Silent Antibodies Start Talking: Enhanced Lateral Flow Serodiagnosis with Two-Stage Incorporation of Labels into Immune Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Materials, and Apparatuses
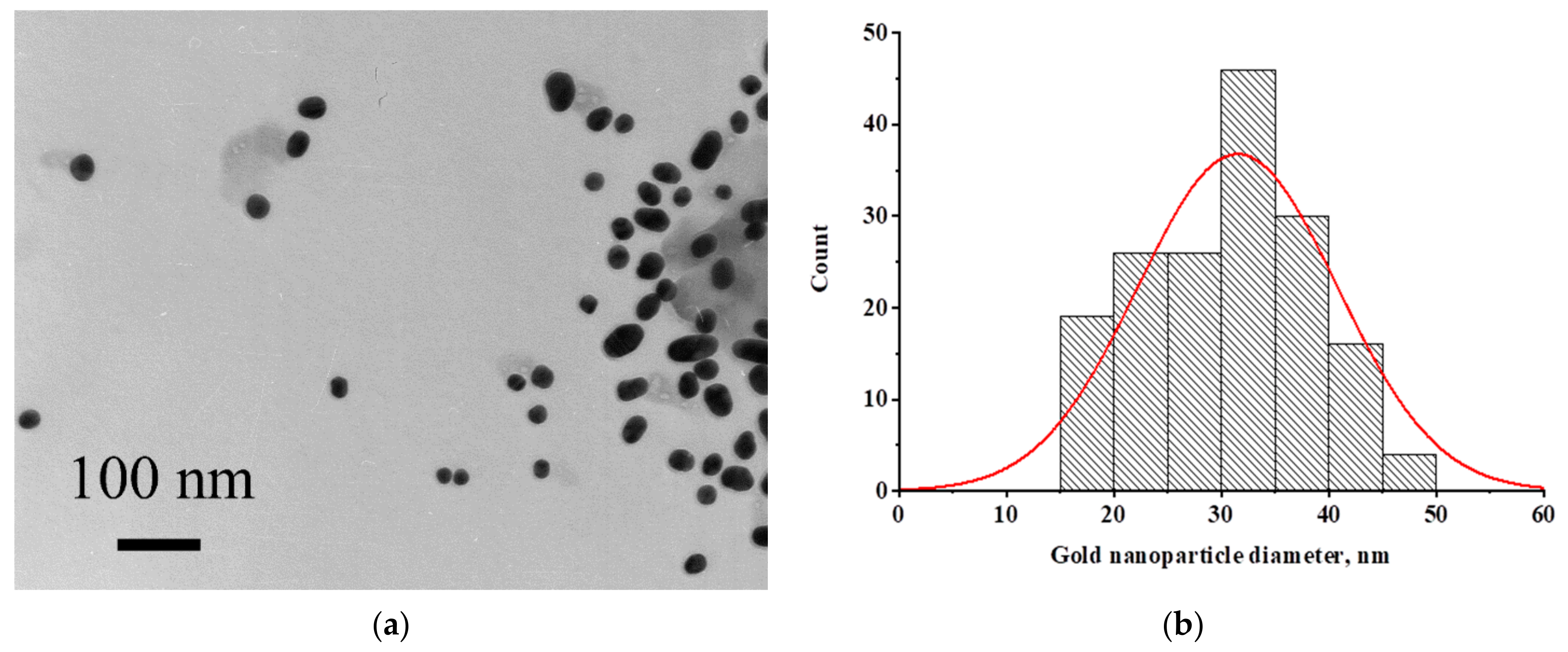
2.2. Characterization of pA–GNP
2.3. Preparation of Test Strips
2.4. Lateral Flow Immunoassay
- −
- The test strip was placed on a horizontal surface;
- −
- 60 µL of a sample was applied to the sample membrane;
- −
- the strip was incubated for 5 min;
- −
- 20 µL of TTBSA buffer (10 mM Tris, 0.25% w/v BSA, 0.25% w/v sucrose, 1.0% v/v Tween 20, 0.05% w/v NaN3, pH 8.5) was applied to the sample membrane;
- −
- the strip was incubated for 5 min.
2.5. ELISA of Human Serum
3. Results and Discussion
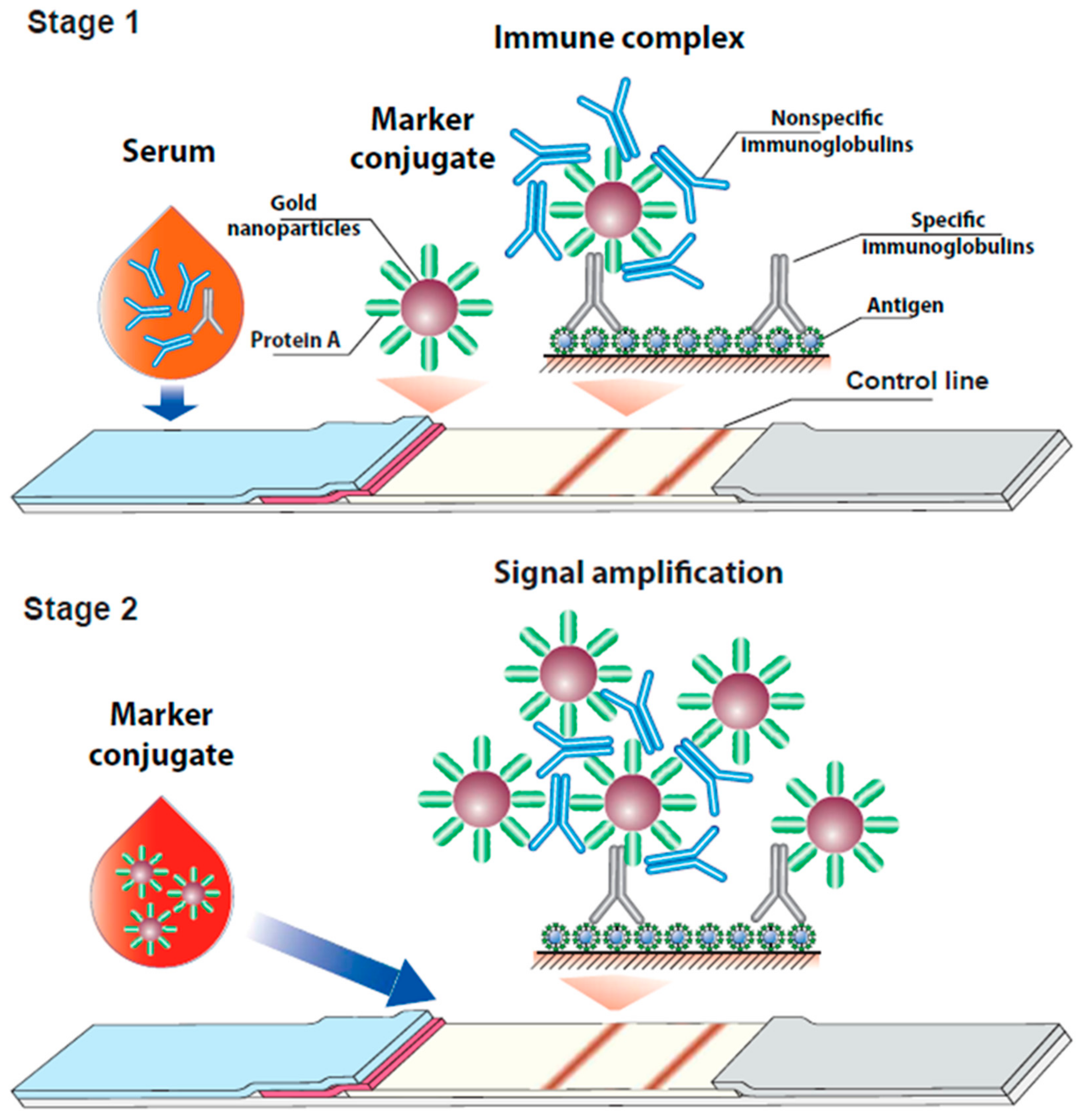
3.1. Consideration of the Proposed Assay Format
3.2. Characterization of the Conjugate of Gold Nanoparticles with Protein A
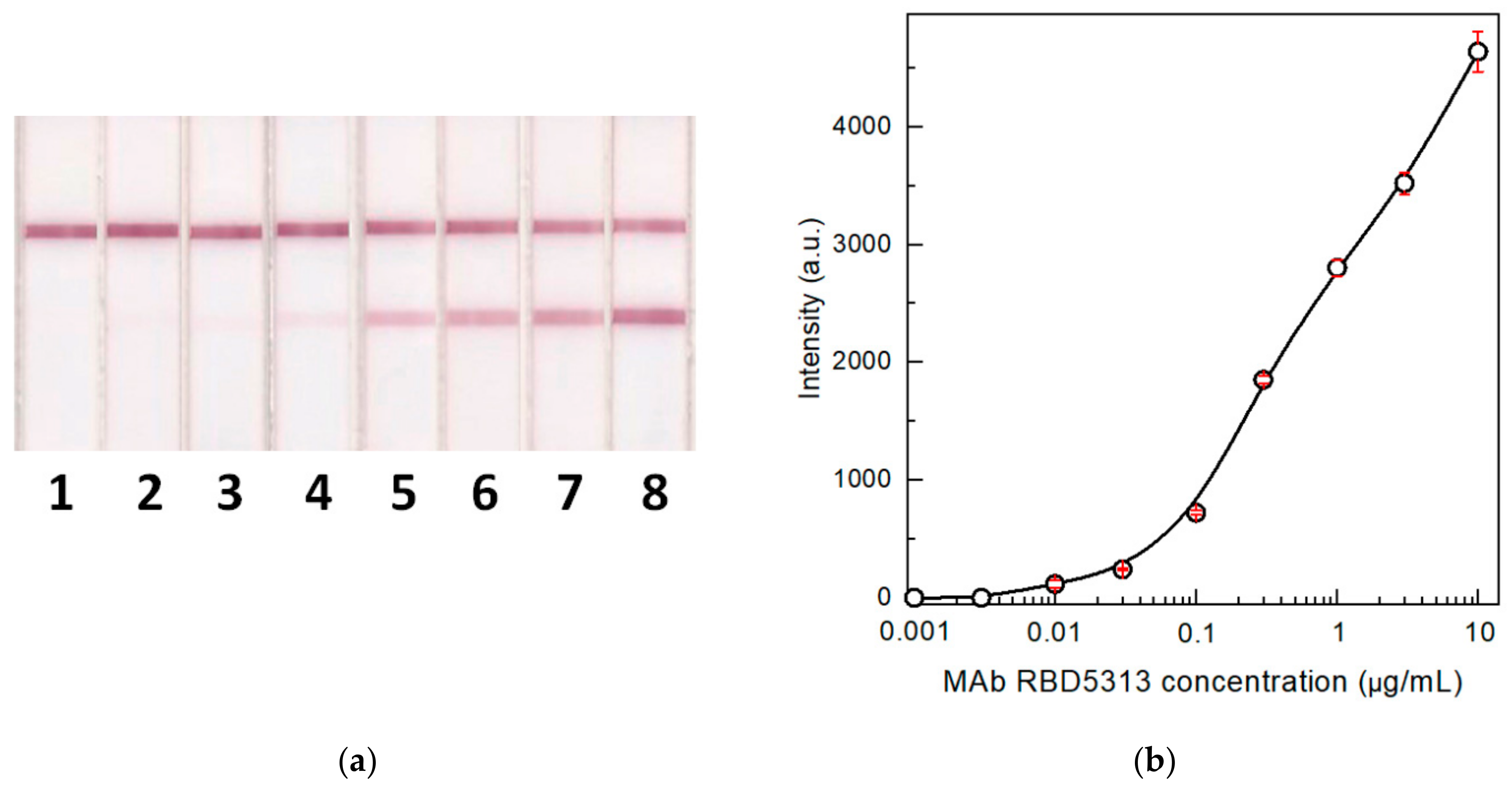
3.3. Detection of Specific IgG in Model Solutions
3.4. The Influence of Serum Components on the LFIA
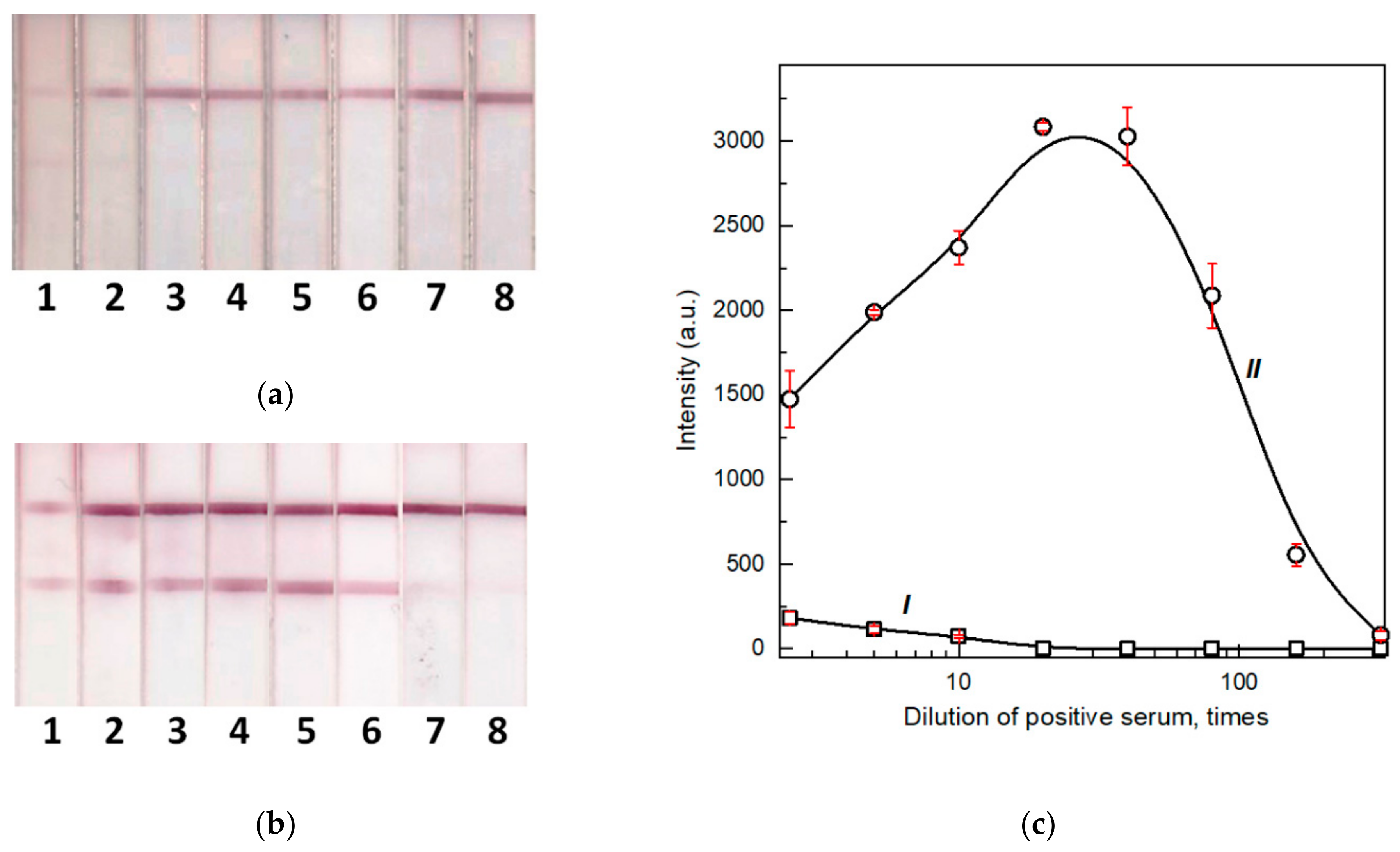
3.5. Comparison of LODs for Two Formats of the LFIA
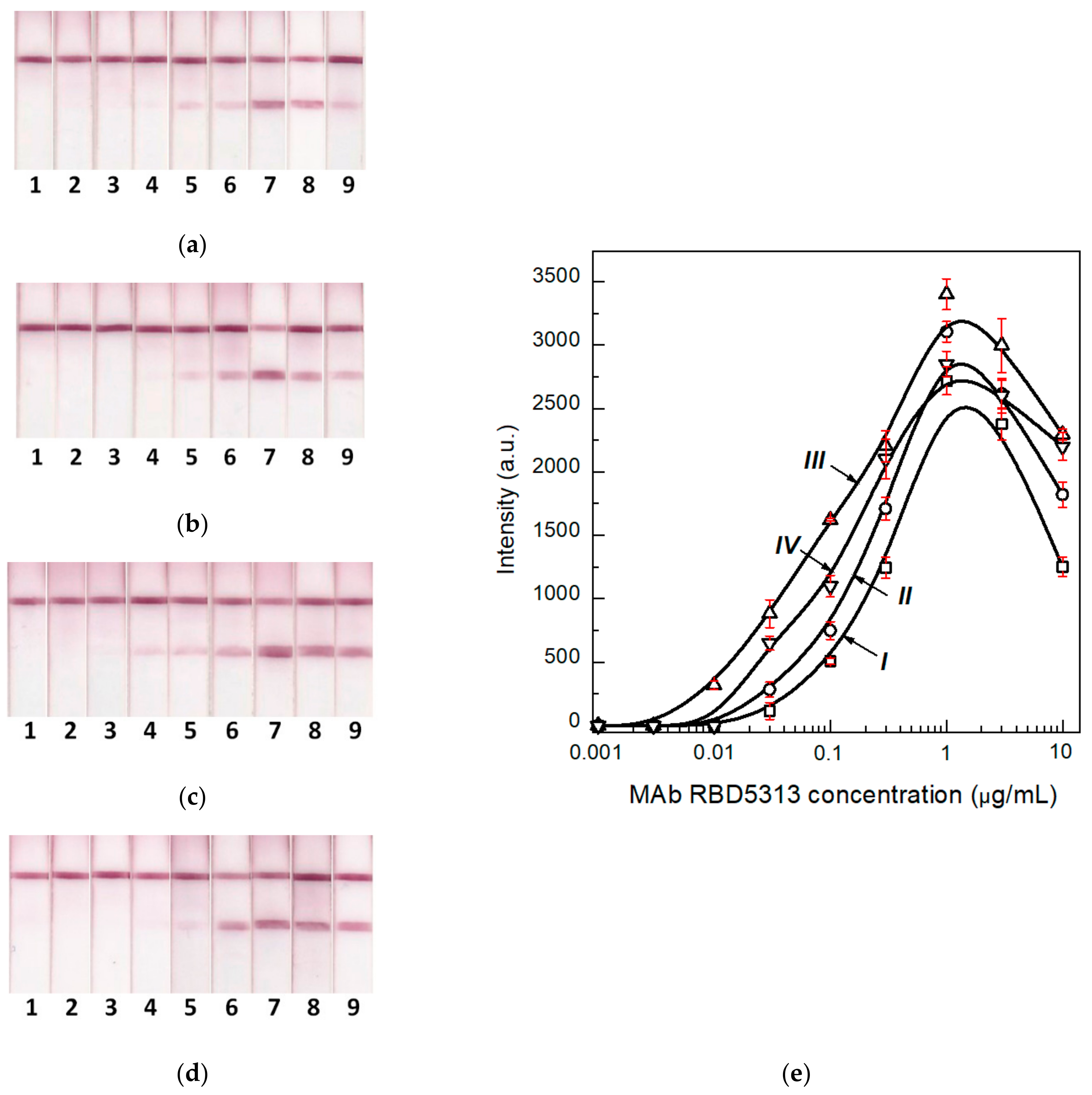
3.6. Determination of the Optimal Concentration of the Label Conjugate
3.7. Testing of the Developed LFIAs on Blood Samples of Patients with a Confirmed COVID-19 Diagnosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Champoux, J.J.; Lawrence, D.W.; Neidhardt, F.C.; Plorde, J.J. Sherris Medical Microbiology: An Introduction to Infectious Diseases, 4th ed.; McGraw-Hill: New York, NY, USA, 2004; pp. 247–249. [Google Scholar]
- Lau, S.K.P.; He, Z.; Tsang, C.C.; Chan, T.T.Y.; Luk, H.K.H.; Chan, E.; Li, K.S.M.; Fung, J.; Chow, F.W.N.; Tam, A.R.; et al. A sensitive and specific competitive enzyme-linked immunosorbent assay for serodiagnosis of COVID-19 in animals. Microorganisms 2021, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Elschner, M.C.; Laroucau, K.; Singha, H.; Tripathi, B.N.; Saqib, M.; Gardner, I.; Saini, S.; Kumar, S.; El-Adawy, H.; Melzer, F.; et al. Evaluation of the comparative accuracy of the complement fixation test, Western blot and five enzyme-linked immunosorbent assays for serodiagnosis of glanders. PLoS ONE 2019, 14, e0214963. [Google Scholar] [CrossRef] [PubMed]
- Fortes-Gabriel, E.; Guedes, M.S.; Shetty, A.; Gomes, C.K.; Carreira, T.; Vieira, M.L.; Esteves, L.; Mota-Vieira, L.; Gomes-Solecki, M. Enzyme immunoassays (EIA) for serodiagnosis of human leptospirosis: Specific IgG3/IgG1 isotyping may further inform diagnosis of acute disease. PLoS Negl. Trop. Dis. 2022, 16, e0010241. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, I.Y.; Lenain, P.; de Saeger, S. Nanosized labels for rapid immunotests. TrAC Trends Anal. Chem. 2013, 46, 30–43. [Google Scholar] [CrossRef]
- Kwizera, R.; Bongomin, F.; Olum, R.; Worodria, W.; Bwanga, F.; Meya, D.B.; Kirenga, B.J.; Gore, R.; Denning, D.W.; Fowler, S.J. Evaluation of an Aspergillus IgG/IgM lateral flow assay for serodiagnosis of fungal asthma in Uganda. PLoS ONE 2021, 16, e0252553. [Google Scholar] [CrossRef]
- Lu, J.; Wu, Z.; Liu, B.; Wang, C.; Wang, Q.; Zhang, L.; Wang, Z.; Chen, C.; Fu, Y.; Li, C.; et al. A time-resolved fluorescence lateral flow immunoassay for rapid and quantitative serodiagnosis of Brucella infection in humans. J. Pharm. Biomed. Anal. 2021, 200, 114071. [Google Scholar] [CrossRef]
- Jain, M.; Singh, A.K.; Kumar, A.; Gupta, S.; Polavarapu, R.; Sohal, J.S. Comparative performance of different antigens on the lateral flow assay (LFA) platform for the rapid serodiagnosis of paratuberculosis. J. Microbiol. Methods 2021, 192, 106367. [Google Scholar] [CrossRef]
- Lempp, F.A.; Roggenbach, I.; Nkongolo, S.; Sakin, V.; Schlund, F.; Schnitzler, P.; Wedemeyer, H.; le Gal, F.; Gordien, E.; Yurdaydin, C.; et al. A rapid point-of-care test for the serodiagnosis of hepatitis delta virus infection. Viruses 2021, 13, 2371. [Google Scholar] [CrossRef]
- Anil, M.; Navaneetha, P.; Reddy, P.V.J.; Chand, M.P.; Kumar, A.V.; Kishor, P.B.K.; Rao, K.R.S.S.; Rathnagiri, P. Development of point-of-care lateral flow immunochromatographic assay for foot and mouth disease diagnosis. Curr. Trends Biotechnol. Pharm. 2021, 15, 15–21. [Google Scholar] [CrossRef]
- Higgins, R.L.; Rawlings, S.A.; Case, J.; Lee, F.Y.; Chan, C.W.; Barrick, B.; Burger, Z.C.; Yeo, K.J.; Marrinucci, D. Longitudinal SARS-CoV-2 antibody study using the Easy Check COVID-19 IgM/IgG™ lateral flow assay. PLoS ONE 2021, 16, e0247797. [Google Scholar] [CrossRef]
- Srivastav, S.; Dankov, A.; Adanalic, M.; Grzeschik, R.; Tran, V.; Pagel-Wieder, S.; Gessler, F.; Spreitzer, I.; Scholz, T.; Schnierle, B.; et al. Rapid and sensitive SERS-based lateral flow test for SARS-CoV2-specific IgM/IgG antibodies. Anal. Chem. 2021, 93, 12391–12399. [Google Scholar] [CrossRef]
- Fu, Y.; Pan, Y.; Li, Z.; Li, Y. The utility of specific antibodies against SARS-CoV-2 in laboratory diagnosis. Front. Microbiol. 2021, 11, 603058. [Google Scholar] [CrossRef]
- Vengesai, A.; Midzi, H.; Kasambala, M.; Mutandadzi, H.; Mduluza-Jokonya, T.L.; Rusakaniko, S.; Mutapi, F.; Naicker, T.; Mduluza, T. A systematic and meta-analysis review on the diagnostic accuracy of antibodies in the serological diagnosis of COVID-19. Syst. Rev. 2021, 10, 155. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Lateral flow serodiagnosis in the double-antigen sandwich format: Theoretical consideration and confirmation of advantages. Sensors 2021, 21, 39. [Google Scholar] [CrossRef]
- Cavalera, S.; di Nardo, F.; Chiarello, M.; Serra, T.; Colitti, B.; Guiotto, C.; Fagioli, F.; Cagnazzo, C.; Denina, M.; Palazzo, A.; et al. Bacterial ligands as flexible and sensitive detectors in rapid tests for antibodies to SARS-CoV-2. Anal. Bioanal. Chem. 2022, 1–10. [Google Scholar] [CrossRef]
- Yunus, M.H.; Arifin, N.; Balachandra, D.; Anuar, N.S.; Noordin, R. Lateral flow dipstick test for serodiagnosis of strongyloidiasis. Am. J. Trop. Med. Hyg. 2019, 101, 432–435. [Google Scholar] [CrossRef]
- Prakash, C.; Kumar, B.; Singh, R.P.; Singh, P.G.; Shrinet, G.; Das, A.R.; Ashmi, M.; Abhishek, S.K.P.; Singh, M.; Gupta, V.K. Development and evaluation of a gold nanoparticle based lateral flow assay (LFA) strip test for detection of Brucella spp. J. Microbiol. Methods 2021, 184, 106185. [Google Scholar] [CrossRef]
- Manasa, M.; Revathi, P.; Chand, M.P.; Maroudam, V.; Navaneetha, P.; Raj, G.D.; Kishor, P.B.K.; De, B.; Rathnagiri, P. Protein-G-based lateral flow assay for rapid serodiagnosis of brucellosis in domesticated animals. J. Immunoass. Immunochem. 2019, 40, 149–158. [Google Scholar] [CrossRef]
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten years of lateral flow immunoassay technique applications: Trends, challenges and future perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Mathematical model of serodiagnostic immunochromatographic assay. Anal. Chem. 2017, 89, 4419–4427. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Berlina, A.N.; Zherdev, A.V.; Eskendirova, S.Z.; Mukanov, K.K.; Ramankulov, Y.R.; Mukantayev, K.N.; Dzantiev, B.B. Comparison of three schemes of quantum dots-based immunochromatography for serodiagnosis of brucellosis in cattle. J. Eng. Appl. Sci. 2019, 14, 3711–3718. [Google Scholar] [CrossRef][Green Version]
- Karakus, C.; Salih, B.A. Comparison of the lateral flow immunoassays (LFIA) for the diagnosis of Helicobacter pylori infection. J. Immunol. Methods 2013, 396, 8–14. [Google Scholar] [CrossRef]
- Cavalera, S.; Di Nardo, F.; Forte, L.; Marinoni, F.; Chiarello, M.; Baggiani, C.; Anfossi, L. Switching from multiplex to multimodal colorimetric lateral flow immunosensor. Sensors 2020, 20, 6609. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Theoretical and experimental comparison of different formats of immunochromatographic serodiagnostics. Sensors 2018, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sernández, V.; Muiño, L.; Perteguer, M.J.; Gárate, T.; Mezo, M.; González-Warleta, M.; Muro, A.; da Costa, J.M.C.; Romarís, F.; Ubeira, F.M. Development and evaluation of a new lateral flow immunoassay for serodiagnosis of human fasciolosis. PLoS Negl. Trop. Dis. 2011, 5, e1376. [Google Scholar] [CrossRef]
- Ben-Selma, W.; Harizi, H.; Boukadida, J. Immunochromatographic IgG/IgM test for rapid diagnosis of active tuberculosis. Clin. Vaccine Immunol. 2011, 18, 2090–2094. [Google Scholar] [CrossRef]
- Wu, H.S.; Chiu, S.C.; Tseng, T.C.; Lin, S.F.; Lin, J.H.; Hsu, Y.H.; Wang, M.C.; Lin, T.L.; Yang, W.Z.; Ferng, T.L.; et al. Serologic and molecular biologic methods for SARS-associated coronavirus infection, Taiwan. Emerg. Infect. Dis. 2004, 10, 304–310. [Google Scholar] [CrossRef]
- Sato, N.S.; de Melo, C.S.; Zerbini, L.C.; Silveira, E.P.; Fagundes, L.J.; Ueda, M. Assessment of the rapid test based on an immunochromatography technique for detecting anti-Treponema pallidum antibodies. Rev. Inst. Med. Trop. Sao Paulo 2003, 45, 319–322. [Google Scholar] [CrossRef][Green Version]
- Vrublevskaya, V.V.; Afanasyev, V.N.; Grinevich, A.A.; Skarga, Y.Y.; Gladyshev, P.P.; Ibragimova, S.A.; Krylsky, D.V.; Dezhurov, S.V.; Morenkov, O.S. A sensitive and specific lateral flow assay for rapid detection of antibodies against glycoprotein B of Aujeszky’s disease virus. J. Virol. Methods 2017, 249, 175–180. [Google Scholar] [CrossRef]
- Jia, J.; Ao, L.; Luo, Y.; Liao, T.; Huang, L.; Zhuo, D.; Jiang, C.; Wang, J.; Hu, J. Quantum dots assembly enhanced and dual-antigen sandwich structured lateral flow immunoassay of SARS-CoV-2 antibody with simultaneously high sensitivity and specificity. Biosens. Bioelectron. 2022, 198, 113810. [Google Scholar] [CrossRef]
- Liu, H.; Dai, E.; Xiao, R.; Zhou, Z.; Zhang, M.; Bai, Z.; Shao, Y.; Qi, K.; Tu, J.; Wang, C.; et al. Development of a SERS-based lateral flow immunoassay for rapid and ultra-sensitive detection of anti-SARS-CoV-2 IgM/IgG in clinical samples. Sens. Actuators B Chem. 2021, 329, 129196. [Google Scholar] [CrossRef] [PubMed]
- Panferov, V.G.; Byzova, N.A.; Biketov, S.F.; Zherdev, A.V.; Dzantiev, B.B. Comparative study of in situ techniques to enlarge gold nanoparticles for highly sensitive lateral flow immunoassay of SARS-CoV-2. Biosensors 2021, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Sotnikov, D.V.; Barshevskaya, L.V.; Zherdev, A.V.; Eskendirova, S.Z.; Mukanov, K.K.; Mukantayev, K.K.; Ramanculov, Y.M.; Dzantiev, B.B. Immunochromatographic system for serodiagnostics of cattle brucellosis using gold nanoparticles and signal amplification with quantum dots. Appl. Sci. 2020, 10, 738. [Google Scholar] [CrossRef]
- Sinegubova, M.V.; Orlova, N.A.; Kovnir, S.V.; Dayanova, L.K.; Vorobiev, I.I. High-level expression of the monomeric SARS-CoV-2 S protein RBD 320-537 in stably transfected CHO cells by the EEF1A1-based plasmid vector. PLoS ONE 2021, 16, e0242890. [Google Scholar] [CrossRef]
- Meulenbroek, A.J. Human IgG Subclasses Useful Diagnostic Markers for Immunocompetence, 3rd ed.; Sanquin: Amsterdam, The Netherlands, 2008; 55p. [Google Scholar]
- Sotnikov, D.V.; Byzova, N.A.; Zherdev, A.V.; Dzantiev, B.B. Retention of activity by antibodies immobilized on gold nanoparticles of different sizes: Fluorometric method of determination and comparative evaluation. Nanomaterials 2021, 11, 3117. [Google Scholar] [CrossRef]
- Choi, D.H.; Lee, S.K.; Oh, Y.K.; Bae, B.W.; Lee, S.D.; Kim, S.; Shin, Y.-B.; Kim, M.G. A dual gold nanoparticle conjugate-based lateral flow assay (LFA) method for the analysis of troponin I. Biosens. Bioelectron. 2010, 25, 1999–2002. [Google Scholar] [CrossRef]
- Taranova, N.A.; Urusov, A.E.; Sadykhov, E.G.; Zherdev, A.V.; Dzantiev, B.B. Bifunctional gold nanoparticles as an agglomeration-enhancing tool for highly sensitive lateral flow tests: A case study with procalcitonin. Microchim. Acta 2017, 184, 4189–4195. [Google Scholar] [CrossRef]
- Kemper, M.J.; Altrogge, H.; Ganschow, R.; Müller-Wiefel, D.E. Serum levels of immunoglobulins and IgG subclasses in steroid sensitive nephrotic syndrome. Pediatr. Nephrol. 2002, 17, 413–417. [Google Scholar] [CrossRef]
- Tuerlinckx, D.; Florkin, B.; Ferster, A.; De Schutter, I.; Chantrain, C.; Haerynck, F.; Philippet, P.; Strengers, P.; Laub, R. Pneumococcal antibody levels in children with PID receiving immunoglobulin. Pediatrics 2014, 133, e154–e162. [Google Scholar] [CrossRef]
- Turner, P.; Turner, C.; Green, N.; Ashton, L.; Lwe, E.; Jankhot, A.; Day, N.P.; White, N.J.; Nosten, F.; Goldblatt, D. Serum antibody responses to pneumococcal colonization in the first 2 years of life: Results from an SE Asian longitudinal cohort study. Clin. Microbiol. Infect. 2013, 19, E551–E558. [Google Scholar] [CrossRef]
- Gong, F.; Wei, H.X.; Li, Q.; Liu, L.; Li, B. Evaluation and comparison of serological methods for COVID-19 diagnosis. Front. Mol. Biosci. 2021, 8, 682405. [Google Scholar] [CrossRef]
- Bastos, M.L.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for COVID-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef] [PubMed]
- Mohit, E.; Rostami, Z.; Vahidi, H.A. A comparative review of immunoassays for COVID-19 detection. Expert Rev. Clin. Immunol. 2021, 17, 573–599. [Google Scholar] [CrossRef]


| Positive Sera | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Common LFIA |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| I (a.u.) | 208 | 135 | 140 | 135 | 155 | 130 | 142 | 180 | 90 | 85 | 0 | 0 | 0 | 0 | 0 | 35 |
| + | + | + | + | + | + | + | + | + | - | - | - | - | - | - | - | |
| Enhanced LFIA |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| I (a.u.) | 585 | 635 | 175 | 525 | 1650 | 1285 | 880 | 2520 | 775 | 1780 | 125 | 956 | 1430 | 785 | 2350 | 3575 |
| + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| ELISA (A 450) | 0.807 | 1.034 | 0.299 | 1.004 | 1.114 | 0.957 | 0.747 | 1.038 | 0.848 | 1.124 | 0.256 | 0.730 | 0.932 | 0.659 | 0.899 | 1.072 |
| Negative Sera | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||||||||
| Common LFIA |  |  |  |  |  |  |  |  | ||||||||
| I (a.u.) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| - | - | - | - | - | - | - | - | |||||||||
| Enhanced LFIA |  |  |  |  |  |  |  |  | ||||||||
| I (a.u.) | 0 | 65 | 75 | 0 | 55 | 0 | 0 | 0 | ||||||||
| - | - | - | - | - | - | - | - | |||||||||
| ELISA (A 450) | 0.125 | 0.184 | 0.198 | 0.093 | 0.128 | 0.120 | 0.191 | 0.228 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotnikov, D.V.; Byzova, N.A.; Zherdev, A.V.; Xu, Y.; Dzantiev, B.B. Silent Antibodies Start Talking: Enhanced Lateral Flow Serodiagnosis with Two-Stage Incorporation of Labels into Immune Complexes. Biosensors 2022, 12, 434. https://doi.org/10.3390/bios12070434
Sotnikov DV, Byzova NA, Zherdev AV, Xu Y, Dzantiev BB. Silent Antibodies Start Talking: Enhanced Lateral Flow Serodiagnosis with Two-Stage Incorporation of Labels into Immune Complexes. Biosensors. 2022; 12(7):434. https://doi.org/10.3390/bios12070434
Chicago/Turabian StyleSotnikov, Dmitriy V., Nadezhda A. Byzova, Anatoly V. Zherdev, Youchun Xu, and Boris B. Dzantiev. 2022. "Silent Antibodies Start Talking: Enhanced Lateral Flow Serodiagnosis with Two-Stage Incorporation of Labels into Immune Complexes" Biosensors 12, no. 7: 434. https://doi.org/10.3390/bios12070434
APA StyleSotnikov, D. V., Byzova, N. A., Zherdev, A. V., Xu, Y., & Dzantiev, B. B. (2022). Silent Antibodies Start Talking: Enhanced Lateral Flow Serodiagnosis with Two-Stage Incorporation of Labels into Immune Complexes. Biosensors, 12(7), 434. https://doi.org/10.3390/bios12070434








