Transducer Technologies for Biosensors and Their Wearable Applications
Abstract
:1. Introduction
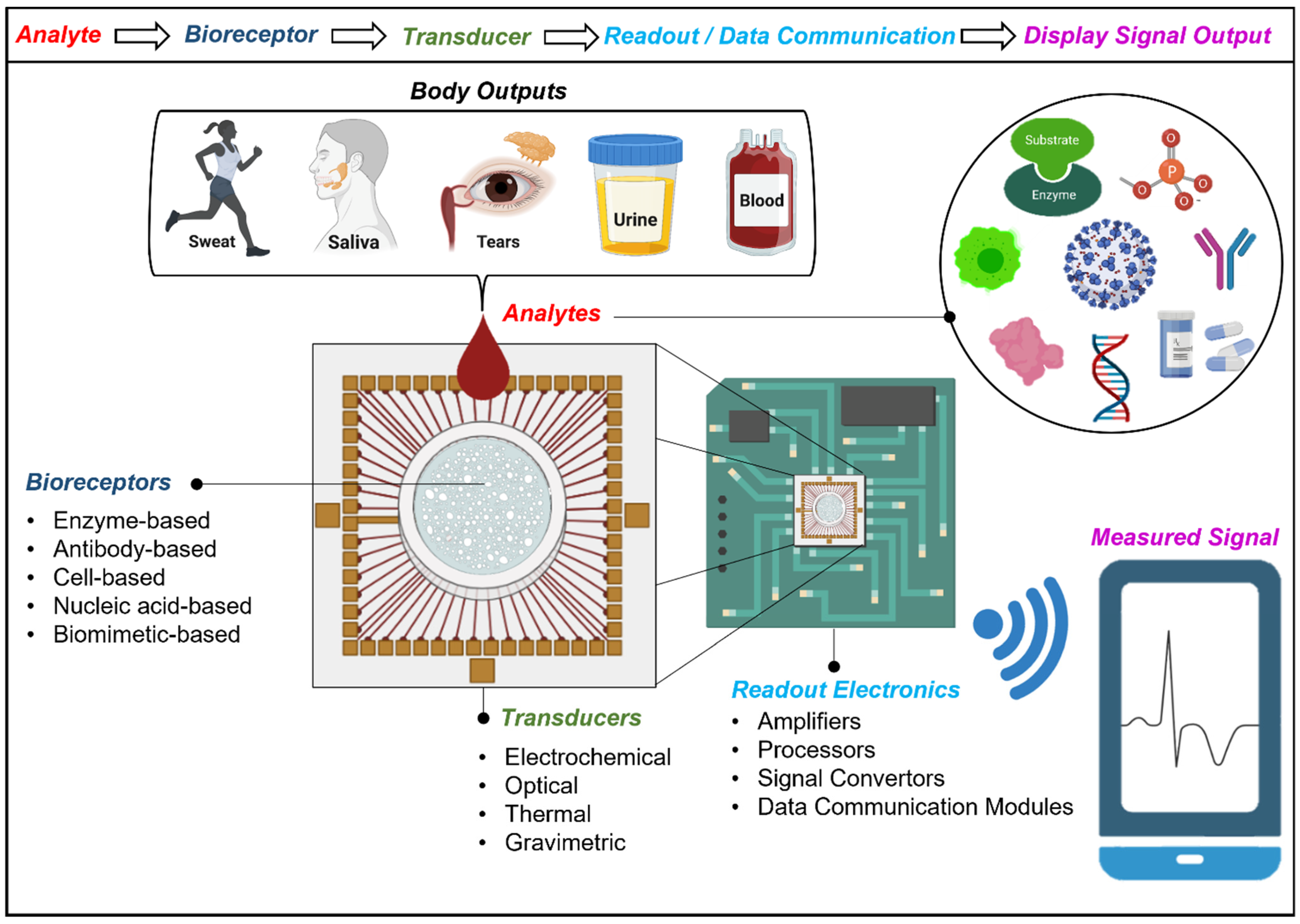
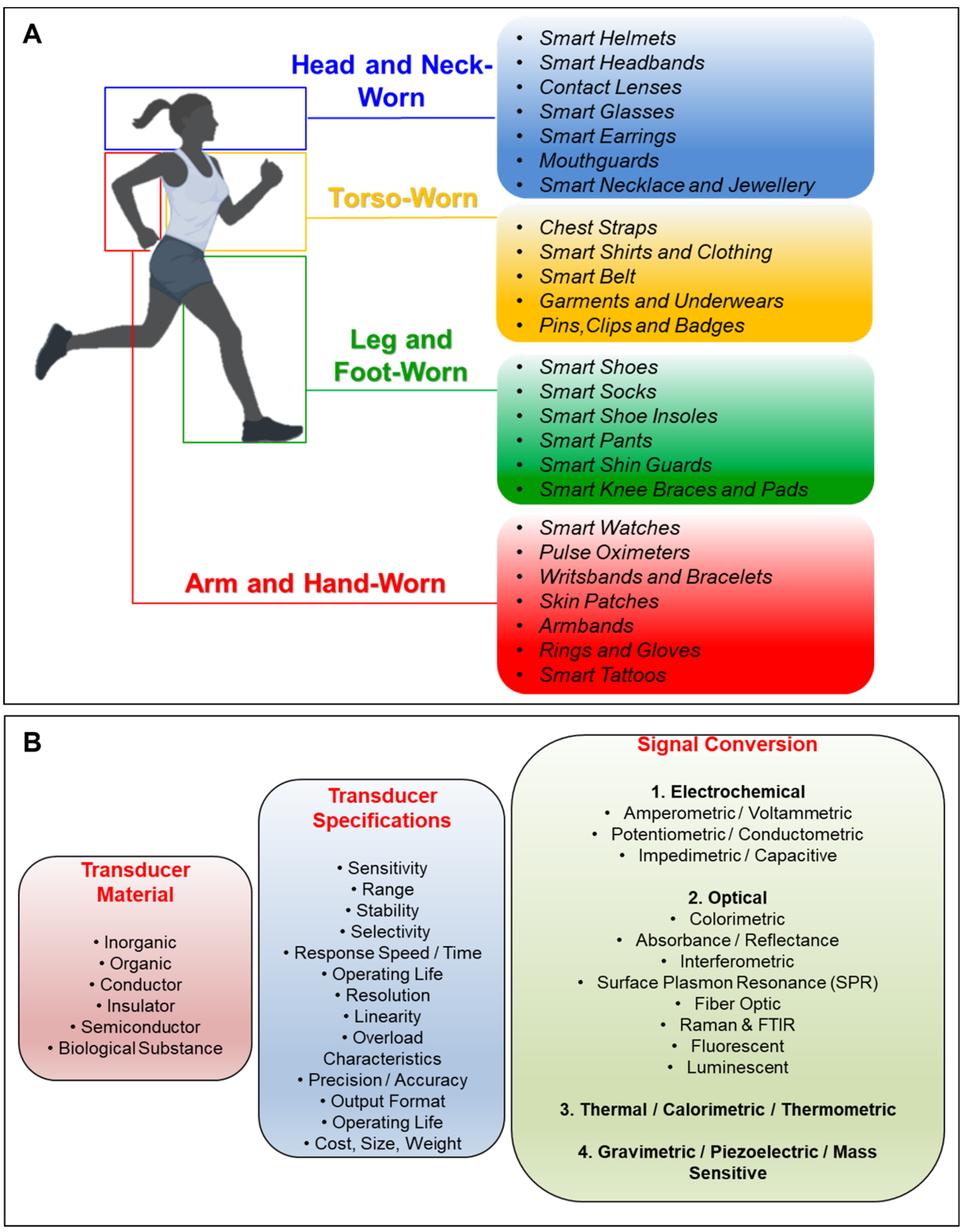
2. Construction and Classification of Transducers for Biosensors and Wearables
2.1. Electrochemical Biosensors
2.1.1. Amperometric and Voltammetric Biosensors
2.1.2. Potentiometric and Conductometric Biosensors
2.1.3. Impedimetric and Capacitive Biosensors
2.2. Optical Biosensors

2.3. Thermal/Calorimetric/Thermometric Biosensors
2.4. Gravimetric/Piezoelectric/Mass-Sensitive Biosensors
3. Supplementary Technologies for Wearable Biosensing
3.1. Microfluidics and Biomedical Microelectromechanical Systems (Bio-MEMS)
3.1.1. Energy Sources and Detection Mechanisms
3.1.2. Data Transmission
3.1.3. Biocompatibility
3.2. Location and Position Services
4. Conclusions, Discussion, and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, G.G.; Montalvo, J.G. Urea-Specific Enzyme Electrode. J. Am. Chem. Soc. 1969, 91, 2164–2165. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.-H.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable Electrochemical Biosensors in North America. Biosens. Bioelectron. 2021, 172, 112750. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing Analytes in Biofluids for Peripheral Biochemical Monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, D.J.; Cai, A.; Dorval Courchesne, N.M. The Evolving Role of Proteins in Wearable Sweat Biosensors. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, D.; Tipparaju, V.V.; Jung, W.; Xian, X. Detection of Transdermal Biomarkers Using Gradient-Based Colorimetric Array Sensor. Biosens. Bioelectron. 2022, 195, 113650. [Google Scholar] [CrossRef]
- Zamkah, A.; Hui, T.; Andrews, S.; Dey, N.; Shi, F.; Sherratt, R.S. Identification of Suitable Biomarkers for Stress and Emotion Detection for Future Personal Affective Wearable Sensors. Biosensors 2020, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Cesewski, E.; Johnson, B.N. Electrochemical Biosensors for Pathogen Detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Nehra, M.; Lettieri, M.; Dilbaghi, N.; Kumar, S.; Marrazza, G. Nano-Biosensing Platforms for Detection of Cow’s Milk Allergens: An Overview. Sensors 2019, 20, 32. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Guo, Q.; Zhang, C.; Sun, Z.; Weng, X. Microfluidic Origami Nano-Aptasensor for Peanut Allergen Ara H1 Detection. Food Chem. 2021, 365, 130511. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhu, J.; Wu, W.; Wang, N.; Wang, J.; Wu, J.; Wu, Q.; Wang, X.; Yu, C.; Wei, G.; et al. Wearable Sweat Biosensors Refresh Personalized Health/Medical Diagnostics. Research 2021, 2021, 9757126. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.; McKee, S.A. Mobile Alcohol Biosensors and Pharmacotherapy Development Research. Alcohol 2019, 81, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Soft, Skin-Integrated Multifunctional Microfluidic Systems for Accurate Colorimetric Analysis of Sweat Biomarkers and Temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef]
- Tseng, R.; Chen, C.-C.; Hsu, S.-M.; Chuang, H.-S. Contact-Lens Biosensors. Sensors 2018, 18, 2651. [Google Scholar] [CrossRef] [Green Version]
- Shilaih, M.; Goodale, B.M.; Falco, L.; Kübler, F.; de Clerck, V.; Leeners, B. Modern Fertility Awareness Methods: Wrist Wearables Capture the Changes in Temperature Associated with the Menstrual Cycle. Biosci. Rep. 2018, 38, BSR20171279. [Google Scholar] [CrossRef] [Green Version]
- Vescio, B.; Quattrone, A.; Nisticò, R.; Crasà, M.; Quattrone, A. Wearable Devices for Assessment of Tremor. Front. Neurol. 2021, 12, 680011. [Google Scholar] [CrossRef]
- Adans-Dester, C.P.; Bamberg, S.; Bertacchi, F.P.; Caulfield, B.; Chappie, K.; Demarchi, D.; Erb, M.K.; Estrada, J.; Fabara, E.E.; Freni, M.; et al. Can MHealth Technology Help Mitigate the Effects of the COVID-19 Pandemic? IEEE Open J. Eng. Med. Biol. 2020, 1, 243–248. [Google Scholar] [CrossRef]
- Xie, J.; Tong, Z.; Guan, X.; Du, B.; Qiu, H.; Slutsky, A.S. Critical Care Crisis and Some Recommendations during the COVID-19 Epidemic in China. Intensive Care Med. 2020, 46, 837–840. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-Care Diagnostics for Infectious Diseases: From Methods to Devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, G.; Chai, H.; Qu, L.; Zhang, X. Flexible Biosensors Based on Colorimetry, Fluorescence, and Electrochemistry for Point-of-Care Testing. Front. Bioeng. Biotechnol. 2021, 9, 753692. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.; Nilsson, E.J.; Cirovic, S.; Tudosoiu, B.; Shleev, S. Wearable Electronic Tongue for Non-Invasive Assessment of Human Sweat. Sensors 2021, 21, 7311. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; He, H.; Zhang, L.; Dong, C.; Zeng, H.; Dai, Y.; Xing, L.; Zhang, Y.; Xue, X. A Self-Powered Wearable Noninvasive Electronic-Skin for Perspiration Analysis Based on Piezo-Biosensing Unit Matrix of Enzyme/ZnO Nanoarrays. ACS Appl. Mater. Interfaces 2017, 9, 29526–29537. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Promphet, N.; Ummartyotin, S.; Ngeontae, W.; Puthongkham, P.; Rodthongkum, N. Non-Invasive Wearable Chemical Sensors in Real-Life Applications. Anal. Chim. Acta 2021, 1179, 338643. [Google Scholar] [CrossRef]
- Gowri, A.; Ashwin Kumar, N.; Suresh Anand, B.S. Recent Advances in Nanomaterials Based Biosensors for Point of Care (PoC) Diagnosis of COVID-19—A Minireview. TrAC Trends Anal. Chem. 2021, 137, 116205. [Google Scholar] [CrossRef]
- Research and Markets Wearable Technology Market Size, Share & Trends Analysis Report by Product (Wrist-Wear, Eye-Wear & Head-Wear, Foot-Wear, Neck-Wear, Body-Wear), by Application, by Region, and Segment Forecasts, 2021–2028. Available online: https://www.researchandmarkets.com/reports/5124989/wearable-technology-market-size-share-and-trends (accessed on 28 February 2022).
- Malhotra, B.D.; Chaubey, A. Biosensors for Clinical Diagnostics Industry. Sens. Actuators B Chem. 2003, 91, 117–127. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Agarwal, A.; Lang, J. Foundations of Analog and Digital Electronic Circuits; Elsevier Inc.: Amsterdam, The Netherlands, 2005; ISBN 1-55860-735-8. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology, 2nd ed.; (the “Gold Book”); Blackwell Science: Oxford, UK, 1997. [Google Scholar]
- Kissinger, P.T. Biosensors—A Perspective. Biosens. Bioelectron. 2005, 20, 2512–2516. [Google Scholar] [CrossRef]
- Polat, E.O. Seamlessly Integrable Optoelectronics for Clinical Grade Wearables. Adv. Mater. Technol. 2021, 6, 2000853. [Google Scholar] [CrossRef]
- Saleem, K.; Shahzad, B.; Orgun, M.A.; Al-Muhtadi, J.; Rodrigues, J.J.P.C.; Zakariah, M. Design and Deployment Challenges in Immersive and Wearable Technologies. Behav. Inf. Technol. 2017, 36, 687–698. [Google Scholar] [CrossRef]
- Rodrigues, J.J.P.C.; de Rezende Segundo, D.B.; Junqueira, H.A.; Sabino, M.H.; Prince, R.M.; Al-Muhtadi, J.; de Albuquerque, V.H.C. Enabling Technologies for the Internet of Health Things. IEEE Access 2018, 6, 13129–13141. [Google Scholar] [CrossRef]
- White, R.M. A Sensor Classification Scheme. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1987, 34, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Ding, J.; Qin, W. Potentiometric Sensing of Nuclease Activities and Oxidative Damage of Single-Stranded DNA Using a Polycation-Sensitive Membrane Electrode. Biosens. Bioelectron. 2013, 47, 559–565. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Zhang, X.; Ju, H.; Wang, J. Electrochemical Sensors, Biosensors and Their Biomedical Applications; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Kamikawa, T.L.; Mikolajczyk, M.G.; Kennedy, M.; Zhang, P.; Wang, W.; Scott, D.E.; Alocilja, E.C. Nanoparticle-Based Biosensor for the Detection of Emerging Pandemic Influenza Strains. Biosens. Bioelectron. 2010, 26, 1346–1352. [Google Scholar] [CrossRef]
- Oja, S.M.; Fan, Y.; Armstrong, C.M.; Defnet, P.; Zhang, B. Nanoscale Electrochemistry Revisited. Anal. Chem. 2016, 88, 414–430. [Google Scholar] [CrossRef]
- Park, W.J.; Yi, Y.; Lee, J.; Lee, B.C.; Park, O.K.; Lee, H.J.; Lee, H. N-Heterocyclic Carbene-Silver Complex as a Novel Reference Electrode in Electrochemical Applications. Talanta 2010, 81, 482–485. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Luo, F.; Wang, P.; Lin, Z. Miniaturized Electrochemical Sensors and Their Point-of-Care Applications. Chin. Chem. Lett. 2020, 31, 589–600. [Google Scholar] [CrossRef]
- Silvestrini, M.; Fruk, L.; Moretto, L.M.; Ugo, P. Detection of DNA Hybridization by Methylene Blue Electrochemistry at Activated Nanoelectrode Ensembles. J. Nanosci. Nanotechnol. 2015, 15, 3437–3442. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Soldatkin, O.O.; Dzyadevych, S.V.; Soldatkin, A.P. Electrochemical Biosensors Based on Multienzyme Systems: Main Groups, Advantages and Limitations—A Review. Analytica Chimica Acta 2020, 1111, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caballero, A.; Ugarte, A.; Sánchez-Ortega, A.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Molecularly Imprinted Poly[Tetra(o-Aminophenyl)Porphyrin] as a Stable and Selective Coating for the Development of Voltammetric Sensors. J. Electroanal. Chem. 2010, 638, 246–253. [Google Scholar] [CrossRef]
- Toghill, K.E.; Compton, R.G. Electrochemical Non-Enzymatic Glucose Sensors: A Perspective and an Evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Guo, C.; Wang, Y.; Zhao, Y.; Xu, C. Non-Enzymatic Glucose Sensor Based on Three Dimensional Nickel Oxide for Enhanced Sensitivity. Anal. Methods 2013, 5, 1644–1647. [Google Scholar] [CrossRef]
- Oswald, B.; Lehmann, F.; Simon, L.; Terpetschnig, E.; Wolfbeis, O.S. Red Laser-Induced Fluorescence Energy Transfer in an Immunosystem. Anal. Biochem. 2000, 280, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Mishra, A.; Narang, J. Electrochemical Sensor Method for Food Quality Evaluation. In Evaluation Technologies for Food Quality; Elsevier: Amsterdam, The Netherlands, 2019; pp. 793–815. [Google Scholar]
- Pollap, A.; Kochana, J. Electrochemical Immunosensors for Antibiotic Detection. Biosensors 2019, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarocka, U.; Wąsowicz, M.; Radecka, H.; Malinowski, T.; Michalczuk, L.; Radecki, J. Impedimetric Immunosensor for Detection of Plum Pox Virus in Plant Extracts. Electroanalysis 2011, 23, 2197–2204. [Google Scholar] [CrossRef]
- Haji-Hashemi, H.; Safarnejad, M.R.; Norouzi, P.; Ebrahimi, M.; Shahmirzaie, M.; Ganjali, M.R. Simple and Effective Label Free Electrochemical Immunosensor for Fig Mosaic Virus Detection. Anal. Biochem. 2019, 566, 102–106. [Google Scholar] [CrossRef]
- Ning, S.; Zhou, M.; Liu, C.; Waterhouse, G.I.N.; Dong, J.; Ai, S. Ultrasensitive Electrochemical Immunosensor for Avian Leukosis Virus Detection Based on a β-Cyclodextrin-Nanogold-Ferrocene Host-Guest Label for Signal Amplification. Anal. Chim. Acta 2019, 1062, 87–93. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chem. Soc. Rev. 2010, 39, 1747. [Google Scholar] [CrossRef] [PubMed]
- Eggins, B.R. Analytical Techniques in the Science-Chemical Sensor and Biosensor; Jonhn Wiley & Sons Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Luppa, P.B.; Sokoll, L.J.; Chan, D.W. Immunosensors-Principles and Applications to Clinical Chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Bueno, P.R. Nanobiosensors for Personalized and Onsite Biomedical Diagnosis; Chandra, P., Ed.; Institution of Engineering and Technology: London, UK, 2016; ISBN 9781849199506. [Google Scholar]
- Wang, J. Analytical Electrochemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 9780471790303. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Rishpon, J.; Ivnitski, D. An Amperometric Enzyme-Channeling Immunosensor. Biosens. Bioelectron. 1997, 12, 195–204. [Google Scholar] [CrossRef]
- Hendry, S.P.; Higgins, I.J.; Bannister, J.V. Amperometric Biosensors. J. Biotechnol. 1990, 15, 229–238. [Google Scholar] [CrossRef]
- Chaubey, A.; Malhotra, B.D. Mediated Biosensors. Biosens. Bioelectron. 2002, 17, 441–456. [Google Scholar] [CrossRef]
- Fang, L.; Ren, H.; Mao, X.; Zhang, S.; Cai, Y.; Xu, S.; Zhang, Y.; Li, L.; Ye, X.; Liang, B. Differential Amperometric Microneedle Biosensor for Wearable Levodopa Monitoring of Parkinson’s Disease. Biosensors 2022, 12, 102. [Google Scholar] [CrossRef]
- Shiwaku, R.; Matsui, H.; Nagamine, K.; Uematsu, M.; Mano, T.; Maruyama, Y.; Nomura, A.; Tsuchiya, K.; Hayasaka, K.; Takeda, Y.; et al. A Printed Organic Circuit System for Wearable Amperometric Electrochemical Sensors. Sci. Rep. 2018, 8, 6368. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable Salivary Uric Acid Mouthguard Biosensor with Integrated Wireless Electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A Graphene-Based Electrochemical Device with Thermoresponsive Microneedles for Diabetes Monitoring and Therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors-Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Heyrovský, J. The Development of Polarographic Analysis. Analyst 1956, 81, 189–192. [Google Scholar] [CrossRef]
- Pohanka, M.; Skládal, P. Electrochemical Biosensors-Principles and Applications. J. Appl. Biomed. 2008, 6, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Katz, E.; Willner, I. Probing Biomolecular Interactions at Conductive and Semiconductive Surfaces by Impedance Spectroscopy: Routes to Impedimetric Immunosensors, DNA-Sensors, and Enzyme Biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Kueng, A.; Kranz, C.; Mizaikoff, B. Amperometric ATP Biosensor Based on Polymer Entrapped Enzymes. Biosens. Bioelectron. 2004, 19, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Mahanthesha, K.; Swamy, K.; Chandra, U.; Reddy, S.; Pai, K.V. Sodium Dodecyl Sulphate/Polyglycine/Phthalamide/Carbon Paste Electrode Based Voltammetric Sensors for Detection of Dopamine in the Presence of Ascorbic Acid and Uric Acid. Chem. Sens. 2014, 4, 10. [Google Scholar]
- Ji, D.; Liu, Z.; Liu, L.; Low, S.S.; Lu, Y.; Yu, X.; Zhu, L.; Li, C.; Liu, Q. Smartphone-Based Integrated Voltammetry System for Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid with Graphene and Gold Nanoparticles Modified Screen-Printed Electrodes. Biosens. Bioelectron. 2018, 119, 55–62. [Google Scholar] [CrossRef]
- Bustos, E.; Godínez, L.A. Modified Surfaces with Nano-Structured Composites of Prussian Blue and Dendrimers. New Materials for Advanced Electrochemical Applications. Int. J. Electrochem. Sci. 2011, 6, 36. [Google Scholar]
- Güell, A.G.; Meadows, K.E.; Unwin, P.R.; Macpherson, J.V. Trace Voltammetric Detection of Serotonin at Carbon Electrodes: Comparison of Glassy Carbon, Boron Doped Diamond and Carbon Nanotube Network Electrodes. Phys. Chem. Chem. Phys. 2010, 12, 10108. [Google Scholar] [CrossRef]
- Apetrei, C.; Apetrei, I.M.; de Saja, J.A.; Rodriguez-Mendez, M.L. Carbon Paste Electrodes Made from Different Carbonaceous Materials: Application in the Study of Antioxidants. Sensors 2011, 11, 1328–1344. [Google Scholar] [CrossRef]
- Lim, C.X.; Hoh, H.Y.; Ang, P.K.; Loh, K.P. Direct Voltammetric Detection of DNA and PH Sensing on Epitaxial Graphene: An Insight into the Role of Oxygenated Defects. Anal. Chem. 2010, 82, 7387–7393. [Google Scholar] [CrossRef] [PubMed]
- Koncki, R. Recent Developments in Potentiometric Biosensors for Biomedical Analysis. Anal. Chim. Acta 2007, 599, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Qin, W. Recent Advances in Potentiometric Biosensors. TrAC Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Smith, J.T.; Shah, S.S.; Goryll, M.; Stowell, J.R.; Allee, D.R. Flexible ISFET Biosensor Using IGZO Metal Oxide TFTs and an ITO Sensing Layer. IEEE Sens. J. 2014, 14, 937–938. [Google Scholar] [CrossRef]
- Dzyadevych, S.V.; Soldatkin, A.P.; El’skaya, A.V.; Martelet, C.; Jaffrezic-Renault, N. Enzyme Biosensors Based on Ion-Selective Field-Effect Transistors. Anal. Chim. Acta 2006, 568, 248–258. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Kougianos, E. Biosensors: A Tutorial Review. IEEE Potentials 2006, 25, 35–40. [Google Scholar] [CrossRef]
- Casans, S.; Muñoz, D.R.; Navarro, A.E.; Salazar, A. ISFET Drawbacks Minimization Using a Novel Electronic Compensation. Sens. Actuators B Chem. 2004, 99, 42–49. [Google Scholar] [CrossRef]
- Van der Schoot, B.H.; Bergveld, P. ISFET Based Enzyme Sensors. Biosensors 1987, 3, 161–186. [Google Scholar] [CrossRef] [Green Version]
- Covington, A.K.; Whalley, P.D. Recent Advances in Microelectronic Ion-Sensitive Devices (ISFETs). Oper. Transducer. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 1209. [Google Scholar] [CrossRef]
- Parrilla, M.; Ortiz-Gómez, I.; Cánovas, R.; Salinas-Castillo, A.; Cuartero, M.; Crespo, G.A. Wearable Potentiometric Ion Patch for On-Body Electrolyte Monitoring in Sweat: Toward a Validation Strategy to Ensure Physiological Relevance. Anal. Chem. 2019, 91, 8644–8651. [Google Scholar] [CrossRef] [Green Version]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable Potentiometric Ion Sensors. TrAC Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Manjakkal, L.; Dang, W.; Yogeswaran, N.; Dahiya, R. Textile-Based Potentiometric Electrochemical PH Sensor for Wearable Applications. Biosensors 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzyadevych, S.; Jaffrezic-Renault, N. Conductometric Biosensors. In Biological Identification; Elsevier: Amsterdam, The Netherlands, 2014; pp. 153–193. [Google Scholar]
- Dzyadevich, S.V.; Zhylyak, G.A.; Soldatkin, A.P.; El’skaya, A.V. Conductometric Urease Microbiosensor Based on Thin-Film Interdigitated Electrodes for Urea Determination. Biopolym. Cell 1996, 12, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Guiseppi-Elie, A. Impedimetric Biosensors for Nano- and Microfluidics. In Encyclopedia of Microfluidics and Nanofluidics; Springer: Boston, MA, USA, 2008; pp. 811–823. [Google Scholar]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Valdes, J.J.; Wall, J.G.; Chambers, J.P.; Eldefrawi, M.E. Receptor-Based Capacitive Biosensor; Johns Hopkins APL Technical Digest; Applied Physics Laboratory: Baltimore, MD, USA, 1988; Volume 9. [Google Scholar]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive Biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Berney, H. Capacitance Affinity Biosensors. In Ultrathin Electrochemical Chemo- and Biosensors; Springer: Berlin/Heidelberg, Germany, 2004; pp. 43–65. [Google Scholar]
- Bontidean, I.; Ahlqvist, J.; Mulchandani, A.; Chen, W.; Bae, W.; Mehra, R.K.; Mortari, A.; Csöregi, E. Novel Synthetic Phytochelatin-Based Capacitive Biosensor for Heavy Metal Ion Detection. Biosens. Bioelectron. 2003, 18, 547–553. [Google Scholar] [CrossRef]
- Mattiasson, B.; Hedström, M. Capacitive Biosensors for Ultra-Sensitive Assays. TrAC Trends Anal. Chem. 2016, 79, 233–238. [Google Scholar] [CrossRef]
- Piro, B. Electronic Devices for Biomarker Monitoring. In The Detection of Biomarkers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 183–207. [Google Scholar]
- Alhoshany, A.; Sivashankar, S.; Mashraei, Y.; Omran, H.; Salama, K.N. A Biosensor-CMOS Platform and Integrated Readout Circuit in 0.18-Μm CMOS Technology for Cancer Biomarker Detection. Sensors 2017, 17, 1942. [Google Scholar] [CrossRef] [Green Version]
- Ghafar-Zadeh, E.; Sawan, M.; Shabani, A.; Zourob, M.; Chodavarapu, V. Bacteria Growth Monitoring through an On-Chip Capacitive Sensor. In Proceedings of the 2008 IEEE 14th International Mixed-Signals, Sensors, and Systems Test Workshop, Vancouver, BC, Canada, 18–20 June 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 1–4. [Google Scholar]
- Lu, M.S.-C. Capacitive DNA Hybridization Detection. In Handbook of Biochips; Springer: New York, NY, USA, 2015; pp. 1–9. [Google Scholar]
- Ghafar-Zadeh, E. Wireless Integrated Biosensors for Point-of-Care Diagnostic Applications. Sensors 2015, 15, 3236–3261. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Si, Y.; Zhu, Z.; Guo, Y.; Zhang, Y.; Pan, N.; Sun, G.; Pan, T. Supercapacitive Iontronic Nanofabric Sensing. Adv. Mater. 2017, 29, 1700253. [Google Scholar] [CrossRef]
- Pan, L.; Chortos, A.; Yu, G.; Wang, Y.; Isaacson, S.; Allen, R.; Shi, Y.; Dauskardt, R.; Bao, Z. An Ultra-Sensitive Resistive Pressure Sensor Based on Hollow-Sphere Microstructure Induced Elasticity in Conducting Polymer Film. Nat. Commun. 2014, 5, 3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.; Lee, T.-I.; Shim, J.; Ryu, S.; Kim, M.S.; Kim, S.; Kim, T.-S.; Park, I. Highly Sensitive, Flexible, and Wearable Pressure Sensor Based on a Giant Piezocapacitive Effect of Three-Dimensional Microporous Elastomeric Dielectric Layer. ACS Appl. Mater. Interfaces 2016, 8, 16922–16931. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneyra, A.; López-Villanueva, J.A. Recent Advances in Printed Capacitive Sensors. Micromachines 2020, 11, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Cong, Y.; Fu, J. Stretchable and Tough Conductive Hydrogels for Flexible Pressure and Strain Sensors. J. Mater. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef] [PubMed]
- Tee, B.C.-K.; Chortos, A.; Dunn, R.R.; Schwartz, G.; Eason, E.; Bao, Z. Tunable Flexible Pressure Sensors Using Microstructured Elastomer Geometries for Intuitive Electronics. Adv. Funct. Mater. 2014, 24, 5427–5434. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.B.; Tee, B.C.-K.; Stoltenberg, R.M.; Chen, C.V.H.-H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly Sensitive Flexible Pressure Sensors with Microstructured Rubber Dielectric Layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible Polymer Transistors with High Pressure Sensitivity for Application in Electronic Skin and Health Monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef]
- Joo, Y.; Byun, J.; Seong, N.; Ha, J.; Kim, H.; Kim, S.; Kim, T.; Im, H.; Kim, D.; Hong, Y. Silver Nanowire-Embedded PDMS with a Multiscale Structure for a Highly Sensitive and Robust Flexible Pressure Sensor. Nanoscale 2015, 7, 6208–6215. [Google Scholar] [CrossRef]
- Guo, X.; Huang, Y.; Cai, X.; Liu, C.; Liu, P. Capacitive Wearable Tactile Sensor Based on Smart Textile Substrate with Carbon Black /Silicone Rubber Composite Dielectric. Meas. Sci. Technol. 2016, 27, 045105. [Google Scholar] [CrossRef]
- Chen, S.; Zhuo, B.; Guo, X. Large Area One-Step Facile Processing of Microstructured Elastomeric Dielectric Film for High Sensitivity and Durable Sensing over Wide Pressure Range. ACS Appl. Mater. Interfaces 2016, 8, 20364–20370. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A Bioinspired Mineral Hydrogel as a Self-Healable, Mechanically Adaptable Ionic Skin for Highly Sensitive Pressure Sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wu, P. Zwitterionic Skins with a Wide Scope of Customizable Functionalities. ACS Nano 2018, 12, 12860–12868. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, G.; Kim, T.; Lee, S.; Kang, D.; Hwang, M.-S.; Chae, Y.; Kang, S.; Lee, H.; Park, H.-G.; et al. Transparent, Flexible, Conformal Capacitive Pressure Sensors with Nanoparticles. Small 2018, 14, 1703432. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Peng, S.; Sun, W.; Gu, G.; Zhang, Q.; Guo, X. Scalable Processing Ultrathin Polymer Dielectric Films with a Generic Solution Based Approach for Wearable Soft Electronics. Adv. Mater. Technol. 2019, 4, 1800681. [Google Scholar] [CrossRef]
- Yang, J.; Luo, S.; Zhou, X.; Li, J.; Fu, J.; Yang, W.; Wei, D. Flexible, Tunable, and Ultrasensitive Capacitive Pressure Sensor with Microconformal Graphene Electrodes. ACS Appl. Mater. Interfaces 2019, 11, 14997–15006. [Google Scholar] [CrossRef]
- Luo, Y.; Shao, J.; Chen, S.; Chen, X.; Tian, H.; Li, X.; Wang, L.; Wang, D.; Lu, B. Flexible Capacitive Pressure Sensor Enhanced by Tilted Micropillar Arrays. ACS Appl. Mater. Interfaces 2019, 11, 17796–17803. [Google Scholar] [CrossRef]
- Wang, A.; Feng, J.; Li, Y.; Zou, P. Beyond Fluorescent Proteins: Hybrid and Bioluminescent Indicators for Imaging Neural Activities. ACS Chem. Neurosci. 2018, 9, 639–650. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical Biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.; Shankar, P.M.; Mutharasan, R. A Review of Fiber-Optic Biosensors. Sens. Actuators B Chem. 2007, 125, 688–703. [Google Scholar] [CrossRef]
- Lai, M.; Slaughter, G. Label-Free MicroRNA Optical Biosensors. Nanomaterials 2019, 9, 1573. [Google Scholar] [CrossRef] [Green Version]
- Mondal, H.S.; Hossain, M.M.; Mahasin, M.M.H.; Mondal, P.K.; Rahaman, M.E. Emerging Applications of Optical Bio-Sensors. J. Biomim. Biomater. Biomed. Eng. 2019, 40, 41–55. [Google Scholar] [CrossRef]
- Homola, J. Present and Future of Surface Plasmon Resonance Biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Garcia, M.N. Optical Biosensors for Probing at the Cellular Level: A Review of Recent Progress and Future Prospects. Semin. Cell Dev. Biol. 2009, 20, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.; Mutharasan, R. Review of Biosensors for Foodborne Pathogens and Toxins. Sens. Actuators B Chem. 2013, 183, 535–549. [Google Scholar] [CrossRef]
- Pospíšilová, M.; Kuncová, G.; Trögl, J. Fiber-Optic Chemical Sensors and Fiber-Optic Bio-Sensors. Sensors 2015, 15, 25208–25259. [Google Scholar] [CrossRef]
- Benito-Peña, E.; Valdés, M.G.; Glahn-Martínez, B.; Moreno-Bondi, M.C. Fluorescence Based Fiber Optic and Planar Waveguide Biosensors. A Review. Anal. Chim. Acta 2016, 943, 17–40. [Google Scholar] [CrossRef]
- Eltzov, E.; Marks, R.S. Fiber-Optic Based Cell Sensors. In Whole Cell Sensing Systems I; Springer: Berlin/Heidelberg, Germany, 2009; pp. 131–154. [Google Scholar]
- Tagit, O.; Hildebrandt, N. Fluorescence Sensing of Circulating Diagnostic Biomarkers Using Molecular Probes and Nanoparticles. ACS Sens. 2017, 2, 31–45. [Google Scholar] [CrossRef]
- Soloducho, J.; Cabaj, J. Electrochemical and Optical Biosensors in Medical Applications. In Biosensors—Micro and Nanoscale Applications; InTech: Vienna, Austria, 2015. [Google Scholar]
- Ong, J.J.; Pollard, T.D.; Goyanes, A.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Optical Biosensors—Illuminating the Path to Personalized Drug Dosing. Biosens. Bioelectron. 2021, 188, 113331. [Google Scholar] [CrossRef]
- Henriksen, A.; Mikalsen, M.H.; Woldaregay, A.Z.; Muzny, M.; Hartvigsen, G.; Hopstock, L.A.; Grimsgaard, S. Using Fitness Trackers and Smartwatches to Measure Physical Activity in Research: Analysis of Consumer Wrist-Worn Wearables. J. Med. Internet Res. 2018, 20, e9157. [Google Scholar] [CrossRef]
- Ahmad Tarar, A.; Mohammad, U.K.; Srivastava, S. Wearable Skin Sensors and Their Challenges: A Review of Transdermal, Optical, and Mechanical Sensors. Biosensors 2020, 10, 56. [Google Scholar] [CrossRef]
- Weiler, D.T.; Villajuan, S.O.; Edkins, L.; Cleary, S.; Saleem, J.J. Wearable Heart Rate Monitor Technology Accuracy in Research: A Comparative Study Between PPG and ECG Technology. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2017, 61, 1292–1296. [Google Scholar] [CrossRef]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating Sources of Inaccuracy in Wearable Optical Heart Rate Sensors. NPJ Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, T.B. Soleil et Peau. J. Med. Esthet. 1975, 2, 33–34. [Google Scholar]
- Addison, P.S.; Watson, J.N.; Mestek, M.L.; Ochs, J.P.; Uribe, A.A.; Bergese, S.D. Pulse Oximetry-Derived Respiratory Rate in General Care Floor Patients. J. Clin. Monit. Comput. 2014, 29, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, K.; Ward, T.; Markham, C. Noncontact Simultaneous Dual Wavelength Photoplethysmography: A Further Step toward Noncontact Pulse Oximetry. Rev. Sci. Instrum. 2007, 78, 044304. [Google Scholar] [CrossRef] [PubMed]
- Hosanee, M.; Chan, G.; Welykholowa, K.; Cooper, R.; Kyriacou, P.A.; Zheng, D.; Allen, J.; Abbott, D.; Menon, C.; Lovell, N.H.; et al. Cuffless Single-Site Photoplethysmography for Blood Pressure Monitoring. J. Clin. Med. 2020, 9, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Q.Y.; Redmond, S.J.; Chan, G.S.; Middleton, P.M.; Steel, E.; Malouf, P.; Critoph, C.; Flynn, G.; O’Lone, E.; Lovell, N.H. Estimation of Cardiac Output and Systemic Vascular Resistance Using a Multivariate Regression Model with Features Selected from the Finger Photoplethysmogram and Routine Cardiovascular Measurements. BioMedical Eng. OnLine 2013, 12, 19. [Google Scholar] [CrossRef] [Green Version]
- Allen, J. Photoplethysmography and Its Application in Clinical Physiological Measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [Green Version]
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible Organic Photonic Skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Salvatore, G.A.; Araki, H.; Chiarelli, A.M.; Xie, Z.; Banks, A.; Sheng, X.; Liu, Y.; Lee, J.W.; Jang, K.I.; et al. Battery-Free, Stretchable Optoelectronic Systems for Wireless Optical Characterization of the Skin. Sci. Adv. 2016, 2, e1600418. [Google Scholar] [CrossRef] [Green Version]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-Free, Skin-Interfaced Microfluidic/Electronic Systems for Simultaneous Electrochemical, Colorimetric, and Volumetric Analysis of Sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable Sensors: Modalities, Challenges, and Prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, A.; Yeom, J.; Shanker, R.; Kim, M.P.; Kang, S.; Ko, H. Stretchable and Wearable Colorimetric Patches Based on Thermoresponsive Plasmonic Microgels Embedded in a Hydrogel Film. NPG Asia Mater. 2018, 10, 912–922. [Google Scholar] [CrossRef]
- Hackney, A.C. Exercise, Sport, and Bioanalytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Gaddes, D.E.; Demirel, M.C.; Reeves, W.B.; Tadigadapa, S. Remote Calorimetric Detection of Urea via Flow Injection Analysis. Analyst 2015, 140, 8033–8040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Arya, A.; Gangwar, A.; Kumar, A. Biosensors in Animal Biotechnology. In Nanotechnology in Modern Animal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 75–95. [Google Scholar]
- Sabr, A.K. Biosensors. Glob. J. Res. Eng. J. 2016, 16, 1–12. [Google Scholar]
- Grime, J.K. Analytical Solution Calorimetry; Wiley: New York, NY, USA, 1985; Volume 79. [Google Scholar]
- Ramanathan, K.; Danielsson, B. Principles and Applications of Thermal Biosensors. Biosens. Bioelectron. 2001, 16, 417–423. [Google Scholar] [CrossRef]
- Xie, B.; Mecklenburg, M.; Danielsson, B.; Öhman, O.; Norlin, P.; Winquist, F. Development of an Integrated Thermal Biosensor for the Simultaneous Determination of Multiple Analytes. Analyst 1995, 120, 155–160. [Google Scholar] [CrossRef]
- Xie, B.; Ramanathan, K.; Danielsson, B. Principles of Enzyme Thermistor Systems: Applications to Biomedical and Other Measurements. In Thermal Biosensors, Bioactivity, Bioaffinitty; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–33. [Google Scholar]
- Weetall, H.H. [10] Covalent Coupling Methods for Inorganic Support Materials. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1976; pp. 134–148. [Google Scholar]
- Yakovleva, M.; Bhand, S.; Danielsson, B. The Enzyme Thermistor—A Realistic Biosensor Concept. A Critical Review. Anal. Chim. Acta 2013, 766, 1–12. [Google Scholar] [CrossRef]
- Justino, C.; Duarte, A.; Rocha-Santos, T. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [Green Version]
- Mousa, S. Biosensors: The New Wave in Cancer Diagnosis. Nanotechnol. Sci. Appl. 2010, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchner, P.; Oberländer, J.; Friedrich, P.; Berger, J.; Rysstad, G.; Keusgen, M.; Schöning, M.J. Realisation of a Calorimetric Gas Sensor on Polyimide Foil for Applications in Aseptic Food Industry. Sens. Actuators B Chem. 2012, 170, 60–66. [Google Scholar] [CrossRef]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the Medical Revolution. Pers. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilaih, M.; de Clerck, V.; Falco, L.; Kübler, F.; Leeners, B. Pulse Rate Measurement During Sleep Using Wearable Sensors, and Its Correlation with the Menstrual Cycle Phases, A Prospective Observational Study. Sci. Rep. 2017, 7, 1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poh, M.-Z.; Loddenkemper, T.; Reinsberger, C.; Swenson, N.C.; Goyal, S.; Sabtala, M.C.; Madsen, J.R.; Picard, R.W. Convulsive Seizure Detection Using a Wrist-Worn Electrodermal Activity and Accelerometry Biosensor. Epilepsia 2012, 53, e93–e97. [Google Scholar] [CrossRef]
- De Zambotti, M.; Rosas, L.; Colrain, I.M.; Baker, F.C. The Sleep of the Ring: Comparison of the ŌURA Sleep Tracker against Polysomnography. Behav. Sleep Med. 2019, 17, 124–136. [Google Scholar] [CrossRef]
- Cooper, M.A. Label-Free Screening of Bio-Molecular Interactions. Anal. Bioanal. Chem. 2003, 377, 834–842. [Google Scholar] [CrossRef]
- Canh, T.M. Biosensors; Chapman & Hall: London, UK, 1993; ISBN 0412481901/9780412481901. [Google Scholar]
- Janshoff, A.; Galla, H.-J.; Steinem, C. Piezoelectric Mass-Sensing Devices as Biosensors—An Alternative to Optical Biosensors? Angew. Chem. 2000, 39, 4004–4032. [Google Scholar] [CrossRef]
- Narita, F.; Wang, Z.; Kurita, H.; Li, Z.; Shi, Y.; Jia, Y.; Soutis, C. A Review of Piezoelectric and Magnetostrictive Biosensor Materials for Detection of COVID-19 and Other Viruses. Adv. Mater. 2021, 33, 2005448. [Google Scholar] [CrossRef]
- Su, L.; Zou, L.; Fong, C.-C.; Wong, W.-L.; Wei, F.; Wong, K.-Y.; Wu, R.S.S.; Yang, M. Detection of Cancer Biomarkers by Piezoelectric Biosensor Using PZT Ceramic Resonator as the Transducer. Biosens. Bioelectron. 2013, 46, 155–161. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.; Yu, F.; Jiang, X.; Lee, H.-Y.; Luo, J.; Shrout, T.R. Recent Developments in Piezoelectric Crystals. J. Korean Ceram. Soc. 2018, 55, 419–439. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.D.; Buttry, D.A. In Situ Interfacial Mass Detection with Piezoelectric Transducers. Science 1990, 249, 1000–1007. [Google Scholar] [CrossRef]
- Tombelli, S. Piezoelectric Biosensors for Medical Applications. In Biosensors for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2012; pp. 41–64. [Google Scholar]
- Dullah, E.C.; Ongkudon, C.M. Current Trends in Endotoxin Detection and Analysis of Endotoxin–Protein Interactions. Crit. Rev. Biotechnol. 2017, 37, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.L.; Wang, S.M.; Zhang, Y.; Chen, L.C. Detection of Endotoxin Concentration Using Piezoelectric Based Biosensor System. Appl. Mech. Mater. 2012, 195–196, 874–878. [Google Scholar] [CrossRef]
- Narwal, V.; Deswal, R.; Batra, B.; Kalra, V.; Hooda, R.; Sharma, M.; Rana, J.S. Cholesterol Biosensors: A Review. Steroids 2019, 143, 6–17. [Google Scholar] [CrossRef]
- Pramanik, S.; Pingguan-Murphy, B.; Osman, N.A. Developments of immobilized surface modified piezoelectric crystal biosensors for advanced applications. Int. J. Electrochem. Sci. 2013, 8, 8863–8892. [Google Scholar]
- Pundir, C.S.; Malik, A.; Preety. Bio-Sensing of Organophosphorus Pesticides: A Review. Biosens. Bioelectron. 2019, 140, 111348. [Google Scholar] [CrossRef]
- Loo, L.; Capobianco, J.A.; Wu, W.; Gao, X.; Shih, W.Y.; Shih, W.-H.; Pourrezaei, K.; Robinson, M.K.; Adams, G.P. Highly Sensitive Detection of HER2 Extracellular Domain in the Serum of Breast Cancer Patients by Piezoelectric Microcantilevers. Anal. Chem. 2011, 83, 3392–3397. [Google Scholar] [CrossRef] [Green Version]
- Arif, S.; Qudsia, S.; Urooj, S.; Chaudry, N.; Arshad, A.; Andleeb, S. Blueprint of Quartz Crystal Microbalance Biosensor for Early Detection of Breast Cancer through Salivary Autoantibodies against ATP6AP1. Biosens. Bioelectron. 2015, 65, 62–70. [Google Scholar] [CrossRef]
- Tombelli, S.; Minunni, M.; Mascini, M. Piezoelectric Biosensors: Strategies for Coupling Nucleic Acids to Piezoelectric Devices. Methods 2005, 37, 48–56. [Google Scholar] [CrossRef]
- Su, Y.; Chen, C.; Pan, H.; Yang, Y.; Chen, G.; Zhao, X.; Li, W.; Gong, Q.; Xie, G.; Zhou, Y.; et al. Muscle Fibers Inspired High-Performance Piezoelectric Textiles for Wearable Physiological Monitoring. Adv. Funct. Mater. 2021, 31, 2010962. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Qiu, Y.; Wu, H.; Qin, W.; Liao, Y.; Yu, Q.; Cheng, H. Stretchable Piezoelectric Energy Harvesters and Self-Powered Sensors for Wearable and Implantable Devices. Biosens. Bioelectron. 2020, 168, 112569. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Hwang, S.-W.; Su, Y.; Kim, S.; Cheng, H.; Gur, O.; Haney, R.; Omenetto, F.G.; Huang, Y.; Rogers, J.A. Transient, Biocompatible Electronics and Energy Harvesters Based on ZnO. Small 2013, 9, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yang, R.; Wang, S.; Wang, Z.L. Flexible High-Output Nanogenerator Based on Lateral ZnO Nanowire Array. Nano Lett. 2010, 10, 3151–3155. [Google Scholar] [CrossRef] [Green Version]
- Yeo, J.C.; Kenry, K.; Lim, C.T. Emergence of Microfluidic Wearable Technologies. Lab Chip 2016, 16, 4082–4090. [Google Scholar] [CrossRef]
- Ardalan, S.; Hosseinifard, M.; Vosough, M.; Golmohammadi, H. Towards Smart Personalized Perspiration Analysis: An IoT-Integrated Cellulose-Based Microfluidic Wearable Patch for Smartphone Fluorimetric Multi-Sensing of Sweat Biomarkers. Biosens. Bioelectron. 2020, 168, 112450. [Google Scholar] [CrossRef]
- Lin, H.; Tan, J.; Zhu, J.; Lin, S.; Zhao, Y.; Yu, W.; Hojaiji, H.; Wang, B.; Yang, S.; Cheng, X.; et al. A Programmable Epidermal Microfluidic Valving System for Wearable Biofluid Management and Contextual Biomarker Analysis. Nat. Commun. 2020, 11, 4405. [Google Scholar] [CrossRef]
- Gualandi, I.; Marzocchi, M.; Achilli, A.; Cavedale, D.; Bonfiglio, A.; Fraboni, B. Textile Organic Electrochemical Transistors as a Platform for Wearable Biosensors. Sci. Rep. 2016, 6, 33637. [Google Scholar] [CrossRef]
- Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.M.V. Fully Printed Wearable Microfluidic Devices for High-Throughput Sweat Sampling and Multiplexed Electrochemical Analysis. ACS Sens. 2021, 6, 1174–1186. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A Soft, Wearable Microfluidic Device for the Capture, Storage, and Colorimetric Sensing of Sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [Green Version]
- Nyein, H.Y.Y.; Tai, L.-C.; Ngo, Q.P.; Chao, M.; Zhang, G.B.; Gao, W.; Bariya, M.; Bullock, J.; Kim, H.; Fahad, H.M.; et al. A Wearable Microfluidic Sensing Patch for Dynamic Sweat Secretion Analysis. ACS Sens. 2018, 3, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A Laser-Engraved Wearable Sensor for Sensitive Detection of Uric Acid and Tyrosine in Sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Liu, S.; Hu, Z.; Zhang, X.; Yi, N.; Tang, K.; Dexheimer, M.G.; Lian, X.; Wang, Q.; Yang, J.; et al. Laser-Induced Graphene Non-Enzymatic Glucose Sensors for on-Body Measurements. Biosens. Bioelectron. 2021, 193, 113606. [Google Scholar] [CrossRef]
- Shitanda, I.; Mitsumoto, M.; Loew, N.; Yoshihara, Y.; Watanabe, H.; Mikawa, T.; Tsujimura, S.; Itagaki, M.; Motosuke, M. Continuous Sweat Lactate Monitoring System with Integrated Screen-Printed MgO-Templated Carbon-Lactate Oxidase Biosensor and Microfluidic Sweat Collector. Electrochim. Acta 2021, 368, 137620. [Google Scholar] [CrossRef]
- Kim, S.; Lee, B.; Reeder, J.T.; Seo, S.H.; Lee, S.-U.; Hourlier-Fargette, A.; Shin, J.; Sekine, Y.; Jeong, H.; Oh, Y.S.; et al. Soft, Skin-Interfaced Microfluidic Systems with Integrated Immunoassays, Fluorometric Sensors, and Impedance Measurement Capabilities. Proc. Natl. Acad. Sci. USA 2020, 117, 27906–27915. [Google Scholar] [CrossRef]
- Song, Y.; Min, J.; Yu, Y.; Wang, H.; Yang, Y.; Zhang, H.; Gao, W. Wireless Battery-Free Wearable Sweat Sensor Powered by Human Motion. Sci. Adv. 2020, 6, eaay9842. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, T.; Fan, C.; Zhang, X. Wearable Strain Sensor for Real-Time Sweat Volume Monitoring. iScience 2021, 24, 102028. [Google Scholar] [CrossRef]
- Ziaie, B. Hard and Soft Micromachining for BioMEMS: Review of Techniques and Examples of Applications in Microfluidics and Drug Delivery. Adv. Drug Deliv. Rev. 2004, 56, 145–172. [Google Scholar] [CrossRef]
- Hofmann-Wellenhof, B.; Lichtenegger, H.; Collins, J. Global Positioning System: Theory and Practice; Hofmann-Wellenhof, B., Lichtenegger, H., Collins, J., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; ISBN 9783211835340. [Google Scholar]
- Djuknic, G.M.; Richton, R.E. Geolocation and Assisted GPS. Computer 2001, 34, 123–125. [Google Scholar] [CrossRef]
- Huang, H.; Gartner, G. A Survey of Mobile Indoor Navigation Systems. In Cartography in Central and Eastern Europe; Springer: Berlin/Heidelberg, Germany, 2009; pp. 305–319. [Google Scholar]
- Liu, H.; Darabi, H.; Banerjee, P.; Liu, J. Survey of Wireless Indoor Positioning Techniques and Systems. IEEE Trans. Syst. Man Cybern. Part C (Appl. Rev.) 2007, 37, 1067–1080. [Google Scholar] [CrossRef]
- Ram, S.; Sharf, J. The People Sensor: A Mobility Aid for the Visually Impaired. In Proceedings of the Digest of Papers. Second International Symposium on Wearable Computers (Cat. No.98EX215), Pittsburgh, PA, USA, 19–20 October 1998; IEEE Computer Society: Washington, DC, USA, 1998; pp. 166–167. [Google Scholar]
- Gu, Y.; Lo, A.; Niemegeers, I. A Survey of Indoor Positioning Systems for Wireless Personal Networks. IEEE Commun. Surv. Tutor. 2009, 11, 13–32. [Google Scholar] [CrossRef] [Green Version]
- Komine, T.; Nakagawa, M. Fundamental Analysis for Visible-Light Communication System Using LED Lights. IEEE Trans. Consum. Electron. 2004, 50, 100–107. [Google Scholar] [CrossRef]
- Kumar, N.; Lourenco, N.; Spiez, M.; Aguiar, R. Visible Light Communication Systems Conception and VIDAS. IETE Tech. Rev. (Inst. Electron. Telecommun. Eng. India) 2008, 25, 359–367. [Google Scholar] [CrossRef]
- Galván-Tejada, C.; García-Vázquez, J.; Brena, R. Magnetic Field Feature Extraction and Selection for Indoor Location Estimation. Sensors 2014, 14, 11001–11015. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.; Zhao, F.; Wang, C.; Luo, H.; Muhammad Zahid, T.; Wang, Q.; Li, D. Location Fingerprint Extraction for Magnetic Field Magnitude Based Indoor Positioning. J. Sens. 2016, 2016, 1945695. [Google Scholar] [CrossRef]
- Alsindi, N.A.; Alavi, B.; Pahlavan, K. Measurement and Modeling of Ultrawideband TOA-Based Ranging in Indoor Multipath Environments. IEEE Trans. Veh. Technol. 2009, 58, 1046–1058. [Google Scholar] [CrossRef]
- Breed, G. A Summary of FCC Rules for Ultra Wideband Communications. High Freq. Electron. 2005, 4, 42–44. [Google Scholar]
- Kopta, V.; Farserotu, J.; Enz, C. FM-UWB: Towards a Robust, Low-Power Radio for Body Area Networks. Sensors 2017, 17, 1043. [Google Scholar] [CrossRef] [Green Version]
- Nasr, K.M. Hybrid Channel Modelling for Ultra-Wideband Portable Multimedia Applications. IET Microw. Antennas Propag. 2008, 2, 229. [Google Scholar] [CrossRef]
- De Santis, V.; Feliziani, M.; Maradei, F. Safety Assessment of UWB Radio Systems for Body Area Network by the FD2TD Method. IEEE Trans. Magn. 2010, 46, 3245–3248. [Google Scholar] [CrossRef]
- Fort, A.; Ryckaert, J.; Desset, C.; de Doncker, P.; Wambacq, P.; van Biesen, L. Ultra-Wideband Channel Model for Communication around the Human Body. IEEE J. Sel. Areas Commun. 2006, 24, 927–933. [Google Scholar] [CrossRef]
- Rowe, N.C.; Fathy, A.E.; Kuhn, M.J.; Mahfouz, M.R. A UWB Transmit-Only Based Scheme for Multi-Tag Support in a Millimeter Accuracy Localization System. In Proceedings of the 2013 IEEE Topical Conference on Wireless Sensors and Sensor Networks (WiSNet), Austin, TX, USA, 20–23 January 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 7–9. [Google Scholar]
- Kuhn, M.J.; Mahfouz, M.R.; Turnmire, J.; Wang, Y.; Fathy, A.E. A Multi-Tag Access Scheme for Indoor UWB Localization Systems Used in Medical Environments. In Proceedings of the 2011 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems, Phoenix, AZ, USA, 16–19 January 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 75–78. [Google Scholar]
- Mahfouz, M.R.; Kuhn, M.J.; Wang, Y.; Turnmire, J.; Fathy, A.E. Towards Sub-Millimeter Accuracy in UWB Positioning for Indoor Medical Environments. In Proceedings of the 2011 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems, Phoenix, AZ, USA, 16–19 January 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 83–86. [Google Scholar]
- Arsan, T.; Kepez, O. Early Steps in Automated Behavior Mapping via Indoor Sensors. Sensors 2017, 17, 2925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghtadaiee, V.; Dempster, A.G. Determining the Best Vector Distance Measure for Use in Location Fingerprinting. Pervasive Mob. Comput. 2015, 23, 59–79. [Google Scholar] [CrossRef]
- Alarifi, A.; Al-Salman, A.; Alsaleh, M.; Alnafessah, A.; Al-Hadhrami, S.; Al-Ammar, M.; Al-Khalifa, H. Ultra Wideband Indoor Positioning Technologies: Analysis and Recent Advances. Sensors 2016, 16, 707. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Skibniewski, M.J.; Yuan, Y.; Sun, C.; Lu, Y. Ultra-Wide Band Applications in Industry: A Critical Review/Ultraplačios Juostos Bangų Taikymas Pramonėje: Kritinė Apžvalga. J. Civ. Eng. Manag. 2011, 17, 437–444. [Google Scholar] [CrossRef]
- Polat, E.O.; Uzlu, H.B.; Balci, O.; Kakenov, N.; Kovalska, E.; Kocabas, C. Graphene-Enabled Optoelectronics on Paper. ACS Photonics 2016, 3, 964–971. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, G.; Ehrmann, A. Textile-Based Sensors for Biosignal Detection and Monitoring. Sensors 2021, 21, 6042. [Google Scholar] [CrossRef]
- Tao, H.; Brenckle, M.A.; Yang, M.; Zhang, J.; Liu, M.; Siebert, S.M.; Averitt, R.D.; Mannoor, M.S.; McAlpine, M.C.; Rogers, J.A.; et al. Silk-Based Conformal, Adhesive, Edible Food Sensors. Adv. Mater. 2012, 24, 1067–1072. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-Layer MoS2 Transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]

| Material | Sensitivity Range (kPa) | Pressure Sensitivity (kPa−1) | Reference |
|---|---|---|---|
| Ecoflex | 0–5 | 0.601 | [108] |
| PDMS square pyramid microstructure | 0–2 | 0.55 | [112] |
| PDMS (microstructure)/PiI2T-Si | 0–8 | 8.2 | [113] |
| PMMA/PDMS/PVP/Silver | 45–500 | 3.8 | [114] |
| Carbon/Silicon | 0–700 | 0.025 | [115] |
| PDMS porous structure | 0–0.33 | 0.26 | [116] |
| ACC/PAA/Alginate | 0–1 | 0.17 | [117] |
| MAA/DMAPS | 0–5 | 9 | [118] |
| PEDOT:PSS/PDMS/silica | 0–10 | 1 | [119] |
| AgNW-PMMA | 0–1 | 2.76 | [120] |
| Graphene Micropyramid | 0–4 | 7.68 | [121] |
| Au/PET/PDMS micropillar | 0–16 | 0.42 | [122] |
| Analyte | Ranges | Limit of Detection | Sensitivity | Literature |
|---|---|---|---|---|
| Glucose | 0–400 μM | 1.5–7 μM | 1.08–3.5 mA mM−1 cm−2 | [191,192,195,198,200] |
| Lactate | 0–100 mM | 0.2–2 mM | 36.2 μA μM−1 cm−2 | [191,192,194,195,199] |
| pH | 4–8.5 | – | 71.4 mV pH−1 | [191,194,196,201] |
| Chloride | 0–625 mM | 5–39 mM | – | [191,195,196] |
| Creatinine | 0–1000 μM | 15.6 μM | – | [195] |
| Tyrosine | 0–160 μM | 3.6 μM | 0.61 μA μM−1 cm−2 | [197] |
| Uric Acid | 0–140 μM | 0.74 μM | 3.50 μA μM−1 cm−2 | [196] |
| Potassium | 0.1–100 mM | – | – | [194,195] |
| Sodium | 0.2–200 mM | – | 56 mV dec−1 | [194,196,201] |
| Ascorbic Acid | 0.02–10 mM | 0.013–10 μM | 0.78 × 105 C mol−1 | [193,200] |
| Cortisol/Cortisol-BSA | 5–100 ng/mL | – | – | [200] |
| 1–8 mg/mL | – | – | ||
| Dopamine | 1–100 μM | 0.05–1 μM | 1.1 × 105 C mol−1 | [193] |
| Adrenaline | 10–500 μM | 2–10 μM | 0.8 × 105 C mol−1 | [193] |
| Microfluidic Drive | Reported Pressure Values | Literature | ||
| Pressure of Sweat Glands | 70–72 kPa | [191,192,193,194,195,196,197,198,199,200,201,202] | ||
| Capillary Force (pressure difference) | 100–400 Pa | [191,194,195,198] | ||
| Active Valves (thermo-responsive hydrogels) | 15–300 mmHg | [192] | ||
| Passive Valves (bursting valves) | Laplace-Young Equation | [200] | ||
| Energy Source | Literature |
|---|---|
| Electrochemical Conversion (Rechargeable Battery Pack) | [192,194,196,197] |
| Energy Scavenging (Radio Frequency) | [200] |
| Energy Scavenging (Mechanical Motion) | [201] |
| Natural Pressure Difference (~70 kPa) | [191,192,194,195,196,197,198,199,200,201,202] |
| Detection Mechanism Literature | |
| Colorimetric (Fluorescent and visible light) | [191,195,200] |
| Strain (Swelling) | [202] |
| Galvanic (Capacitance) | [196] |
| Electrochemical (Mediator molecules) | [192,193,196,197,199] |
| Materials | Literature |
| PDMS | [196,198,199,201] |
| Silicone Rubber | [194] |
| Medical Adhesive | [197] |
| Polyimide | [197,198] |
| Polyethylene Terephthalate | [197] |
| Hydrogel | [202] |
| Ecoflex | [198] |
| Paper | [191] |
| Cotton | [193] |
| Poly(N-isopropylacrylamide) | [192] |
| Fabrication Technique | Literature |
| Photolithography | [195,196,199,203] |
| Screen printing | [194] |
| Laser Engraving | [197,201] |
| Laying Cotton Fibers | [191] |
| Embossment | [195] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Bilget Güven, E.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. https://doi.org/10.3390/bios12060385
Polat EO, Cetin MM, Tabak AF, Bilget Güven E, Uysal BÖ, Arsan T, Kabbani A, Hamed H, Gül SB. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors. 2022; 12(6):385. https://doi.org/10.3390/bios12060385
Chicago/Turabian StylePolat, Emre Ozan, M. Mustafa Cetin, Ahmet Fatih Tabak, Ebru Bilget Güven, Bengü Özuğur Uysal, Taner Arsan, Anas Kabbani, Houmeme Hamed, and Sümeyye Berfin Gül. 2022. "Transducer Technologies for Biosensors and Their Wearable Applications" Biosensors 12, no. 6: 385. https://doi.org/10.3390/bios12060385
APA StylePolat, E. O., Cetin, M. M., Tabak, A. F., Bilget Güven, E., Uysal, B. Ö., Arsan, T., Kabbani, A., Hamed, H., & Gül, S. B. (2022). Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors, 12(6), 385. https://doi.org/10.3390/bios12060385








