Amelioration of Full-Thickness Wound Using Hesperidin Loaded Dendrimer-Based Hydrogel Bandages
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Hesperidin Loaded PAMAM Dendrimer (Hsp-PAMAM) Based Hydrogel Bandages
2.3. Drug Loading and Entrapment Efficiency
2.4. Characterization of Hesperidin PAMAM Dendrimer
2.4.1. Fourier Transform Infrared Spectroscopy (FT-IR)
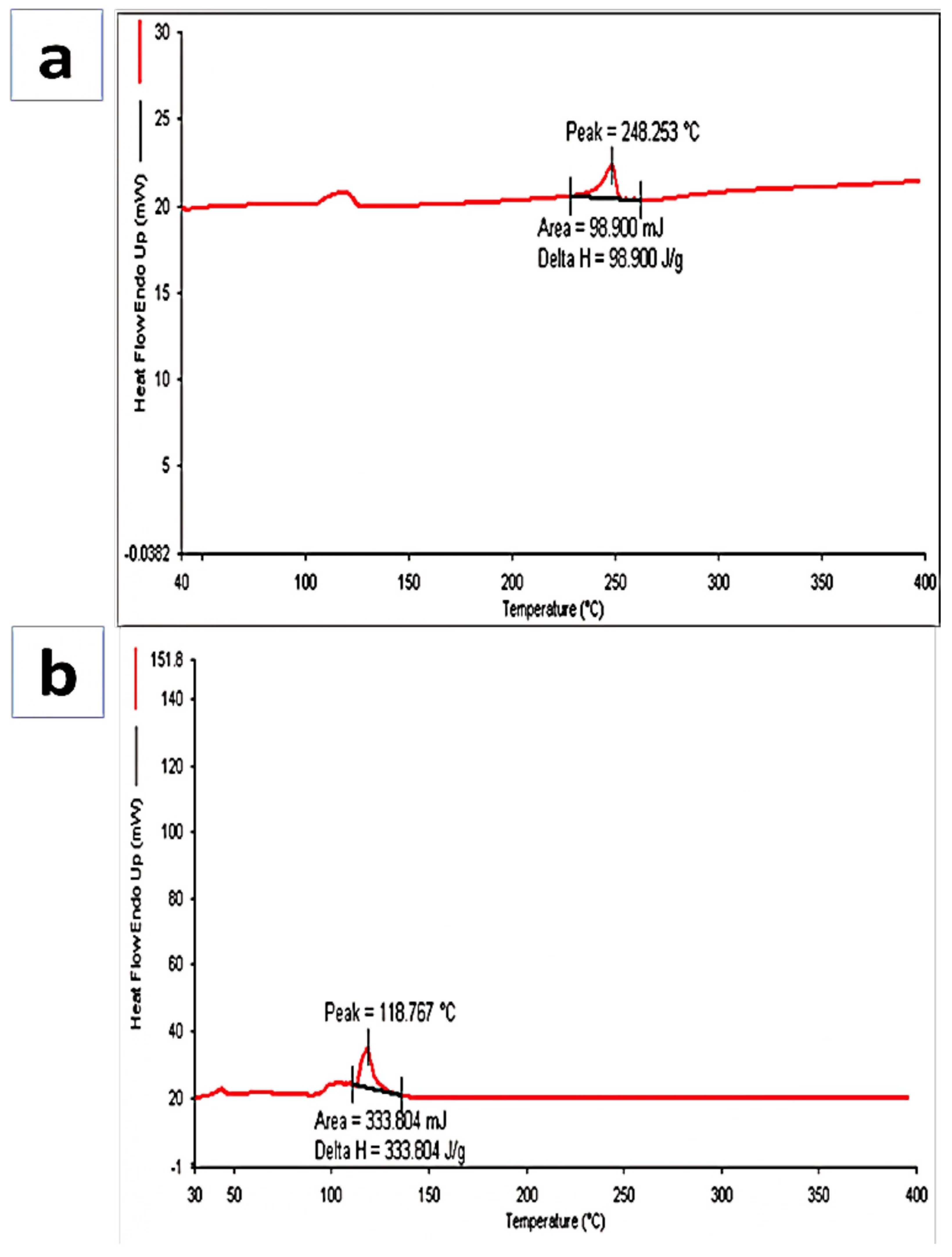
2.4.2. Differential Scanning Colorimetry (DSC)
2.4.3. Particle Size, Zeta Potential, and PDI Determination
2.4.4. Morphology Examination (Transmission Electron Microscopy)
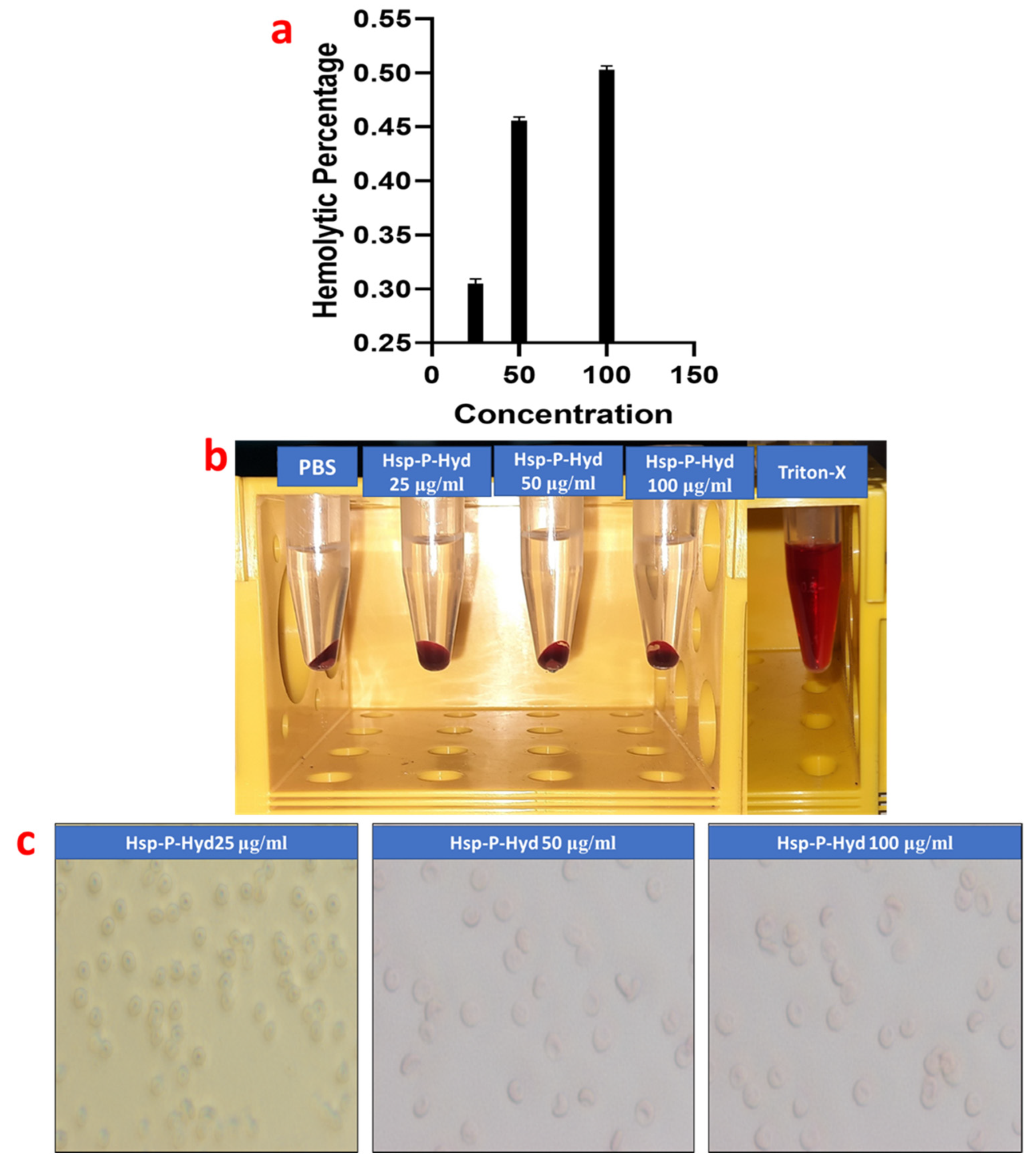
2.5. Hemcompatibility Study
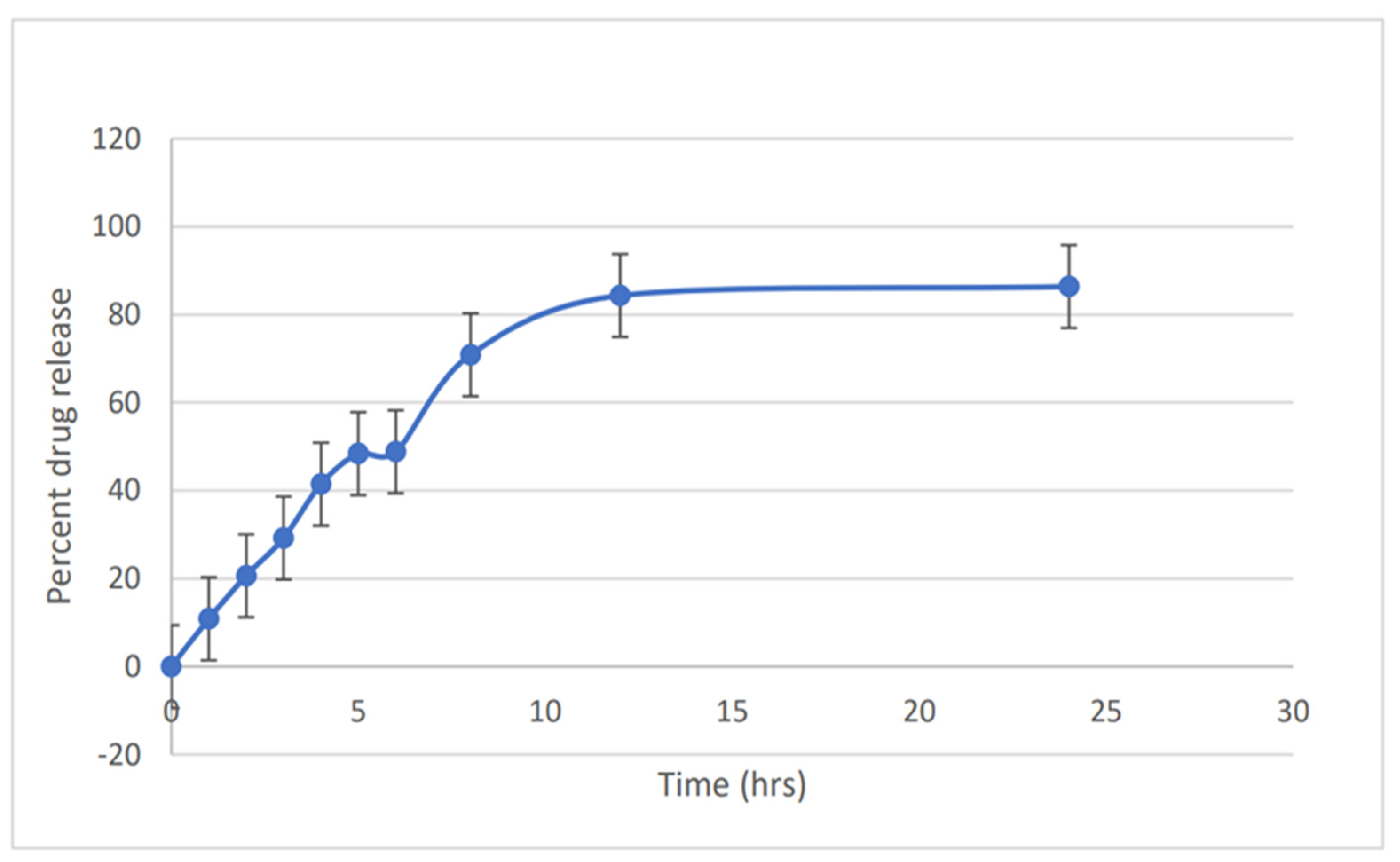
2.6. In Vitro Drug Release
2.7. Ex Vivo Drug Permeation Study
2.8. Skin Permeation Enhancement Study
2.9. In Vivo Wound Healing Activity
2.10. Histopathological Study
2.11. Skin Irritation Study
3. Results and Discussion
3.1. Preparation of Hesperidin Loaded PAMAM Dendrimer (Hsp-PAMAM) Based Hydrogel Bandages
3.2. FT-IR
3.3. Differential Scanning Calorimetry (DSC)
3.4. Particle Size, Zeta Potential, PDI Determination, and Transmission Electron Microscopy (TEM)
3.5. Hemolysis Study
3.6. In Vitro Release Study
3.7. Ex Vivo Permeation Study
3.8. Skin Permeation Enhancement Study
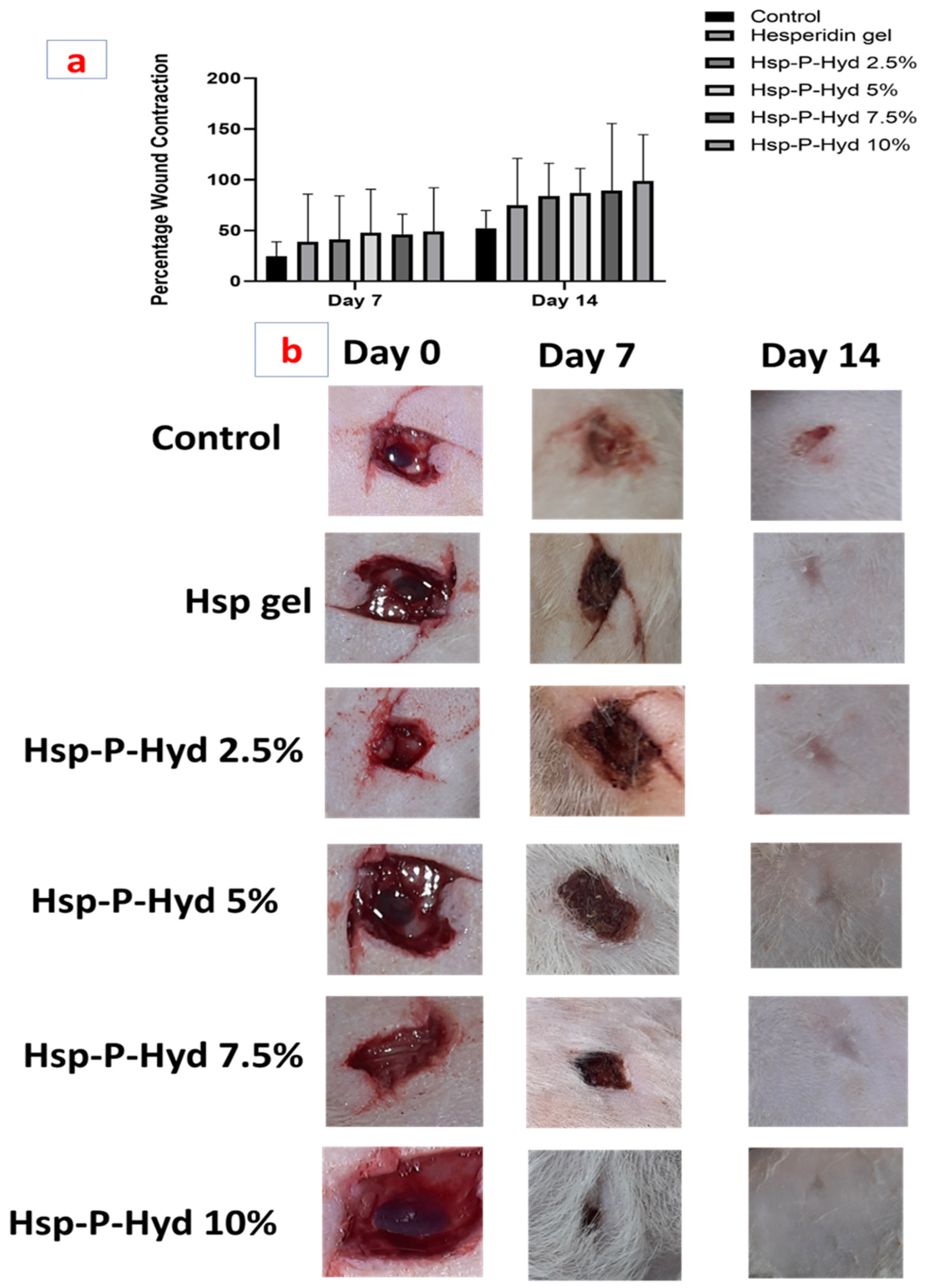
3.9. In Vivo Wound Healing Activity
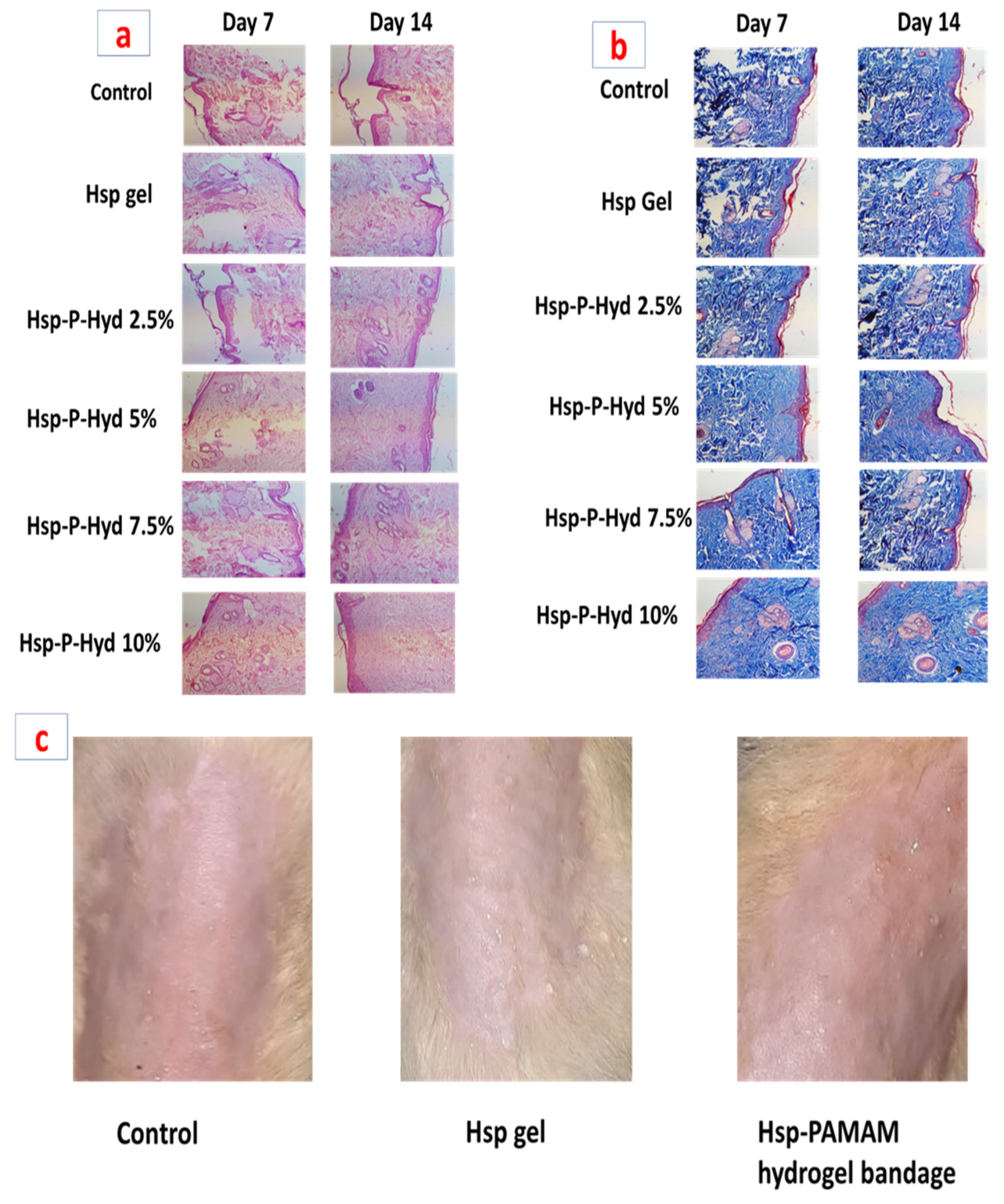
3.10. Histopathological Study
3.11. Skin Irritation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Ashram, S.; El-Samad, L.M.; Basha, A.A.; El Wakil, A. Naturally-derived targeted therapy for wound healing: Beyond classical strategies. Pharmacol. Res. 2021, 170, 105749. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Alqahtani, A.; Kazi, M.; Ahmad, M.Z.; Alahmari, A.; Alsenaidy, M.A.; Syed, R. Wound-healing potential of curcumin loaded lignin nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 60, 102020. [Google Scholar] [CrossRef]
- Medina-Cruz, D.; Saleh, B.; Vernet-Crua, A.; Ajo, A.; Roy, A.K.; Webster, T.J. Drug-delivery nanocarriers for skin wound-healing applications. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 439–488. [Google Scholar]
- Bennett, N.T.; Sehultz, G.S.; Schultz, S. Growth Factors and Wound Healing: Part II. Role in Normal and Chronic Wound Healing. Am. J. Surg. 1993, 166, 74–81. [Google Scholar] [CrossRef]
- Bagher, Z.; Ehterami, A.; Nasrolahi, M.; Azimi, M.; Salehi, M. Hesperidin promotes peripheral nerve regeneration based on tissue engineering strategy using alginate/chitosan hydrogel: In vitro and in vivo study. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 299–308. [Google Scholar] [CrossRef]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Sheikh, A.; Md, S.; Alhakamy, N.A.; Kesharwani, P. Recent development of aptamer conjugated chitosan nanoparticles as cancer therapeutics. Int. J. Pharm. 2022, 620, 121751. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gajbhiye, V.; Jain, N.K. A review of nanocarriers for the delivery of small interfering RNA. Biomaterials 2012, 33, 7138–7150. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Kesharwani, P. An Insight into Aptamer Engineered Dendrimer for Cancer Therapy. Eur. Polym. J. 2021, 159, 110746. [Google Scholar] [CrossRef]
- Kesharwani, P.; Banerjee, S.; Gupta, U.; Amin, M.C.I.M.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater. Today 2015, 18, 565–572. [Google Scholar] [CrossRef]
- Kesharwani, P.; Iyer, A.K. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov. Today 2015, 20, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Ujjwal, R.R.; Naqvi, S.; Verma, R.K.; Tiwari, S.; Kesharwani, P.; Shukla, R. Formulation development of tocopherol polyethylene glycol nanoengineered polyamidoamine dendrimer for neuroprotection and treatment of Alzheimer disease. J. Drug Target. 2022, 1–15. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gothwal, A.; Kesharwani, P.; Alsaab, H.; Iyer, A.K.; Gupta, U. Dendrimer Nanoarchitectures for Cancer Diagnosis and Anticancer Drug Delivery. Drug Discov. Today 2017, 22, 314–326. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Gancarz, R.; Wilk, K.A. Polysaccharide hydrogel particles for enhanced delivery of hesperidin: Fabrication, characterization and in vitro evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 48–56. [Google Scholar] [CrossRef]
- Li, W.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: Role of TGF-B/SMADS and ANG-1/TIE-2 signaling pathways. EXCLI J. 2018, 17, 399–419. [Google Scholar]
- Musa, A.E.; Omyan, G.; Esmaely, F.; Shabeeb, D. Radioprotective effect of hesperidin: A systematic review. Medicina 2019, 55, 370. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Tanabe, F.; Arai, N.; Mitsuzumi, H.; Miwa, Y.; Kubota, M.; Chaen, H.; Kibata, M. Bioavailability of glucosyl hesperidin in rats. Biosci. Biotechnol. Biochem. 2006, 70, 1386–1394. [Google Scholar] [CrossRef]
- Vabeiryureilai, M.; Lalrinzuali, K.; Jagetia, G.C. NF-κB and COX-2 repression with topical application of hesperidin and naringin hydrogels augments repair and regeneration of deep dermal wounds. Burns 2022, 48, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Srirangam, R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: A natural bioflavonoid. Pharm. Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.H.; Sulaiman, G.M.; Al-Halbosiy, M.M.F.; Jabir, M.S.; Hameed, A.H. Fabrication of hesperidin nanoparticles loaded by poly lactic co-Glycolic acid for improved therapeutic efficiency and cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 378–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, A.; Md, S.; Kesharwani, P. RGD engineered dendrimer nanotherapeutic as an emerging targeted approach in cancer therapy. J. Control. Release 2021, 340, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Choudhary, P.K.; Shukla, R.; Sahebkar, A.; Kesharwani, P. Recent advances in nanotechnology based combination drug therapy for skin cancer. J. Biomater. Sci. Polym. Ed. 2022, 1–34. [Google Scholar] [CrossRef]
- Sheikh, A.; Md, S.; Kesharwani, P. Aptamer grafted nanoparticle as targeted therapeutic tool for the treatment of breast cancer. Biomed. Pharmacother. 2022, 146, 112530. [Google Scholar] [CrossRef]
- Fatima, M.; Sheikh, A.; Hasan, N.; Sahebkar, A.; Riadi, Y.; Kesharwani, P. Folic acid conjugated poly(amidoamine) dendrimer as a smart nanocarriers for tracing, imaging, and treating cancers over-expressing folate receptors. Eur. Polym. J. 2022, 170, 111156. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained wound healing activity of curcumin loaded oleic acid based polymeric bandage in a rat model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef]
- Singh, V.; Md, S.; Alhakamy, N.A.; Kesharwani, P. Taxanes loaded polymersomes as an emerging polymeric nanocarrier for cancer therapy. Eur. Polym. J. 2022, 162, 110883. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Thermal analysis study of flavonoid solid dispersions having enhanced solubility. J. Therm. Anal. Calorim. 2006, 83, 283–290. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Parenterally administrable nano-micelles of 3,4-difluorobenzylidene curcumin for treating pancreatic cancer. Colloids Surf. B Biointerfaces 2015, 132, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, C.; Chen, K.; Ni, J.; Cai, Y.; Guo, X.; Wu, X.Y. A New Method for Evaluating Actual Drug Release Kinetics of Nanoparticles inside Dialysis Devices via Numerical Deconvolution. J. Control. Release 2016, 243, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sarah Sujitha, Y.; Indira Muzib, Y.; Sarah Sujitha, Y. Preparation of Topical Nano Gel Loaded with Hesperidin Emulsomes: In vitro and in vivo Studies. Int. J. Pharm. Investig. 2020, 10, 500–505. [Google Scholar] [CrossRef]
- Terhorst, D.; Maltusch, A.; Stockfleth, E.; Lange-Asschenfeldt, S.; Sterry, W.; Ulrich, M.; Lange-Asschenfeldt, B. Reflectance confocal microscopy for the evaluation of acute epidermal wound healing. Wound Repair Regen. 2011, 19, 671–679. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Iqubal, A.; Imtiyaz, K.; Rizvi, M.M.A.; Gupta, M.M.; Ali, J.; Baboota, S. Combinatorial lipid-nanosystem for dermal delivery of 5-fluorouracil and resveratrol against skin cancer: Delineation of improved dermatokinetics and epidermal drug deposition enhancement analysis. Eur. J. Pharm. Biopharm. 2021, 163, 223–239. [Google Scholar] [CrossRef]
- Vashisth, I.; Ahad, A.; Aqil, M.; Agarwal, S.P. Investigating the potential of essential oils as penetration enhancer for transdermal losartan delivery: Effectiveness and mechanism of action. Asian J. Pharm. Sci. 2014, 9, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Moglad, E.H.; Hamad, A.M.; Fatima, F.; Devanathadesikan Seshadri, V.; Naz, M. Antimicrobial and wound healing activities of certain Sudanese medicinal plants. Saudi J. Biol. Sci. 2020, 27, 1766–1772. [Google Scholar] [CrossRef]
- Aramwit, P.; Sangcakul, A. The effects of sericin cream on wound healing in rats. Biosci. Biotechnol. Biochem. 2007, 71, 2473–2477. [Google Scholar] [CrossRef] [Green Version]
- Yadav, E.; Yadav, P.; Verma, A. Amelioration of full thickness dermal wounds by topical application of biofabricated zinc oxide and iron oxide nano-ointment in albino Wistar rats. J. Drug Deliv. Sci. Technol. 2021, 66, 102833. [Google Scholar] [CrossRef]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Rekha, S.S.; Pradeepkiran, J.A.; Bhaskar, M. Bioflavonoid hesperidin possesses the anti-hyperglycemic and hypolipidemic property in STZ induced diabetic myocardial infarction (DMI) in male Wister rats. J. Nutr. Intermed. Metab. 2019, 15, 58–64. [Google Scholar] [CrossRef]
- Man, M.Q.; Yang, B.; Elias, P.M. Benefits of Hesperidin for Cutaneous Functions. Evid. Based. Complement. Alternat. Med. 2019, 2019, 2676307. [Google Scholar] [CrossRef] [Green Version]
- Maingi, V.; Kumar, M.V.S.; Maiti, P.K. PAMAM dendrimer-drug interactions: Effect of pH on the binding and release pattern. J. Phys. Chem. B 2012, 116, 4370–4376. [Google Scholar] [CrossRef] [PubMed]
- Borowska, K.; Wołowiec, S.; Rubaj, A.; Głowniak, K.; Sieniawska, E.; Radej, S. Effect of polyamidoamine dendrimer G3 and G4 on skin permeation of 8-methoxypsoralene—In vivo study. Int. J. Pharm. 2012, 426, 280–283. [Google Scholar] [CrossRef]
- Bae, E.A.; Han, M.J.; Lee, M.; Kim, D.H. In vitro inhibitory effect of some flavonoids on rotavirus infectivity. Biol. Pharm. Bull. 2000, 23, 1122–1124. [Google Scholar] [CrossRef] [Green Version]
- Ohtsuki, K.; Abe, A.; Mitsuzumi, H.; Kondo, M.; Uemura, K.; Iwasaki, Y.; Kondo, Y. Glucosyl Hesperidin Improves Serum Cholesterol Composition and Inhibits Hypertrophy in Vasculature. J. Nutr. Sci. Vitaminol. 2003, 49, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Jagetia, G.C. Topical Application of Hesperidin, a Citrus Bioflavanone Accelerates Healing of Full Thickness Dermal Excision Wounds in Mice Exposed to 6 Gy of Whole Body Γ-Radiation. J. Clin. Res. Dermatol. 2017, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Haddadi, G.; Abbaszadeh, A.; Mosleh-Shirazi, M.A.; Okhovat, M.A.; Salajeghe, A.; Ghorbani, Z. Evaluation of the effect of hesperidin on vascular endothelial growth factor gene expression in rat skin animal models following cobalt-60 gamma irradiation. J. Cancer Res. Ther. 2018, 14, S1098–S1104. [Google Scholar]
- Surekha, B.; Kommana, N.S.; Dubey, S.K.; Kumar, A.V.P.; Shukla, R.; Kesharwani, P. PAMAM dendrimer as a talented multifunctional biomimetic nanocarrier for cancer diagnosis and therapy. Colloids Surf. B Biointerfaces 2021, 204, 111837. [Google Scholar] [CrossRef]
- Chen, H.; Xing, X.; Tan, H.; Jia, Y.; Zhou, T.; Chen, Y.; Ling, Z.; Hu, X. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater. Sci. Eng. C 2017, 70, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Patwary, B.N. Formulation and in Vitro Evaluation of Hesperidin-Phospholipid Complex and Its Antioxidant Potential. Curr. Drug ther. 2019, 15, 28–36. [Google Scholar] [CrossRef]




| (a): Distribution of Animals for In Vivo Wound Healing Study | ||||||||
| Groups | Treatment | No of Animals Required/Group | Route | |||||
| 1 | Control | 6 | Topical | |||||
| 2 | Hesperidin gel | 6 | Topical | |||||
| 3 | Hsp-P-Hyd—10% | 6 | Topical | |||||
| 4 | Hsp-P-Hyd—7.5% | 6 | Topical | |||||
| 5 | Hsp-P-Hyd—5% | 6 | Topical | |||||
| 6 | Hsp-P-Hyd—2.5% | 6 | Topical | |||||
| Total number of animals = 36 | ||||||||
| (b): Percentage wound contraction evaluation post treatment with Hesperidin gel, Hsp-P-Hyd 2.5%, Hsp-P-Hyd 5%, Hsp-P-Hyd 7.5%, Hsp-P-Hyd 10%. Wounds not treated were considered as control | ||||||||
| Groups | Day 7 | Day 14 | ||||||
| Control | 24.665 ± 0.94 | 52 ± 1.41 | ||||||
| Hesperidin gel | 38.995 ± 0.47 | 74.998 ± 0.46 | ||||||
| Hsp-P-Hyd 2.5% | 41 ± 0.48 | 83.93 ± 0.098 | ||||||
| Hsp-P-Hyd 5% | 47.665 ± 0.47 | 87.263 ± 0.85 | ||||||
| Hsp-P-Hyd 7.5% | 46.33 ± 0.46 | 89.16 ± 0.14 | ||||||
| Hsp-P-Hyd 10% | 49.105 ± 0.62 | 98.9 ± 0.42 | ||||||
| (c): Skin irritation evaluation after treatment with Hsp gel and Hsp-P-Hyd 10%. | ||||||||
| Rat group | Score after days | Mean score | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hesperidin gel | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hsp-P-Hyd 10% | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, P.; Sheikh, A.; Abourehab, M.A.S.; Kesharwani, P. Amelioration of Full-Thickness Wound Using Hesperidin Loaded Dendrimer-Based Hydrogel Bandages. Biosensors 2022, 12, 462. https://doi.org/10.3390/bios12070462
Gupta P, Sheikh A, Abourehab MAS, Kesharwani P. Amelioration of Full-Thickness Wound Using Hesperidin Loaded Dendrimer-Based Hydrogel Bandages. Biosensors. 2022; 12(7):462. https://doi.org/10.3390/bios12070462
Chicago/Turabian StyleGupta, Praveen, Afsana Sheikh, Mohammed A. S. Abourehab, and Prashant Kesharwani. 2022. "Amelioration of Full-Thickness Wound Using Hesperidin Loaded Dendrimer-Based Hydrogel Bandages" Biosensors 12, no. 7: 462. https://doi.org/10.3390/bios12070462
APA StyleGupta, P., Sheikh, A., Abourehab, M. A. S., & Kesharwani, P. (2022). Amelioration of Full-Thickness Wound Using Hesperidin Loaded Dendrimer-Based Hydrogel Bandages. Biosensors, 12(7), 462. https://doi.org/10.3390/bios12070462





