificial Interstitial Fluid. Catalysts 2019, 9, 580. [Google Scholar] [CrossRef] [Green Version]
- Bojang, A.A.; Wu, H.S. Characterization of Electrode Performance in Enzymatic Biofuel Cells Using Cyclic Voltammetry and Electrochemical Impedance Spectroscopy. Catalysts 2020, 10, 782. [Google Scholar] [CrossRef]
- Aini, B.N.; Siddiquee, S.; Ampon, K.; Rodrigues, K.F.; Suryani, S. Development of glucose biosensor based on ZnO nanoparticles film and glucose oxidase-immobilized eggshell membrane. Sens. Bio-Sens. Res. 2015, 4, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Lović, J.; Stevanović, S.; Nikolić, N.D.; Petrović, S.; Vuković, D.; Prlainović, N.; Mijin, D.; Ivić, M.A.; Avramov, I. Glucose sensing using glucose oxidase-glutaraldehyde-cysteine modified gold electrode. Int. J. Electrochem. Sci. 2017, 12, 5806–5817. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Du, X.; Li, Y.; Tang, W. A highly sensitive glucose sensor based on a gold nanoparticles/polyaniline/multi-walled carbon nanotubes composite modified glassy carbon electrode. New J. Chem. 2018, 42, 11944–11953. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, Y.; Shao, C.; Wan, L.; Jiao, K. Unadulterated Glucose Biosensor Based on Direct Electron Transfer of Glucose Oxidase Encapsulated Chitosan Modified Glassy Carbon Electrode. Electroanalysis 2008, 20, 520–526. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q.; Guan, Q.-M.; Wu, J.; Li, H.; Yan, J.-J. Enhanced direct electrochemistry of glucose oxidase and biosensing for glucose via synergy effect of graphene and CdS nanocrystals. Biosens. Bioelectron. 2011, 26, 2252–2257. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, X.; Li, Y.; Zheng, H.; Liu, Y.; Suye, S.-I. A Glucose Biosensor Based on Direct Electron Transfer of Glucose Oxidase on PEDOT Modified Microelectrode. J. Electrochem. Soc. 2020, 167, 067502. [Google Scholar] [CrossRef]
- Zhong, Y.; Shi, T.; Liu, Z.; Cheng, S.; Huang, Y.; Tao, X.; Liao, G.; Tang, Z. Ultrasensitive non-enzymatic glucose sensors based on different copper oxide nanostructures by in-situ growth. Sens. Actuators B Chem. 2016, 236, 326–333. [Google Scholar] [CrossRef] [Green Version]


| Electrode Materials | Sensitivity (μA mM−1 cm−2) | Linear Range (mM) | Limit of Detection (mM) | Reference |
|---|---|---|---|---|
| Go/Co/chitosan | 0.14 | 1.0–15 | 2.7 | This work |
| Go/chitosan/GCE | 0.233 | 0.6–2.8 | 1.0 | [32] |
| Graphene-Cds-Go/GCE | 1.76 | 2.0–16 | 7.0 | [33] |
| Go/PEDOT | 8.5 | 0.5–15 | 6.5 | [34] |
| CuO nanowires Non-enzyme | 1.2 | Up to 1 | 1.0 | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.S.; Yang, X.; Lee, J.H.; Yoo, H.Y.; Park, C.; Kim, S.W.; Lee, J. Development of GO/Co/Chitosan-Based Nano-Biosensor for Real-Time Detection of D-Glucose. Biosensors 2022, 12, 464. https://doi.org/10.3390/bios12070464
Kim DS, Yang X, Lee JH, Yoo HY, Park C, Kim SW, Lee J. Development of GO/Co/Chitosan-Based Nano-Biosensor for Real-Time Detection of D-Glucose. Biosensors. 2022; 12(7):464. https://doi.org/10.3390/bios12070464
Chicago/Turabian StyleKim, Dong Sup, Xiaoguang Yang, Ja Hyun Lee, Hah Young Yoo, Chulhwan Park, Seung Wook Kim, and Jinyoung Lee. 2022. "Development of GO/Co/Chitosan-Based Nano-Biosensor for Real-Time Detection of D-Glucose" Biosensors 12, no. 7: 464. https://doi.org/10.3390/bios12070464
APA StyleKim, D. S., Yang, X., Lee, J. H., Yoo, H. Y., Park, C., Kim, S. W., & Lee, J. (2022). Development of GO/Co/Chitosan-Based Nano-Biosensor for Real-Time Detection of D-Glucose. Biosensors, 12(7), 464. https://doi.org/10.3390/bios12070464







