Sensitive and Stable Electrochemical Sensor for Folic Acid Determination Using a ZIF-67/AgNWs Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
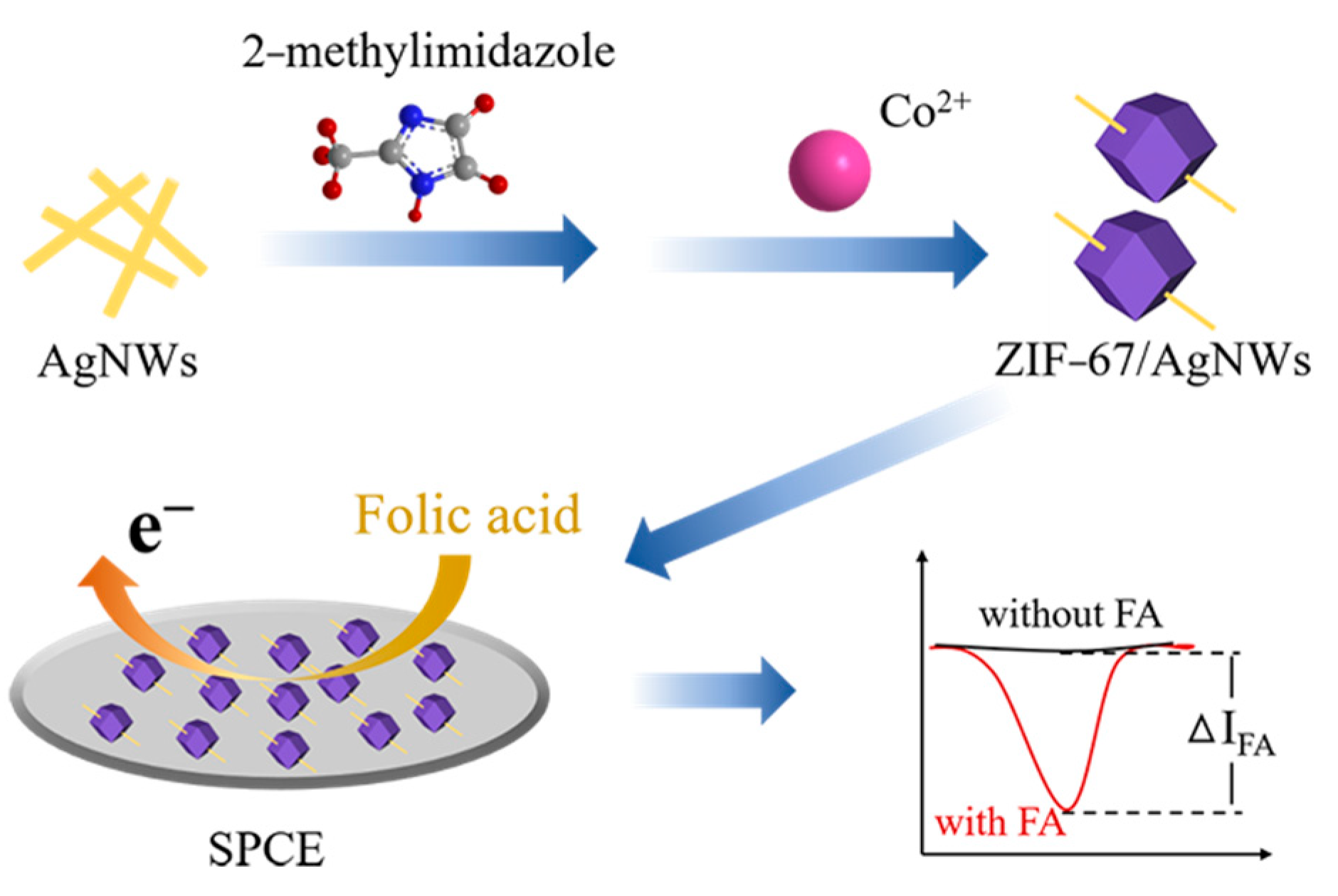
2.3. Preparation of the ZIF-67/AgNWs Nanocomposites
2.4. Preparation of ZIF-67/AgNWs@SPCE
2.5. Electrochemical Measurements and Electrochemical Sensor Fabrication
2.6. Selectivity, Stability, and Practicality of the Electrochemical Sensor
3. Results and Discussion
3.1. Microscopic Morphological Characterization
3.2. Spectral Characterizations
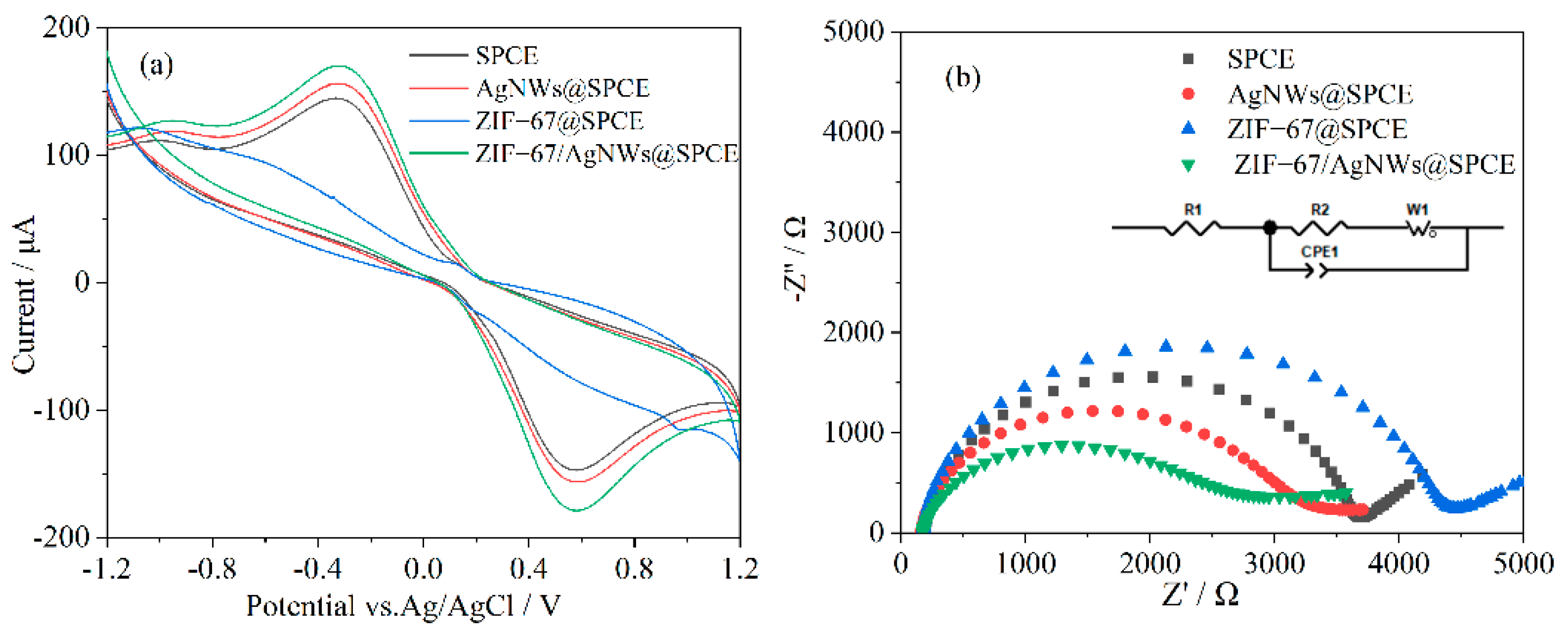
3.3. Electrochemical Performance of the Modified Electrode
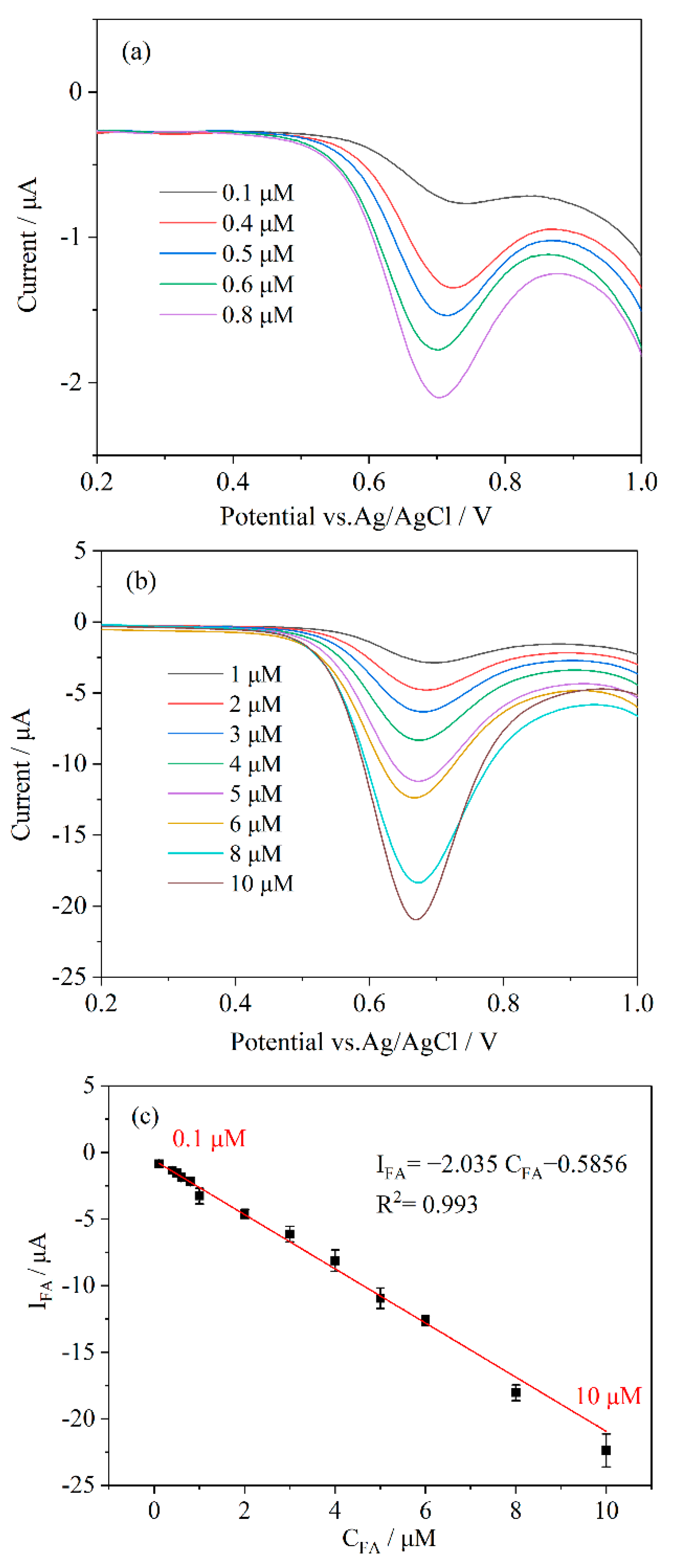
3.4. Sensitivity of the Electrochemical Sensor
3.5. Selectivity of the Electrochemical Sensor
3.6. Stability of the Electrochemical Sensor
3.7. Determination Performance of the Electrochemical Sensor in Real Samples
3.8. Comparison of the Electrochemical Sensor with Other Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Liu, T.; Liu, X.; Yan, Y.; Huang, Y. Metal-organic framework-based materials for flexible supercapacitor application. Co-ord. Chem. Rev. 2021, 452, 214300. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. The Application of ZIF-67 and Its Derivatives: Adsorption, Separation, Electrochemistry and Catalysts. J. Mater. Chem. A 2017, 6, 1887–1899. [Google Scholar] [CrossRef]
- Bennett, T.D.; Tan, J.-C.; Moggach, S.A.; Galvelis, R.; Mellot-Draznieks, C.; Reisner, B.A.; Thirumurugan, A.; Allan, D.R.; Cheetham, A.K. Mechanical properties of dense zeolitic imidazolate frameworks (zifs): A high-pressure x-ray diffraction, nanoindentation and computational study of the zinc framework zn(im)2, and its lithium-boron analogue, LiB(Im)4. Chem. A Eur. J. 2010, 16, 10684–10690. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, S.; Zhao, Q.; Lu, R.; Hang, C.; Chen, Z.; Zheng, H. Selective separation of methyl orange from water using magnetic ZIF-67 composites. Chem. Eng. J. 2018, 333, 49–57. [Google Scholar] [CrossRef]
- Tran, N.T.; Kwon, H.T.; Kim, W.-S.; Kim, J. Counter-diffusion-based in situ synthesis of ZIF-67 membranes for propylene/propane separation. Mater. Lett. 2020, 271, 127777. [Google Scholar] [CrossRef]
- Ping, E.; Ji, H.; Zhang, L.; Zhou, Y.; Ding, C.; Chen, X. Kinetic and Chemical Affinity Quantum Sieving Effects of ZIF-67 for H2 and D2 and Gas Chromatographic Separation of Hydrogen Isotopes on Columns Packed with ZIF-67@NH2-γ-Al2O3. ACS Appl. Energy Mater. 2021, 4, 10857–10866. [Google Scholar] [CrossRef]
- Sun, S.; Tang, Y.; Wu, C.; Wan, C. Phytic acid functionalized ZIF-67 decorated graphene nanosheets with remarkably boosted electrochemical sensing performance. Anal. Chim. Acta 2020, 1107, 55–62. [Google Scholar] [CrossRef]
- Bibi, S.; Pervaiz, E.; Ali, M. Synthesis and applications of metal oxide derivatives of ZIF-67: A mini-review. Chem. Pap. 2021, 75, 2253–2275. [Google Scholar] [CrossRef]
- Zhao, J.; Calin, B.; Han, J.; Lu, J.P. Quantum transport properties of ultrathin silver nanowires. Nanotechnology 2003, 14, 501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Engholm, M. Recent Progress on the Fabrication and Properties of Silver Nanowire-Based Transparent Electrodes. Nanomaterials 2018, 8, 628. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Shaikh, M.O.; Chuang, C.H. Silver Nanowire Synthesis and Strategies for Fabricating Transparent Conducting Electrodes. Nanomaterials 2021, 11, 693. [Google Scholar] [CrossRef]
- Abbasi, N.M.; Yu, H.; Wang, L.; Abdin, Z.U.; Amer, W.; Akram, M.; Khalid, H.; Chen, Y.; Saleem, M.; Sun, R.; et al. Preparation of silver nanowires and their application in conducting polymer nanocomposites. Mater. Chem. Phys. 2015, 166, 1–15. [Google Scholar] [CrossRef]
- Jones, R.S.; Draheim, R.R.; Roldo, M. Silver Nanowires: Synthesis, Antibacterial Activity and Biomedical Applications. Appl. Sci. 2018, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Amitai, Y.; Koren, G. The Folic Acid Rescue Strategy: High-Dose Folic Acid Supplementation in Early Pregnancy. JAMA Pediatr. 2015, 169, 1083–1084. [Google Scholar] [CrossRef]
- Pereira, D.F.; Santana, E.R.; Spinelli, A. Electrochemical paper-based analytical devices containing magnetite nanoparticles for the determination of vitamins B2 and B6. Microchem. J. 2022, 179, 107588. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Obeid, R.; Kirsch, S.H.; Kasoha, M.; Eckert, R.; Herrmann, W. Concentrations of unmetabolized folic acid and primary folate forms in plasma after folic acid treatment in older adults. Metabolism 2011, 60, 673–680. [Google Scholar] [CrossRef]
- Kumar, A.A.; Anusree, V.R.; Satheesh, G.; Vijayakumar, G.; Chandran, M.; Simon, L.; Lakshmi, S.; Pillai, M.R.; Jaleel, A. Hyperhomocysteinemia-related serum metabolome alterations not normalized by short-term folic acid treatment. Metabolomics 2021, 17, 47. [Google Scholar] [CrossRef]
- Van der Windt, M.; Schoenmakers, S.; van Rijn, B.; Galjaard, S.; Steegers-Theunissen, R.; van Rossem, L. Epidemiology and (Patho)Physiology of Folic Acid Supplement Use in Obese Women before and during Pregnancy. Nutrients 2021, 13, 331. [Google Scholar] [CrossRef]
- Sweeney, M.R.; McPartlin, J.; Weir, D.G.; Scott, J.M. Measurements of sub-nanomolar concentrations of unmetabolised folic acid in serum. J. Chromatogr. B 2003, 788, 187–191. [Google Scholar] [CrossRef]
- Modupe, O.; Maurras, J.B.; Diosady, L.L. A spectrophotometric method for determining the amount of folic acid in fortified salt. J. Agric. Food Res. 2020, 2, 100060. [Google Scholar] [CrossRef]
- Kanjilal, G.; Mahajan, S.N.; Rao, G.R. Colorimetric determination of folic acid in pharmaceutical preparations. Analyst 1975, 100, 19–24. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Miao, H.; Wang, L.; Wu, S.; Yang, X. Fluorescent carbon dots derived from lactose for assaying folic acid. Sci. China Ser. B Chem. 2015, 59, 487–492. [Google Scholar] [CrossRef]
- Batra, B.; Narwal, V.; Kalra, V.; Sharma, M.; Rana, J. Folic acid biosensors: A review. Process Biochem. 2020, 92, 343–354. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification*. Anal. Lett. 2001, 34, 635–659. [Google Scholar] [CrossRef] [Green Version]
- Pundir, C.S.; Narwal, V.; Batra, B. Determination of lactic acid with special emphasis on biosensing methods: A review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef]
- Venu, M. Simultaneous Determination of Dopamine, Uric Acid and Folic Acid with Electrochemical Techniques Based on Co3O4/rGO/CTAB Modified Carbon Paste Electrode. Int. J. Electrochem. Sci. 2018, 13, 11702–11719. [Google Scholar] [CrossRef]
- Abdelwahab, A.A.; Shim, Y.-B. Simultaneous determination of ascorbic acid, dopamine, uric acid and folic acid based on activated graphene/MWCNT nanocomposite loaded Au nanoclusters. Sens. Actuators B Chem. 2015, 221, 659–665. [Google Scholar] [CrossRef]
- Wang, Q.; Si, H.; Zhang, L.; Li, L.; Wang, X.; Wang, S. A fast and facile electrochemical method for the simultaneous detection of epinephrine, uric acid and folic acid based on ZrO2/ZnO nanocomposites as sensing material. Anal. Chim. Acta 2020, 1104, 69–77. [Google Scholar] [CrossRef]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H. Highly sensitive square wave voltammetric sensor employing CdO/SWCNTs and room temperature ionic liquid for analysis of vanillin and folic acid in food samples. J. Food Compos. Anal. 2017, 62, 254–259. [Google Scholar] [CrossRef]
- Raoof, J.B.; Teymoori, N.; Khalilzadeh, M.A.; Ojani, R. A high sensitive electrochemical nanosensor for simultaneous determination of glutathione, NADH and folic acid. Mater. Sci. Eng. C 2015, 47, 77–84. [Google Scholar] [CrossRef]
- Benvidi, A.; Dehghani-Firouzabadi, A.; Mazloum-Ardakani, M.; Mirjalili, B.-B.F.; Zare, R. Electrochemical deposition of gold nanoparticles on reduced graphene oxide modified glassy carbon electrode for simultaneous determination of levodopa, uric acid and folic acid. J. Electroanal. Chem. 2015, 736, 22–29. [Google Scholar] [CrossRef]
- Erçarıkcı, E.; Aksu, Z.; Kıranşan, K.D.; Topçu, E. Graphene paper with electrodeposited NiCo2S4 nanoparticles as a novel flexible sensor for simultaneous detection of folic acid and ascorbic acid. Diam. Relat. Mater. 2021, 121, 108713. [Google Scholar] [CrossRef]
- Winiarski, J.P.; Rampanelli, R.; Bassani, J.C.; Mezalira, D.Z.; Jost, C.L. Multi-walled carbon nanotubes/nickel hydroxide composite applied as electrochemical sensor for folic acid (vitamin B9) in food samples. J. Food Compos. Anal. 2020, 92, 103511. [Google Scholar] [CrossRef]
- Sun, D.; Yang, D.; Wei, P.; Liu, B.; Chen, Z.; Zhang, L.; Lu, J. One-Step Electrodeposition of Silver Nanostructures on 2D/3D Metal-Organic Framework ZIF-67: Comparison and Application in Electrochemical Detection of Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2020, 12, 41960–41968. [Google Scholar] [CrossRef]
- Li, Q.; Gong, S.; Zhang, H.; Huang, F.; Zhang, L.; Li, S. Tailored necklace-like Ag@ZIF-8 core/shell heterostructure nanowires for high-performance plasmonic SERS detection. Chem. Eng. J. 2019, 371, 26–33. [Google Scholar] [CrossRef]
- Wang, C.; Yang, F.; Sheng, L.; Yu, J.; Yao, K.; Zhang, L.; Pan, Y. Zinc-substituted ZIF-67 nanocrystals and polycrystalline membranes for propylene/propane separation. Chem. Commun. 2016, 52, 12578–12581. [Google Scholar] [CrossRef]
- Usov, P.M.; McDonnell-Worth, C.; Zhou, F.; MacFarlane, D.; D’Alessandro, D. The Electrochemical Transformation of the Zeolitic Imidazolate Framework ZIF-67 in Aqueous Electrolytes. Electrochim. Acta 2015, 153, 433–438. [Google Scholar] [CrossRef]
- Gu, L.; Li, S.; Peng, L.; Zhang, J. Anodic Voltammetric Determination of Folic Acid Using Carbon Paste Electrode Modified with Amino-silicon Oil. PTCA 2010, 46, 503–505, 508. [Google Scholar]




| a Sample | Spiked/μM | b Detected/μM | c RSD/% | Recovery/% |
|---|---|---|---|---|
| Serum 1 | 0 | Not Found | - | - |
| Serum 2 | 1 | 1.021 ± 0.02510 | 2.459 | 102.1 |
| Serum 3 | 10 | 10.09 ± 0.3092 | 3.065 | 100.9 |
| Electrode | Modifier | Linear Range/μM | LOD/μM | Ref. |
|---|---|---|---|---|
| CPE | Co3O4/RGO/PEI/CD | 19–94 | 19 | [27] |
| GCE | AuNCs/AGR/MWCNT | 10–170 | 0.09 | [28] |
| GCE | ZrO2/ZnO | 2–480 | 0.037 | [29] |
| CPE | CdO/SWCNTs/DPIB | 0.1–1200 | 0.06 | [30] |
| CPE | ZnO/CNTs/BCB | 3–700 | 1 | [31] |
| GCE | DHB/AuNPs/RGO | 1–500 | 0.6 | [32] |
| PE | NiCo2S4/rGO | 0.1–3600 | 0.0016 | [33] |
| GCE | f-MWCNT-Ni (OH)2-Si4Pic+Cl− | 0.5–26 | 0.095 | [34] |
| SPCE | ZIF-67/AgNWs | 0.1–10 | 0.03 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, X.; Zhang, H. Sensitive and Stable Electrochemical Sensor for Folic Acid Determination Using a ZIF-67/AgNWs Nanocomposite. Biosensors 2022, 12, 382. https://doi.org/10.3390/bios12060382
Sun Y, Wang X, Zhang H. Sensitive and Stable Electrochemical Sensor for Folic Acid Determination Using a ZIF-67/AgNWs Nanocomposite. Biosensors. 2022; 12(6):382. https://doi.org/10.3390/bios12060382
Chicago/Turabian StyleSun, Yujiao, Xue Wang, and Hao Zhang. 2022. "Sensitive and Stable Electrochemical Sensor for Folic Acid Determination Using a ZIF-67/AgNWs Nanocomposite" Biosensors 12, no. 6: 382. https://doi.org/10.3390/bios12060382
APA StyleSun, Y., Wang, X., & Zhang, H. (2022). Sensitive and Stable Electrochemical Sensor for Folic Acid Determination Using a ZIF-67/AgNWs Nanocomposite. Biosensors, 12(6), 382. https://doi.org/10.3390/bios12060382





