High Fundamental Frequency (HFF) Monolithic Quartz Crystal Microbalance with Dissipation Array for the Simultaneous Detection of Pesticides and Antibiotics in Complex Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honey Samples, Chemicals and Immunoreagents
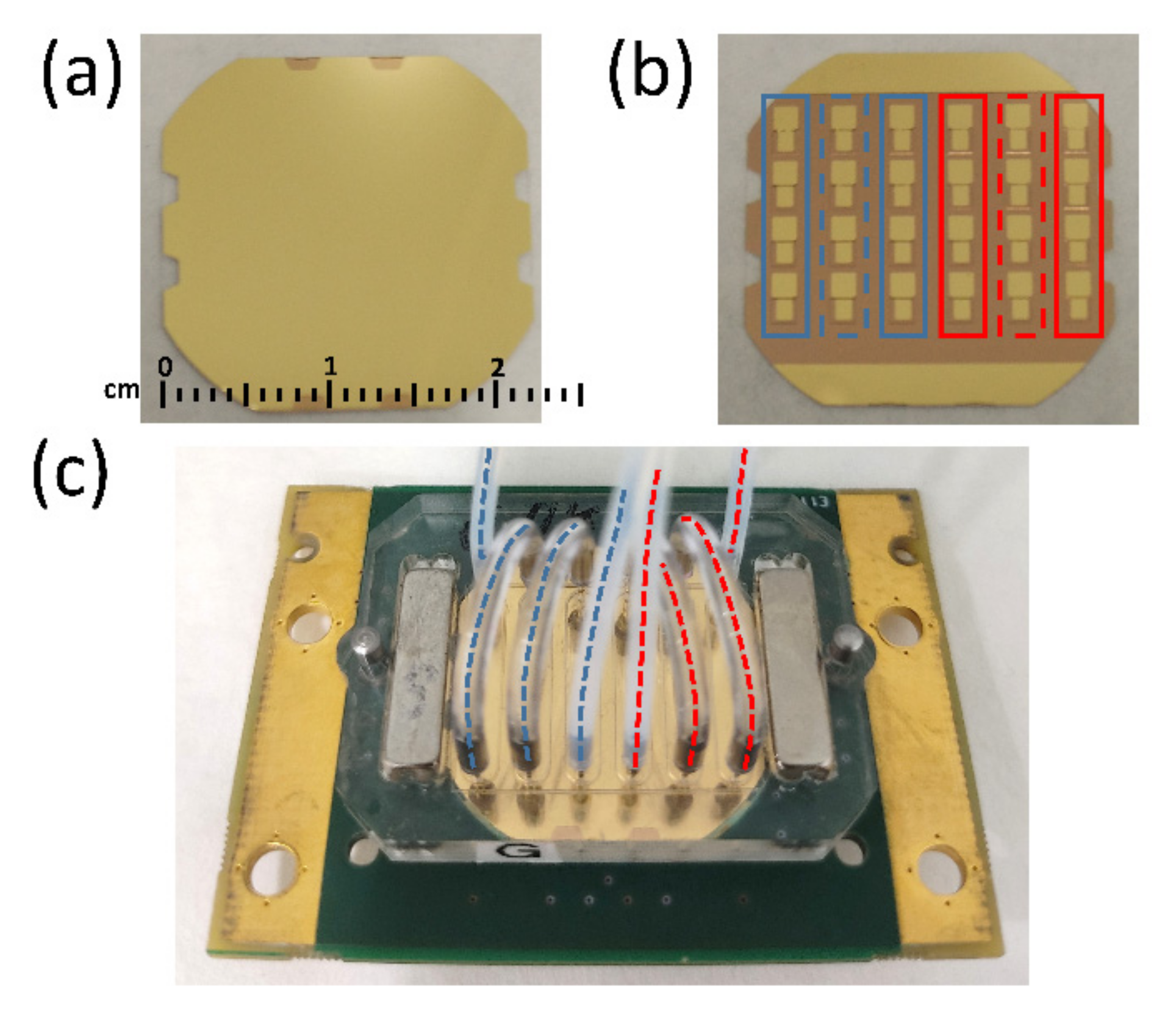
2.2. HFF-QCMD Array Methodology
2.2.1. HFF-QCMD Array Immunosensor Setup
2.2.2. Detection Format and Array Sensor Functionalization
2.2.3. Immunoassay Protocol
3. Results and Discussion
3.1. Immunoassay Optimization: Selection of the Immunoreagent Concentrations
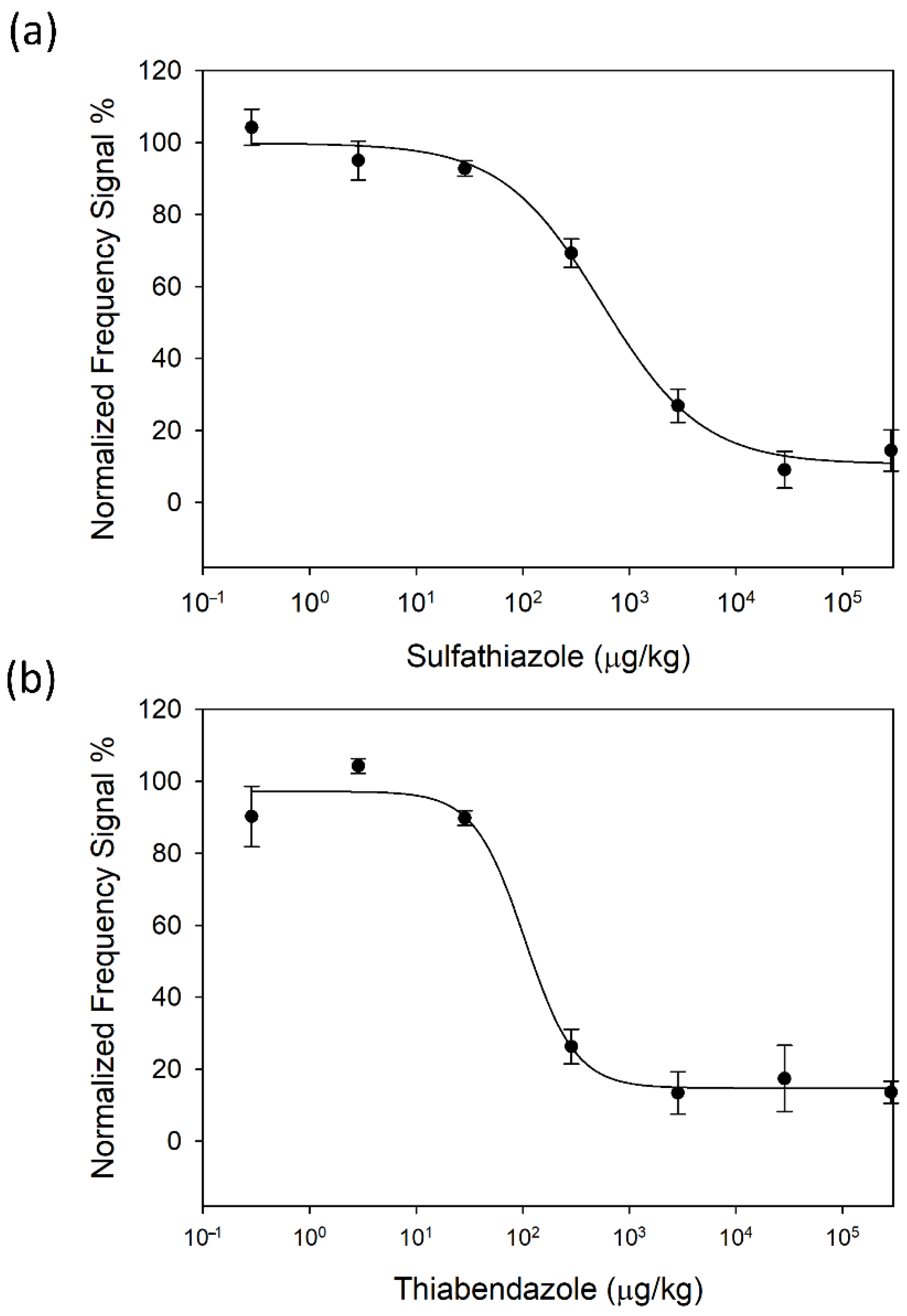
3.2. Standard Calibration Curves: Sample Pre-Treatment and Immunoassay Sensitivity
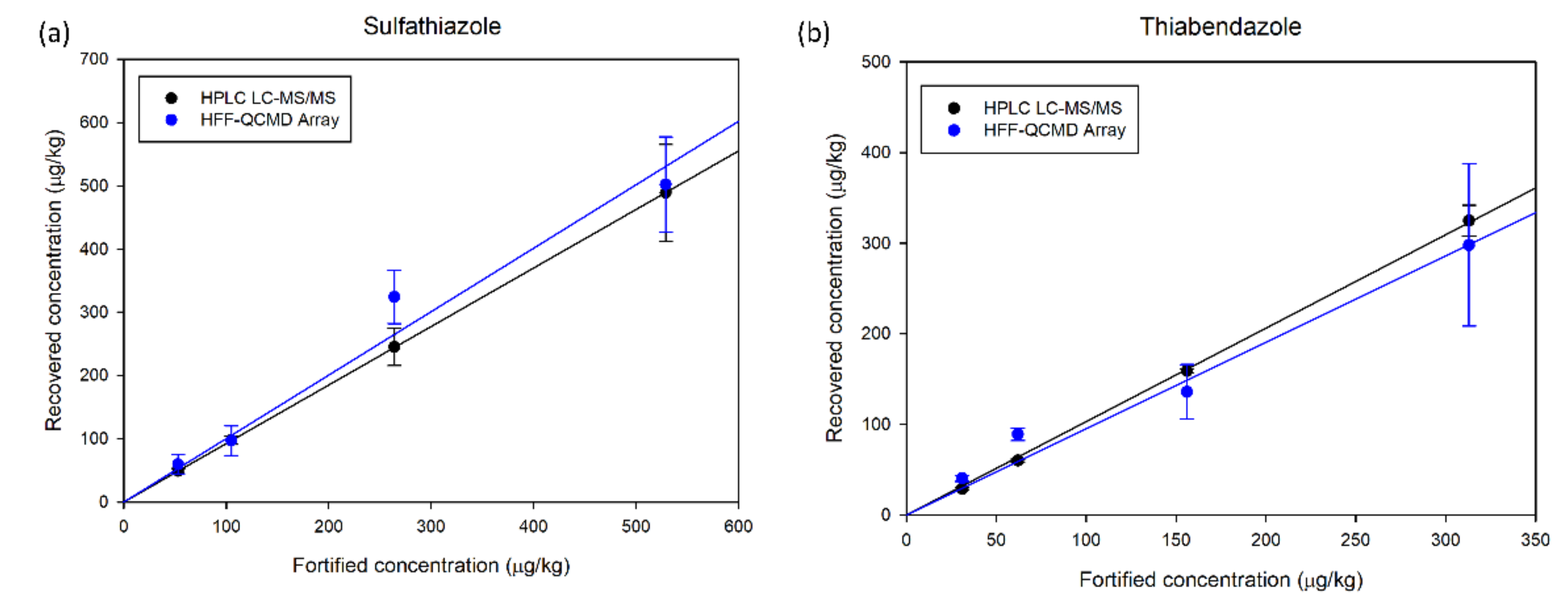
3.3. HFF-QCMD Array Validation with HPLC LC-MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Van Klaveren, J.D.; Boon, P.E. Probabilistic risk assessment of dietary exposure to single and multiple pesticide residues or contaminants: Summary of the work performed within the SAFE FOODS project. Food Chem. Toxicol. 2009, 47, 2879–2882. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.C. Risk assessment of chemicals in European traditional foods. Trends Food Sci. Technol. 2006, 17, 471–481. [Google Scholar] [CrossRef]
- Dorne, J.L.C.M.; Dorne, J.L.C.M.; Bordajandi, L.R.; Amzal, B.; Ferrari, P.; Verger, P. Combining analytical techniques, exposure assessment and biological effects for risk assessment of chemicals in food. TrAC Trends Anal. Chem. 2009, 28, 695–707. [Google Scholar] [CrossRef]
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.D.; Pettis, J.; Vanengelsdorp, D. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012, 58, 613–620. [Google Scholar] [CrossRef]
- Daisley, B.A.; Chmiel, J.A.; Pitek, A.P.; Thompson, G.J.; Reid, G. Missing Microbes in Bees: How Systematic Depletion of Key Symbionts Erodes Immunity. Trends Microbiol. 2020, 28, 1010–1021. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.-F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple Routes of Pesticide Exposure for Honey Bees Living Near Agricultural Fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, Pesticide, and Microbial Contaminants of Honey: Human Health Hazards. Sci. World J. 2012, 2012, 930849. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32005R0396 (accessed on 20 November 2021).
- Juan-Borrás, M.; Domenech, E.; Escriche, I. Mixture-risk-assessment of pesticide residues in retail polyfloral honey. Food Control 2016, 67, 127–134. [Google Scholar] [CrossRef]
- Souza Tette, P.A.; Guidi, L.R.; De Abreu Glória, M.B.; Fernandes, C. Pesticides in honey: A review on chromatographic analytical methods. Talanta 2016, 149, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Masiá, A.; Suarez-Varela, M.M.; Llopis-Gonzalez, A.; Picó, Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: A review. Anal. Chim. Acta 2016, 936, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Bargańska, Ż.; Namieśnik, J.; Ślebioda, M. Determination of antibiotic residues in honey. TrAC Trends Anal. Chem. 2011, 30, 1035–1041. [Google Scholar] [CrossRef]
- Debayle, D.; Dessalces, G.; Grenier-Loustalot, M.F. Multi-residue analysis of traces of pesticides and antibiotics in honey by HPLC-MS-MS. Anal. Bioanal. Chem. 2008, 391, 1011–1020. [Google Scholar] [CrossRef]
- Abad, A.; Montoya, A. Development of an Enzyme-Linked Immunosorbent Assay to Carbaryl. 2. Assay Optimization and Application to the Analysis of Water Samples. J. Agric. Food Chem. 1997, 45, 1495–1501. [Google Scholar] [CrossRef]
- González-Martínez, M.A.; Morais, S.; Puchades, R.; Maquieira, A.; Abad, A.; Montoya, A. Development of an automated controlled-pore glass flow-through immunosensor for carbaryl. Anal. Chim. Acta 1997, 347, 199–205. [Google Scholar] [CrossRef]
- Marco, M.P.; Gee, S.J.; Cheng, H.M.; Liang, Z.Y.; Hammock, B.D. Development of an Enzyme-Linked Immunosorbent Assay for Carbaryl. J. Agric. Food Chem. 1993, 41, 423–430. [Google Scholar] [CrossRef]
- Nunes, G.S.; Toscano, I.A.; Barceló, D. Analysis of pesticides in food and environmental samples by enzyme- linked immunosorbent assays. TrAC Trends Anal. Chem. 1998, 17, 79–87. [Google Scholar] [CrossRef]
- Qian, G.; Wang, L.; Wu, Y.; Zhang, Q.; Sun, Q.; Liu, Y.; Liu, F. A monoclonal antibody-based sensitive enzyme-linked immunosorbent assay (ELISA) for the analysis of the organophosphorous pesticides chlorpyrifos-methyl in real samples. Food Chem. 2009, 117, 364–370. [Google Scholar] [CrossRef]
- Barrasso, R.; Bonerba, E.; Savarino, A.; Ceci, E.; Bozzo, G.; Tantillo, G. Simultaneous Quantitative Detection of Six Families of Antibiotics in Honey Using A Biochip Multi-Array Technology. Vet. Sci. 2018, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Robert, S. Charm II System-Comprehensive residue analysis system for honey. Apiacta 2003, 38, 198–206. [Google Scholar]
- Nirschl, M.; Reuter, F.; Vörös, J. Review of Transducer Principles for Label-Free Biomolecular Interaction Analysis. Biosensors 2011, 1, 70–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudin, V. Advances in biosensor development for the screening of antibiotic residues in food products of animal origin—A comprehensive review. Biosens. Bioelectron. 2017, 90, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Johannsmann, D. The Quartz Crystal Microbalance in Soft Matter Research. In Soft and Biological Matter; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-07835-9. [Google Scholar]
- March, C.; García, J.V.; Sánchez, Á.; Arnau, A.; Jiménez, Y.; García, P.; Manclús, J.J.; Montoya, Á. High-frequency phase shift measurement greatly enhances the sensitivity of QCM immunosensors. Biosens. Bioelectron. 2015, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; March, C.; Montagut, Y.; Moreno, M.; Manclus, J.; Arnau, A.; Jimenez, Y.; Jaramillo, M.; Marin, P.; Torres, R. A High Fundamental Frequency (HFF)-based QCM Immunosensor for Tuberculosis Detection. Curr. Top. Med. Chem. 2017, 17, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, B.; Lucklum, R.; Hauptmann, P.; Rabe, J.; Büttgenbach, S. Electrical characterisation of high-frequency thickness-shear-mode resonators by impedance analysis. Sens. Actuators B Chem. 2001, 76, 47–57. [Google Scholar] [CrossRef]
- Fernández, R.; García, P.; García, M.; García, J.; Jiménez, Y.; Arnau, A. Design and Validation of a 150 MHz HFFQCM Sensor for Bio-Sensing Applications. Sensors 2017, 17, 2057. [Google Scholar] [CrossRef]
- Cervera-Chiner, L.; March, C.; Arnau, A.; Jiménez, Y.; Montoya, Á. Detection of DDT and carbaryl pesticides in honey by means of immunosensors based on high fundamental frequency quartz crystal microbalance (HFF-QCM). J. Sci. Food Agric. 2020, 100, 2468–2472. [Google Scholar] [CrossRef]
- Cervera-Chiner, L.; Juan-Borrás, M.; March, C.; Arnau, A.; Escriche, I.; Montoya, Á.; Jiménez, Y. High Fundamental Frequency Quartz Crystal Microbalance (HFF-QCM) immunosensor for pesticide detection in honey. Food Control 2018, 92, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cervera-Chiner, L.; Jiménez, Y.; Montoya, Á.; Juan-Borrás, M.; Pascual, N.; Arnau, A.; Escriche, I. High Fundamental Frequency Quartz Crystal Microbalance (HFF-QCMD) Immunosensor for detection of sulfathiazole in honey. Food Control 2020, 115, 107296. [Google Scholar] [CrossRef]
- Fernandez, R.; Calero, M.; Reiviakine, I.; Garcia, J.V.; Rocha-Gaso, M.I.; Arnau, A.; Jimenez, Y. High Fundamental Frequency (HFF) Monolithic Resonator Arrays for Biosensing Applications: Design, Simulations, Experimental Characterization. IEEE Sens. J. 2020, 21, 284–295. [Google Scholar] [CrossRef]
- Tuantranont, A.; Wisitsora-at, A.; Sritongkham, P.; Jaruwongrungsee, K. A review of monolithic multichannel quartz crystal microbalance: A review. Anal. Chim. Acta 2011, 687, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Hung, V.N.; Abe, T.; Minh, P.N.; Esashi, M. Miniaturized, highly sensitive single-chip multichannel quartz-crystal microbalance. Appl. Phys. Lett. 2002, 81, 5069–5071. [Google Scholar] [CrossRef]
- Abe, T.; Esashi, M. One-chip multuchannel quartz crystal microbalance fabricated by deep RIE. Sens. Actuators A Phys. 2000, 82, 139–143. [Google Scholar] [CrossRef]
- Abad, A.; Manclús, J.J.; Moreno, M.J.; Montoya, A. Determination of Thiabendazole in Fruit Juices by a New Monoclonal Enzyme Immunoassay. J. AOAC Int. 2001, 84, 156–161. [Google Scholar] [CrossRef]
- Calero, M.; Fernández, R.; García, P.; García, J.V.; García, M.; Gamero-Sandemetrio, E.; Reviakine, I.; Arnau, A.; Jiménez, Y. A Multichannel Microfluidic Sensing Cartridge for Bioanalytical Applications of Monolithic Quartz Crystal Microbalance. Biosensors 2020, 10, 189. [Google Scholar] [CrossRef]
- Fernandez, R.; Calero, M.; Garcia-Narbon, J.V.; Reviakine, I.; Arnau, A.; Jimenez, Y. A Fast Method for Monitoring the Shifts in Resonance Frequency and Dissipation of the QCM Sensors of a Monolithic Array in Biosensing Applications. IEEE Sens. J. 2021, 21, 6643–6651. [Google Scholar] [CrossRef]
- SANCO, D.G. European Comission, Health.& Consumer Protection Directorate General. Available online: https://ec.europa.eu/health/ph_overview/strategy/docs/consultation_frep_en.pdf (accessed on 20 May 2022).


| SFZ (µg/kg) * | TBZ (µg/kg) * | |
|---|---|---|
| 554.6 | 104.6 | |
| LOD | 53 | 31.3 |
| LOQ | 122.8 | 48.9 |
| WR | 122.8 to 2490.7 | 48.9 to 223.7 |
| Spiked Level (μg/kg) | Recovered (μg/kg) | Recovery (%) | CV (%) | Detected (μg/kg) | Recovery (%) | CV (%) |
|---|---|---|---|---|---|---|
| HFF-QCMD array 1 | LC-MS/MS 2 | |||||
| 0 | <LOD | No false positives | <LOD | No false positives | ||
| 31 | 40 ± 3 | 130 | 8 | 29 ± 1.2 | 94 | 4 |
| 62 | 89 ± 6 | 143 | 7 | 60 ± 1.6 | 97 | 2.6 |
| 156 | 135 ± 30 | 87 | 22 | 159 ± 2.4 | 102 | 1.5 |
| 313 | 297 ± 89 | 95 | 30 | 352 ± 17.2 | 104 | 5.3 |
| Spiked Level (μg/kg) | Recovered (μg/kg) | Recovery (%) | CV (%) | Detected (μg/kg) | Recovery (%) | CV (%) |
|---|---|---|---|---|---|---|
| HFF-QCMD array 1 | LC-MS/MS 2 | |||||
| 0 | <LOD | No false positives | <LOD | No false positives | ||
| 53 | 59 ± 14 | 112 | 25 | 49 ± 4 | 94 | 8.2 |
| 105 | 97 ± 23 | 92 | 24 | 98 ± 6.4 | 93 | 6.5 |
| 264 | 324 ± 42 | 122 | 13 | 245 ± 29.4 | 93 | 12 |
| 529 | 502 ± 75 | 94 | 14 | 489 ± 76.3 | 92 | 15.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calero, M.; Fernández, R.; García, M.; Juan-Borrás, M.; Escriche, I.; Arnau, A.; Montoya, Á.; Jiménez, Y. High Fundamental Frequency (HFF) Monolithic Quartz Crystal Microbalance with Dissipation Array for the Simultaneous Detection of Pesticides and Antibiotics in Complex Food. Biosensors 2022, 12, 433. https://doi.org/10.3390/bios12060433
Calero M, Fernández R, García M, Juan-Borrás M, Escriche I, Arnau A, Montoya Á, Jiménez Y. High Fundamental Frequency (HFF) Monolithic Quartz Crystal Microbalance with Dissipation Array for the Simultaneous Detection of Pesticides and Antibiotics in Complex Food. Biosensors. 2022; 12(6):433. https://doi.org/10.3390/bios12060433
Chicago/Turabian StyleCalero, María, Román Fernández, María García, Marisol Juan-Borrás, Isabel Escriche, Antonio Arnau, Ángel Montoya, and Yolanda Jiménez. 2022. "High Fundamental Frequency (HFF) Monolithic Quartz Crystal Microbalance with Dissipation Array for the Simultaneous Detection of Pesticides and Antibiotics in Complex Food" Biosensors 12, no. 6: 433. https://doi.org/10.3390/bios12060433
APA StyleCalero, M., Fernández, R., García, M., Juan-Borrás, M., Escriche, I., Arnau, A., Montoya, Á., & Jiménez, Y. (2022). High Fundamental Frequency (HFF) Monolithic Quartz Crystal Microbalance with Dissipation Array for the Simultaneous Detection of Pesticides and Antibiotics in Complex Food. Biosensors, 12(6), 433. https://doi.org/10.3390/bios12060433





