Towards Development of Molecularly Imprinted Electrochemical Sensors for Food and Drug Safety: Progress and Trends
Abstract
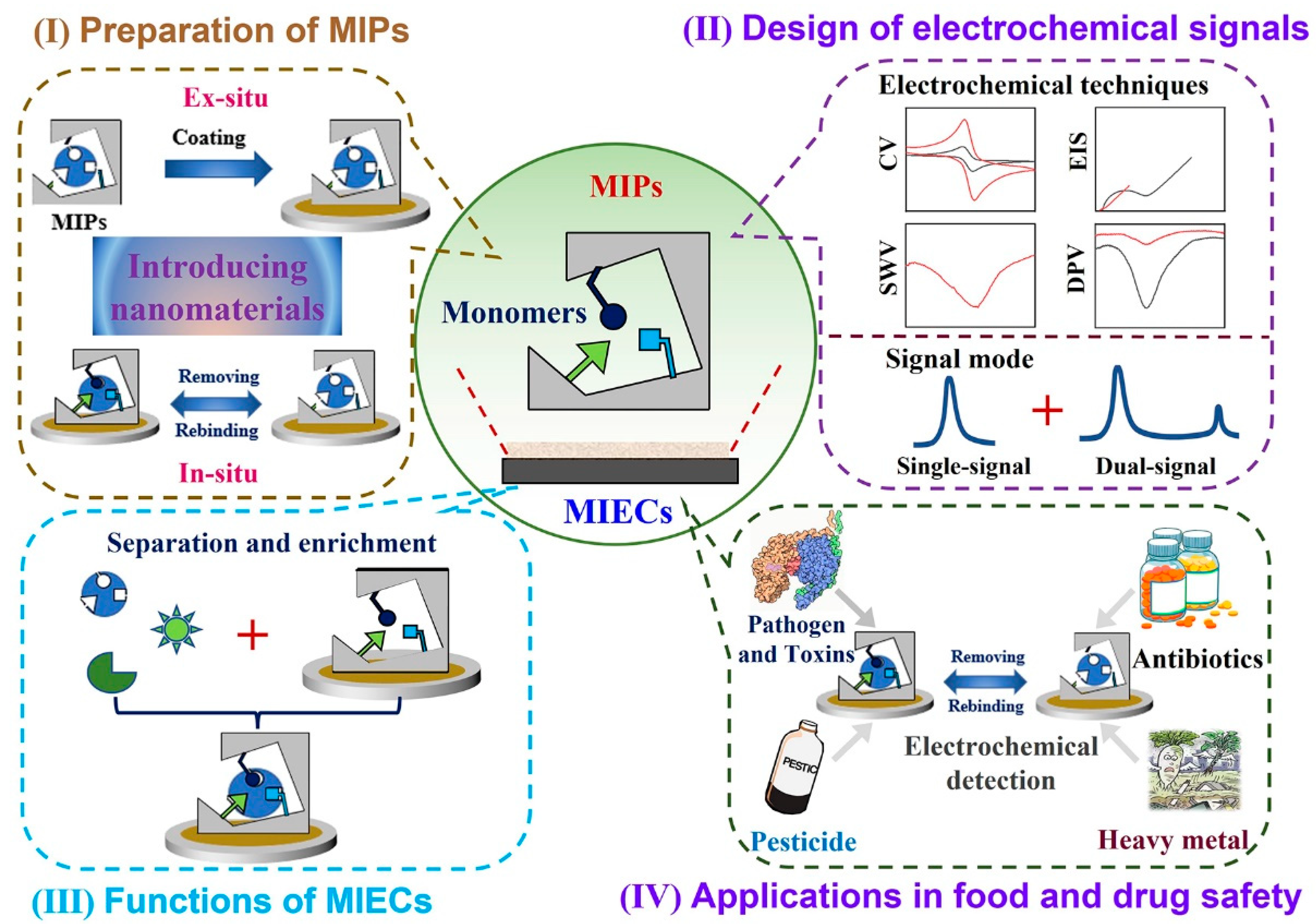
:1. Introduction
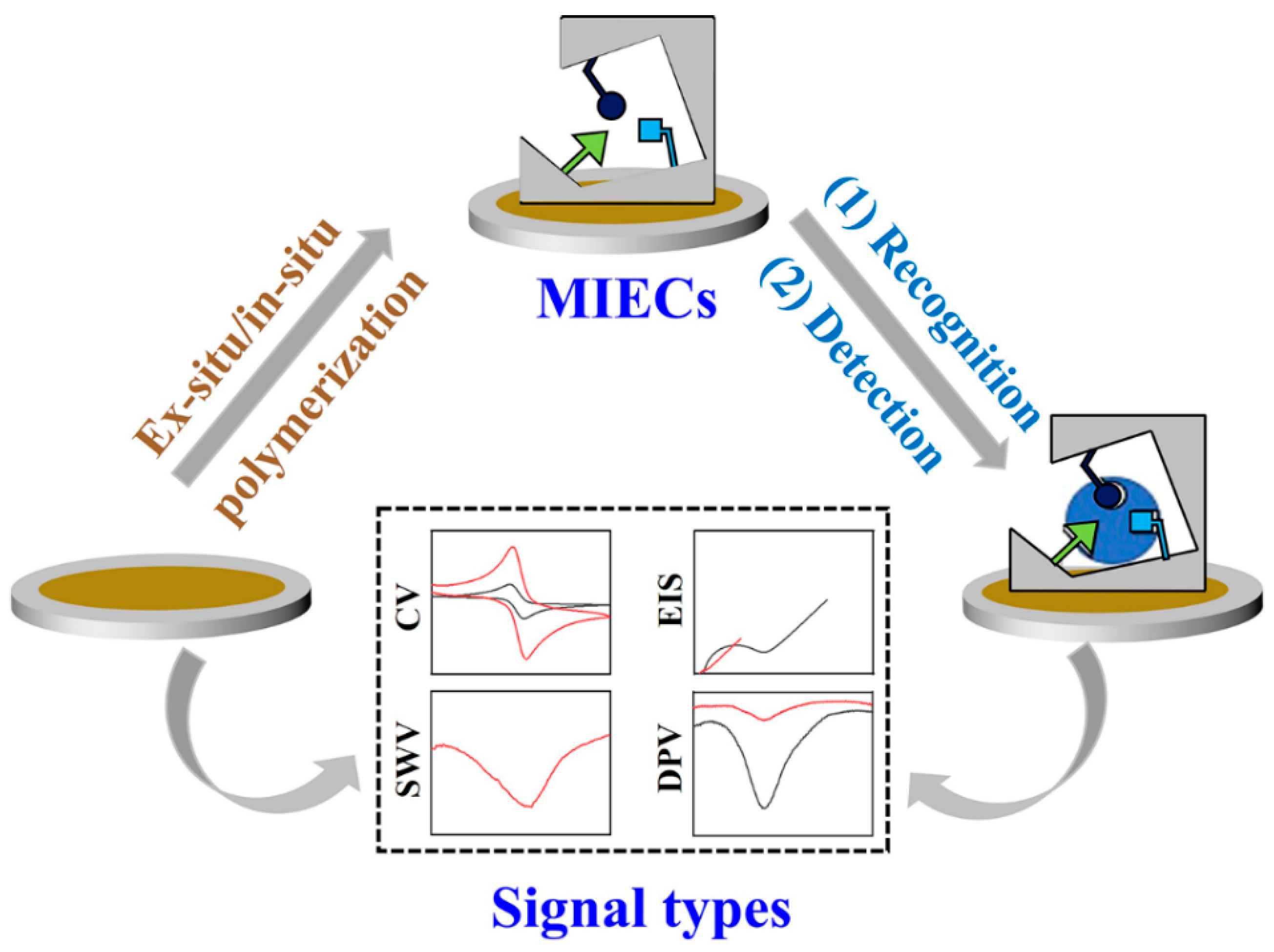
2. Brief Introduction of MIECs
2.1. Principle of MIECs
2.2. The Classification of MIECs
2.3. The Construction of MIECs
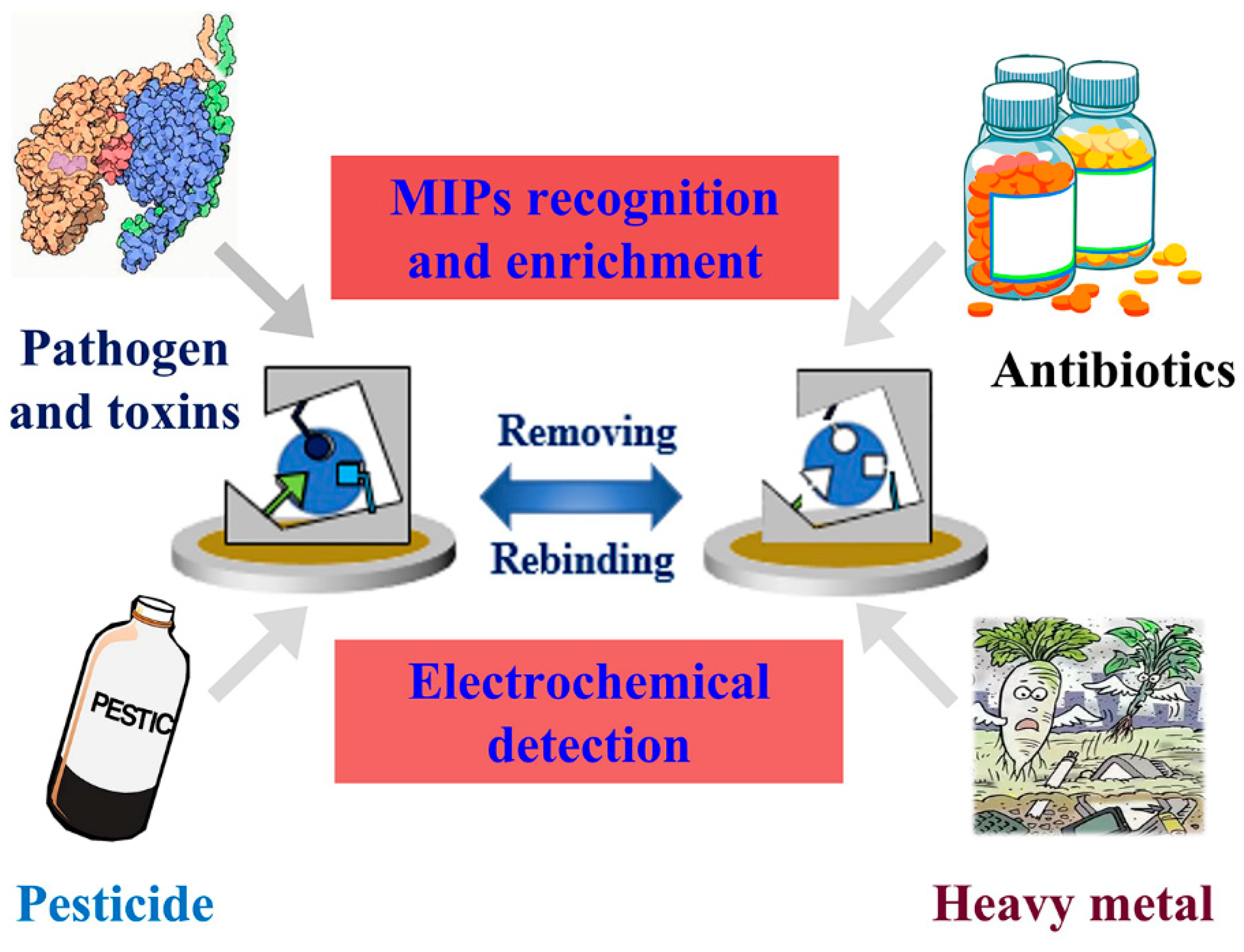
3. Applications of MIECs in Food Safety and Drug Detection
3.1. Pathogen and Toxins
3.2. Pesticide Residue
3.3. Heavy Metal Ions
3.4. Antibiotics Monitoring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mahato, K.; Wang, J. Electrochemical sensors: From the bench to the skin. Sens. Actuators B Chem. 2021, 344, 130178. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, C.; Long, Y.; Chen, Q.; Zhang, W.; Liu, G. Food additives: From functions to analytical methods. Crit. Rev. Food Sci. Nutr. 2021, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Xie, S.; Zhu, J.; Liu, H.; Zhao, Y.; Ni, T.; Wu, L.; Zhu, Y. Mesoporous CoOx/C Nanocomposites Functionalized Electrochemical Sensor for Rapid and Continuous Detection of Nitrite. Coatings 2021, 11, 596. [Google Scholar] [CrossRef]
- Ding, R.; Cheong, Y.H.; Ahamed, A.; Lisak, G. Heavy Metals Detection with Paper-Based Electrochemical Sensors. Anal. Chem. 2021, 93, 1880–1888. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Ahmadi, S.A.; Askari, M.B.; Di Bartolomeo, A. Screen-printed electrode surface modification with NiCo2O4/RGO nanocomposite for hydroxylamine detection. Nanomaterials 2021, 11, 3208. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Zhou, S.; Zhu, Y.; Chen, X. Enzyme-induced Cu2+/Cu+ conversion as the electrochemical signal for sensitive detection of ethyl carbamate. Anal. Chim. Acta 2021, 1151, 338256. [Google Scholar] [CrossRef]
- Wu, L.; Ding, F.; Yin, W.; Ma, J.; Wang, B.; Nie, A.; Han, H. From Electrochemistry to Electroluminescence: Development and Application in a Ratiometric Aptasensor for Aflatoxin B1. Anal. Chem. 2017, 89, 7578–7585. [Google Scholar] [CrossRef]
- Costa-Rama, E.; Fernández-Abedul, M.T. Paper-Based screen-printed electrodes: A new generation of low-cost electroanalytical platforms. Biosensors 2021, 11, 51. [Google Scholar] [CrossRef]
- El-Said, W.A.; Al-Bogami, A.S.; Alshitari, W. Synthesis of gold nanoparticles@ reduced porous graphene-modified ITO electrode for spectroelectrochemical detection of SARS-CoV-2 spike protein. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120237. [Google Scholar] [CrossRef]
- Lu, Q.; Su, T.; Shang, Z.; Jin, D.; Shu, Y.; Xu, Q.; Hu, X. Flexible paper-based Ni-MOF composite/AuNPs/CNTs film electrode for HIV DNA detection. Biosens. Bioelectron. 2021, 184, 113229. [Google Scholar] [CrossRef]
- Daly, R.; Narayan, T.; Shao, H.; O’Riordan, A.; Lovera, P. Platinum-Based Interdigitated Micro-Electrode Arrays for Reagent-Free Detection of Copper. Sensors 2021, 21, 3544. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Orooji, Y.; Ghazanfari, Z.; Karimi, F.; Beitollahi, H.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Nanomaterials modified electrodes for electrochemical detection of Sudan I in food. J. Food Meas. Charact. 2021, 15, 3837–3852. [Google Scholar] [CrossRef]
- Wu, L.; Luo, Y.; Liu, C.; Li, J.; Li, D. Highly Adjustable Three-Dimensional Hollow Pt(Au)Cu Nanonetwork Structures as Enhancing Electrocatalysts for Alcohol Oxidation Reaction. J. Electrochem. Soc. 2020, 167, 066518. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Chen, Z.; Zuo, X. Innovative Electrochemical Sensor Using TiO2 Nanomaterials to Detect Phospho-peptides. Anal. Chem. 2021, 93, 10635–10643. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yan, H.; Wang, J.; Liu, G.; Xie, W. Tyrosinase incorporated with Au-Pt@ SiO2 nanospheres for electrochemical detection of bisphenol A. J. Electrochem. Soc. 2019, 166, B562. [Google Scholar] [CrossRef]
- Ranjan, P.; Singhal, A.; Yadav, S.; Kumar, N.; Murali, S.; Sanghi, S.K.; Khan, R. Rapid diagnosis of SARS-CoV-2 using potential point-of-care electrochemical immunosensor: Toward the future prospects. Int. Rev. Immunol. 2021, 40, 126–142. [Google Scholar] [CrossRef]
- Wu, L.; Yin, W.; Tang, K.; Li, D.; Shao, K.; Zuo, Y.; Han, H. Enzymatic biosensor of horseradish peroxidase immobi-lized on Au-Pt nanotube/Au-graphene for the simultaneous determination of antioxidants. Anal. Chim. Acta 2016, 933, 89–96. [Google Scholar] [CrossRef]
- Elugoke, S.E.; Adekunle, A.S.; Fayemi, O.E.; Akpan, E.D.; Mamba, B.B.; Sherif, E.M.; Ebenso, E.E. Molecularly imprinted polymers (MIPs) based electrochemical sensors for the determination of catecholamine neurotransmitters—Review. Electrochem. Sci. Adv. 2021, 1, e2000026. [Google Scholar] [CrossRef]
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of nano-ELISA in food analysis: Recent advances and challenges. TrAC Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Qureshi, S.; Asif, M.; Sajid, H.; Gilani, M.A.; Ayub, K.; Mahmood, T. First-principles study for electrochemical sensing of neurotoxin hydrazine derivatives via hg-C3N4 quantum dot. Surf. Interfaces 2022, 30101913. [Google Scholar]
- Chen, Q.; Yao, C.; Yang, C.; Liu, Z.; Wan, S. Development of an in-situ signal amplified electrochemical assay for de-tection of Listeria monocytogenes with label-free strategy. Food Chem. 2021, 358, 129894. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Cheng, D.; Li, P.; Xu, Z.; Zhu, X.; Zhang, Y.; Yao, S. Au/Metal–Organic Framework Nanocapsules for Elec-trochemical Determination of Glutathione. ACS Appl. Nano Mater. 2021, 4, 4853–4862. [Google Scholar] [CrossRef]
- Isailović, J.; Vidović, K.; Hočevar, S.B. Simple electrochemical sensors for highly sensitive detection of gaseous hydrogen peroxide using polyacrylic-acid-based sensing membrane. Sens. Actuators B Chem. 2022, 352, 131053. [Google Scholar] [CrossRef]
- Oberhaus, F.V.; Frense, D. Catalysing electropolymerization: High-quality polythiophene films for electrochemical sensors by the utilization of fluorine based Lewis acid catalysts. Electrochim. Acta 2022, 402, 139536. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, S.; Wang, G.; Yun, Y.; Liu, G.; Zhang, W. Nanozyme Applications: A Glimpse of Insight in Food Safety. Front. Bioeng. Biotechnol. 2021, 9, 727886. [Google Scholar] [CrossRef]
- Meng, T.; Zhao, D.; Ye, H.; Feng, Y.; Wang, H.; Zhang, Y. Construction of an ultrasensitive electrochemical sensing platform for microRNA-21 based on interface impedance spectroscopy. J. Colloid Interface Sci. 2020, 578, 164–170. [Google Scholar] [CrossRef]
- Castle, L.M.; Schuh, D.A.; Reynolds, E.E.; Furst, A.L. Electrochemical Sensors to Detect Bacterial Foodborne Pathogens. ACS Sens. 2021, 6, 1717–1730. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, L.; Dai, L.; Wang, Y.; Tian, Y. Nonenzymatic Electrochemical Sensor with Ratiometric Signal Output for Selective Determination of Superoxide Anion in Rat Brain. Anal. Chem. 2021, 93, 5570–5576. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, L.; Yan, H.; Lu, Z.; Yin, W.; Han, H. Enzyme induced molecularly imprinted polymer on SERS substrate for ultrasensitive detection of patulin. Anal. Chim. Acta 2020, 1101, 111–119. [Google Scholar] [CrossRef]
- Wu, L.; Yan, H.; Li, G.; Xu, X.; Zhu, L.; Chen, X.; Wang, J. Surface-Imprinted Gold Nanoparticle-Based Surface-Enhanced Raman Scattering for Sensitive and Specific Detection of Patulin in Food Samples. Food Anal. Methods 2019, 12, 1648–1657. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Amine, A. Recent Advances in Electrochemical Sensors Based on Molecularly Imprinted Polymers and Nanomaterials. Electroanalysis 2019, 31, 188–201. [Google Scholar] [CrossRef]
- Ann Maria, C.G.; Anitha, V.; Nidhin, M. Recent Advances in Nanomaterials Based Molecularly Imprinted Electrochemical Sensors. Crit. Rev. Anal. Chem. 2021, 51, 1–10. [Google Scholar]
- Motia, S.; Bouchikhi, B.; Llobet, E.; El Bari, N. Synthesis and characterization of a highly sensitive and selective elec-trochemical sensor based on molecularly imprinted polymer with gold nanoparticles modified screen-printed electrode for glycerol determination in wastewater. Talanta 2020, 216, 120953. [Google Scholar] [CrossRef] [PubMed]
- Sarpong, K.A.; Zhang, K.; Luan, Y.; Cao, Y.; Xu, W. Development and application of a novel electrochemical sensor based on AuNPS and difunctional monomer-MIPs for the selective determination of Tetrabromobisphenol-S in water samples. Microchem. J. 2020, 154, 104526. [Google Scholar] [CrossRef]
- Hedborg, E.; Winquist, F.; Lundström, I.; Andersson, L.I.; Mosbach, K. Some studies of molecularly-imprinted polymer membranes in combination with field-effect devices. Sens. Actuators A Phys. 1993, 37, 796–799. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Fabrication of electrochemical immunosensor based on acid-substituted poly(pyrrole) polymer modified disposable ITO electrode for sensitive detection of CCR4 cancer biomarker in human serum. Talanta 2021, 222, 121487. [Google Scholar] [CrossRef]
- Kaya, H.K.; Cinar, S.; Altundal, G.; Bayramlı, Y.; Unaleroglu, C.; Kuralay, F. A novel design thia-bilane structure-based molecular imprinted electrochemical sensor for sensitive and selective dopamine determination. Sens. Actuators B Chem. 2021, 346, 130425. [Google Scholar] [CrossRef]
- Dechtrirat, D.; Sookcharoenpinyo, B.; Prajongtat, P.; Sriprachuabwong, C.; Sanguankiat, A.; Tuantranont, A.; Han-nongbua, S. An electrochemical MIP sensor for selective detection of salbutamol based on a graphene/PEDOT: PSS modi-fied screen printed carbon electrode. RSC Adv. 2018, 8, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Petrucci, E.; Orsini, M.; Porcelli, F.; De Santis, S.; Sotgiu, G. Effect of Spin Coating Parameters on the Electrochemical Properties of Ruthenium Oxide Thin Films. Electrochem 2021, 2, 83–94. [Google Scholar] [CrossRef]
- He, S.; Zhang, L.; Bai, S.; Yang, H.; Cui, Z.; Zhang, X.; Li, Y. Advances of molecularly imprinted polymers (MIP) and the application in drug delivery. Eur. Polym. J. 2020, 143, 110179. [Google Scholar] [CrossRef]
- Kadhem, A.J.; Gentile, G.J.; Fidalgo de Cortalezzi, M.M. Molecularly Imprinted Polymers (MIPs) in Sensors for Envi-ronmental and Biomedical Applications: A Review. Molecules 2021, 26, 6233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Zhang, L.; Song, G.; Wang, N.; Xu, W.; Huang, W. A molecularly imprinted electrochemical BPA sensor based on multi-walled carbon nanotubes modified by CdTe quantum dots for the detection of bisphenol A. Microchem. J. 2021, 170, 106737. [Google Scholar] [CrossRef]
- Rezaei, F.; Ashraf, N.; Zohuri, G.H.; Arbab-Zavar, M.H. Water-compatible synthesis of core-shell polysilicate molecularly imprinted polymer on polyvinylpyrrolidone capped gold nanoparticles for electrochemical sensing of uric acid. Microchem. J. 2022, 177, 107312. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection methodologies for pathogen and toxins: A review. Sensors 2017, 17, 1885. [Google Scholar] [CrossRef] [Green Version]
- Anal, A.K.; Koirala, S.; Shrestha, S. Gut Microbiome and Their Possible Roles in Combating Mycotoxins. In Mycotoxins Food Beverages; CRC Press: Boca Raton, FL, USA, 2021; pp. 213–235. [Google Scholar] [CrossRef]
- Banerji, R.; Karkee, A.; Kanojiya, P.; Saroj, S.D. Pore-forming toxins of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2265–2285. [Google Scholar] [CrossRef]
- Hatta, M.; Hanif, E.M.; Chin, S.-F.; Neoh, H.-M. Pathogens and Carcinogenesis: A Review. Biology 2021, 10, 533. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, M.; Wang, Y.; Liu, J. Nanozyme and aptamer- based immunosorbent assay for aflatoxin BJ. Hazard. Mater. 2020, 399, 123154. [Google Scholar] [CrossRef]
- Golabi, M.; Kuralay, F.; Jager, E.W.; Beni, V.; Turner, A.P. Electrochemical bacterial detection using poly(3-aminophenylboronic acid)-based imprinted polymer. Biosens. Bioelectron. 2017, 93, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Guo, Z.; Qiu, X.; Lu, W.; Yang, W.; Wang, Q.; Wu, Q. Simple electrochemical detection of Listeria monocytogenes based on a surface-imprinted polymer-modified electrode. Anal. Methods 2021, 13, 4864–4870. [Google Scholar] [CrossRef]
- Guo, W.; Pi, F.; Zhang, H.; Sun, J.; Zhang, Y.; Sun, X. A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosens. Bioelectron. 2017, 98, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Munawar, H.; Garcia-Cruz, A.; Majewska, M.; Karim, K.; Kutner, W.; Piletsky, S.A. Electrochemical determination of fumonisin B1 using a chemosensor with a recognition unit comprising molecularly imprinted polymer nanoparticles. Sens. Actuators B Chem. 2020, 321, 128552. [Google Scholar] [CrossRef]
- Ferdous, Z.; Zulfiqar, F.; Datta, A.; Hasan, A.K.; Sarker, A. Potential and challenges of organic agriculture in Bangla-desh: A review. J. Crop Improv. 2021, 35, 403–426. [Google Scholar] [CrossRef]
- Cioffi, A.; Mancini, M.; Gioia, V.; Cinti, S. Office Paper-Based Electrochemical Strips for Organophosphorus Pesticide Monitoring in Agricultural Soil. Environ. Sci. Technol. 2021, 55, 8859–8865. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, B.; Mercader, J.V.; Checa-Orrego, B.I.; De La Escosura-Muñiz, A.; Costa-García, A. A monoclonal antibody-based immunosensor for the electrochemical detection of imidacloprid pesticide. Analyst 2019, 144, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Gao, F.; Ma, X.; Zou, J.; Yu, Y.; Li, M.; Qu, F.; Huang, X.; Lu, L. Mxene/carbon nanohorn/β-cyclodextrin-Metal-organic frame-works as high-performance electrochemical sensing platform for sensitive detection of carbendazim pesticide. J. Hazard. Mater. 2020, 396, 122776. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, X.; Wang, Q.; Zhe, T.; Bai, Y.; Bu, T.; Zhang, M.; Wang, L. Electrochemical behavior of reduced graphene ox-ide/cyclodextrins sensors for ultrasensitive detection of imidacloprid in brown rice. Food Chem. 2020, 333, 127495. [Google Scholar] [CrossRef]
- Dai, Y.; Kan, X. From non-electroactive to electroactive species: Highly selective and sensitive detection based on a du-al-template molecularly imprinted polymer electrochemical sensor. Chem. Commun. 2017, 53, 11755–11758. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Yola, M.L.; Atar, N.; Orooji, Y.; Karimi, F.; Kumar, P.S.; Rouhi, J.; Baghayeri, M. A novel detection method for organophosphorus insecticide fenamiphos: Molecularly imprinted electrochemical sensor based on core-shell Co3O4@MOF-74 nanocomposite. J. Colloid Interface Sci. 2021, 592, 174–185. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Vahidinia, A.; Samiee, F.; Faradmal, J.; Rahmani, A.; Javad, M.T.; Leili, M. Mercury, Lead, Cadmium, and Barium Levels in Human Breast Milk and Factors Affecting Their Concentrations in Hamadan, Iran. Biol. Trace Element Res. 2019, 187, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A. Novel imprinted polymeric nanoparticles prepared by sol–gel technique for elec-trochemical detection of toxic cadmium (II) ions. Chem. Eng. J. 2017, 327, 135–141. [Google Scholar] [CrossRef]
- Sebastian, M.; Mathew, B. Ion imprinting approach for the fabrication of an electrochemical sensor and sorbent for lead ions in real samples using modified multiwalled carbon nanotubes. J. Mater. Sci. 2018, 53, 3557–3572. [Google Scholar] [CrossRef]
- Zhang, Z.; Lou, Y.; Guo, C.; Jia, Q.; Song, Y.; Tian, J.-Y.; Zhang, S.; Wang, M.; He, L.; Du, M. Metal–organic frameworks (MOFs) based chemosensors/biosensors for analysis of food contaminants. Trends Food Sci. Technol. 2021, 118, 569–588. [Google Scholar] [CrossRef]
- Majdinasab, M.; Mishra, R.K.; Tang, X.; Marty, J.L. Detection of antibiotics in food: New achievements in the development of biosensors. TrAC Trends Anal. Chem. 2020, 127, 115883. [Google Scholar] [CrossRef]
- Dutta, T.; Yadav, S.; Chatterjee, A. Antibiotics as feed additives for livestock: Human health concerns. Indian J. Anim. Health 2019, 58, 121–136. [Google Scholar] [CrossRef]
- Treiber, F.; Beranek-Knauer, H. Antimicrobial Residues in Food from Animal Origin—A Review of the Literature Focusing on Products Collected in Stores and Markets Worldwide. Antibiotics 2021, 10, 534. [Google Scholar] [CrossRef]
- Henrique, J.M.; Monteiro, M.K.; Cardozo, J.C.; Martinez-Huitle, C.A.; da Silva, D.R.; dos Santos, E.V. Integrated-electrochemical approaches powered by photovoltaic energy for detecting and treating paracetamol in water. J. Electroanal. Chem. 2020, 876, 114734. [Google Scholar] [CrossRef]
- Dai, Y.; Li, X.; Lu, X.; Kan, X. Voltammetric determination of paracetamol using a glassy carbon electrode modified with Prussian Blue and a molecularly imprinted polymer, and ratiometric read-out of two signals. Mikrochim. Acta 2016, 183, 2771–2778. [Google Scholar] [CrossRef]
- Dos Santos, A.M.; Wong, A.; Fatibello-Filho, O. Simultaneous determination of salbutamol and propranolol in biological fluid samples using an electrochemical sensor based on functionalized-graphene, ionic liquid and silver nanoparticles. J. Electroanal. Chem. 2018, 824, 1–8. [Google Scholar] [CrossRef]
- Liu, B.; Lian, H.; Chen, L.; Wei, X.; Sun, X. Differential potential ratiometric sensing platform for enantiorecognition of chiral drugs. Anal. Biochem. 2019, 574, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wei, X.; Wang, X.; Shi, J.; Chen, Z.; Zhang, H.; Zhang, W.; Zou, X. Ratiometric electrochemical analysis on a flexi-bly-fabricated vibratory electrode module for reliable and selective determination of imidacloprid. Sens. Actuators B Chem. 2021, 329, 129228. [Google Scholar] [CrossRef]
- Anirudhan, T.; Mani, A.; Athira, V. Molecularly imprinted electrochemical sensing platform for 2-Aminoadipic acid, a diabetes biomarker. React. Funct. Polym. 2021, 168, 105056. [Google Scholar] [CrossRef]
- Sargazi, S.; Mukhtar, M.; Rahdar, A.; Bilal, M.; Barani, M.; Díez-Pascual, A.M.; Behzadmehr, R.; Pandey, S. Opportunities and challenges of using high-sensitivity nanobiosensors to detect long noncoding RNAs: A preliminary review. Int. J. Biol. Macromol. 2022, 205, 304–315. [Google Scholar] [CrossRef]

| Type of Application | Test Target | R Value | RSD | Linear Range | LOD | References |
|---|---|---|---|---|---|---|
| Pathogen and toxins | S. epidermidis | 0.9730 | N | 103–107 CFU/mL | 7.5 × 10−8 M | [50] |
| LM | N | N | 10–106 CFU/mL | 6 CFU/mL | [51] | |
| Patulin | 0.9953 | 7.3% | 1 × 10-12–1 × 10−9 M | 7.57 × 10−13 M | [52] | |
| FB1 | 0.9899 | N | 1 fM–10 pM | 0.03 fM | [53] | |
| 0.9798 | N | 0.7 fM | ||||
| Pesticide residue | IMI | 0.9987 | 4.5% | 1.0 × 10−7–1.0 × 10−4 M | 6.5 × 10−8 M | [59] |
| FEN | 0.9995 | N | 1.0 × 10−11–1.0 × 10−9 M | 3.0 × 10−12 M | [60] | |
| Heavy metal ions | Cd(II) | 0.9989 | 2.7% | 0.5–40 µg L−1 | 0.15 μg L−1 | [64] |
| Pb(II) | 0.9993 | N | 1–5 ppm | 2 × 10−2 μM | [65] | |
| Antibiotics monitoring | PR | N | 1.2% | 1.0 nM–0.1 mM | 0.53 nM | [71] |
| s/r-Prop | N | N | 50 μM–1000 μM | N | [73] | |
| CPZ | 0.9981 | 0.94% | 0.005–9 μM | 2.6 nM | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Liu, C.; Lin, J.; Zhu, Z.; Hu, B.; Wu, L. Towards Development of Molecularly Imprinted Electrochemical Sensors for Food and Drug Safety: Progress and Trends. Biosensors 2022, 12, 369. https://doi.org/10.3390/bios12060369
Zhou S, Liu C, Lin J, Zhu Z, Hu B, Wu L. Towards Development of Molecularly Imprinted Electrochemical Sensors for Food and Drug Safety: Progress and Trends. Biosensors. 2022; 12(6):369. https://doi.org/10.3390/bios12060369
Chicago/Turabian StyleZhou, Shuhong, Chen Liu, Jianguo Lin, Zhi Zhu, Bing Hu, and Long Wu. 2022. "Towards Development of Molecularly Imprinted Electrochemical Sensors for Food and Drug Safety: Progress and Trends" Biosensors 12, no. 6: 369. https://doi.org/10.3390/bios12060369
APA StyleZhou, S., Liu, C., Lin, J., Zhu, Z., Hu, B., & Wu, L. (2022). Towards Development of Molecularly Imprinted Electrochemical Sensors for Food and Drug Safety: Progress and Trends. Biosensors, 12(6), 369. https://doi.org/10.3390/bios12060369





